Synthesis, Antimicrobial Activities, and Model of Action of Indolyl Derivatives Containing Amino-Guanidinium Moieties
Abstract
1. Introduction
2. Result and Discussion
2.1. Chemistry
2.2. Evaluation of In Vitro Antimicrobial Activity
2.3. 4P Disrupted Bacteria Viability and Morphology
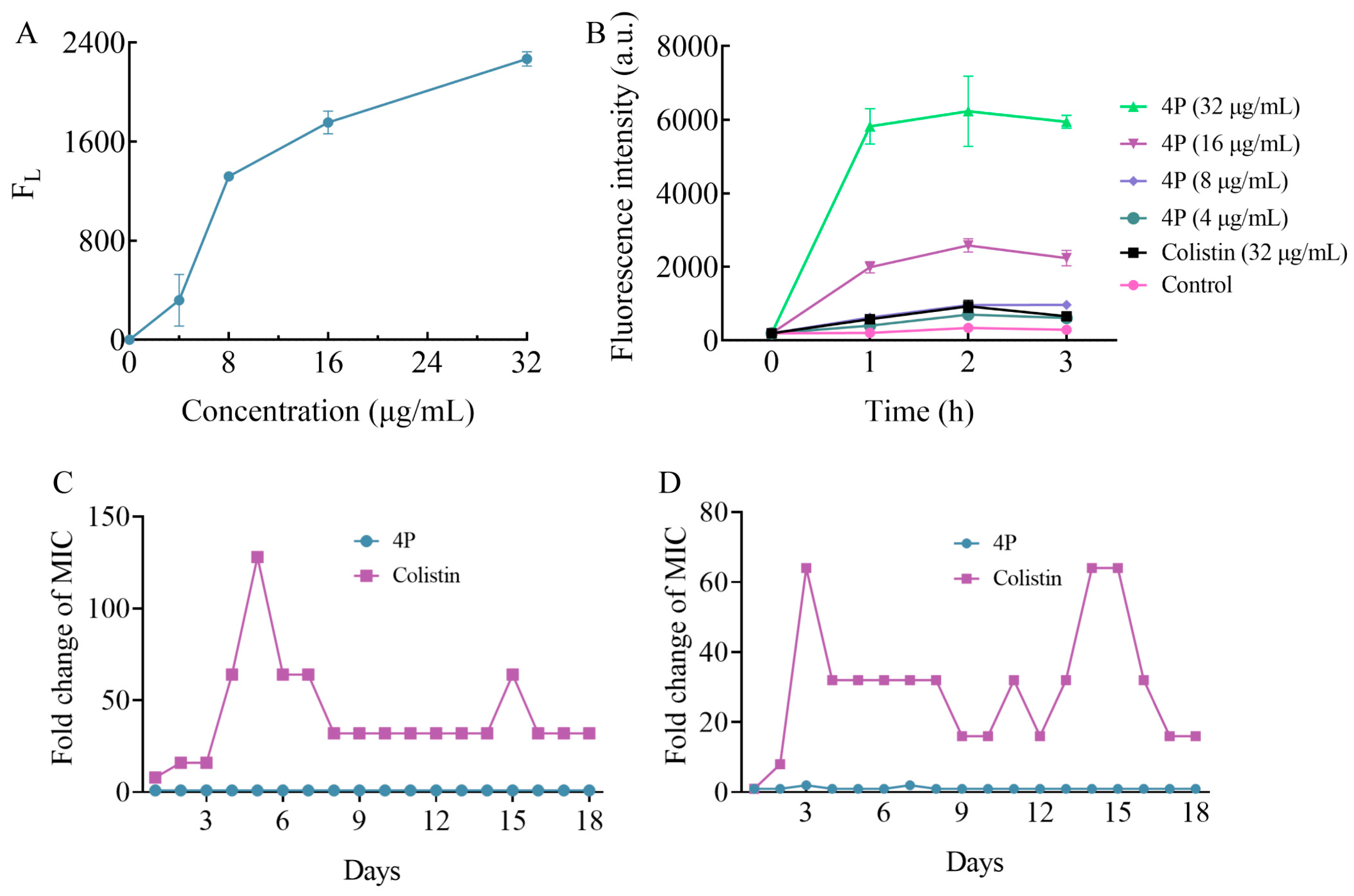
2.4. 4P Exerts Its Antibacterial Action on the Bacterial Membrane
2.5. 4P Was Not Prone to Antibiotic Resistance
2.6. Docking Analysis
2.7. 4P Inhibited DHFR Activities In Vitro
2.8. Hemolytic Activity
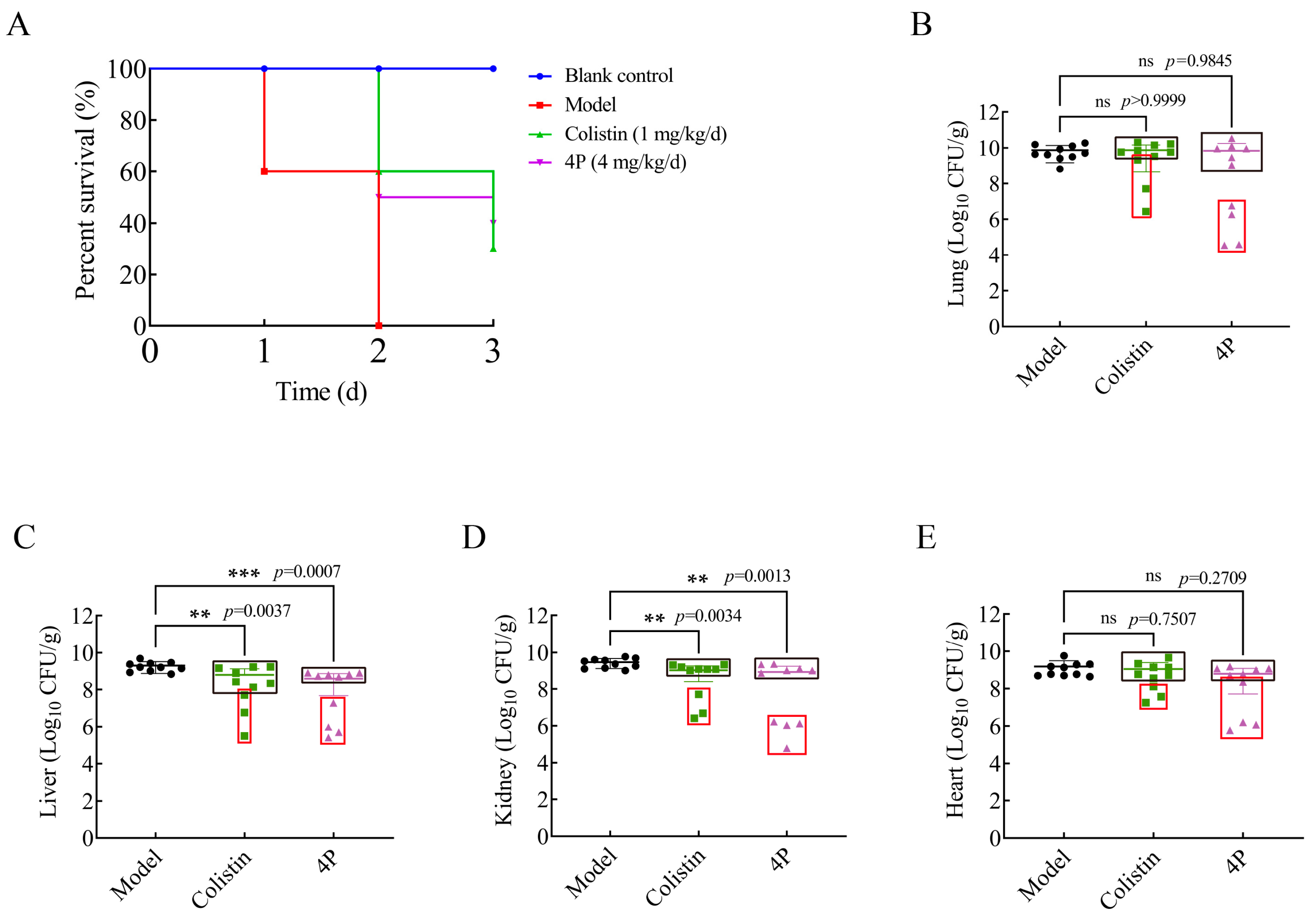
2.9. In Vivo Efficacy
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of Intermediates (2)
3.1.3. General Procedure for the Synthesis of Compounds 3A-3V, 4L-4P, 5L-5P and 6L-6P
3.2. Antibacterial Evaluation
3.2.1. MIC and MBC Testing
3.2.2. Growth Curve and Bactericidal Time-Kill Kinetics Assay
3.3. Mode of Action Study
3.3.1. FC Analysis
3.3.2. Scanning Electron Microscopy (SEM) Analysis
3.3.3. Cytoplasmic Membrane Depolarization Assay
3.3.4. Membrane Integrity Assay
3.3.5. Propensity of Bacterial Resistance Development
3.3.6. Predicted Binding Mode of 4P in DHFR
3.3.7. Inhibition of DHFR Activities In Vitro
3.4. Hemolysis Assay
3.5. In Vivo Infection Model
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| K. pneumoniae | Klebsiella pneumoniae |
| DMF | N,N-Dimethylformamide |
| MDR | Multidrug-resistant |
| MCR-1 | Mobile colistin resistance gene |
| MIC | Minimum inhibitory concentrations |
| CLSI | Clinical and Laboratory Standards Institute |
| PBS | Phosphate-buffered saline |
| CLSM | Confocal laser scanning microscope |
| SEM | Scanning electron microscopy |
| PI | Propidium iodide |
| DiOC3(5) | 3,3′-dipropylthiadicarbocyanine iodides |
References
- Ruzante, J.M.; Harris, B.; Plummer, P.; Raineri, R.R.; Loy, J.D.; Jacob, M.; Sahin, O.; Kreuder, A.J. Surveillance of Antimicrobial Resistance in Veterinary Medicine in the United States: Current Efforts, Challenges, and Opportunities. Front. Vet. Sci. 2022, 9, 1068406. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 24 October 2024).
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE Pathogens in the Environment: Antibiotic Resistance Status, Community-Acquired Infection and Risk to Human Health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella Pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Han, M.; Xu, L.; Zhang, H. Molecular Basis of Klebsiella Pneumoniae Colonization in Host. Microb. Pathog. 2023, 177, 106026. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Zhang, Y.-F.; Xu, Q.; Qiu, Y.; Lu, Q.-B.; Wang, T.; Zhang, X.-A.; Lin, S.-H.; Lv, C.-L.; Jiang, B.-G.; et al. Infection and Co-Infection Patterns of Community-Acquired Pneumonia in Patients of Different Ages in China from 2009 to 2020: A National Surveillance Study. Lancet Microbe 2023, 4, e330–e339. [Google Scholar] [CrossRef]
- Verani, J.R.; Blau, D.M.; Gurley, E.S.; Akelo, V.; Assefa, N.; Baillie, V.; Bassat, Q.; Berhane, M.; Bunn, J.; Cossa, A.C.A.; et al. Child Deaths Caused by Klebsiella Pneumoniae in Sub-Saharan Africa and South Asia: A Secondary Analysis of Child Health and Mortality Prevention Surveillance (CHAMPS) Data. Lancet Microbe 2024, 5, e131–e141. [Google Scholar] [CrossRef] [PubMed]
- Abban, M.K.; Ayerakwa, E.A.; Mosi, L.; Isawumi, A. The Burden of Hospital Acquired Infections and Antimicrobial Resistance. Heliyon 2023, 9, e20561. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Howard-Anderson, J.; Weiss, D.S. Hypervirulent Klebsiella Pneumoniae—Clinical and Molecular Perspectives. J. Intern. Med. 2020, 287, 283–300. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 1 November 2024).
- Gray, D.A.; Wenzel, M. Multitarget Approaches against Multiresistant Superbugs. ACS Infect. Dis. 2020, 6, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ritika, A. Brief Review of the Biological Potential of Indole Derivatives. Future J. Pharm. Sci. 2020, 6, 121. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, S.; Yang, F.; Dong, S. Marine Indole Alkaloids—Isolation, Structure and Bioactivities. Mar. Drugs 2021, 19, 658. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-L.; Liu, J.; Fang, W.-Y.; Ravindar, L.; Rakesh, K.P. Indole-Based Derivatives as Potential Antibacterial Activity against Methicillin-Resistance Staphylococcus Aureus (MRSA). Eur. J. Med. Chem. 2020, 194, 112245. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, S.; Wang, Z.; Fu, Z.; Zhou, S.; Cheng, H.; Liang, Z.; Deng, X. Synthesis, Antimicrobial and Cytotoxic Activities, and Molecular Docking Studies of N-Arylsulfonylindoles Containing an Aminoguanidine, a Semicarbazide, and a Thiosemicarbazide Moiety. Eur. J. Med. Chem. 2019, 166, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Konus, M.; Çetin, D.; Kızılkan, N.D.; Yılmaz, C.; Fidan, C.; Algso, M.; Kavak, E.; Kivrak, A.; Kurt-Kızıldoğan, A.; Otur, Ç.; et al. Synthesis and Biological Activity of New Indole Based Derivatives as Potent Anticancer, Antioxidant and Antimicrobial Agents. J. Mol. Struct. 2022, 1263, 133168. [Google Scholar] [CrossRef]
- Castro, R.A.D.; Borrell, S.; Gagneux, S. The Within-Host Evolution of Antimicrobial Resistance in Mycobacterium Tuberculosis. FEMS Microbiol. Rev. 2021, 45, fuaa071. [Google Scholar] [CrossRef] [PubMed]
- Saxena, D.; Maitra, R.; Bormon, R.; Czekanska, M.; Meiers, J.; Titz, A.; Verma, S.; Chopra, S. Tackling the Outer Membrane: Facilitating Compound Entry into Gram-Negative Bacterial Pathogens. npj Antimicrob. Resist. 2023, 1, 17. [Google Scholar] [CrossRef]
- Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. How to Enter a Bacterium: Bacterial Porins and the Permeation of Antibiotics. Chem. Rev. 2021, 121, 5158–5192. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive Compound Accumulation Rules Yield a Broad-Spectrum Antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.A.; Hergenrother, P.J. Facilitating Compound Entry as a Means to Discover Antibiotics for Gram-Negative Bacteria. Acc. Chem. Res. 2021, 54, 1322–1333. [Google Scholar] [CrossRef]
- Perlmutter, S.J.; Geddes, E.J.; Drown, B.S.; Motika, S.E.; Lee, M.R.; Hergenrother, P.J. Compound Uptake into E. Coli Can Be Facilitated by N-Alkyl Guanidiniums and Pyridiniums. ACS Infect. Dis. 2021, 7, 162–173. [Google Scholar] [CrossRef]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial Drugs Bearing Guanidine Moieties: A Review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.-G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and Its Role in the Era of Antibiotic Resistance: An Extended Review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Geng, X.; Ge, W.; Bai, L.; Li, Z.; Yang, Y.; Li, J. Amino-Guanidine Indole Derivative, and Preparation Method Therefor and Use Thereof. CN 119080667 A, 6 December 2024. [Google Scholar]
- Weiss, A.; Delavenne, E.; Matias, C.; Lagler, H.; Simon, D.; Li, P.; Hansen, J.U.; dos Santos, T.P.; Jana, B.; Priemel, P.; et al. Topical Niclosamide (ATx201) Reduces Staphylococcus Aureus Colonization and Increases Shannon Diversity of the Skin Microbiome in Atopic Dermatitis Patients in a Randomized, Double-blind, Placebo-controlled Phase 2 Trial. Clin. Transl. Med. 2022, 12, e790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Walsh, T.R.; Wang, Y.; Shen, J.; Yang, M. Minimizing Risks of Antimicrobial Resistance Development in the Environment from a Public One Health Perspective. China CDC Wkly. 2022, 4, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kosowska-Shick, K.; Clark, C.; Pankuch, G.A.; McGhee, P.; Dewasse, B.; Beachel, L.; Appelbaum, P.C. Activity of Telavancin against Staphylococci and Enterococci Determined by MIC and Resistance Selection Studies. Antimicrob. Agents Chemother. 2009, 53, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Braspenning, A.J.M.M.; Rajakani, S.G.; Sey, A.; Bounja, M.E.; Lammens, C.; Glupczynski, Y.; Malhotra-Kumar, S. Assessment of Colistin Heteroresistance among Multidrug-Resistant Klebsiella Pneumoniae Isolated from Intensive Care Patients in Europe. Antibiotics 2024, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, I.; Pérez-Nadales, E.; Marín-Sanz, J.A.; García-Martínez, T.; Martínez-Martínez, L. Heteroresistance to Colistin in Wild-Type Klebsiella Pneumoniae Isolates from Clinical Origin. Microbiol. Spectr. 2023, 11, e02238-23. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-J.; Li, M.-Y.; Jin, X.-J.; Zheng, C.-J.; Piao, H.-R. Design, Synthesis and Evaluation of Carbazole Derivatives as Potential Antimicrobial Agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 296. [Google Scholar] [CrossRef] [PubMed]
- Askari, B.S.; Krajinovic, M. Dihydrofolate Reductase Gene Variations in Susceptibility to Disease and Treatment Outcomes. Curr. Genom. 2010, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Kishony, R. Understanding, Predicting and Manipulating the Genotypic Evolution of Antibiotic Resistance. Nat. Rev. Genet. 2013, 14, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Varela, C.L.; Pires, A.S.; Tavares-da-Silva, E.J.; Roleira, F.M.F. Synthetic and Natural Guanidine Derivatives as Antitumor and Antimicrobial Agents: A Review. Bioorganic Chem. 2023, 138, 106600. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at Bactericidal Concentrations Induces Bacterial Membrane Depolarization in Staphylococcus Aureus. Antimicrob. Agents Chemother. 2014, 58, 6819–6827. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and Synthesis of New Norfloxacin-1,3,4-Oxadiazole Hybrids as Antibacterial Agents against Methicillin-Resistant Staphylococcus Aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966. [Google Scholar] [CrossRef]
- Ridder, M.J.; Daly, S.M.; Hall, P.R.; Bose, J.L. Quantitative Hemolysis Assays. In Staphylococcus aureus: Methods and Protocols; Rice, K.C., Ed.; Springer: New York, NY, USA, 2021; pp. 25–30. ISBN 978-1-07-161550-8. [Google Scholar]
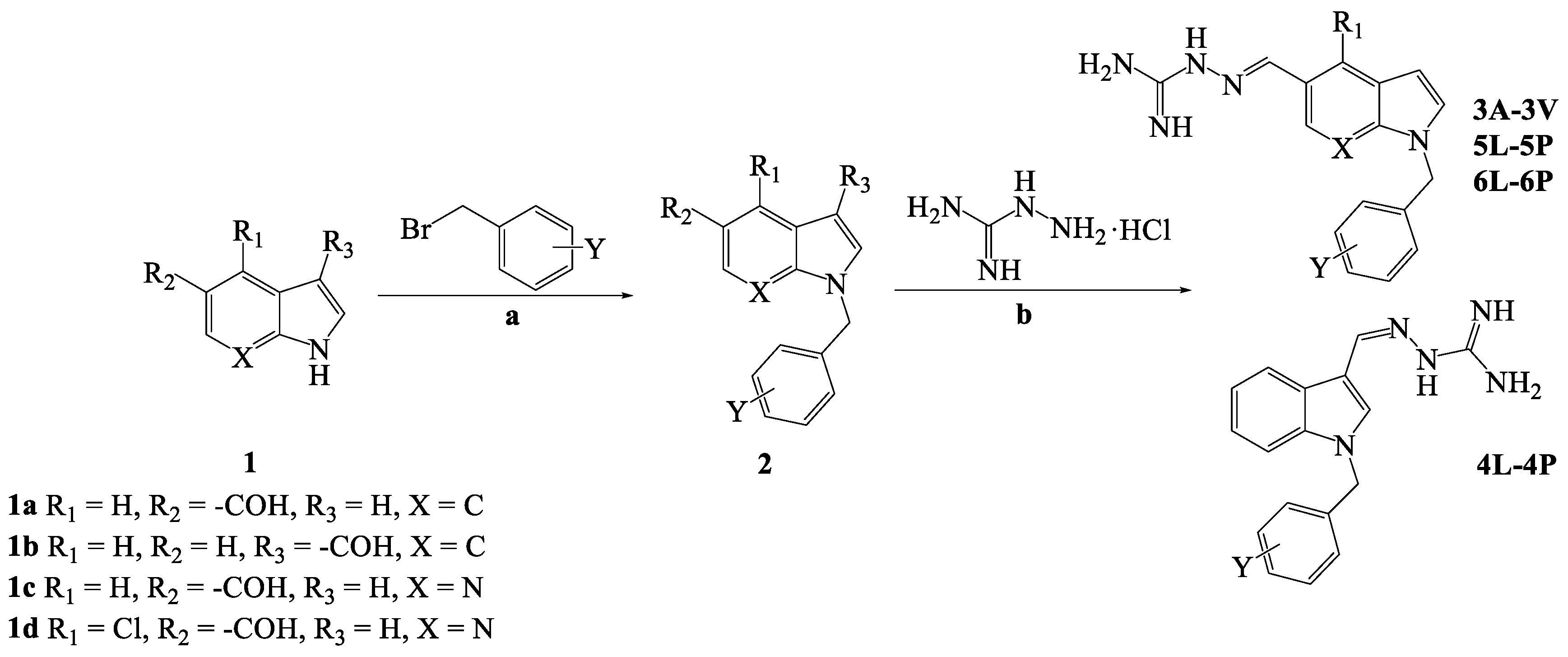




| Structure | Compound | R1 | X | Y | MW |
|---|---|---|---|---|---|
 | 3A | H | C | 2-F | 309.35 |
| 3B | H | C | 3-F | 309.35 | |
| 3C | H | C | 4-F | 309.35 | |
| 3D | H | C | 2-F, 4-F | 327.34 | |
| 3E | H | C | 2-F,5-F | 327.34 | |
| 3F | H | C | 2-F, 6-F | 327.34 | |
| 3G | H | C | 3-F, 4-F | 327.34 | |
| 3H | H | C | 3-F, 5-F | 327.34 | |
| 3I | H | C | 3-CF3 | 359.36 | |
| 3J | H | C | 4-CF3 | 359.36 | |
| 3K | H | C | 3-CF3, 5-CF3 | 427.35 | |
| 3L | H | C | 2-Cl | 325.80 | |
| 3M | H | C | 3-Cl | 325.80 | |
| 3N | H | C | 4-Cl | 325.80 | |
| 3O | H | C | 2-Cl, 4-Cl | 360.24 | |
| 3P | H | C | 3-Cl, 4-Cl | 360.24 | |
| 3Q | H | C | 2-Br | 370.25 | |
| 3R | H | C | 3-Br | 370.25 | |
| 3S | H | C | 4-Br | 370.25 | |
| 3T | H | C | 2-Cl, 4-F | 343.79 | |
| 3U | H | C | 3-Cl, 4-F | 343.79 | |
| 3V | H | C | 4-CN | 316.37 | |
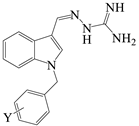 | 4L | H | C | 2-Cl | 325.80 |
| 4M | H | C | 3-Cl | 325.80 | |
| 4N | H | C | 4-Cl | 325.80 | |
| 4O | H | C | 2-Cl, 4-Cl | 360.24 | |
| 4P | H | C | 3-Cl, 4-Cl | 360.24 | |
 | 5L | H | N | 2-Cl | 326.79 |
| 5M | H | N | 3-Cl | 326.79 | |
| 5N | H | N | 4-Cl | 326.79 | |
| 5O | H | N | 2-Cl, 4-Cl | 361.23 | |
| 5P | H | N | 3-Cl, 4-Cl | 361.23 | |
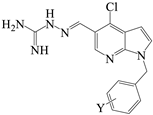 | 6L | Cl | N | 2-Cl | 361.23 |
| 6M | Cl | N | 3-Cl | 361.23 | |
| 6N | Cl | N | 4-Cl | 361.23 | |
| 6O | Cl | N | 2-Cl, 4-Cl | 395.67 | |
| 6P | Cl | N | 3-Cl, 4-Cl | 395.67 |
| Compounds | Strains (MIC/MBC, µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| E. faecium ATCC35667 | S. aureus ATCC29223 | MRSA | K. pneumoniae ATCC700603 | A. baumannii ATCC19606 | P. aeruginosa ATCC27853 | E. coli ATCC25922 | |
| 3A | 8/16 | 8/32 | 16/16 | 32/64 | 8/64 | 16/16 | 32/64 |
| 3B | 8/16 | 8/32 | 16/- | 32/32 | 8/64 | 16/32 | 32/32 |
| 3C | 8/16 | 4/16 | 16/32 | 32/64 | 16/16 | 16/32 | 16/32 |
| 3D | 8/16 | 8/32 | 16/16 | 32/32 | 8/16 | 16/64 | 32/- |
| 3E | 8/16 | 8/16 | 16/16 | 32/64 | 8/64 | 32/64 | 32/32 |
| 3F | 8/16 | 4/64 | >64/- | >64/- | 8/32 | >64/- | 64/- |
| 3G | 8/16 | 8/32 | 16/64 | 32/32 | 8/16 | 16/32 | 32/32 |
| 3H | 8/16 | 4/16 | 16/16 | 32/32 | 8/16 | 16/16 | 16/32 |
| 3I | 4/8 | 4/8 | 8/8 | 16/64 | 4/16 | 16/64 | 8/16 |
| 3J | 4/16 | 2/32 | 8/8 | 8/8 | 4/32 | 16/16 | 8/16 |
| 3K | 4/4 | 2/8 | 8/8 | 8/16 | 4/4 | 32/64 | 8/32 |
| 3L | 4/8 | 2/8 | 8/8 | 16/16 | 8/8 | 16/32 | 16/64 |
| 3M | 4/8 | 4/8 | 16/32 | 16/64 | 4/8 | 16/16 | 16/16 |
| 3N | 4/8 | 4/8 | 8/8 | 8/64 | 4/8 | 16/32 | 16/16 |
| 3O | 4/8 | 4/8 | 4/8 | 8/32 | 4/8 | 16/64 | 4/32 |
| 3P | 4/4 | 2/8 | 4/4 | 8/32 | 2/4 | 16/32 | 4/16 |
| 3Q | 4/16 | 2/8 | 8/64 | 8/32 | 4/16 | 32/64 | 8/16 |
| 3R | 4/8 | 2/32 | 8/8 | 16/16 | 4/4 | 16/32 | 8/16 |
| 3S | 4/32 | 8/32 | 16/32 | 16/64 | 4/16 | 32/- | 16/32 |
| 3T | 8/16 | 4/8 | 8/32 | 8/64 | 4/8 | 16/32 | 16/16 |
| 3U | 4/8 | 4/8 | 8/64 | 16/32 | 4/4 | 16/16 | 8/8 |
| 3V | 32/32 | 8/32 | 64/64 | >64/- | 32/32 | 64/64 | 64/64 |
| 4L | 8/16 | 2/8 | 2/8 | 16/32 | 8/8 | 16/16 | 8/16 |
| 4M | 8/16 | 2/8 | 2/8 | 16/32 | 8/16 | 16/16 | 8/16 |
| 4N | 4/16 | 2/4 | 2/8 | 16/16 | 8/16 | 8/16 | 8/8 |
| 4O | 2/16 | 2/8 | 2/4 | 4/16 | 4/8 | 8/16 | 4/8 |
| 4P | 2/8 | 2/4 | 2/4 | 4/16 | 4/32 | 8/16 | 2/4 |
| 5L | 32/32 | 8/32 | 8/32 | 32/32 | 32/32 | 16/16 | 16/16 |
| 5M | >64/- | 16/64 | 16/32 | >64/- | >64/- | >64/- | >64/- |
| 5N | 16/32 | 4/32 | 8/16 | 16/32 | 16/32 | 16/32 | 16/16 |
| 5O | 16/16 | 2/8 | 4/32 | 16/64 | 16/16 | 16/32 | 8/16 |
| 5P | 16/16 | 4/8 | 4/32 | 8/32 | 16/16 | 16/32 | 8/8 |
| 6L | >64/- | 16/64 | 16/64 | >64/- | >64/- | >64/- | >64/- |
| 6M | 8/64 | 8/16 | 4/32 | >64/- | 8/8 | >64/- | 16/16 |
| 6N | 32/- | 8/32 | 8/16 | >64/- | 16/32 | >64/- | 16/64 |
| 6O | >64/- | >64/- | >64/- | >64/- | >64/- | >64/- | >64/- |
| 6P | 16/32 | 8/64 | 8/64 | >64/- | 32/32 | >64/- | >64/- |
| Colistin | >64/- | >64/- | 64/- | 2/8 | 0.5/0.5 | 1/2 | 1/2 |
| Compounds | Strains (MIC/MBC, µg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K.P. 2102 | K.P. 2105 | K.P. 2107 | K.P. 2108 | K.P. 2109 | K.P. 2112 | K.P. 2118 | K.P. 2125 | K.P. 2134 | K.P. 2135 | K.P. 2138 | |
| 3I | 8/8 | 8/32 | 8/16 | 8/16 | 8/8 | 8/64 | 8/8 | 8/16 | 8/32 | 8/32 | 8/8 |
| 3J | 8/32 | 8/16 | 8/8 | 8/8 | 8/16 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 |
| 3K | 8/64 | 16/64 | 8/16 | 8/32 | 16/32 | 8/32 | 8/16 | 8/16 | 16/32 | 8/16 | 8/32 |
| 3L | 8/16 | 16/16 | 8/8 | 8/8 | 8/16 | 8/8 | 8/8 | 8/16 | 8/8 | 8/8 | 8/8 |
| 3M | 8/16 | 16/16 | 8/16 | 8/8 | 8/8 | 8/8 | 8/64 | 16/16 | 8/16 | 8/32 | 8/8 |
| 3N | 8/8 | 16/32 | 8/16 | 8/8 | 8/16 | 8/32 | 8/64 | 16/16 | 8/16 | 8/8 | 8/8 |
| 3O | 8/8 | 8/32 | 4/16 | 8/16 | 8/64 | 4/64 | 4/4 | 8/32 | 8/64 | 4/8 | 4/16 |
| 3P | 4/16 | 8/16 | 4/8 | 4/16 | 4/64 | 4/8 | 4/4 | 8/16 | 8/8 | 4/8 | 4/8 |
| 3Q | 8/8 | 8/- | 8/8 | 8/8 | 8/8 | 8/8 | 8/32 | 8/8 | 8/8 | 8/16 | 8/16 |
| 3R | 8/8 | 16/32 | 8/8 | 8/8 | 8/8 | 8/8 | 8/64 | 16/16 | 8/8 | 8/32 | 8/8 |
| 3S | 8/16 | 16/32 | 8/16 | 8/64 | 8/16 | 8/16 | 8/8 | 8/16 | 8/8 | 8/8 | 8/16 |
| 3T | 8/8 | 16/16 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/16 |
| 3U | 8/8 | 8/8 | 8//32 | 8/8 | 816 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 |
| 4O | 4/8 | 4/8 | 4/8 | 4/8 | 8/8 | 4/4 | 4/8 | 4/4 | 8/8 | 4/16 | 4/16 |
| 4P | 4/8 | 4/8 | 4/4 | 4/8 | 4/16 | 4/4 | 4/8 | 4/8 | 4/8 | 4/4 | 4/4 |
| 5P | 8/16 | 8/8 | 16/16 | 8/32 | 8/32 | 8/8 | 8/8 | 8/8 | 8/8 | 8/18 | 8/16 |
| Colistin | 2/4 | >64/- | 8/8 | >64/- | >64/- | 4/4 | 8/8 | 8/16 | 8/32 | 0.5/4 | 4/8 |
| Compounds (µg/mL) | |||
|---|---|---|---|
| 3P | 4P | 5P | |
| HC50 | 245.0 | 123.6 | 243.7 |
| SI * | 30.63 | 30.90 | 30.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Geng, X.; Tao, Q.; Hao, R.-C.; Yang, Y.-J.; Liu, X.-W.; Li, J.-Y. Synthesis, Antimicrobial Activities, and Model of Action of Indolyl Derivatives Containing Amino-Guanidinium Moieties. Molecules 2025, 30, 887. https://doi.org/10.3390/molecules30040887
Li Y-X, Geng X, Tao Q, Hao R-C, Yang Y-J, Liu X-W, Li J-Y. Synthesis, Antimicrobial Activities, and Model of Action of Indolyl Derivatives Containing Amino-Guanidinium Moieties. Molecules. 2025; 30(4):887. https://doi.org/10.3390/molecules30040887
Chicago/Turabian StyleLi, Yu-Xi, Xiang Geng, Qi Tao, Ruo-Chen Hao, Ya-Jun Yang, Xi-Wang Liu, and Jian-Yong Li. 2025. "Synthesis, Antimicrobial Activities, and Model of Action of Indolyl Derivatives Containing Amino-Guanidinium Moieties" Molecules 30, no. 4: 887. https://doi.org/10.3390/molecules30040887
APA StyleLi, Y.-X., Geng, X., Tao, Q., Hao, R.-C., Yang, Y.-J., Liu, X.-W., & Li, J.-Y. (2025). Synthesis, Antimicrobial Activities, and Model of Action of Indolyl Derivatives Containing Amino-Guanidinium Moieties. Molecules, 30(4), 887. https://doi.org/10.3390/molecules30040887







