Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection
Abstract
1. Introduction
2. Results and Discussion
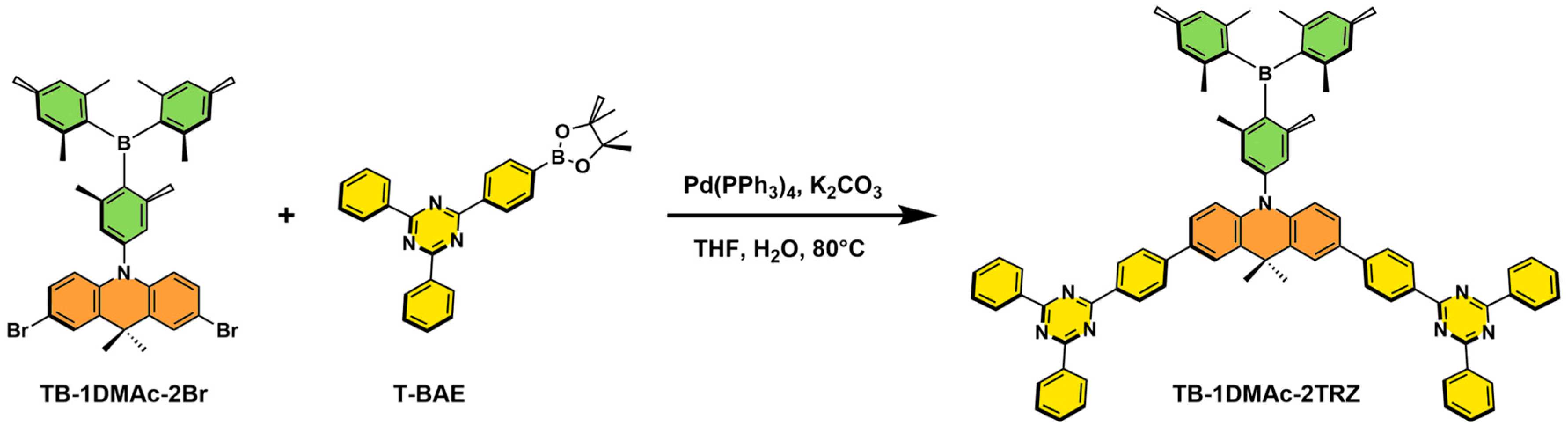
2.1. Synthesis and Characterization
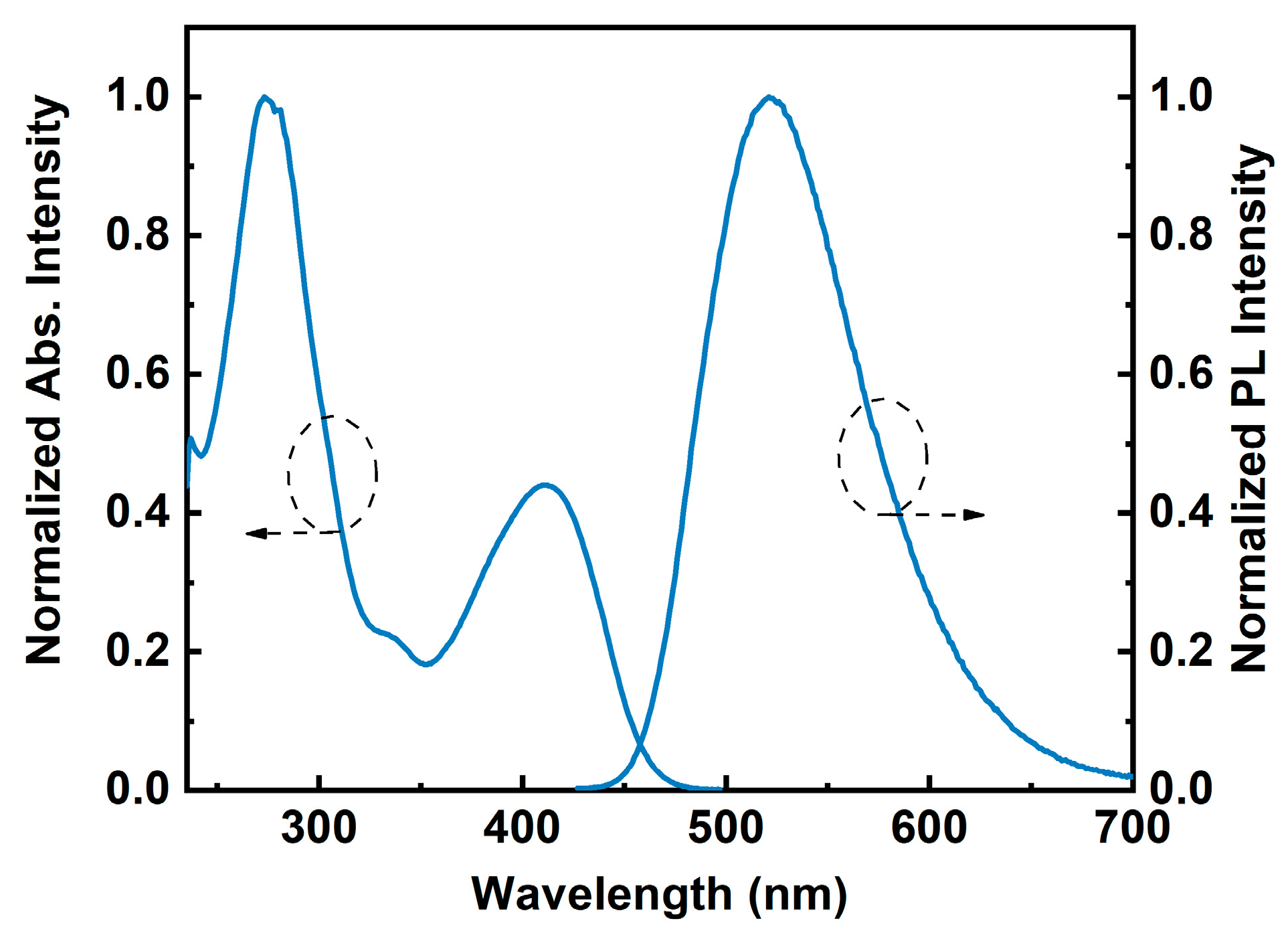
2.2. Photophysical Properties
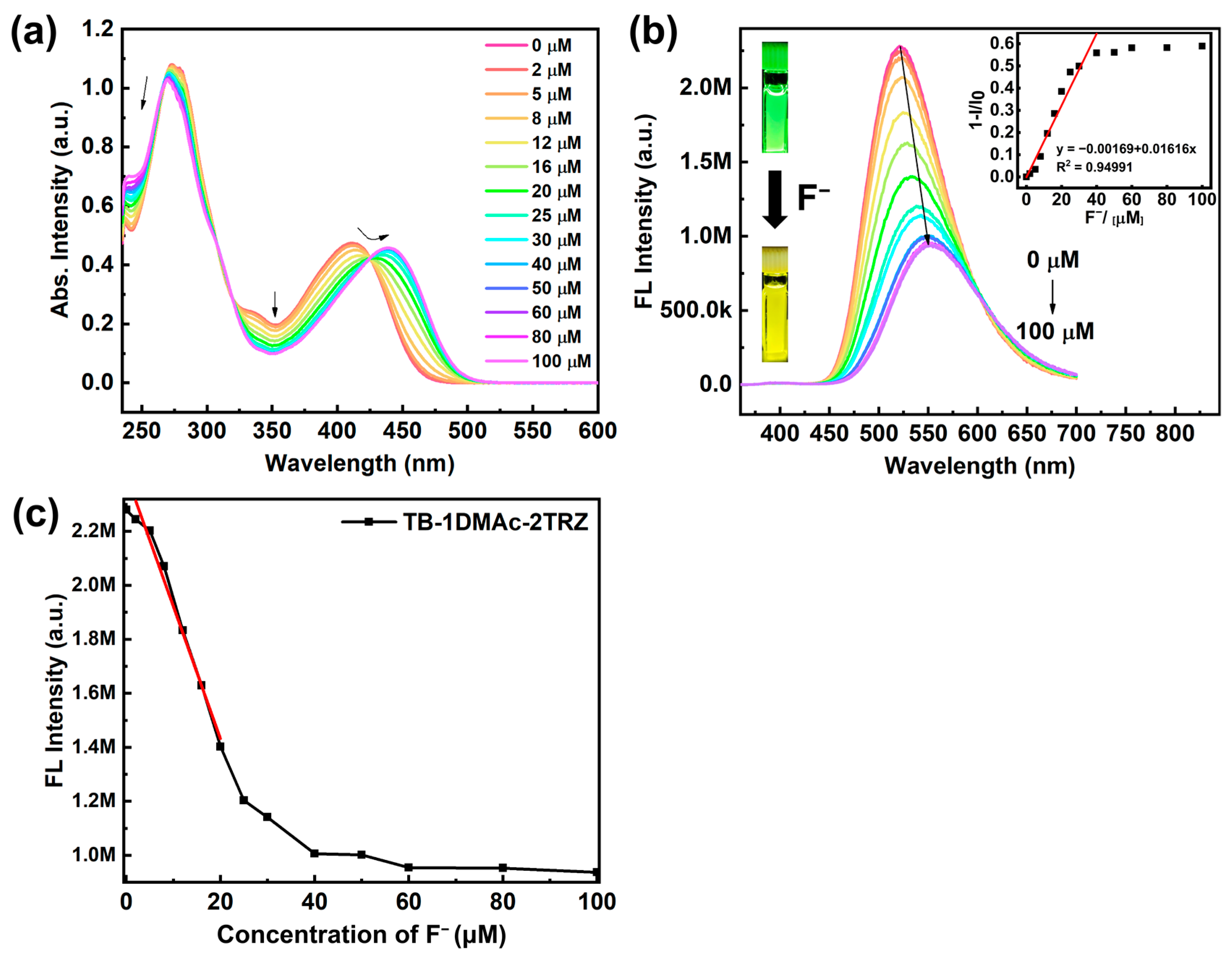
2.3. Detection of Fluoride Ions
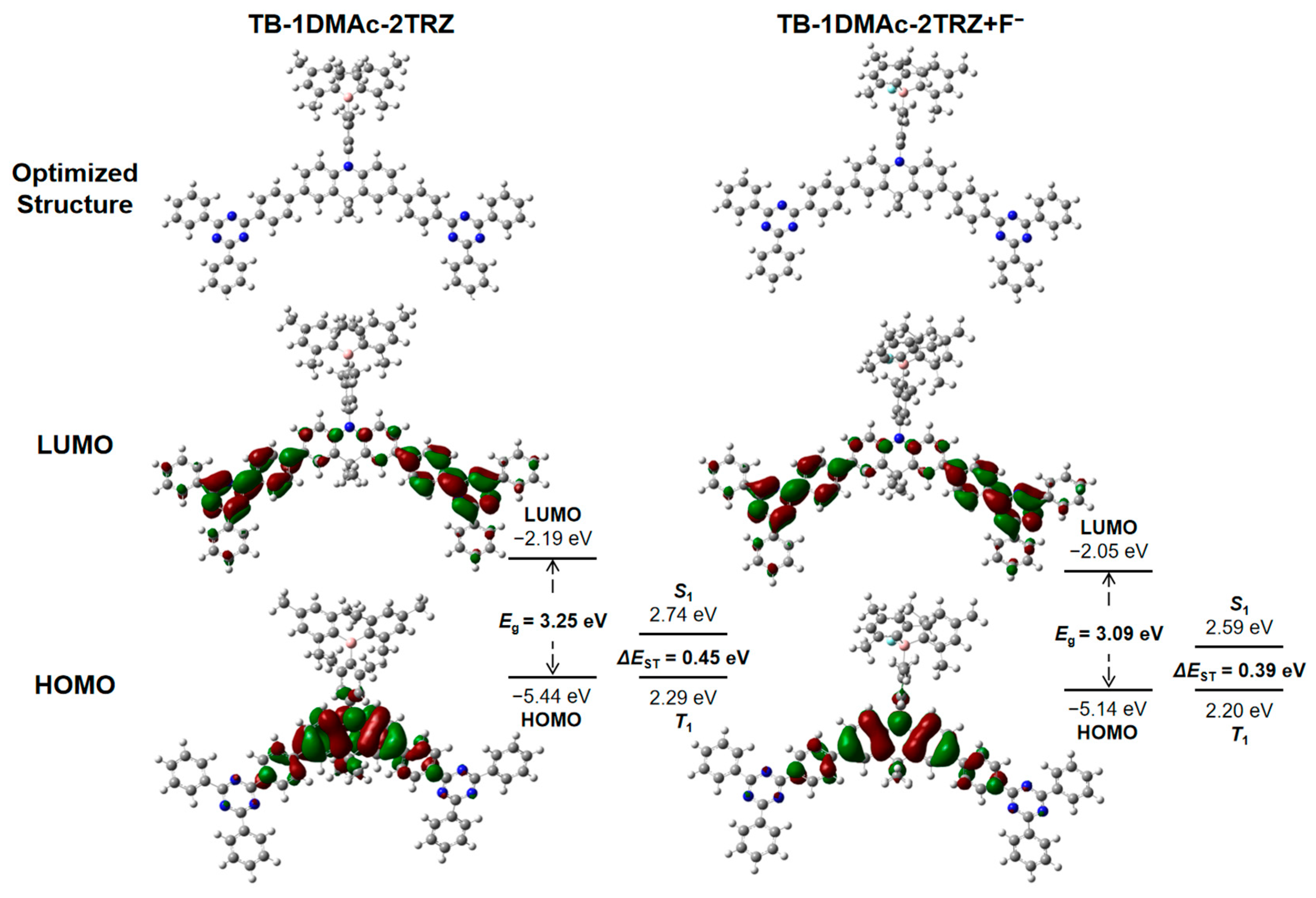
2.4. Theoretical Calculations
2.5. Photostability, Repeatability, Selectivity, and Anti-Interference Analysis
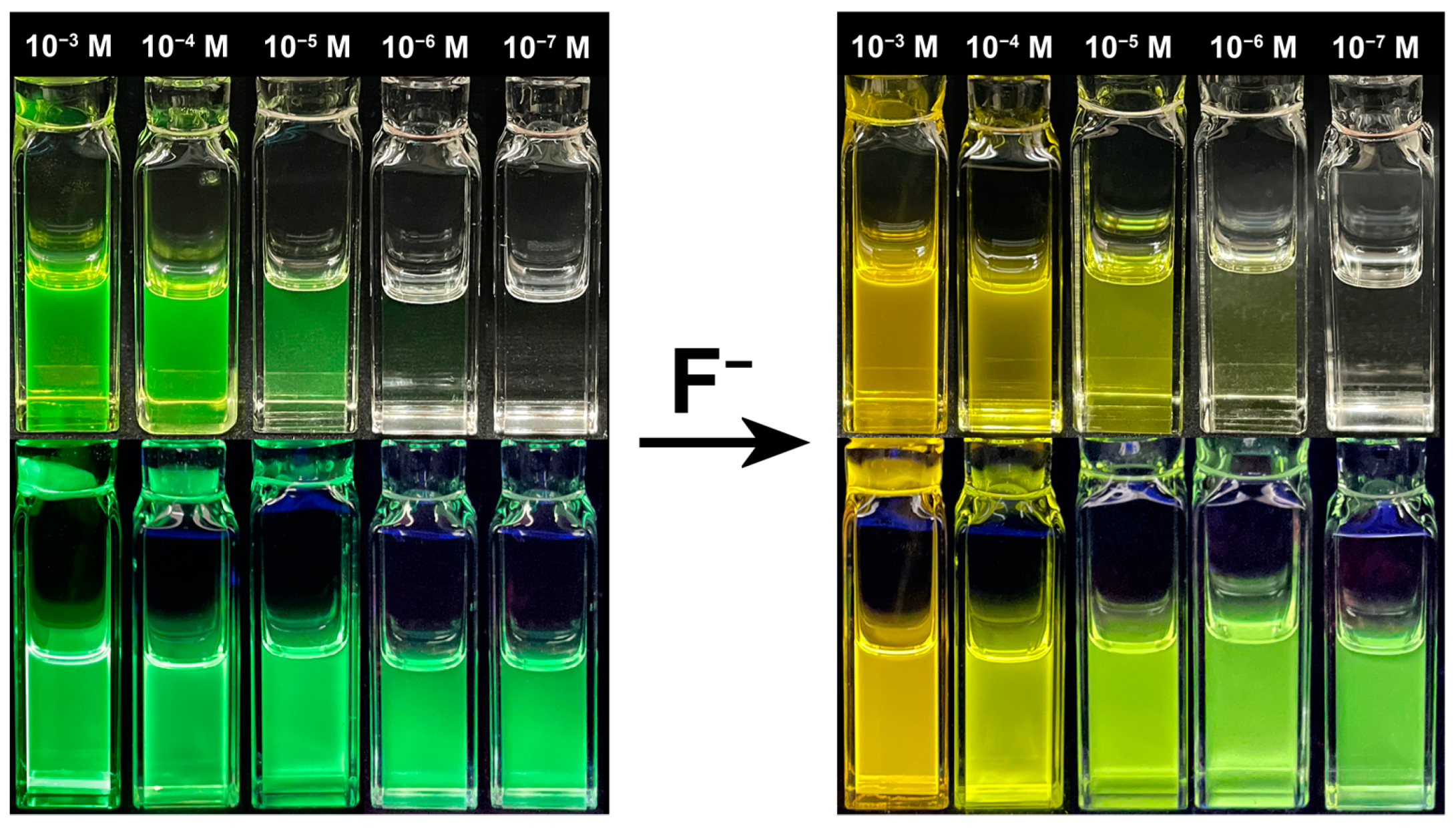
2.6. Visual Applications for Fluoride Ion Detection
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, R.; Dwivedi, S.K.; Mishra, H.; Misra, A. Imidazole-coumarin containing D–A type fluorescent probe: Synthesis photophysical properties and sensing behavior for F− and CN− anion. Dye. Pigm. 2020, 175, 108163. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Z.; Dang, J.; Sun, Y.; Zhou, G.; Wong, W.-Y. High performance solution-processed organic yellow light-emitting devices and fluoride ion sensors based on a versatile phosphorescent Ir(III) complex. Mater. Chem. Front. 2019, 3, 376–384. [Google Scholar] [CrossRef]
- Muthusamy, S.; Rajalakshmi, K.; Ahn, D.-H.; Kannan, P.; Zhu, D.; Nam, Y.-S.; Choi, K.Y.; Luo, Z.; Song, J.-W.; Xu, Y. Spontaneous detection of F− and viscosity using a multifunctional tetraphenylethene-lepidine probe: Exploring environmental applications. Food Chem. 2025, 466, 142147. [Google Scholar] [CrossRef]
- Sha, H.; Yan, B. Terbium-based metal-organic frameworks through energy transfer modulation for visual logical sensing zinc and fluorine ions. Talanta 2023, 257, 124326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Feng, X.; Zhao, Y. An experimental and theoretical study on mechanistic insights into urolithin-based ratiometric fluorescent probe for instant quantitative detection of fluoride ions. Talanta 2024, 276, 126220. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, Z.; Wei, X.; Han, Q.; Wang, H.; Yang, X.; Xu, S.; Wang, X. Engineering a triplet exciton enhanced photoluminescence self-assembled nanoprobe containing block copolymer and Tb3+/Ga3+ complex for fluoride ion detection in serum. Sens. Actuators B 2022, 366, 131975. [Google Scholar] [CrossRef]
- Ali, R.; Gupta, R.C.; Dwivedi, S.K.; Misra, A. Excited state proton transfer (ESIPT) based molecular probe to sense F− and CN− anions through a fluorescence “turn-on” response. New J. Chem. 2018, 42, 11746–11754. [Google Scholar] [CrossRef]
- Ayoob, S.; Gupta, A.K. Fluoride in drinking water: A review on the status and stress effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-A.; Mani, V.; Huang, S.-T. Design and synthesis of ultrasensitive off-on fluoride detecting fluorescence probe via autoinductive signal amplification. Analyst 2015, 140, 346–352. [Google Scholar] [CrossRef]
- Brugnara, A.; Topić, F.; Rissanen, K.; de la Lande, A.; Colasson, B.; Reinaud, O. Selective recognition of fluoride anion in water by a copper(II) center embedded in a hydrophobic cavity. Chem. Sci 2014, 5, 3897–3904. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Recognition and sensing of fluoride anion. Chem. Commun. 2009, 2809–2829. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Highlights on contemporary recognition and sensing of fluoride anion in solution and in the solid state. Chem. Soc. Rev. 2013, 42, 2016–2038. [Google Scholar] [CrossRef] [PubMed]
- Day, J.K.; Bresner, C.; Coombs, N.D.; Fallis, I.A.; Ooi, L.-L.; Aldridge, S. Colorimetric fluoride ion sensing by polyborylated ferrocenes: Structural influences on thermodynamics and kinetics. Inorg. Chem. 2008, 47, 793–804. [Google Scholar] [CrossRef]
- Langton, M.J.; Serpell, C.J.; Beer, P.D. Anion recognition in water: Recent advances from a supramolecular and macromolecular perspective. Angew. Chem. Int. Ed. 2016, 55, 1974–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, Y.; Zheng, J.; Li, J.; Zhao, W.; Yang, S.; Yang, R. Self-assembly of graphene oxide with a silyl-appended spiropyran dye for rapid and sensitive colorimetric detection of fluoride ions. Anal. Chem. 2013, 85, 11456–11463. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Li, W.-Y.; Gu, J.-A.; Lin, C.-M.; Huang, S.-T. Electrochemical off-on ratiometric chemodosimeters for the selective and rapid detection of fluoride. Talanta 2015, 131, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Morés, S.; Monteiro, G.C.; da Silva Santos, F.; Carasek, E.; Welz, B. Determination of fluorine in tea using high-resolution molecular absorption spectrometry with electrothermal vaporization of the calcium mono-fluoride CaF. Talanta 2011, 85, 2681–2685. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef]
- Zhu, B.; Yuan, F.; Li, R.; Li, Y.; Wei, Q.; Ma, Z.; Du, B.; Zhang, X. A highly selective colorimetric and ratiometric fluorescent chemodosimeter for imaging fluoride ions in living cells. Chem. Commun. 2011, 47, 7098–7100. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.T.; Xie, D.; Martinez, D.; Yu, M.; Que, E.L. A dual-responsive probe for detecting cellular hypoxia using 19F magnetic resonance and fluorescence. Chem. Commun. 2019, 55, 8860–8863. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Wang, Y.; Wang, X.; Ma, H.; Li, P.; Song, W.; Xia Han, X.; Zhao, B. Direct detection of fluoride ions in aquatic samples by surface-enhanced raman scattering. Talanta 2018, 178, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Guo, D.; Wang, N.; Wu, S.; Zhang, P.; Zhu, Y. Detection of trace fluoride in serum and urine by online membrane-based distillation coupled with ion chromatography. J. Chromatogr. A 2017, 1500, 145–152. [Google Scholar] [CrossRef]
- Musijowski, J.; Szostek, B.; Koc, M.; Trojanowicz, M. Determination of fluoride as fluorosilane derivative using reversed-phase HPLC with UV detection for determination of total organic fluorine. J. Sep. Sci. 2010, 33, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Yu, Z.; Phillips, B.L.; Wang, H.; Ji, J.; Pan, B.; Li, W. Molecular-scale investigation of fluoride sorption mechanism by nanosized hydroxyapatite using 19F solid-state NMR spectroscopy. J. Colloid Interface Sci. 2019, 557, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhao, Q.; Xu, W.J.; Huang, W. Fluoride probe based on organoboron compounds. Prog. Chem. 2008, 20, 1708–1715. [Google Scholar]
- Qi, Y.; Cao, X.; Zou, Y.; Yang, C. Multi-resonance organoboron-based fluorescent probe for ultra-sensitive, selective and reversible detection of fluoride ions. J. Mater. Chem. C 2021, 9, 1567–1571. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Lin, Q.; Huang, Q.; Liu, Y. Triarylboron-based fluorescent probe exhibiting simultaneous turn-on/turn off color-tunable emission for the highly sensitive detection of fluoride ion. J. Mol. Struct. 2022, 1257, 132537. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, Y.; Du, X.; Li, L.; Li, Q.; Mao, H. Annealing temperature-dependent photoelectrochemical property of zinc oxide/graphene nanocomposite and the application for fabricating a “signal-off” photoelectrochemical aptasensing for ATP. IEEE Sens. J. 2023, 23, 6489–6498. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Y.; Fu, H.L.K.; Chan, A.K.W.; Yam, V.W.W. Aggregation and tunable color emission behaviors of L-glutamine-derived platinum (II) bipyridine complexes by hydrogen-bonding, π–π stacking and metal–metal interactions. Chem. Eur. J. 2019, 25, 5251–5258. [Google Scholar] [CrossRef]
- Cheng, F.; Bonder, E.M.; Jäkle, F. Electron-deficient triarylborane block copolymers: Synthesis by controlled free radical polymerization and application in the detection of fluoride ions. J. Am. Chem. Soc. 2013, 135, 17286–17289. [Google Scholar] [CrossRef]
- Gao, H.; Li, Z.; Pang, Z.; Qin, Y.; Liu, G.; Gao, T.; Dong, X.; Shen, S.; Xie, X.; Wang, P. Rational molecular design strategy for high-efficiency ultrapure blue TADF emitters: Symmetrical and rigid sulfur-bridged boron-based acceptors. ACS Appl. Mater. Interfaces 2023, 15, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kye, M.; Jung, J.Y.; Lim, Y.-b.; Yoon, J. Design for a small molecule based chemosensor containing boron and pyridine moieties to detect HF. Sens. Actuators B 2018, 255, 2621–2627. [Google Scholar] [CrossRef]
- Lee, H.; Jana, S.; Kim, J.; Lee, S.U.; Lee, M.H. Donor-acceptor-appended triarylboron lewis acids: Ratiometric or time-resolved turn-on fluorescence response toward fluoride binding. Inorg. Chem. 2020, 59, 1414–1423. [Google Scholar] [CrossRef]
- Madayanad Suresh, S.; Hall, D.; Beljonne, D.; Olivier, Y.; Zysman-Colman, E. Multiresonant thermally activated delayed fluorescence emitters based on heteroatom-doped nanographenes: Recent advances and prospects for organic light-emitting diodes. Adv. Funct. Mater. 2020, 30, 1908677. [Google Scholar] [CrossRef]
- Tao, L.-M.; Guo, Y.-H.; Huang, X.-M.; Wang, C.-K. Theoretical studies on two-photon absorption properties of newly synthesized triaryl boron-based A-π-A and triaryl nitrogen-based D-π-D quadrupolar compounds. Chem. Phys. Lett. 2006, 425, 10–15. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Triarylborane-based materials for OLED applications. Molecules 2017, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Liu, S.; Yan, Q.; Wei, Y.; Wang, J.; Lan, Y.; Tan, J. Empowering boronic acids as hydroxyl synthons for aryne induced three-component coupling reactions. Chem. Sci. 2023, 14, 4278–4287. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Agou, T.; Kobayashi, J.; Kawashima, T.; Gabbaï, F.P. Fluoride ion complexation by a cationic borane in aqueous solution. Chem. Commun. 2007, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shimoyama, D.; Zhang, N.; Jia, Y.; Hu, G.; Li, C.; Yin, X.; Wang, N.; Jäkle, F.; Chen, P. A new platform of B/N-doped cyclophanes: Access to a π-conjugated block-type B3N3 macrocycle with strong dipole moment and unique optoelectronic properties. Angew. Chem. Int. Ed. 2022, 61, e202200612. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-linked conjugated microporous polymers: Sensing and removal of fluoride ions. Chem. Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-Q.; Zhan, B.; Zhao, J.; Guo, Y.; Zu, B.; Li, Y.; He, C. Modular enantioselective assembly of multi-substituted boron-stereogenic bodipys. Nat. Chem. 2024, 17, 83–91. [Google Scholar] [CrossRef]
- Sun, F.; Tan, S.; Cao, H.-J.; Lu, C.-s.; Tu, D.; Poater, J.; Solà, M.; Yan, H. Facile construction of new hybrid conjugation via boron cage extension. J. Am. Chem. Soc. 2023, 145, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Ahmad, M.; Singla, N.; Kumar, G.; Singh, P.; Luxami, V.; Kaur, N.; Kumar, S. Chemodosimeters for optical detection of fluoride anion. Coord. Chem. Rev. 2020, 405, 213138. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, B.; Chen, J.; Duan, X. Fluorescent sensing of fluoride in cellular system. Theranostics 2015, 5, 173. [Google Scholar] [CrossRef]
- Manjare, S.T.; Kim, Y.; Churchill, D.G. Selenium-and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cao, X.; Zou, Y.; Yang, C. Color-tunable tetracoordinated organoboron complexes exhibiting aggregation-induced emission for the efficient turn-on detection of fluoride ions. Mater. Chem. Front. 2021, 5, 2353–2360. [Google Scholar] [CrossRef]
- Wang, H.; Da, L.; Yang, L.; Chu, S.; Yang, F.; Yu, S.; Jiang, C. Colorimetric fluorescent paper strip with smartphone platform for quantitative detection of cadmium ions in real samples. J. Hazard. Mater. 2020, 392, 122506. [Google Scholar] [CrossRef]
- Hu, K.; Lian, G.; Wang, Y.; Shao, T.; Zhou, M.; Liu, Y.; Jin, G. Rich fluorine trident type rigid donor-acceptor (D-A) tricarbazoles-triazine structures: An effective strong acid affinity fluorescent dye. Dye. Pigm. 2022, 201, 110254. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, S.; Wang, R.; Zhang, L.; Ma, Q.; Ren, X.; Gao, Y.; Wang, Z.; Guo, Z.; Zhang, C. Covalent triazine frameworks for advanced energy storage: Challenges and new opportunities. Energy Environ. Sci. 2023, 16, 3181–3213. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Li, W.; Zhang, J.; Luo, M.; Du, S.; Zhang, X.; Xu, S.; Ge, Z. Multiple charge transfer processes enable blue emitter for highly efficient OLEDs. Adv. Opt. Mater. 2023, 11, 2300017. [Google Scholar] [CrossRef]
- Xie, F.M.; Li, H.Z.; Zhang, K.; Shen, Y.; Zhao, X.; Li, Y.Q.; Tang, J.X. A dislocated twin-locking acceptor-donor-acceptor configuration for efficient delayed fluorescence with multiple through-space charge transfer. Angew. Chem. Int. Ed. 2022, 61, e202213823. [Google Scholar] [CrossRef]
- Suzuki, N.; Suda, K.; Yokogawa, D.; Kitoh-Nishioka, H.; Irle, S.; Ando, A.; Abegão, L.M.G.; Kamada, K.; Fukazawa, A.; Yamaguchi, S. Near infrared two-photon-excited and -emissive dyes based on a strapped excited-state intramolecular proton-transfer (ESIPT) scaffold. Chem. Sci. 2018, 9, 2666–2673. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, D.; Lee, T.; Lee, J.; Jung, J.; Yoo, S.; Lee, M.H. Impact of boryl acceptors in para-acridine-appended triarylboron emitters on blue thermally activated delayed fluorescence OLEDs. Dye. Pigm. 2021, 188, 109224. [Google Scholar] [CrossRef]
- Buss, B.L.; Lim, C.H.; Miyake, G.M. Dimethyl dihydroacridines as photocatalysts in organocatalyzed atom transfer radical polymerization of acrylate monomers. Angew. Chem. Int. Ed. 2020, 59, 3209–3217. [Google Scholar] [CrossRef]
- Wu, J.; Lai, G.; Li, Z.; Lu, Y.; Leng, T.; Shen, Y.; Wang, C. Novel 2,1,3-benzothiadiazole derivatives used as selective fluorescent and colorimetric sensors for fluoride ion. Dye. Pigm. 2016, 124, 268–276. [Google Scholar] [CrossRef]
- Cugnasca, B.S.; Duarte, F.; Petrarca de Albuquerque, J.L.; Santos, H.M.; Luis Capelo-Martínez, J.; Lodeiro, C.; Dos Santos, A.A. Precision detection of cyanide, fluoride, and hydroxide ions using a new tetraseleno-BODIPY fluorescent sensor. J. Photochem. Photobiol. A 2024, 457, 115881. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Pei, X.; Qu, M.; Zhang, J.; Hu, W.; Zhang, Y.; Wu, W.; Pei, S. A multifunctional near-infrared fluorescent probe based on benzothiazole structure for fluoride-ion detection. Spectrochim. Acta Part A 2025, 324, 125009. [Google Scholar] [CrossRef]
- Zhou, M.-G.; Chen, L.; Zhou, J.; Zhong, X.; Yuan, M.-S. A fluorescence probe based on naphthalimide-functionalized triarylboron for fluoride ion detection. Inorg. Chem. Commun. 2024, 170, 113392. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.-C.; Bai, J.; Fang, H.; Wu, F.-Y.; Xiao, Q. A “turn-on” fluorescent probe based on BODIPY dyes for highly selective detection of fluoride ions. Dye. Pigm. 2021, 190, 109347. [Google Scholar] [CrossRef]
- Xiao, L.; Ren, L.; Jing, X.; Li, Z.; Wu, S.; Guo, D. A selective naphthalimide-based colorimetric and fluorescent chemosensor for “naked-eye” detection of fluoride ion. Inorg. Chim. Acta 2020, 500, 119207. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Ding, L.; Gao, J. Reusable colorimetric and fluorescent chemosensors based on 1,8-naphthalimide derivatives for fluoride ion detection. Spectrochim. Acta Part A 2020, 237, 118395. [Google Scholar] [CrossRef]

| Compound | λabs 1 [nm] | λem 1 [nm] | Td 2 [°C] | Tg 3 [°C] | ES/ET 4 [eV] | ΔEST 5 [eV] | HOMO 6/LUMO 7 [eV] | Eg 8 [eV] |
|---|---|---|---|---|---|---|---|---|
| TB-1DMAc-2TRZ | 272/411 | 522 | 432 | 206 | 2.74/2.29 | 0.45 | −5.44/−2.19 | 3.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Wang, J.; Liu, Y. Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection. Molecules 2025, 30, 879. https://doi.org/10.3390/molecules30040879
Tang L, Wang J, Liu Y. Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection. Molecules. 2025; 30(4):879. https://doi.org/10.3390/molecules30040879
Chicago/Turabian StyleTang, Lei, Jiaoyun Wang, and Yuan Liu. 2025. "Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection" Molecules 30, no. 4: 879. https://doi.org/10.3390/molecules30040879
APA StyleTang, L., Wang, J., & Liu, Y. (2025). Triazine-Modified Color-Responsive Triarylboron/Acridine Fluorescent Probe with Multi-Channel Charge Transfer for Highly Sensitive Fluoride Ion Detection. Molecules, 30(4), 879. https://doi.org/10.3390/molecules30040879







