Biocompatible Black Phosphorus Nanosheets-Antimicrobial Peptide Nanocomposites for Enhanced Anti-Infection Therapy
Abstract
1. Introduction
2. Results and Discussion
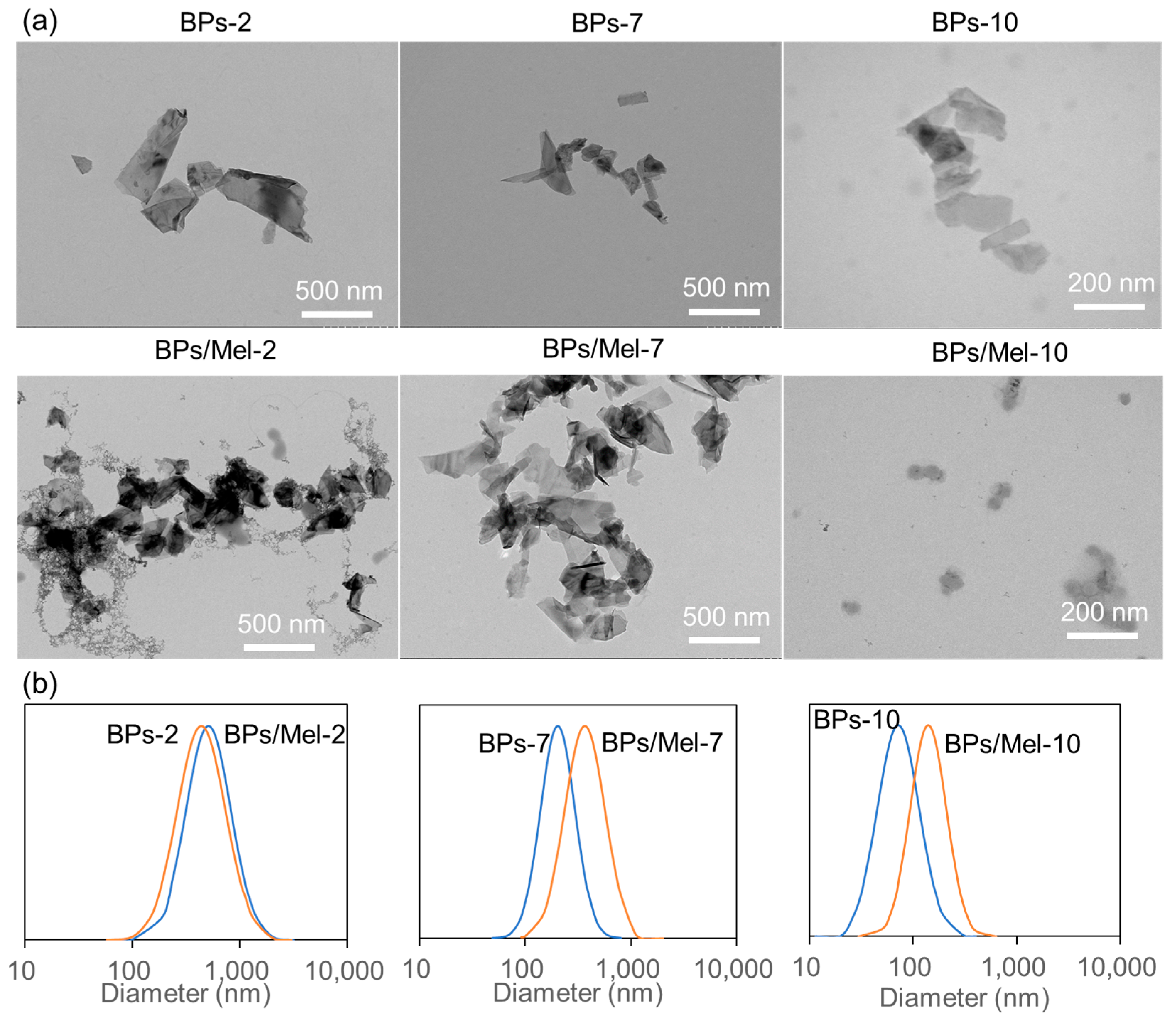
2.1. Characterization of BPs and BPs/Mel Nanocomposites
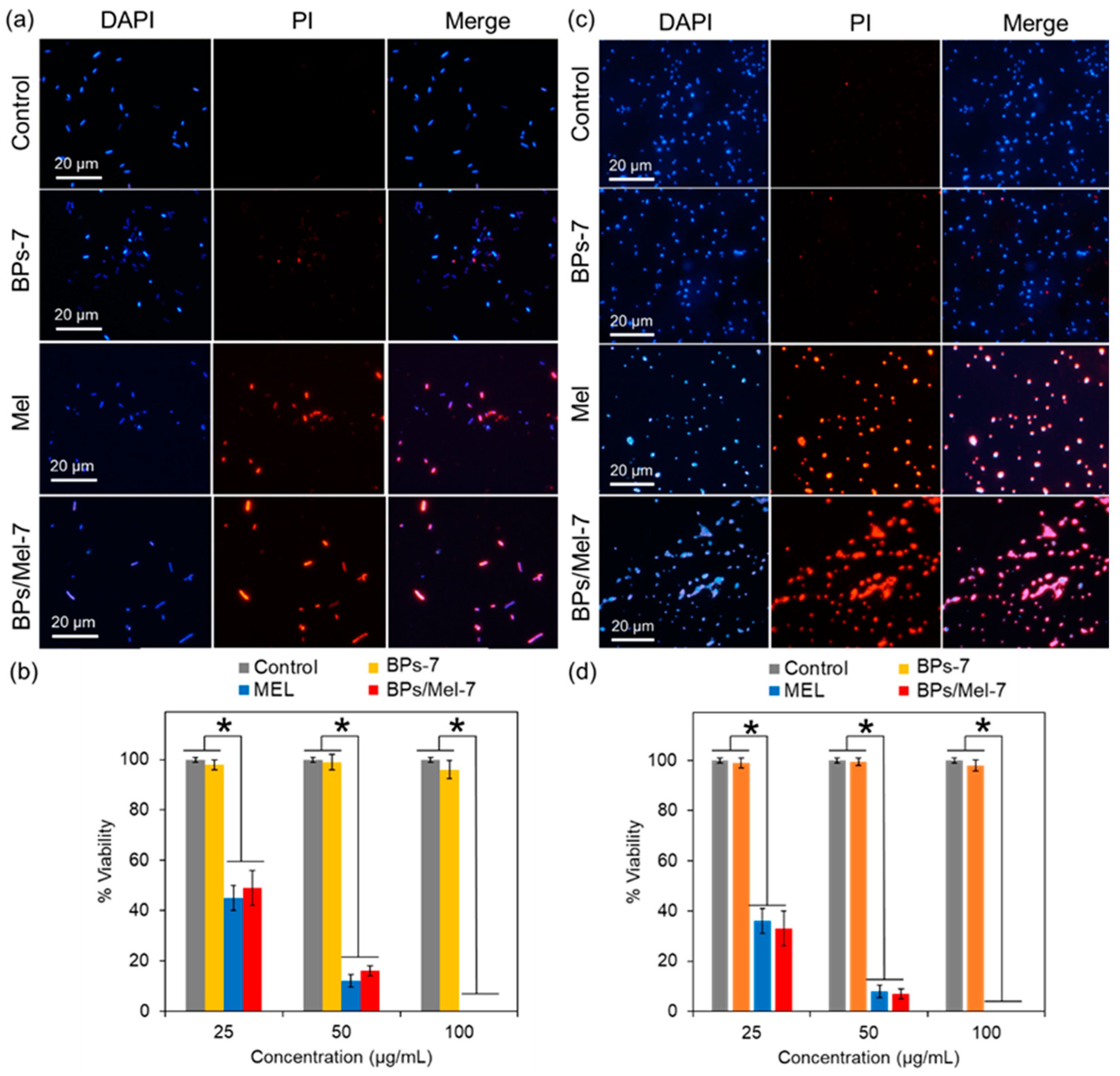
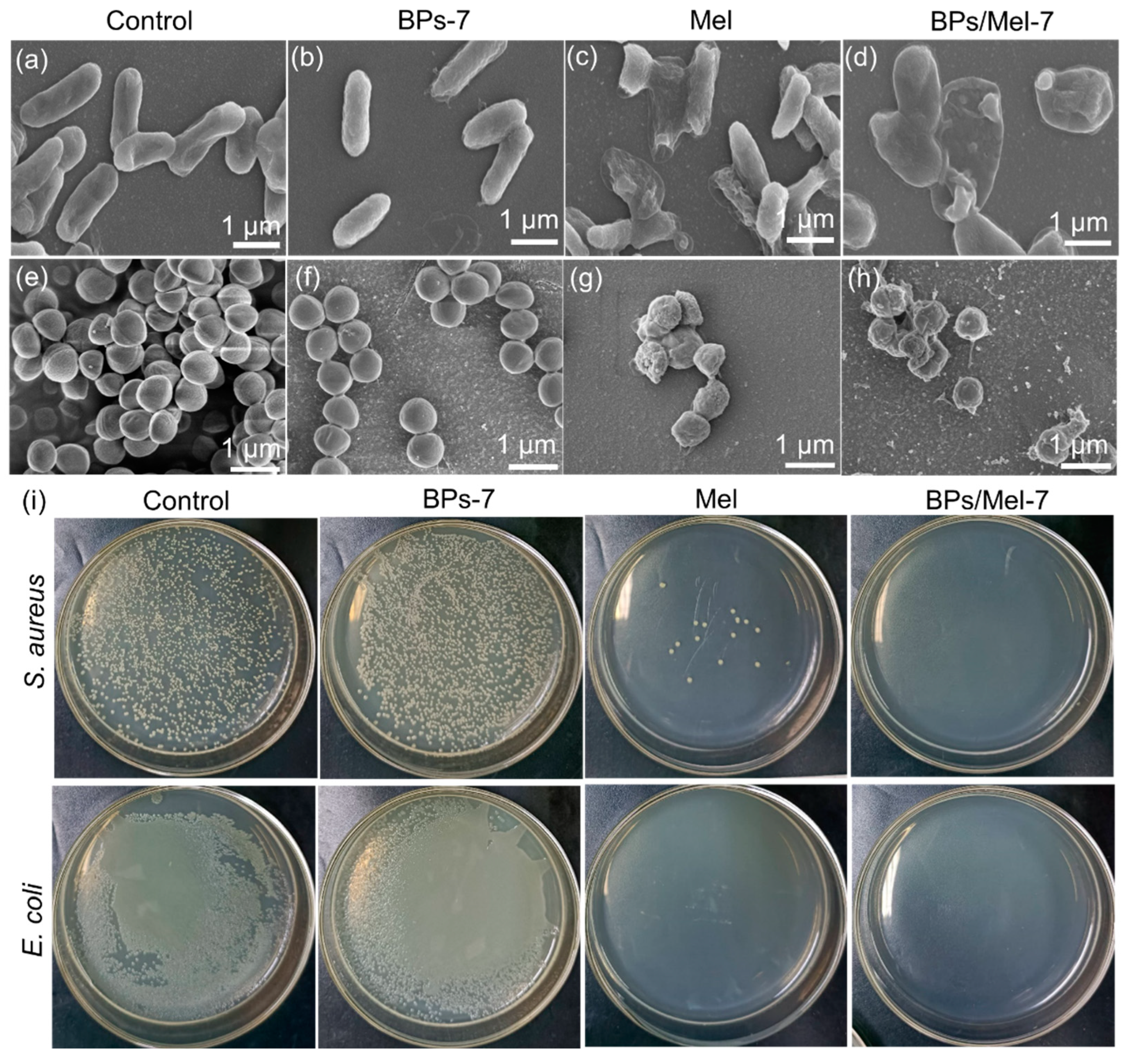
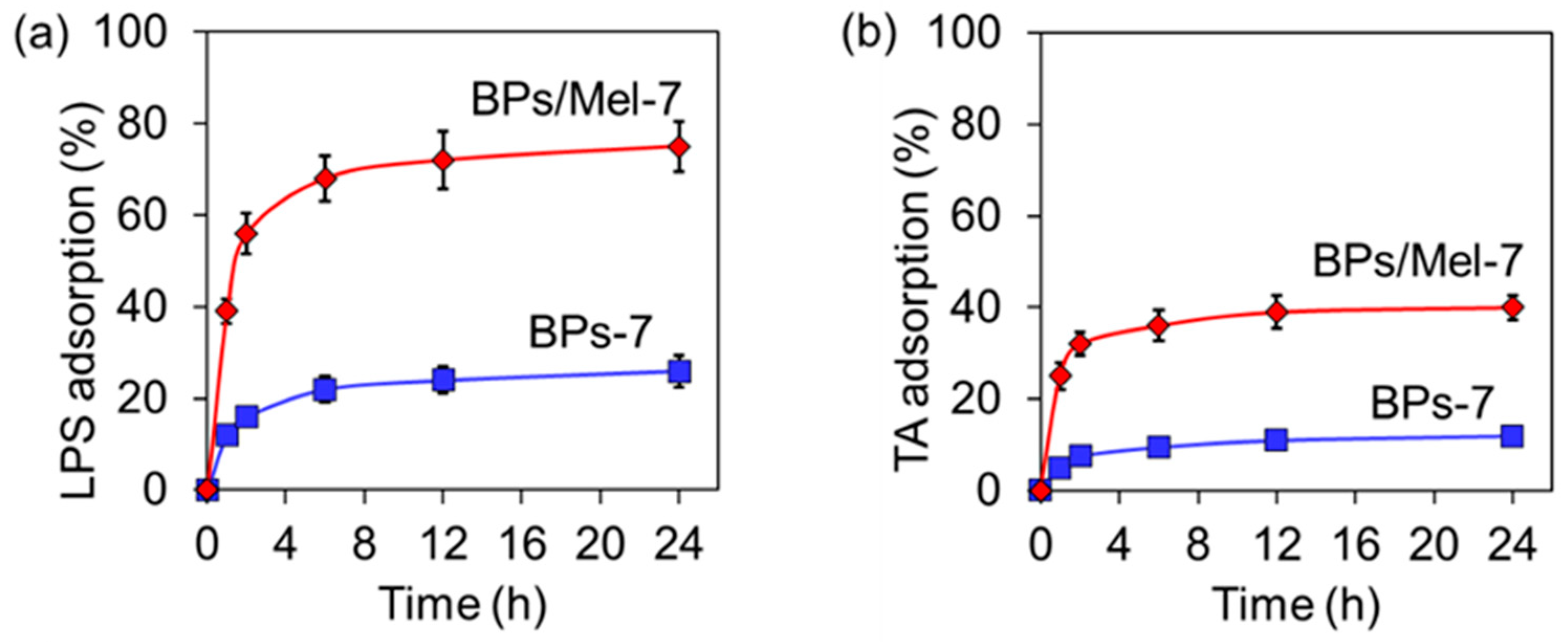
2.2. Antibacterial Activity of BPs/Mel
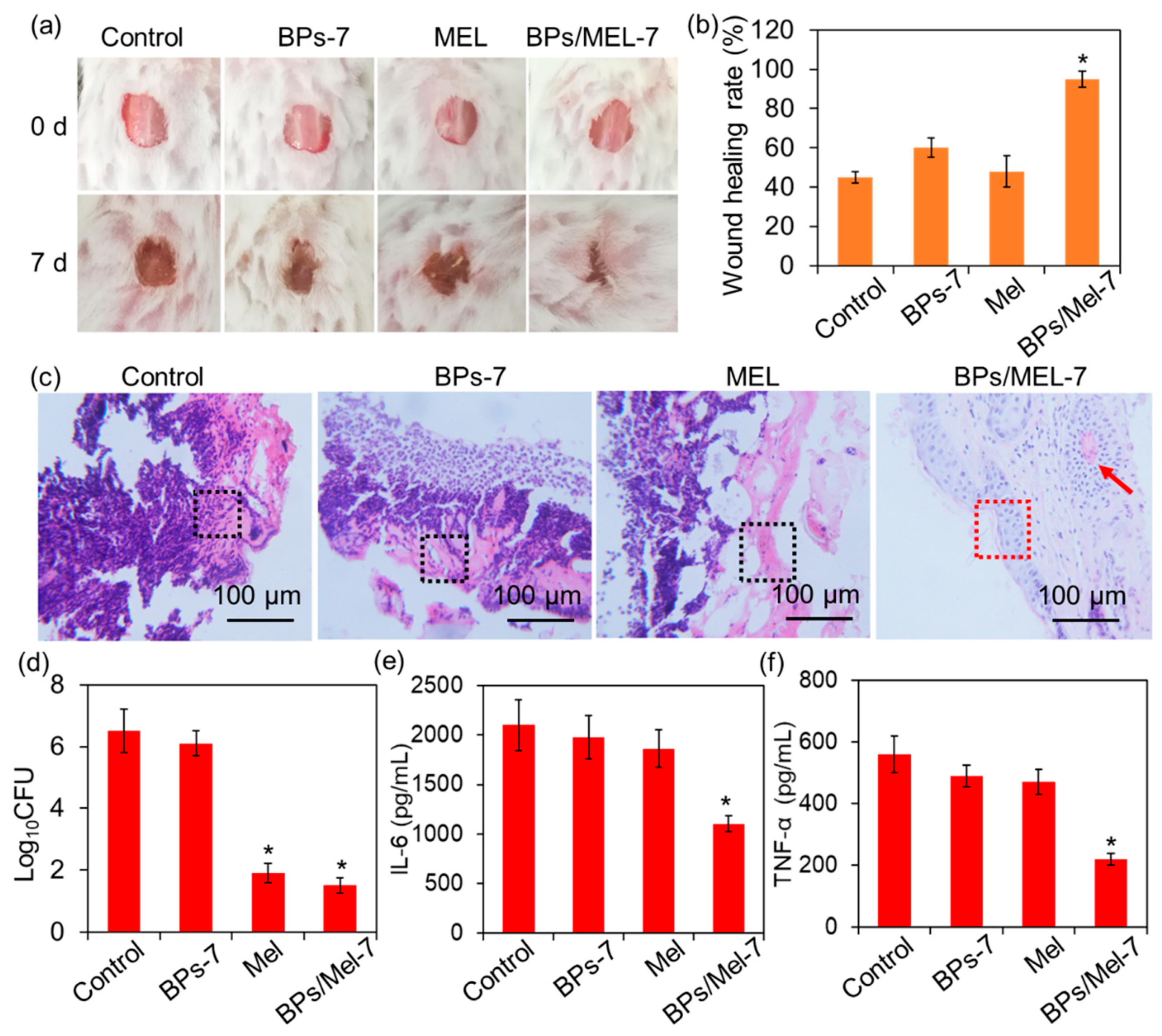
2.3. In Vivo Antibacterial Activity of BPs/Mel-7
3. Materials and Methods
3.1. Materials
3.2. Preparation of BP Nanosheets (BPs) and BPs/Mel with Different Sizes
3.3. Characterization
3.4. Quantitative Method for Loading Amount of Mel on BPs
3.5. Bacterial Culture
3.6. Growth Inhibition Assay
3.7. Cell Viability Test
3.8. In Vitro Antimicrobial Effect of BPs/Mel
3.9. Adsorption Assay
3.10. In Vivo Wound Healing Test
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, W.P.J.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial defences: Mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Pai, L.; Patil, S.; Liu, S.; Wen, F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: Insights from reviews on antibiotic resistance. Front. Cell. Infect. Microbiol. 2023, 13, 1327069. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial peptides: An alternative to antibiotic for mitigating the risks of Antibiotic resistance in aquaculture. Environ. Res. 2024, 251, 118619. [Google Scholar] [CrossRef]
- Li, G.; Lai, Z.; Shan, A. Advances of antimicrobial peptide-based biomaterials for the treatment of bacterial infections. Adv. Sci. 2023, 10, 2206602. [Google Scholar] [CrossRef]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological function of antimicrobial peptides on suppressing pathogens and improving host immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial peptides: Challenging journey to the pharmaceutical, biomedical, and cosmeceutical use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cai, X.; et al. Antibacterial nanomaterials: Mechanisms, impacts on antimicrobial resistance and design principles. Angew. Inter. Ed. Chem. 2023, 62, e202217345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Deng, Y.; Ren, Y.; Poon, H.L.; Chu, W.Y.; Wang, H.; Chan, Y.K. Antibiotics-free nanomaterials against bacterial keratitis: Eliminating infections with reactive oxygen species (ROS). Chem. Eng. J. 2024, 482, 148978. [Google Scholar] [CrossRef]
- Gao, B.; Ye, Q.; Ding, Y.; Wu, Y.; Zhao, X.; Deng, M.; Zhang, J.; Chen, M.; Zhang, Y.; Wei, X.; et al. Metal-based nanomaterials with enzyme-like characteristics for bacterial rapid detection and control. Coordin. Chem. Rev. 2024, 510, 215799. [Google Scholar] [CrossRef]
- Manivasagan, P.; Thambi, T.; Joe, A.; Han, H.; Seo, S.; Jeon, Y.; Conde, J.; Jang, E. Progress in nanomaterial-based synergistic photothermal-enhanced chemodynamic therapy in combating bacterial infections. Prog. Mater. Sci. 2024, 144, 101292. [Google Scholar] [CrossRef]
- Imperlini, E.; Massaro, F.; Buonocore, F. Antimicrobial peptides against bacterial pathogens: Innovative delivery nanosystems for pharmaceutical applications. Antibiotics 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, S.; Feng, W.; Shan, X.; Zhu, X.; Yuan, R.; Cao, Y.; Fan, L.; Yuan, B.; Wang, H.; et al. Antimicrobial peptide-modified liquid metal nanomaterials for enhanced antibacterial photothermal therapy. Adv. Eng. Mater. 2024, 26, 2400189. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, J.; Yang, H. Emerging low-dimensional black phosphorus: From physical-optical properties to biomedical applications. Sci. China Chem. 2023, 66, 406–435. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, C.; Zhang, X.; Kong, N.; Tao, W. Black phosphorus in biological applications: Evolutionary journey from monoelemental materials to composite materials. Acc. Mater. Res. 2021, 2, 489–500. [Google Scholar] [CrossRef]
- Sun, L.; Han, Y.; Zhao, Y.; Cui, J.; Bi, Z.; Liao, S.; Ma, Z.; Lou, F.; Xiao, C.; Feng, W.; et al. Black phosphorus, an advanced versatile nanoparticles of antitumor, antibacterial and bone regeneration for OS therapy. Front. Pharmacol. 2024, 15, 1396975. [Google Scholar] [CrossRef]
- Zhang, L.; You, J.; Lv, H.; Liu, M.; Quni, S.; Liu, X.; Zhou, Y. Black phosphorus—A rising star in the antibacterial materials. Int. J. Nanomed. 2023, 18, 6563–6584. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Zhang, Y.; Wu, C.; Li, L.; Hu, X.; Zhang, J.; Wang, Y. Antibacterial black phosphorus nanosheets for biomedical applications. J. Mater. Chem. B 2023, 11, 7069–7093. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhuang, S.; Qiu, H.; Guo, Y.; Wang, L.; Jin, G.; Lin, W.; Huang, G.; Yang, H. Black phosphorus nanosheets for killing bacteria through nanoknife effect. Part. Part. Syst. Charact. 2020, 37, 2000169. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, M.; Yu, S.; Wu, Q.; Ma, K.; Chen, Y.; Liu, X.; Li, C.; Wang, F. Silver-laden black phosphorus nanosheets for an efficient in vivo antimicrobial application. Small 2020, 16, 1905938. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, X.; Ge, Y.; Guo, Z.; Zeng, Z.; Xu, Q.; Zhang, H.; Yu, X.; Fan, D. Size-dependent nonlinear optical properties of black phosphorus nanosheets and their applications in ultrafast photonics. J. Mater. Chem. C 2017, 5, 3007–3013. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Cheng, G.; Ni, Y.; Xie, A.; Zhu, X.; Yin, C.; Zhang, Y.; Chen, T. Customized intranasal hydrogel delivering methylene blue ameliorates cognitive dysfunction against Alzheimer’s disease. Adv. Mater. 2024, 36, 2307081. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Xu, N.; Liu, W. The current landscape of the antimicrobial peptide melittin and its therapeutic potential. Front. Immunol. 2024, 15, 1326033. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Teng, L.; Ping, J. Graphitic carbon nitride confers bacterial tolerance to antibiotics in wastewater relating to ATP depletion. Molecules 2024, 29, 5780. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ji, Y.; Zhu, H.; Shi, Z.; Li, M.; Yu, Q. Gallium-based metal–organic frameworks loaded with antimicrobial peptides for synergistic killing of drug-resistant bacteria. J. Mater. Chem. B 2023, 11, 10446–10454. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, Y.; Zhang, J.; Yu, Q.; Liu, S.; Li, M. Synthesis of the ternary nanocomposites composed of zinc 2-methylimidazolate frameworks, lactoferrin and melittin for antifungal therapy. J. Mater. Sci. 2022, 57, 16809–16819. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zhang, Y.; Dong, A. Black phosphorus nanosheets counteract bacteria without causing antibiotic resistance. Chem. Eur. J. 2020, 26, 2478–2485. [Google Scholar] [CrossRef]
- Virgo, E.P.; Haidari, H.; Shaw, Z.L.; Huang, L.Z.Y.; Kennewell, T.L.; Smith, L.; Ahmed, T.; Bryant, S.J.; Howarth, G.S.; Walia, S.; et al. Layered black phosphorus nanoflakes reduce bacterial burden and enhance healing of murine infected wounds. Adv. Therap. 2023, 6, 2300235. [Google Scholar] [CrossRef]
- Javed, A.; Balhuizen, M.D.; Pannekoek, A.; Bikker, F.J.; Heesterbeek, D.A.C.; Haagsman, H.P.; Broere, F.; Weingarth, M.; Veldhuizen, E.J.A. Effects of Escherichia coli LPS structure on antibacterial and anti-endotoxin activities of host defense peptides. Pharmaceuticals 2023, 16, 1485. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Z.; Zhang, Y.; Li, Y.; Li, N.; Huang, D.; Xu, B. Interaction with teichoic acids contributes to highly effective antibacterial activity of graphene oxide on Gram-positive bacteria. J. Hazard. Mater. 2021, 412, 125333. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Wang, Y.; Yu, Q.; Li, M. Synthesis of antimicrobial peptide-grafted graphene oxide nanosheets with high antimicrobial efficacy. Mater. Lett. 2019, 235, 42–45. [Google Scholar] [CrossRef]
- Peng, L.; Wei, H.; Tian, L.; Xu, J.; Li, M.; Yu, Q. Phospholipid/protein co-mediated assembly of Cu2O nanoparticles for specific inhibition of growth and biofilm formation of pathogenic fungi. Sci. China Mater. 2021, 64, 759–768. [Google Scholar] [CrossRef]
- Ahmed, W.; Li, S.; Liang, M.; Kang, Y.; Liu, X.; Gao, C. Multifunctional drug- and AuNRs-loaded ROS-responsive Selenium-containing polyurethane nanofibers for smart wound healing. ACS Biomater. Sci. Eng. 2024, 10, 3946–3957. [Google Scholar] [CrossRef]
- Yu, J.R.; Varrey, P.; Liang, B.J.; Huang, H.-C.; Fisher, J.P. Liposomal SDF-1 alpha delivery in nanocomposite hydrogels promotes macrophage phenotype changes and skin tissue regeneration. ACS Biomater. Sci. Eng. 2021, 7, 5230–5241. [Google Scholar] [CrossRef] [PubMed]
- Andoy, N.M.O.; Jeon, K.; Kreis, C.T.; Sullan, R.M.A. Multifunctional and stimuli-responsive polydopamine nanoparticle-based platform for targeted antimicrobial applications. Adv. Func. Mater. 2020, 30, 2004503. [Google Scholar] [CrossRef]
| IC50 (μg/mL) | BPs-2 | BPs/Mel-2 | BPs-7 | BPs/Mel-7 | Mel |
|---|---|---|---|---|---|
| E. coli | >200 | 62.1 ± 2.1 | >200 | 19.1 ± 2.8 | 16.5 ± 2.2 |
| S. aureus | >200 | 53.2 ± 1.5 | >200 | 12.6 ± 1.3 | 11.8 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Shi, Z.; Teng, L.; Nie, J.; Zhang, L. Biocompatible Black Phosphorus Nanosheets-Antimicrobial Peptide Nanocomposites for Enhanced Anti-Infection Therapy. Molecules 2025, 30, 872. https://doi.org/10.3390/molecules30040872
Liu S, Shi Z, Teng L, Nie J, Zhang L. Biocompatible Black Phosphorus Nanosheets-Antimicrobial Peptide Nanocomposites for Enhanced Anti-Infection Therapy. Molecules. 2025; 30(4):872. https://doi.org/10.3390/molecules30040872
Chicago/Turabian StyleLiu, Shuo, Zhishang Shi, Lin Teng, Junlian Nie, and Libing Zhang. 2025. "Biocompatible Black Phosphorus Nanosheets-Antimicrobial Peptide Nanocomposites for Enhanced Anti-Infection Therapy" Molecules 30, no. 4: 872. https://doi.org/10.3390/molecules30040872
APA StyleLiu, S., Shi, Z., Teng, L., Nie, J., & Zhang, L. (2025). Biocompatible Black Phosphorus Nanosheets-Antimicrobial Peptide Nanocomposites for Enhanced Anti-Infection Therapy. Molecules, 30(4), 872. https://doi.org/10.3390/molecules30040872







