Abstract
Soluble organics (SBC-L) from Santanghu bituminous coal (SBC) were obtained by extracting the coal with a mixed solvent of CS2 and acetone (v/v′ = 1:1). Catalytic hydrogenation of SBC-L was carried out using isopropanol as the solvent and prepared bimetallic material (Ni-Mo/γ-Al2O3) as the catalyst, and the hydrogenation product (SBC-LIP320) was obtained. Gas chromatography-mass spectrometry (GC-MS) was used to compare the difference in the composition and distribution of SBC-L and SBC-LIP320; thus, the effect of the used catalyst on the hydrogenation performance and heteroatom removal of SBC-L can be investigated. Results showed that the organic compounds in SBC-L and SBC-LIP320 could be classified into aliphatic hydrocarbons (AHS), arenes, oxygen-containing organic compounds (OCOCs), nitrogen-containing organics (NCOCs), and compounds containing other heteroatoms (OHACOCs). The relative contents of AHS and arenes detected in SBC-LIP320 were higher than those of SBC-L, while the contents of OCOCs, NCOCs, and OHACOCs decreased, and no S-containing compounds could be detected in SBC-LIP320. It can be concluded that the prepared catalyst presents good de-oxygenation, de-sulfurization, de-nitrogenation, and hydrocracking performance.
1. Introduction
Coal hydrogenation liquefaction technology is one of the important technologies to solve China’s future energy issues. This technology can achieve a high conversion rate and oil yield under mild conditions, providing a new way for the efficient graded conversion and utilization of low-rank coal [1,2,3]. The Santanghu coalfield is an important coal production and processing base in the Hami region of Xinjiang, China, and the coal type is mainly long-flame coal (one of the low-rank coals), with the coalification degree slightly higher than that of lignite. How to remove coal-based soluble heteroatom-containing organic compounds, such as various forms of heteroatoms (O, N, and S) in coal-based liquid fuel, has become one of the keys to the efficient utilization of low-rank coal [4,5].
Solvent extraction is an effective method for obtaining soluble organic compounds from coal, which makes it possible to transition low-rank coal from utilization characterized as “high pollution, low efficiency, and low added value” to one that is “clean, efficient, and high in added value” [6,7,8]. Ya-Ya Ma et al. [9] used petroleum ether, methanol, carbon disulfide, and other solvents to perform sequential five-stage extraction for Hefeng (Xinjiang) sub-bituminous coal. Results showed that methanol gave a higher extraction rate, while the isometric carbon disulfide/acetone mixture solvent was more conducive to the dissolution and diffusion of alcohol compounds. Catalytic hydrogenation deoxygenation of isopropanol-soluble organic compounds from Dongming lignite was also investigated by this group. Results showed that the Co-Mo/γ-Al2O3 catalyst for the conversion of soluble compounds could achieve the effects of hydrogenation, hydrocracking, and heteroatom removal [10].
In the process of coal hydrogenation, the catalyst plays an essential role. On the one hand, catalysts can improve reaction rate and reduce reaction temperature and pressure. On the other hand, the addition of appropriate catalysts can remove some heteroatoms (S and N) to reduce environmental pollution. More importantly, the addition of a catalyst can improve the cracking and conversion of coal to obtain high-added-value liquid fuel oils and chemical products [11,12]. Common coal hydrogenation catalysts include metal catalysts, metal oxide catalysts, metal sulfide catalysts, acidic catalysts, and basic catalysts, etc. [13,14,15,16,17,18]. Efficient and low-cost catalysts are conducive to the removal of heteroatoms in the conversion of low-rank coal. Qi S C et al. [19] used an in situ de-composition method to load carbonyl nickel onto an HZSM-5 carrier to prepare a highly active Ni/HZSM-5 catalyst, which was used for deep hydrogenation and removal of heavy aromatic hydrocarbons (HAs) from coal tar to obtain clean liquid fuel. Kang Y H et al. [20] synthesized a nickel-based Y/ZCM-5 catalyst and applied it to the catalytic hydrogenation of Hecaogou sub-bituminous coal, resulting in clean liquid fuel, primarily composed of alkanes, alkenes, and hydrogenated aromatics. Li W T et al. [21] used prepared Ni/Z5A catalyst for the catalytic hydrogenation of methanol-soluble organic compounds from Xiaolongtan lignite, and it is found that the catalyst played a dual role in breaking >CH-O- and hydrogenating aromatics.
The above results indicate that by obtaining soluble organic compounds through extraction or thermal dissolution, followed by catalytic hydrogenation and heteroatom removal of the soluble organics, it is possible to produce clean liquid fuels, high-density fuels, base oils for high-grade lubricants, as well as high value-added chemicals. However, how to enhance the efficiency of the extraction and catalytic hydrogenation process to obtain clean fuels or value-added chemicals warrants further exploration.
In this work, soluble organic compounds (SBC-L) from Santanghu bituminous coal (SBC) were obtained by extracting the coal with a mixed solvent of CS2 and acetone (v/v = 1:1). Low-boiling-point and low-viscosity isopropanol was selected as the hydrogenation solvent, and gas chromatography-mass spectrometry (GC-MS) was used to compare the difference in the composition and distribution of soluble organic matter (SBC-L) and its hydrogenation products (SBC-LIP320). Thus, the influence of prepared Ni-Mo/γ-Al2O3 bimetallic catalyst on the catalytic hydrogenation of soluble organic matter, especially the removal of heteroatoms, could be explored.
2. Materials and Methods
2.1. Materials
The coal sample (SBC) was selected from the Santanghu coal mine in Hami, Xinjiang, China. After crushing, it was sifted through a 200-mesh sieve (<75 μm) and dried naturally for 24 h. Proximate and ultimate analyses of SBC are shown in Table 1 [22]. All the solvents are analytical reagents and are distilled in a rotary evaporator before use.

Table 1.
Proximate and Ultimate analyses (wt.%) of SBC.
2.2. SBC-L from SBC
Under ultrasonic conditions, a mixed solvent of carbon disulfide (CS2) and acetone (v/v′ = 1:1) is used to extract and separate the easily soluble organic components from the coal sample, resulting in the extraction yield of 5.05%.
2.3. Preparation of Ni-Mo/γ-Al2O3 Bimetallic Catalyst
As shown in Figure 1, a mixed solution was formed by dissolving (NH4)6Mo7O24·4H2O and Ni(NO3)2 (Ni:Mo = 1:2.4) in an appropriate amount of deionized water using the impregnation method. γ-Al2O3 was added with Ni/Mo loading of 10 wt.%, standing for 12 h, dried at 110 °C for 12 h, and calcined in a tube furnace at 550 °C for 4 h to obtain Ni-Mo/γ-Al2O3 bimetallic catalyst.
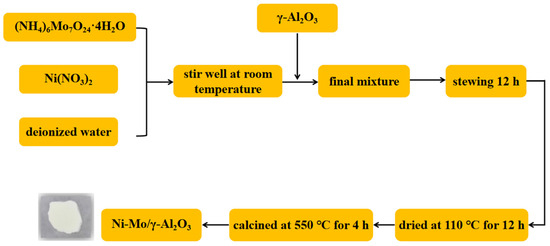
Figure 1.
Preparation process of the catalyst.
2.4. Catalytic Hydrogenation
As shown in Figure 2, 2 g of dried SBC-L, 0.2 g of Ni-Mo/γ-Al2O3 catalyst, and 20 mL of isopropanol (IP) were added into the autoclave. After purging the autoclave with H2 to displace air three times, the autoclave was heated to 320 °C under an initial H2 pressure of 0.4 MPa and maintained for 2 h. Upon completion of the hydrogenation process, the autoclave was cooled to room temperature, and the mixture was filtered to separate into filtrate and residue. The residue was washed repeatedly with IP until the filtrate became colorless. The used solvent was recycled by rotary evaporation, and the hydrogenation products would also be obtained, which is labeled as SBC-LIP320.
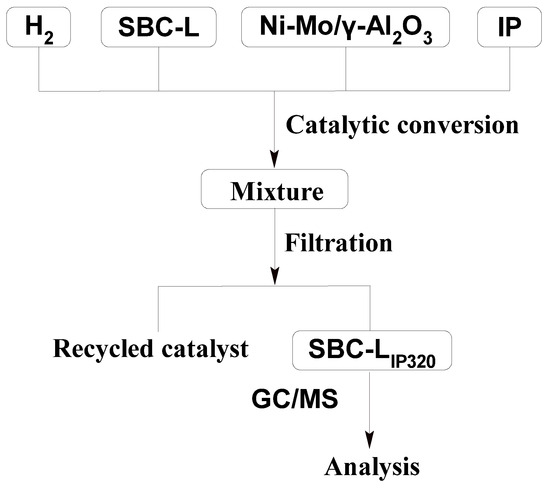
Figure 2.
Catalytic hydrogenation process for SBC-L.
2.5. Analytical Methods
The products SBC-L and SBC-LIP320 were analyzed using Agilent 7890A/5795C GC/MS. The instrument was equipped with a DB-17MS (30.0 m × 250 μm, 0.25 μm) chromatographic column, and high-purity helium gas (1 mL/min) was used as the carrier gas. The inlet initial temperature was set as 60 °C for 3 min, followed by a ramp rate of 10 °C/min up to 300 °C for 10 min. The split ratio was 20:1, and the mass scan range was set from 30 to 500 amu.
3. Results and Discussion
3.1. Composition Characteristics of SBC-L
Figure 3 and Figure 4 show the total ion chromatogram of SBC-L, with a retention time of 2–28 min selected. The number of compounds detected in SBC-L by GC/MS is 38, which can be divided into 5 categories: alkanes, aromatics, oxygen-containing organic compounds (OCOCs), nitrogen-containing compounds (NCOCs), and other heteroatomic-containing organics (OHACOCs).
Figure 3.
Total ion chromatogram of SBC-L.
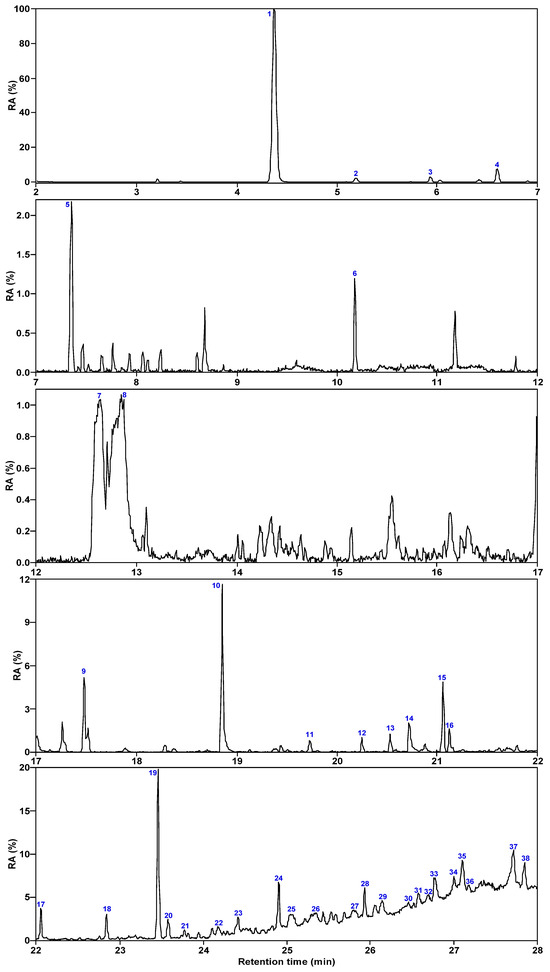
Figure 4.
Relative content of each component in SBC-L.
Table 2 lists all the organic compounds detected in SBC-L along with their relative contents. SBC-L mainly contains 2 types of alkanes, 13 types of aromatic hydrocarbons, 13 types of oxygen-containing organic compounds, 8 types of nitrogen-containing compounds, and 2 types of organics with other heteroatoms. Among them, aromatic hydrocarbons are primarily dominated by polycyclic aromatics (relative content of 9.48%), followed by monocyclic ones (6.16%). The relative content of NCOCs (15.07%) is high, and nitrogen in these compounds is present in the form of C-N bonds. And OHACOCs (3.61%) are low in content with sulfur-containing compounds dominating.

Table 2.
Organic compounds detected in SBC-L by GC/MS.
Figure 5 presents the distribution of the group composition of SBC-L. As can be seen from the figure, SBC-L is predominantly composed of oxygen-containing compounds with a relative content of 63.41%, followed by aromatic hydrocarbons (15.64%). Among them, the oxygen-containing organics mainly include alcohols, aldehydes, ketones, esters, and carboxylic acids, with aldehydes being the dominant group (46.07%).
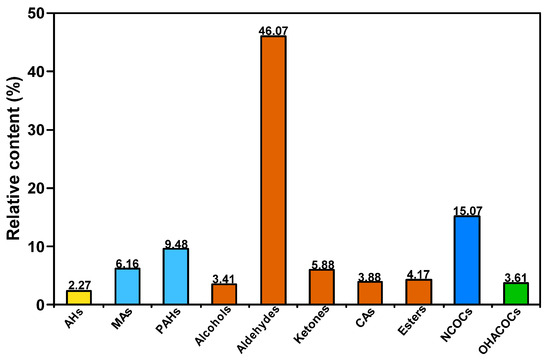
Figure 5.
Distribution of group composition in SBC-L.
3.2. Composition Characteristics of SBC-LIP320
Figure 6 and Figure 7 present the total ion flow chromatograms of SBC-LIP320 with a retention time of 2–30 min. Through GC/MS analysis, 72 organic compounds were identified in SBC-LIP320, including alkanes, aromatic hydrocarbons, OCOCs, and NCOCs.
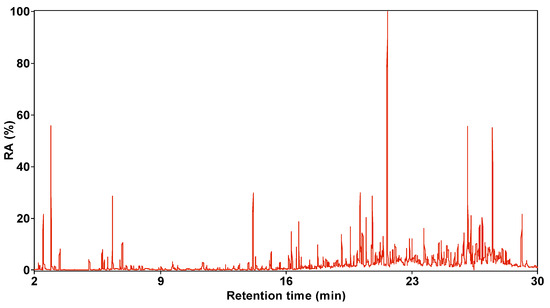
Figure 6.
Total ion chromatogram of SBC-LIP320.
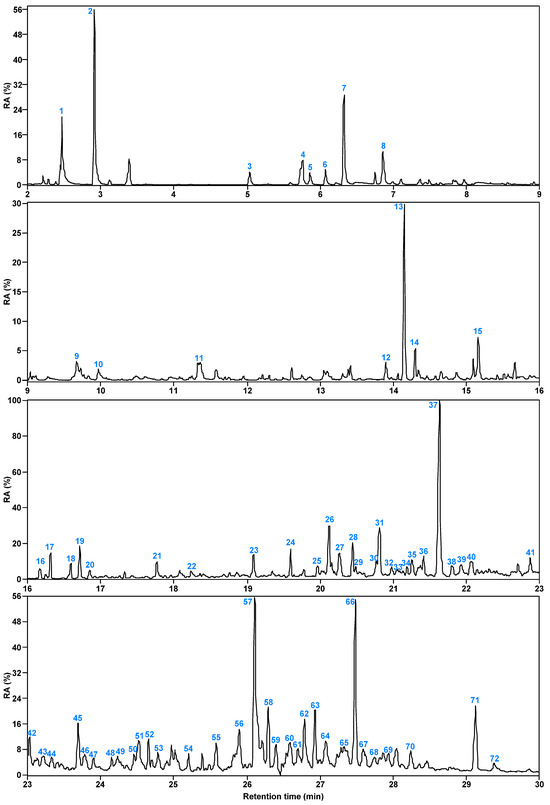
Figure 7.
Relative content of each compound in SBC-LIP320.
Table 3 lists the organic compounds detected in SBC-LIP320 along with their relative contents. According to the table, SBC-LIP320 contains 11 alkanes, 26 aromatic hydrocarbons, 29 OCOCs, and 6 NCOCs. Among them, hydrocarbons are primarily alkenes with a relative content of 14.91%, followed by alkanes (4.14%); aromatic hydrocarbons are mainly polycyclic aromatics (25.44%), followed by monocyclic ones (3.83%).

Table 3.
Organic compounds detected in SBC-LIP320 by GC/MS.
Figure 8 provides the distribution of the group composition of SBC-LIP320. As can be seen from the figure, OCOCs are predominant in SBC-LIP320 (relative content of 42.07%), followed by aromatic hydrocarbons (29.27%). The OCOCs group includes alcohols, phenols, ethers, aldehydes, ketones, esters, and carboxylic acids, with carboxylic acids (19.26%) being the higher content group.
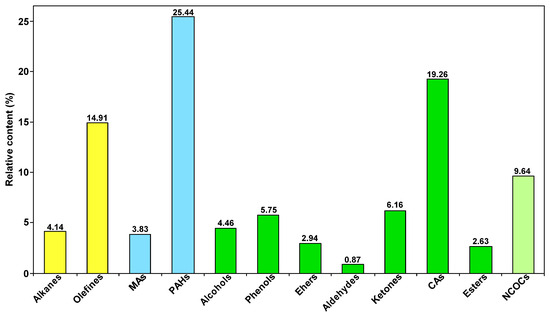
Figure 8.
Distribution of group composition in SBC-LIP320.
Figure 9 presents the distributions of the group composition of SBC-L and its catalytically hydrogenated products, SBC-LIP320. As shown in the figure, compared to SBC-L, the contents of alkanes and aromatic hydrocarbons detected in SBC-LIP320 increased, while the contents of OCOCs, NCOCs, and OHACOCs decreased, indicating that the catalyst of Ni-Mo/γ-Al2O3 might improve side-chain cracking and ring-opening reactions, increasing the contents of alkanes and arenes. The content of OCOCs shows the most significant change, indicating that Ni-Mo/γ-Al2O3 could effectively break C-O and/or C=O bonds. Thus, catalytic hydrogenolysis and hydrogenative deoxygenation and denitrogenation can simultaneously convert the oxygen- and nitrogen-containing organic compounds (in SBC-L) into other products (alkanes and arenes) and effectively remove the heteroatoms.
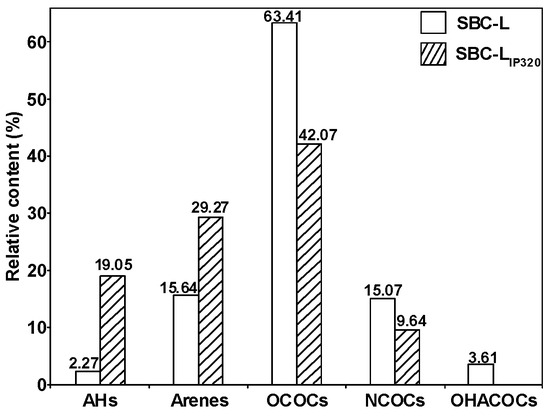
Figure 9.
Distribution of group compositions for SBC-L and SBC-LIP320.
The scheme of the catalytic transformation process for SBC-L is shown in Figure 10. For compounds containing one or more oxygen atoms, the cleavage of carbon–oxygen and carbon–carbon bonds can induce the formation of new compounds, which may be one of the reasons for the increase in the content of aromatic hydrocarbons. At the same time, the low initial hydrogen pressure (0.4 MPa) might be beneficial for the breakage of bridge bonds between aromatic rings, contributing to the increase in arenes.
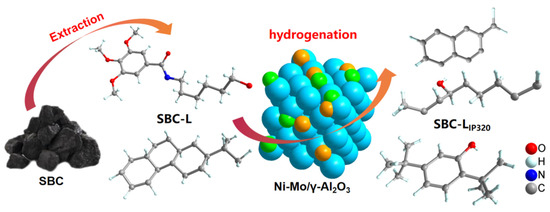
Figure 10.
Schematic diagram of the catalytic hydrogenation process by Ni-Mo/γ-Al2O3.
Figure 11 illustrates the potential catalytic hydrogenation pathways for quinoline and thiophene groups in SBC-L. The quinoline group primarily undergoes transformation through two possible pathways [23]. One pathway involves the initial hydrogenation of the nitrogen ring in quinoline, followed by an aromatic ring, and followed by ring-opening and removal of the nitrogen atom, resulting in the formation of alkanes. Other pathways involve firstly the hydrogenation of the aromatic ring, followed by its opening, after which the nitrogen ring is hydrogenated, and the nitrogen-containing bond, C-N, is broken to produce aromatic hydrocarbons. Further hydrogenation in this process could lead to the formation of alkanes. The catalytic hydrogenation process of the thiophene group might probably begin with a hydrogenation process [24,25], followed by the cleavage of the C-S bond, leading to the formation of alkanes and H2S resulting in the increase in alkanes in SBC-LIP320.
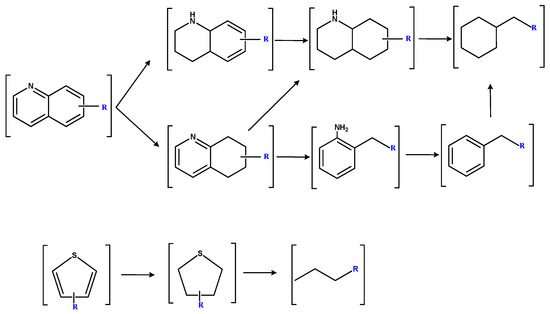
Figure 11.
Possible hydrogenation pathways for quinoline and thiophene groups in SBC-L [22,23,24].
4. Conclusions
In this paper, soluble compounds (SBC-L) from Santanghu bituminous coal (SBC) were extracted with a mixed solvent, and its catalytic hydrogenation products (SBC-LIP320) were obtained by the Ni-Mo/γ-Al2O3 catalyst. Both SBC-L and SBC-LIP320 are primarily composed of alkanes, aromatic hydrocarbons, oxygen-containing compounds (OCOCs), nitrogen-containing organics (NCOCs), and other heteroatom-containing compounds (OHACOCs). Compared to SBC-L, the content of alkanes and aromatic hydrocarbons detected in SBC-LIP320 increased, which may be derived from the ability of the Ni-Mo/γ-Al2O3 catalyst to hydrogenate the bridge bonds between aromatic rings, leading to the formation of alkanes and arenes. Meanwhile, the relative content of OCOCs, NCOCs, and OHACOCs decreased obviously, and no sulfur-containing compounds were detected, suggesting that the Ni-Mo/γ-Al2O3 catalyst can effectively remove oxygen, sulfur, and nitrogen atoms, presenting a good impurity removal effect. The obtained SBC-LIP320 has the potential to be used in the production of clean liquid fuels, high-grade lubricant base oils, and high-value chemicals. However, further research is needed to explore more efficient methods for producing high-purity and high-value chemicals.
Author Contributions
Conceptualization, Y.M.; Methodology, Y.M. and X.F.; Formal analysis, G.Z. and M.Z.; Resources, A.N., W.M. and S.H.; Data curation, J.G., Y.R. and A.A.; Writing-original draft, J.G.; Writing—review & editing, G.Z. and Y.M.; Supervision, A.N., X.W. and W.M.; Project administration, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the special project for regional collaborative innovation of Xinjiang Uyghur Autonomous Region (2022E01057), Tianshan Talents Project of Xinjiang (20243123968), and the science and technology project of Hami (HMKJJH202307).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors acknowledge Xinjiang University for providing the experimental platform.
Conflicts of Interest
Authors Jia Guo was employed by the company Xinjiang Energy Co., Ltd. and Shihao Hao was employed by the company Hami Quality and Metrology Testing Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Nomenclature
| RC | Relative content |
| CHC | Catalytic hydrogenation |
| AHs | Aliphatic hydrocarbon |
| MAs | Monocyclic aromatics |
| PAHs | Polycyclic aromatic hydrocarbon |
| CAs | Carboxylic acids |
| OCOCs | Oxygenated compounds |
| NCOCs | Nitrogen-containing organic compounds |
| OHACOCs | Compounds containing other heteroatoms |
References
- He, X.Q.; Mo, W.L.; Wang, Q.; Ma, Y.Y.; Ma, F.Y.; Fan, X.; Wei, X.Y. Effect of swelling treatment by organic solvent on the structure and pyrolysis performance of the direct coal liquefaction residue. Energy Fuels 2020, 34, 8685–8696. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, W.; Liu, G.H.; An, H.D.; Ma, Y.J.; Kang, Y.H.; Gao, Y.; Bai, N.; Wang, D.K.; Wei, X.Y.; et al. Compositional changes of CH, O, and N moieties in petroleum ether-extractable portions from the three-step mild degradation of Dongming lignite. Fuel 2023, 336, 127132. [Google Scholar] [CrossRef]
- Wei, X.Y.; Bai, X.; Ma, F.Y.; Zong, Z.M.; Zhao, W.; Ni, Z.H.; Fan, X.; Sun, L.B.; Cao, J.P.; Zhao, Y.P.; et al. Advances in Catalytic Hydroconversion of Typical Heavy Carbon Resources under Mild Conditions. Energy Fuels 2023, 37, 12570–12588. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.Y.; Shao, C.W.; Li, Z.; Li, J.H.; Fan, Z.C.; Lu, K.L.; Liu, F.J.; Kong, Q.Q.; Zhao, J.; et al. Catalytic hydroconversion of an organic waste oil to cyclanes over a supported nickel catalyst. Fuel 2023, 331, 125679. [Google Scholar] [CrossRef]
- Zou, H.X.; Bai, X.; Fan, X.; Wang, M.H.; Xu, Y.Y.; Ma, F.Y.; Wei, X.Y.; Kuznetsov, P.N. Infrared spectroscopic evaluation for catalytic hydrogenation of biomass and coal using unsupervised and supervised algorithms. Fuel 2023, 353, 129211. [Google Scholar] [CrossRef]
- Zou, L.; Jin, L.; Li, Y.; Zhu, S.; Hu, H. Effect of tetrahydrofuran extraction on lignite pyrolysis under nitrogen. J. Anal. Appl. Pyrolysis 2015, 112, 113–120. [Google Scholar] [CrossRef]
- Zhu, P.; Luo, A.; Zhang, F.; Lei, Z.; Zhang, J.; Zhang, J. Effects of extractable compounds on the structure and pyrolysis behaviours of two Xinjiang coals. J. Anal. Appl. Pyrolysis 2018, 133, 128–135. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.; Lv, P.; Ren, L.; Kong, J.; Wang, J.; Li, F.; Bai, Y. Effect of n-hexane extraction on the formation of light aromatics from coal pyrolysis and catalytic upgrading. J. Energy Inst. 2020, 3, 1242–1249. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Ma, F.Y.; Mo, W.L.; Wang, Q. Five-stage sequential extraction of Hefeng coal and direct liquefaction performance of the extraction residue. Fuel 2020, 266, 117039. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Fan, X.; Mo, W.L.; Li, G.S.; Ma, F.Y.; Wei, X.Y. Catalytic hydrogenation and heteroatom removal for isopropanol soluble organic matter of Dongming lignite. Fuel Process. Technol. 2021, 211, 106589–106595. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Fan, X.; Mo, W.L.; Muhammad, T.; Bai, X.; Saikia, B.K.; Wei, X.Y.; Ma, F.Y. Advanced separation of soluble organic matter in a low-rank coal and evaluation using unsupervised analyses. Fuel 2022, 328, 125212. [Google Scholar] [CrossRef]
- Liu, G.H.; Zong, Z.M.; Liu, F.J.; Ma, Z.H.; Wei, X.Y.; Kang, Y.H.; Fan, X.; Ma, F.Y.; Liu, J.L.; Mo, W.L. Two-step catalytic degradations of Dahuangshan lignite and directional upgrading of the resulting petroleum ether-extractable portions. Energy Fuel 2020, 34, 5457–5465. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, W.X.; Bai, J.J.; Zhu, Q.Y.; Li, X.L.; Liu, G.H.; Wei, X.Y.; Kang, Y.H.; Li, Y.J.; Ma, X.R.; et al. Investigation on the catalytic performance of magnetic copper ferrite nanoparticles in the catalytic hydroconversion of Hanglaiwan long flame coal. Fuel 2023, 353, 129173. [Google Scholar] [CrossRef]
- Xie, T.; Cao, J.-P.; Zhu, C.; Zhao, X.-Y.; Zhao, M.; Zhao, Y.-P.; Wei, X.-Y. Selective cleavage of C-O bond in benzyl phenyl ether over Pd/AC at room temperature. Fuel Process. Technol. 2019, 188, 190–196. [Google Scholar] [CrossRef]
- Li, X.K.; Zong, Z.M.; Chen, Y.F.; Yang, Z.; Liu, G.H.; Liu, F.J.; Wei, X.Y.; Wang, B.J.; Ma, F.Y.; Liu, J.M. Catalytic hydroconversion of Yinggemajianfeng lignite over difunctional NiMg2Si/γ-Al2O3. Fuel 2019, 249, 496–502. [Google Scholar] [CrossRef]
- Liu, X.X.; Zong, Z.M.; Li, W.T.; Li, X.; Li, Z.K.; Wang, S.K.; Wei, X.Y. A recyclable and highly active magnetic solid superbase for hydrocracking C-O bridged bonds in sawdust. Fuel Process. Technol. 2017, 159, 396–403. [Google Scholar] [CrossRef]
- Xu, M.L.; Wei, X.Y.; Wu, Q.C.; Zhao, Y.P.; Li, F.H.; Liu, G.H.; Liu, F.Z.; Zong, Z.M.; Zhang, M.; Li, S.; et al. Enhanced hydrogenation of aromatic rings and hydrocracking of >Car-O bridged bonds in the extraction residue from Piliqing subbituminous coal over a magnetic difunctional solid superbase. J. Anal. Appl. Pyrol. 2020, 146, 104695. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wei, X.Y.; Liu, F.J.; Liu, G.H.; Zong, Z.M. Catalytic hydroconversion of Yiwu lignite over solid superacid and solid superbase. Fuel 2019, 238, 473–482. [Google Scholar] [CrossRef]
- Qi, S.C.; Wei, X.Y.; Zong, Z.M.; Hayashi, J.I.; Yuan, X.H.; Sun, L.B. A highly active Ni/ZSM-5 catalyst for complete hydrogenation of polymethylbenzenes. Chem. Cat. Chem. 2013, 5, 3543–3547. [Google Scholar] [CrossRef]
- Kang, Y.H.; Wei, X.Y.; Liu, G.H.; Gao, Y.; Li, Y.J.; Ma, X.R.; Zhang, Z.F.; Zong, Z.M. Catalytic hydroconversion of soluble portion in the extraction from Hecaogou subbituminous coal to clean liquid fuel over a Y/ZSM-5 composite zeolite-supported nickel catalyst. Fuel 2020, 269, 117326. [Google Scholar] [CrossRef]
- Li, W.T.; Wei, X.Y.; Liu, X.X.; Guo, L.L.; Qi, S.C.; Li, Z.K.; Zhang, D.D.; Zong, Z.M. Catalytic hydroconversion of methanol-soluble portion from Xiaolongtan lignite over difunctional Ni/Z5A. Fuel Process. Technol. 2016, 148, 146–154. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, M.; Mo, W.; Wang, Y.; Yuan, J.; Wu, R.; Niu, J.; Ma, K.; Guo, W.; Wei, X.; et al. Effect of Solvent Treatment on the Composition and Structure of Santanghu Long Flame Coal and Its Rapid Pyrolysis Products. Molecules 2023, 28, 7074. [Google Scholar] [CrossRef] [PubMed]
- Cocchetto, J.F.; Satterfield, C.N. Chemical equilibriums among quinoline and its reaction products in hydrodenitrogenation. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 49–53. [Google Scholar] [CrossRef]
- Yao, X.Q.; Li, Y.W.; Jiao, H.J. Mechanistic aspects of catalyzed benzothiophene hydrodesulfurization. A density functional theory study. J. Mol. Struct. THEOCHEM 2005, 726, 67–80. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Okuhara, T. Regulation of intermediates on sulfide nickel and MoS2 catalysts. Catal. Rev.-Sci. Eng. 1997, 15, 249–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).