Catalytic Hydrogenation and Heteroatom Removal for the Soluble Organics from Santanghu Bituminous Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
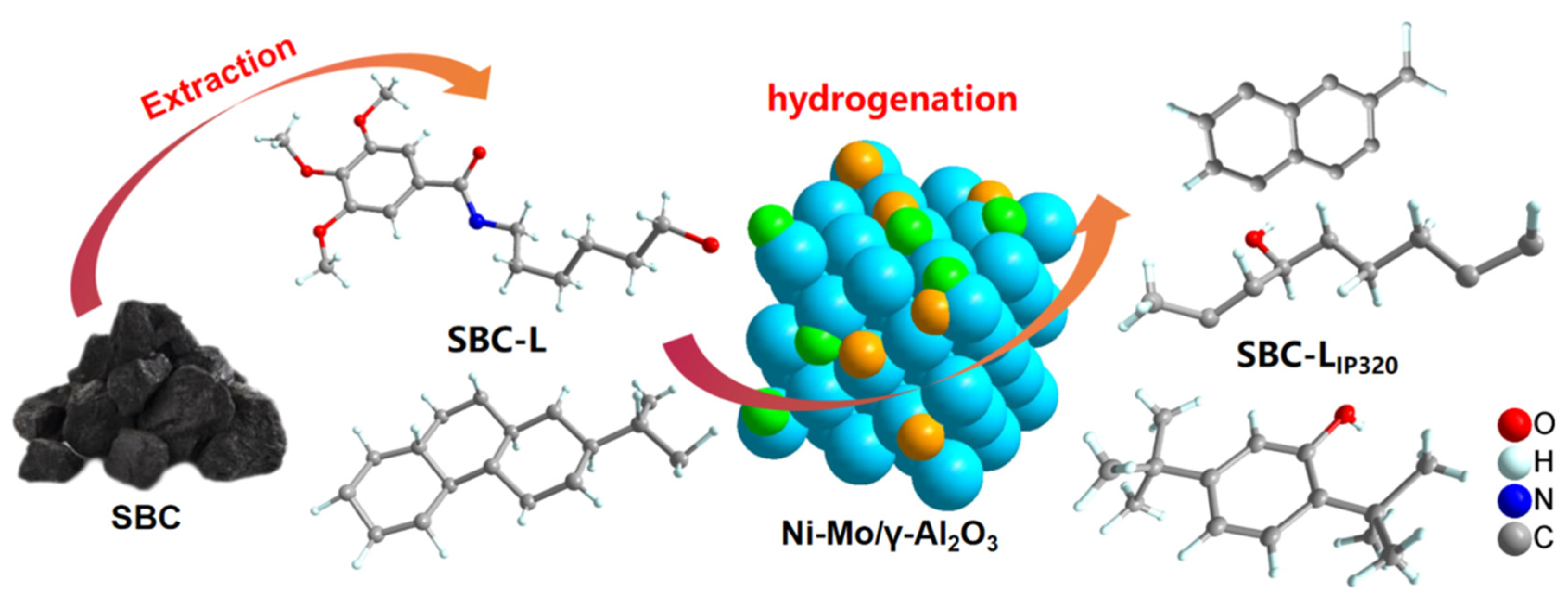
2.2. SBC-L from SBC
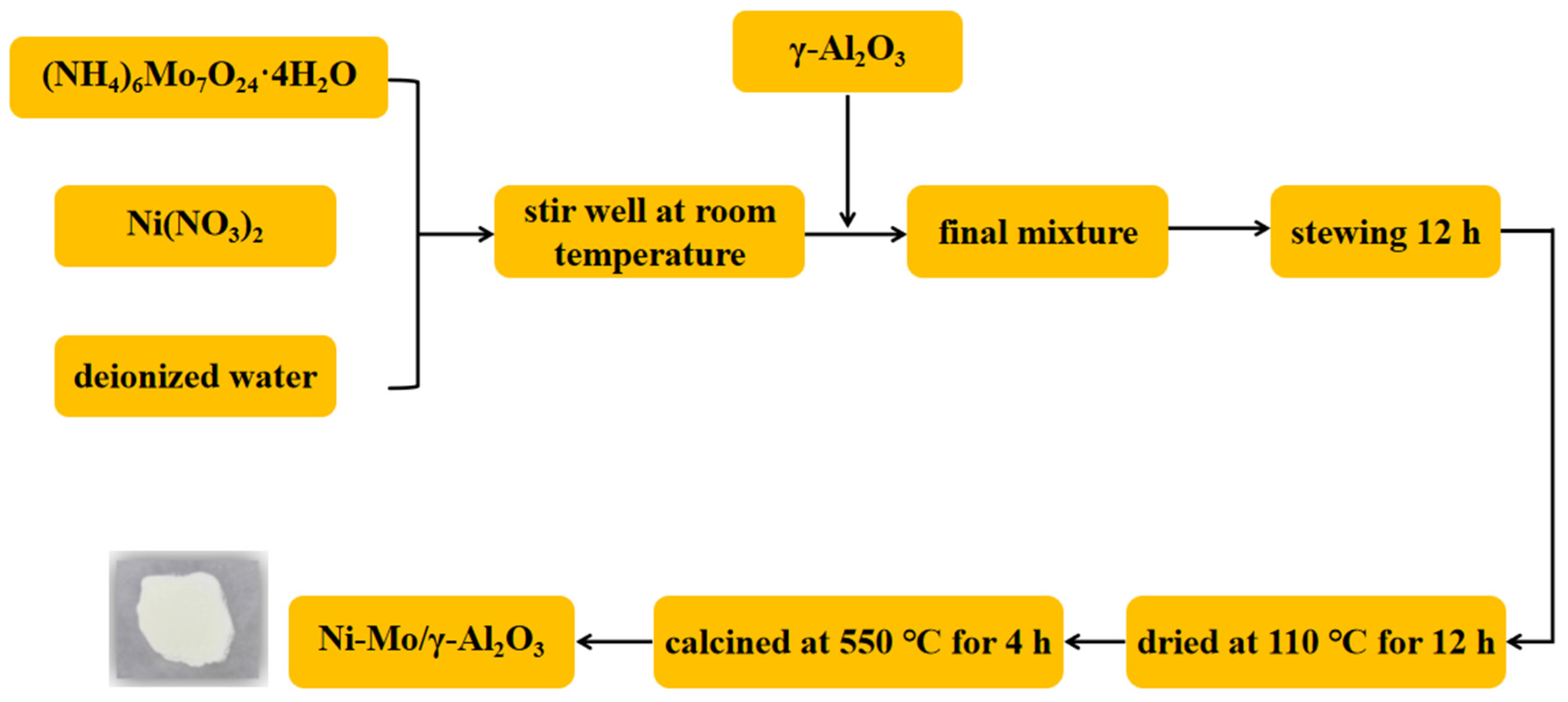
2.3. Preparation of Ni-Mo/γ-Al2O3 Bimetallic Catalyst
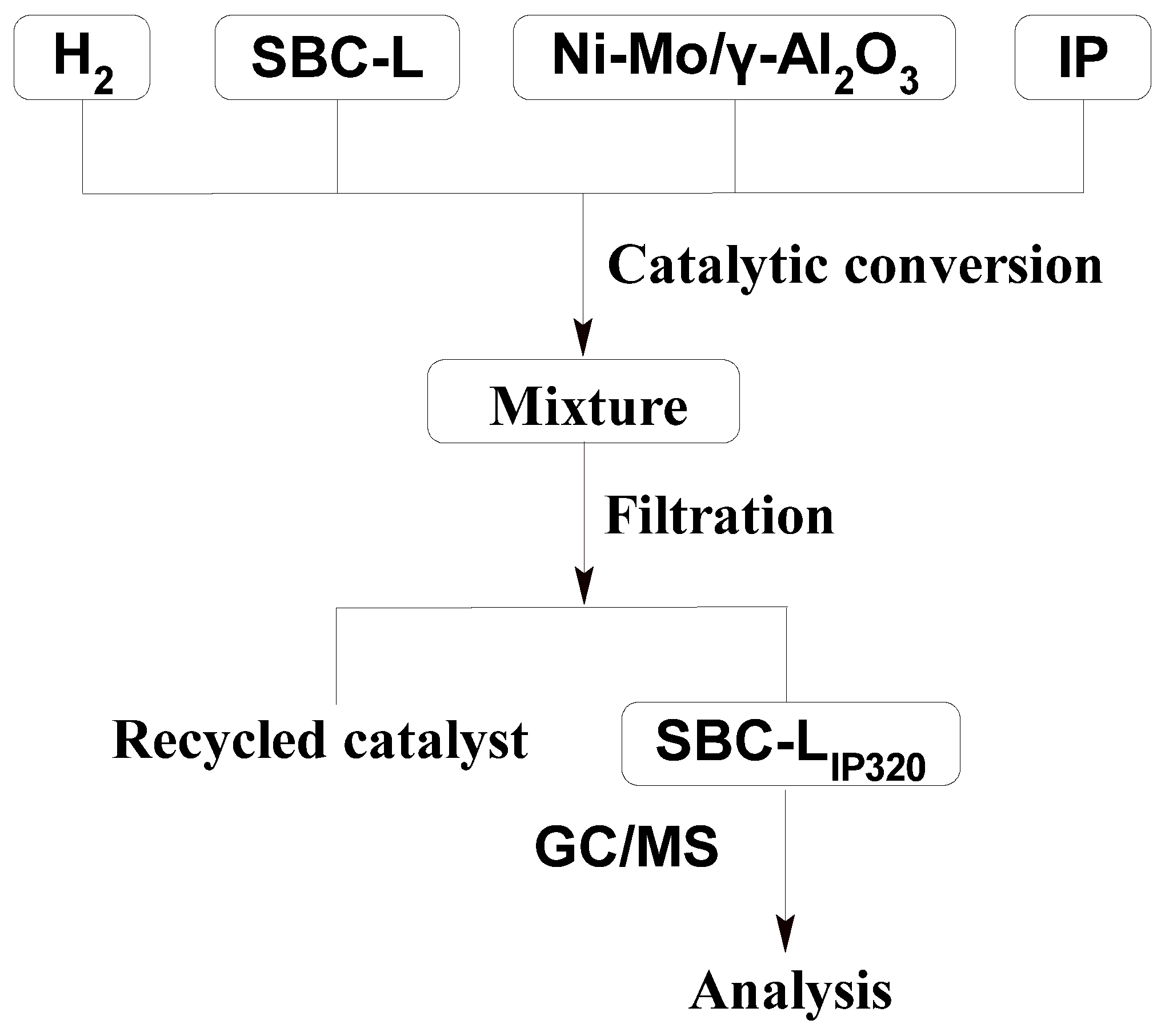
2.4. Catalytic Hydrogenation
2.5. Analytical Methods
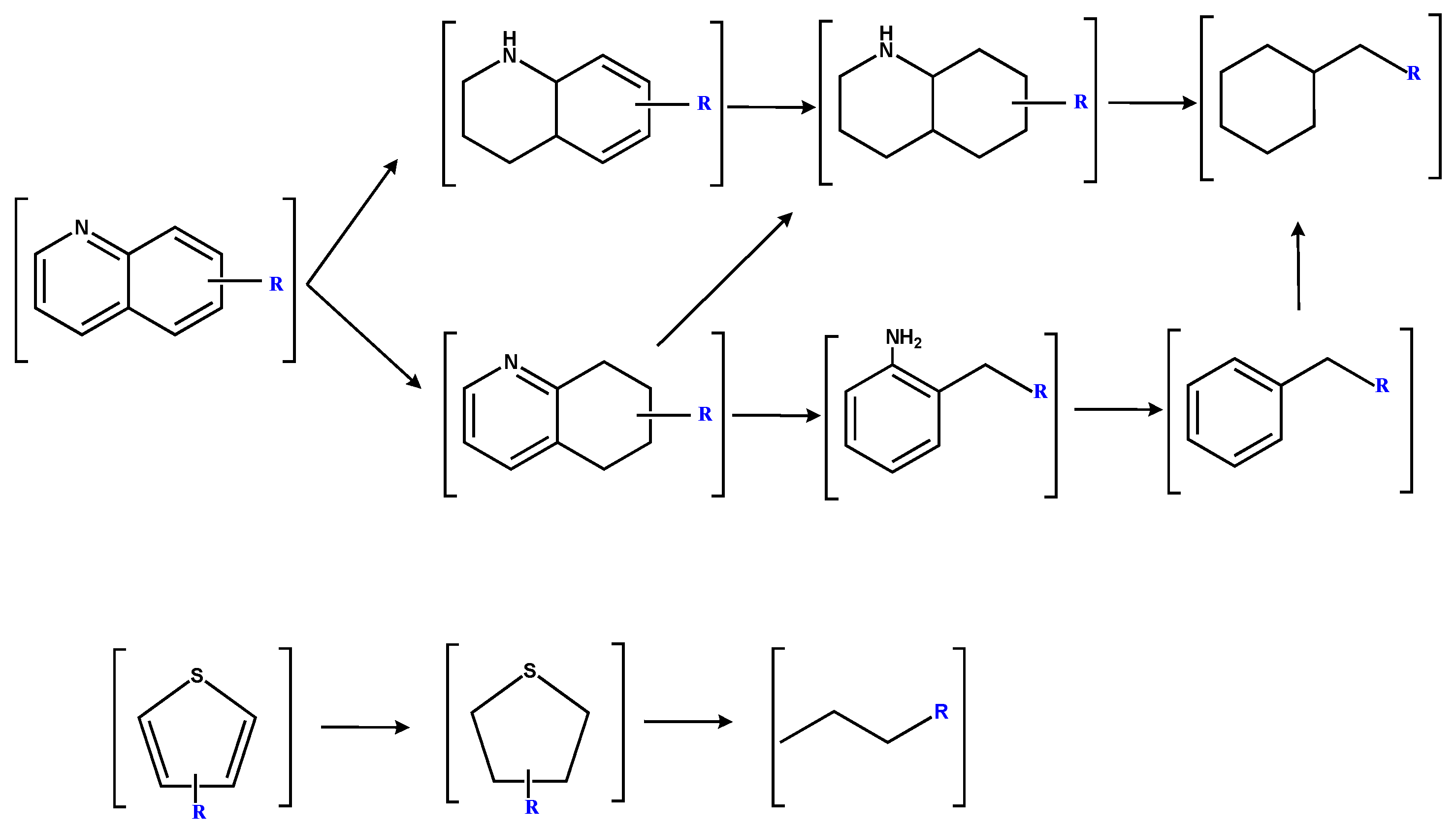
3. Results and Discussion
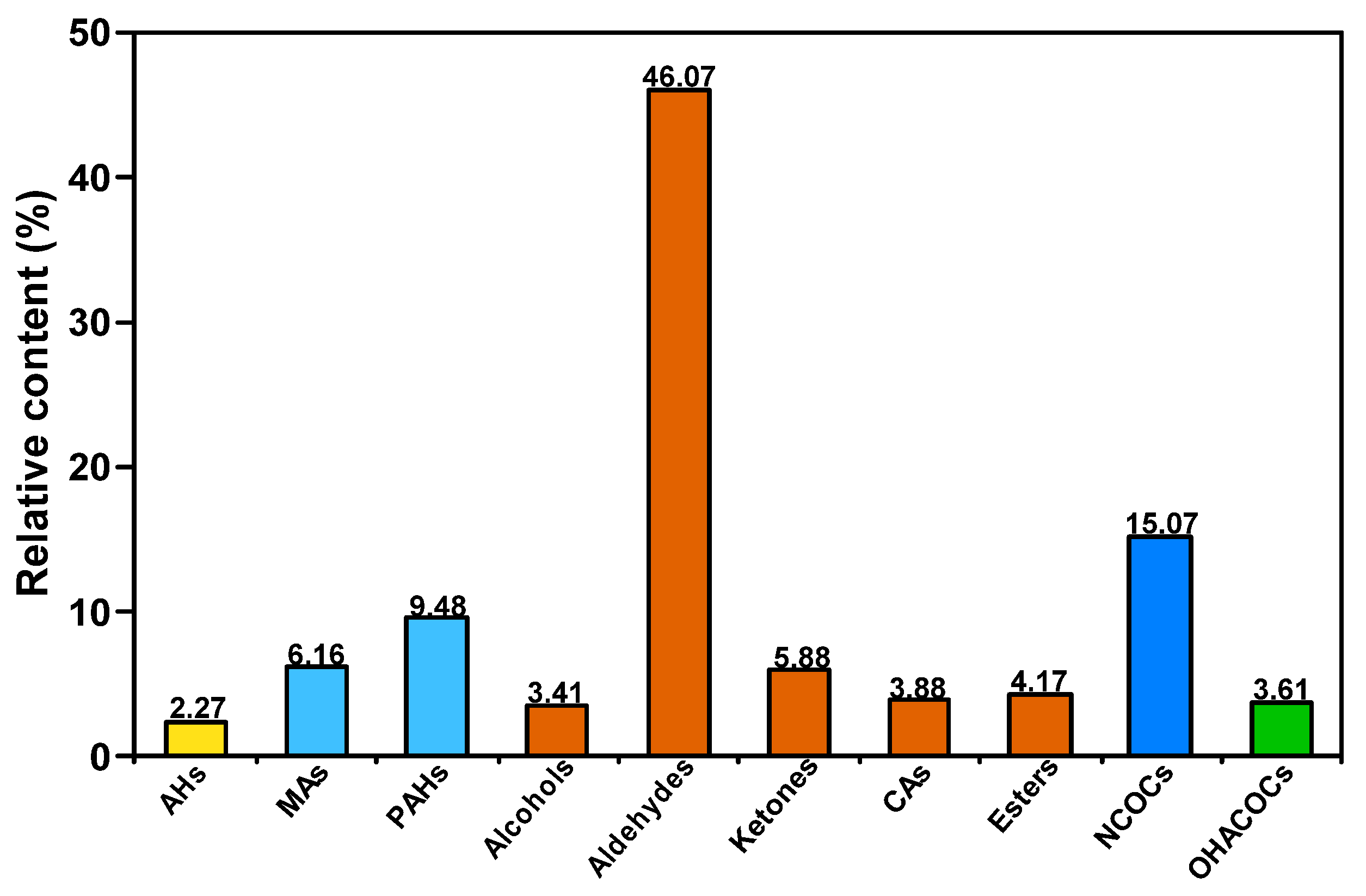
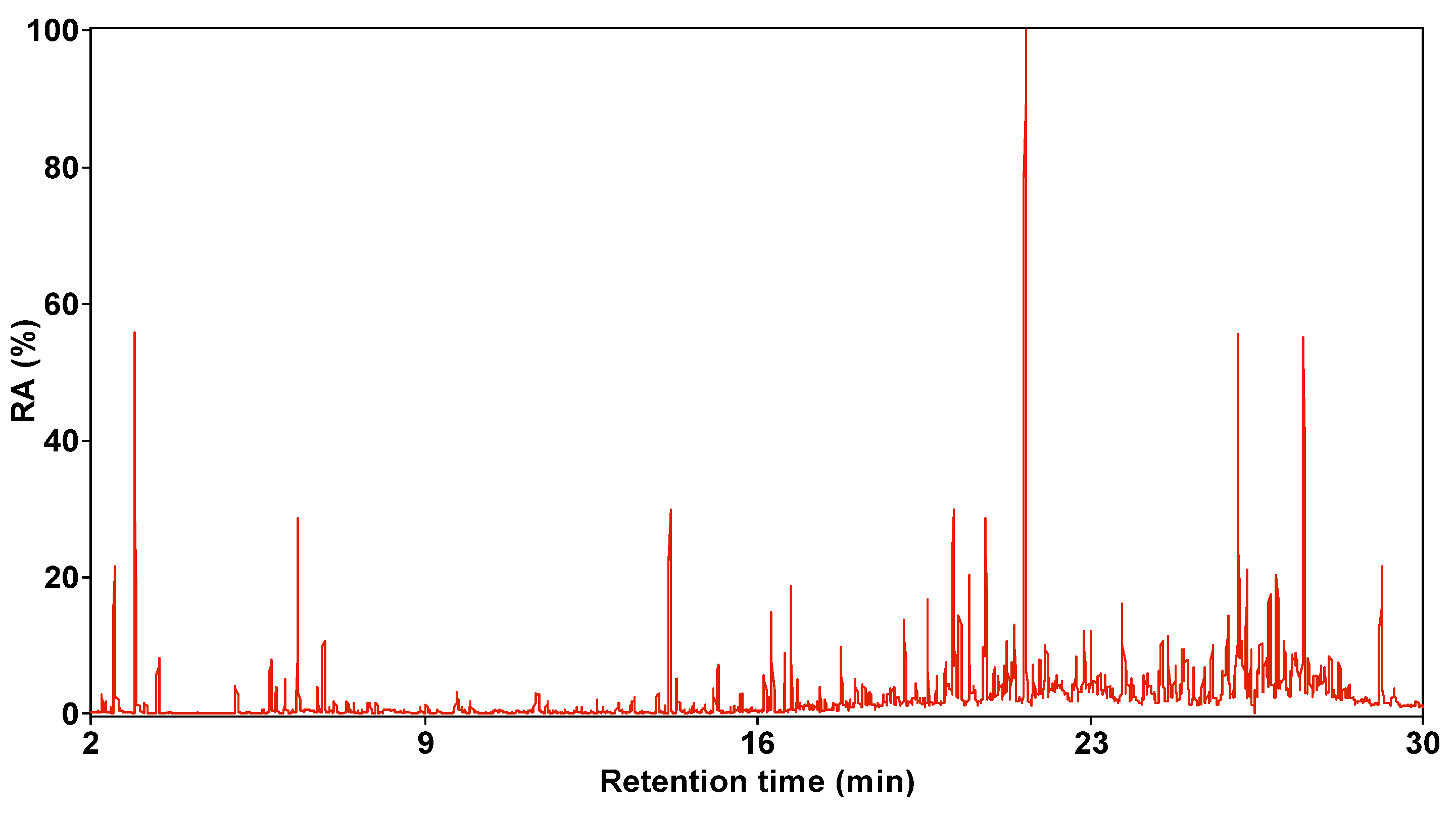
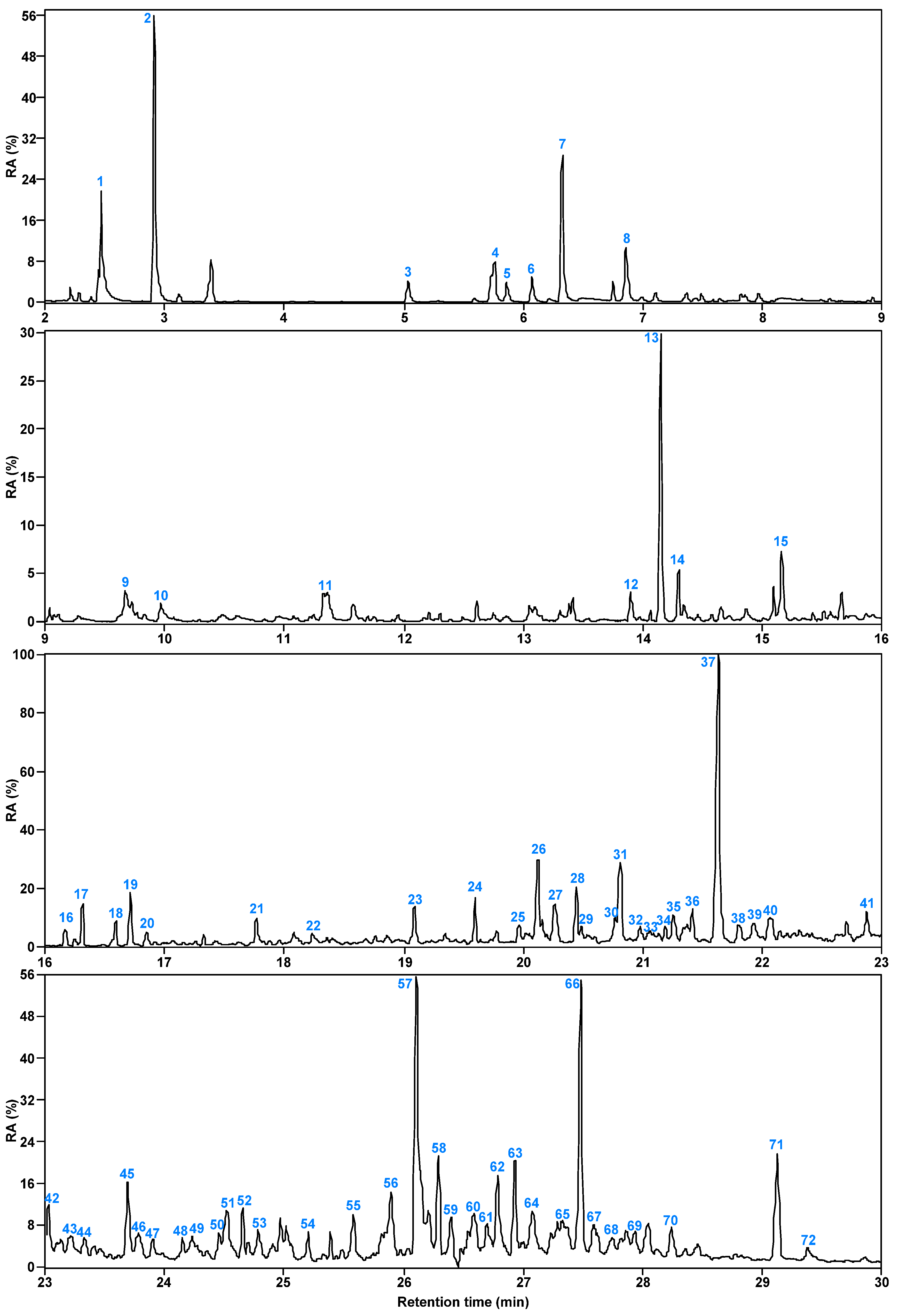
3.1. Composition Characteristics of SBC-L
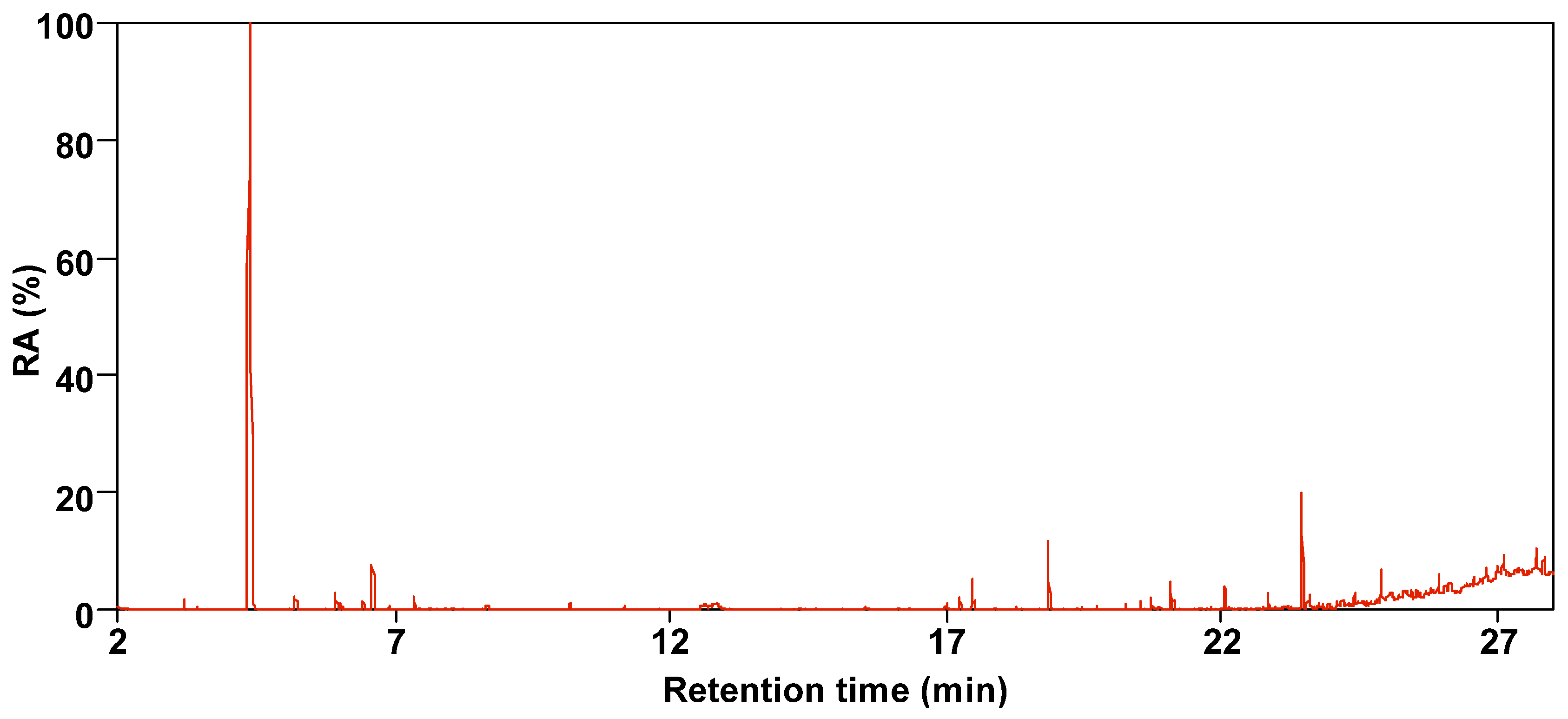
3.2. Composition Characteristics of SBC-LIP320
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| RC | Relative content |
| CHC | Catalytic hydrogenation |
| AHs | Aliphatic hydrocarbon |
| MAs | Monocyclic aromatics |
| PAHs | Polycyclic aromatic hydrocarbon |
| CAs | Carboxylic acids |
| OCOCs | Oxygenated compounds |
| NCOCs | Nitrogen-containing organic compounds |
| OHACOCs | Compounds containing other heteroatoms |
References
- He, X.Q.; Mo, W.L.; Wang, Q.; Ma, Y.Y.; Ma, F.Y.; Fan, X.; Wei, X.Y. Effect of swelling treatment by organic solvent on the structure and pyrolysis performance of the direct coal liquefaction residue. Energy Fuels 2020, 34, 8685–8696. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, W.; Liu, G.H.; An, H.D.; Ma, Y.J.; Kang, Y.H.; Gao, Y.; Bai, N.; Wang, D.K.; Wei, X.Y.; et al. Compositional changes of CH, O, and N moieties in petroleum ether-extractable portions from the three-step mild degradation of Dongming lignite. Fuel 2023, 336, 127132. [Google Scholar] [CrossRef]
- Wei, X.Y.; Bai, X.; Ma, F.Y.; Zong, Z.M.; Zhao, W.; Ni, Z.H.; Fan, X.; Sun, L.B.; Cao, J.P.; Zhao, Y.P.; et al. Advances in Catalytic Hydroconversion of Typical Heavy Carbon Resources under Mild Conditions. Energy Fuels 2023, 37, 12570–12588. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.Y.; Shao, C.W.; Li, Z.; Li, J.H.; Fan, Z.C.; Lu, K.L.; Liu, F.J.; Kong, Q.Q.; Zhao, J.; et al. Catalytic hydroconversion of an organic waste oil to cyclanes over a supported nickel catalyst. Fuel 2023, 331, 125679. [Google Scholar] [CrossRef]
- Zou, H.X.; Bai, X.; Fan, X.; Wang, M.H.; Xu, Y.Y.; Ma, F.Y.; Wei, X.Y.; Kuznetsov, P.N. Infrared spectroscopic evaluation for catalytic hydrogenation of biomass and coal using unsupervised and supervised algorithms. Fuel 2023, 353, 129211. [Google Scholar] [CrossRef]
- Zou, L.; Jin, L.; Li, Y.; Zhu, S.; Hu, H. Effect of tetrahydrofuran extraction on lignite pyrolysis under nitrogen. J. Anal. Appl. Pyrolysis 2015, 112, 113–120. [Google Scholar] [CrossRef]
- Zhu, P.; Luo, A.; Zhang, F.; Lei, Z.; Zhang, J.; Zhang, J. Effects of extractable compounds on the structure and pyrolysis behaviours of two Xinjiang coals. J. Anal. Appl. Pyrolysis 2018, 133, 128–135. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.; Lv, P.; Ren, L.; Kong, J.; Wang, J.; Li, F.; Bai, Y. Effect of n-hexane extraction on the formation of light aromatics from coal pyrolysis and catalytic upgrading. J. Energy Inst. 2020, 3, 1242–1249. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Ma, F.Y.; Mo, W.L.; Wang, Q. Five-stage sequential extraction of Hefeng coal and direct liquefaction performance of the extraction residue. Fuel 2020, 266, 117039. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Fan, X.; Mo, W.L.; Li, G.S.; Ma, F.Y.; Wei, X.Y. Catalytic hydrogenation and heteroatom removal for isopropanol soluble organic matter of Dongming lignite. Fuel Process. Technol. 2021, 211, 106589–106595. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Fan, X.; Mo, W.L.; Muhammad, T.; Bai, X.; Saikia, B.K.; Wei, X.Y.; Ma, F.Y. Advanced separation of soluble organic matter in a low-rank coal and evaluation using unsupervised analyses. Fuel 2022, 328, 125212. [Google Scholar] [CrossRef]
- Liu, G.H.; Zong, Z.M.; Liu, F.J.; Ma, Z.H.; Wei, X.Y.; Kang, Y.H.; Fan, X.; Ma, F.Y.; Liu, J.L.; Mo, W.L. Two-step catalytic degradations of Dahuangshan lignite and directional upgrading of the resulting petroleum ether-extractable portions. Energy Fuel 2020, 34, 5457–5465. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, W.X.; Bai, J.J.; Zhu, Q.Y.; Li, X.L.; Liu, G.H.; Wei, X.Y.; Kang, Y.H.; Li, Y.J.; Ma, X.R.; et al. Investigation on the catalytic performance of magnetic copper ferrite nanoparticles in the catalytic hydroconversion of Hanglaiwan long flame coal. Fuel 2023, 353, 129173. [Google Scholar] [CrossRef]
- Xie, T.; Cao, J.-P.; Zhu, C.; Zhao, X.-Y.; Zhao, M.; Zhao, Y.-P.; Wei, X.-Y. Selective cleavage of C-O bond in benzyl phenyl ether over Pd/AC at room temperature. Fuel Process. Technol. 2019, 188, 190–196. [Google Scholar] [CrossRef]
- Li, X.K.; Zong, Z.M.; Chen, Y.F.; Yang, Z.; Liu, G.H.; Liu, F.J.; Wei, X.Y.; Wang, B.J.; Ma, F.Y.; Liu, J.M. Catalytic hydroconversion of Yinggemajianfeng lignite over difunctional NiMg2Si/γ-Al2O3. Fuel 2019, 249, 496–502. [Google Scholar] [CrossRef]
- Liu, X.X.; Zong, Z.M.; Li, W.T.; Li, X.; Li, Z.K.; Wang, S.K.; Wei, X.Y. A recyclable and highly active magnetic solid superbase for hydrocracking C-O bridged bonds in sawdust. Fuel Process. Technol. 2017, 159, 396–403. [Google Scholar] [CrossRef]
- Xu, M.L.; Wei, X.Y.; Wu, Q.C.; Zhao, Y.P.; Li, F.H.; Liu, G.H.; Liu, F.Z.; Zong, Z.M.; Zhang, M.; Li, S.; et al. Enhanced hydrogenation of aromatic rings and hydrocracking of >Car-O bridged bonds in the extraction residue from Piliqing subbituminous coal over a magnetic difunctional solid superbase. J. Anal. Appl. Pyrol. 2020, 146, 104695. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wei, X.Y.; Liu, F.J.; Liu, G.H.; Zong, Z.M. Catalytic hydroconversion of Yiwu lignite over solid superacid and solid superbase. Fuel 2019, 238, 473–482. [Google Scholar] [CrossRef]
- Qi, S.C.; Wei, X.Y.; Zong, Z.M.; Hayashi, J.I.; Yuan, X.H.; Sun, L.B. A highly active Ni/ZSM-5 catalyst for complete hydrogenation of polymethylbenzenes. Chem. Cat. Chem. 2013, 5, 3543–3547. [Google Scholar] [CrossRef]
- Kang, Y.H.; Wei, X.Y.; Liu, G.H.; Gao, Y.; Li, Y.J.; Ma, X.R.; Zhang, Z.F.; Zong, Z.M. Catalytic hydroconversion of soluble portion in the extraction from Hecaogou subbituminous coal to clean liquid fuel over a Y/ZSM-5 composite zeolite-supported nickel catalyst. Fuel 2020, 269, 117326. [Google Scholar] [CrossRef]
- Li, W.T.; Wei, X.Y.; Liu, X.X.; Guo, L.L.; Qi, S.C.; Li, Z.K.; Zhang, D.D.; Zong, Z.M. Catalytic hydroconversion of methanol-soluble portion from Xiaolongtan lignite over difunctional Ni/Z5A. Fuel Process. Technol. 2016, 148, 146–154. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, M.; Mo, W.; Wang, Y.; Yuan, J.; Wu, R.; Niu, J.; Ma, K.; Guo, W.; Wei, X.; et al. Effect of Solvent Treatment on the Composition and Structure of Santanghu Long Flame Coal and Its Rapid Pyrolysis Products. Molecules 2023, 28, 7074. [Google Scholar] [CrossRef] [PubMed]
- Cocchetto, J.F.; Satterfield, C.N. Chemical equilibriums among quinoline and its reaction products in hydrodenitrogenation. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 49–53. [Google Scholar] [CrossRef]
- Yao, X.Q.; Li, Y.W.; Jiao, H.J. Mechanistic aspects of catalyzed benzothiophene hydrodesulfurization. A density functional theory study. J. Mol. Struct. THEOCHEM 2005, 726, 67–80. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Okuhara, T. Regulation of intermediates on sulfide nickel and MoS2 catalysts. Catal. Rev.-Sci. Eng. 1997, 15, 249–292. [Google Scholar] [CrossRef]



| Sample | Proximate Analysis | Ultimate Analysis | H/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | VMad | FCad | Cdaf | Hdaf | Odaf * | Ndaf | St,d | ||
| SBC | 9.48 | 5.81 | 26.59 | 58.12 | 69.10 | 3.39 | >25.88 | 0.91 | 0.72 | 0.59 |
| Species | Peak | Compound | CAS | RC(%) |
|---|---|---|---|---|
| AHS | 7 | Cyclobutene, bis(1-methylethylidene)- | 003642-14-6 | 1.04 |
| 9 | 2-Phenyl-1,3-cyclohexadiene | 015619-34-8 | 1.23 | |
| Monocyclic aromatics (MAs) | 2 | Benzene, 1-ethyl-3-methyl- | 000620-14-4 | 0.95 |
| 3 | Benzene, 1,2,4-trimethyl- | 000095-63-6 | 0.95 | |
| 4 | Benzene, 1,2,3-trimethyl- | 000526-73-8 | 2.46 | |
| 5 | Mesitylene | 000108-67-8 | 0.66 | |
| 15 | Benzene, 1,2,3,4-tetramethyl-4-(1-methylethenyl)- | 061142-76-5 | 1.14 | |
| Polycyclic aromatic hydrocarbons (PAHs) | 11 | Anthracene | 000120-12-7 | 0.19 |
| 16 | Pyridine, 3-(2,4,6-trimethylphenyl)- | 075601-34-2 | 0.38 | |
| 18 | Fluoranthene | 000206-44-0 | 0.85 | |
| 19 | Retene | 000483-65-8 | 5.31 | |
| 20 | Pyrene | 000129-00-0 | 0.76 | |
| 21 | 11H-Benzo[a]fluorene | 000238-84-6 | 0.38 | |
| 22 | Pyrene, 1-methyl- | 002381-21-7 | 0.57 | |
| 31 | Chrysene | 000218-01-9 | 1.04 | |
| Alcohols | 23 | 1-Pyrenemethanol | 024463-15-8 | 0.76 |
| 28 | Hexadeca-2,6,10,14-tetraen-1-ol, 3,7,11,16-tetramethyl- | 007614-21-3 | 1.23 | |
| 36 | 2-[2-Quinolyl] methylene quinuclidin-3-ol | 1000256-36-9 | 1.42 | |
| Aldehydes | 1 | 2-Pentanone, 4-hydroxy-4-methyl- | 000123-42-2 | 46.07 |
| Ketones | 17 | Acetophenone, 4,4′-methylene | 000790-82-9 | 0.85 |
| 25 | 6-Tridecanone | 022026-12-6 | 1.52 | |
| 35 | 1,2-Dihydropyrido(3,2,1-kl)phenothiazin-3-one | 069513-42-4 | 3.51 | |
| Carboxylic acids (CAs) | 10 | n-Decanoic acid | 000334-48-5 | 2.94 |
| 13 | Benzenebutanoicacid, 2-carboxy-.gamma.-oxo- | 027415-09-4 | 0.28 | |
| 14 | Tetradecanoic acid | 000544-63-8 | 0.66 | |
| Esters | 24 | Dicyclohexyl phthalate | 000084-61-7 | 1.80 |
| 29 | Isonipecotic acid, N-isobutoxycarbonyl-, heptyl ester | 1000322-06-6 | 0.95 | |
| NCOCs | 6 | 3-Acetyl-2-oxo-1,3-oxazolidine | 001432-43-5 | 0.28 |
| 12 | Benzoic acid, 2-phenylhydrazide | 000532-96-7 | 0.19 | |
| 26 | [1,1′-Biphenyl]-4-carbonitrile, 4′-propoxy- | 052709-86-1 | 0.95 | |
| 27 | Mirtazapine | 085650-52-8 | 1.04 | |
| 30 | Benzamide, 2,3-dinitro- | 019613-80-0 | 1.14 | |
| 33 | 9-Octadecenamide, (Z)- | 000301-02-0 | 2.94 | |
| 34 | 2H-1,4-Benzodiazepin-2-one, 7-amino-1,3,4,5-tetrahydro-5-phenyl- | 1000362-81-4 | 3.03 | |
| 37 | Benzamide, N-cyclododecyl-3,4,5-trimethoxy- | 1000311-05-7 | 5.50 | |
| OHACOCs | 8 | 1,3-Dithiolane, 2,2-dimethyl- | 006008-78-2 | 1.52 |
| 38 | 2-[2-Thienyl]-4-acetyl quinoline | 027302-83-6 | 2.09 |
| Species | Peak | Compound | CAS | RC (%) |
|---|---|---|---|---|
| Alkanes | 17 | Pentadecane, 2,6,10,14-tetramethyl- | 001921-70-6 | 0.93 |
| 19 | Cyclopentane, ethyl- | 001640-89-7 | 1.22 | |
| 34 | Pentadecane | 000629-62-9 | 0.52 | |
| 50 | Heptadecane | 000629-78-7 | 0.64 | |
| 54 | Hexadecane | 000544-76-3 | 0.83 | |
| Olefins | 5 | Benzene, (1-methylethyl)- | 000098-82-8 | 0.29 |
| 18 | 4-Nonene | 002198-23-4 | 0.66 | |
| 32 | Benzo[b]naphtho[2,3-d]furan | 000243-42-5 | 0.87 | |
| 37 | Retene | 000483-65-8 | 9.59 | |
| 41 | 6,6-Diphenylfulvene | 002175-90-8 | 1.72 | |
| 51 | 9-Tricosene, (Z)- | 027519-02-4 | 1.78 | |
| Monocyclic aromatics (MAs) | 3 | Benzene, 1-ethyl-3-methyl- | 000620-14-4 | 0.31 |
| 6 | Benzene, 1-ethyl-4-methyl- | 000622-96-8 | 0.35 | |
| 7 | Benzene, 1,2,3-trimethyl- | 000526-73-8 | 1.97 | |
| 8 | Benzene, 1,2,4-trimethyl- | 000095-63-6 | 0.89 | |
| 14 | 1H-Indene, 2,3-dihydro-1,1,2,3,3-pentamethyl- | 001203-17-4 | 0.31 | |
| Polycyclic aromatic hydrocarbons (PAHs) | 9 | Naphthalene | 000091-20-3 | 0.39 |
| 15 | Naphthalene, 2,3,6-trimethyl- | 000829-26-5 | 0.58 | |
| 16 | Naphthalene, 1,6-dimethyl-4-(1-methylethyl)- | 000483-78-3 | 0.42 | |
| 20 | 1,4,5,8-Tetramethylnaphthalene | 002717-39-7 | 0.39 | |
| 26 | 7-Isopropyl-1,1,4a-trimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthrene | 1000210-28-9 | 2.24 | |
| 27 | Pyrene | 000129-00-0 | 1.64 | |
| 28 | 7-Butyl-1-hexylnaphthalene | 055000-55-0 | 1.43 | |
| 30 | Fluoranthene | 000206-44-0 | 0.98 | |
| 38 | Pyrene, 1-methyl- | 002381-21-7 | 0.93 | |
| 39 | 1,2,3,4-Tetrahydrobenz[a]anthracene | 004483-98-1 | 1.35 | |
| 40 | 1-benzyl-3-methylnaphthalene | 1000379-93-5 | 1.47 | |
| 42 | 8-Isopropyl-1,3-dimethylphenanthrene | 135886-06-5 | 1.16 | |
| 43 | Benzo[ghi]fluoranthene | 000203-12-3 | 1.22 | |
| 45 | Benz[a]anthracene | 000056-55-3 | 1.60 | |
| 46 | Triphenylene | 000217-59-4 | 1.06 | |
| 49 | Benz[a]anthracene, 5-methyl- | 002319-96-2 | 1.27 | |
| 61 | Benzo[a]pyrene | 000050-32-8 | 1.33 | |
| 62 | 1,1′:3′,1″:3″,1‴:3‴,1⁗-Quinquephenyl | 016716-13-5 | 2.18 | |
| 65 | 13H-Dibenzo[a,h]fluorene | 000239-85-0 | 1.76 | |
| 70 | 1,1′:2′,″-Terphenyl,3′,4′-dimethyl-5′,6′-diphenyl- | 020143-37-7 | 1.06 | |
| 72 | Benzo[ghi]perylene | 000191-24-2 | 0.98 | |
| Alcohols | 25 | 4,4,5,8-Tetramethylchroman-2-ol | 082391-05-7 | 0.62 |
| 36 | Isopropyl stearate | 000112-10-7 | 1.06 | |
| 64 | 2R-2,8-dimethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2H-1-benzopyran-6-ol | 000119-13-1 | 1.76 | |
| 68 | .beta.-Tocopherol | 000148-03-8 | 1.02 | |
| Phenols | 11 | Phenol, m-tert-butyl- | 000585-34-2 | 0.52 |
| 13 | Phenol, 2,5-bis(1,1-dimethylethyl)- | 005875-45-6 | 1.83 | |
| 69 | Cannabidiol | 013956-29-1 | 0.95 | |
| 71 | Phenol,4,4′-methylenebis[2,6-bis(1,1-dimethylethyl)- | 000118-82-1 | 2.45 | |
| Ethers | 44 | 1,1′-Biphenyl, 2-phenoxy- | 006738-04-1 | 0.68 |
| 47 | Benzo[2,1-b:3,4-b′]bisbenzofuran | 000222-23-1 | 0.79 | |
| 60 | Dinaphtho[2,1-b:1′,2′-d]furan | 000194-63-8 | 1.47 | |
| Aldehydes | 33 | 9-Ethyl-10-methylanthracene | 019713-49-6 | 0.87 |
| Ketones | 2 | 3-Penten-2-one, 4-methyl- | 000141-79-7 | 3.61 |
| 4 | Acetophenone | 000098-86-2 | 0.85 | |
| 12 | Ethanone,1-[4-(1-methyl-2-propenyl)phenyl]- | 109586-49-4 | 0.21 | |
| 67 | 4,6-Dimethyl-4′-hydroxy-2-benzylidene-coumaran-3-one | 002567-73-9 | 1.49 | |
| Carboxylic acids (CAs) | 22 | 4′-Ethyl-4-biphenylcarboxylic acid | 005731-13-5 | 0.56 |
| 24 | n-Hexadecanoic acid | 000057-10-3 | 1.08 | |
| 35 | 6-Octadecenoic acid, (Z)- | 000593-39-5 | 1.14 | |
| 52 | Docosanoic acid | 000112-85-6 | 0.79 | |
| 57 | Tetracosanoic acid | 000557-59-5 | 5.79 | |
| 58 | Phthalic acid, 3,4-dimethylphenyl 3,5-dimethylphenyl ester | 1000315-70-6 | 1.87 | |
| 59 | Phthalic acid, di(2,3-dimethylphenyl) ester | 1000357-09-2 | 1.10 | |
| 63 | Phthalic acid, 3,4-dimethylphenyl 3,5-dimethylphenyl ester | 1000315-70-6 | 1.85 | |
| 66 | Hexacosanoic acid | 000506-46-7 | 5.08 | |
| Esters | 10 | Benzoic acid, 1-methylethyl ester | 000939-48-0 | 0.23 |
| 23 | Phthalic acid, butyl isohexyl ester | 1000309-03-6 | 1.24 | |
| 29 | Benzoic acid, 6-formyl-2,3-dimethoxy-, ethyl ester | 1000196-80-7 | 0.54 | |
| 48 | Phthalic acid, monocyclohexyl ester | 007517-36-4 | 0.62 | |
| NCOCS | 1 | Benzonitrile, 4-hydroxy- | 000767-00-0 | 1.99 |
| 21 | 4-Methyl-3,6-decandione | 1000374-13-2 | 0.95 | |
| 31 | 10H-Benzo[b][1,8]naphthyridin-5-one, 7-ethyl-2,4-dimethyl- | 343780-17-6 | 2.39 | |
| 53 | 2,2′-Biquinoline | 000119-91-5 | 0.97 | |
| 55 | Benzo[f]quinoline, 5,6-dihydro-3-phenyl- | 022877-22-1 | 1.31 | |
| 56 | 13-Docosenamide, (Z)- | 000112-84-5 | 2.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhao, G.; Naeem, A.; Ma, Y.; Zhu, M.; Ren, Y.; Mo, W.; Wei, X.; Fan, X.; Hao, S.; et al. Catalytic Hydrogenation and Heteroatom Removal for the Soluble Organics from Santanghu Bituminous Coal. Molecules 2025, 30, 849. https://doi.org/10.3390/molecules30040849
Guo J, Zhao G, Naeem A, Ma Y, Zhu M, Ren Y, Mo W, Wei X, Fan X, Hao S, et al. Catalytic Hydrogenation and Heteroatom Removal for the Soluble Organics from Santanghu Bituminous Coal. Molecules. 2025; 30(4):849. https://doi.org/10.3390/molecules30040849
Chicago/Turabian StyleGuo, Jia, Guihan Zhao, Akram Naeem, Yaya Ma, Meixia Zhu, Yuan Ren, Wenlong Mo, Xanyong Wei, Xing Fan, Shihao Hao, and et al. 2025. "Catalytic Hydrogenation and Heteroatom Removal for the Soluble Organics from Santanghu Bituminous Coal" Molecules 30, no. 4: 849. https://doi.org/10.3390/molecules30040849
APA StyleGuo, J., Zhao, G., Naeem, A., Ma, Y., Zhu, M., Ren, Y., Mo, W., Wei, X., Fan, X., Hao, S., & Ali, A. (2025). Catalytic Hydrogenation and Heteroatom Removal for the Soluble Organics from Santanghu Bituminous Coal. Molecules, 30(4), 849. https://doi.org/10.3390/molecules30040849