Exploration of Regulation of Alcohol Dehydrogenation Reaction of Dibenzimidazole-Based Ruthenium Complexes
Abstract
1. Introduction
2. Results and Discussion
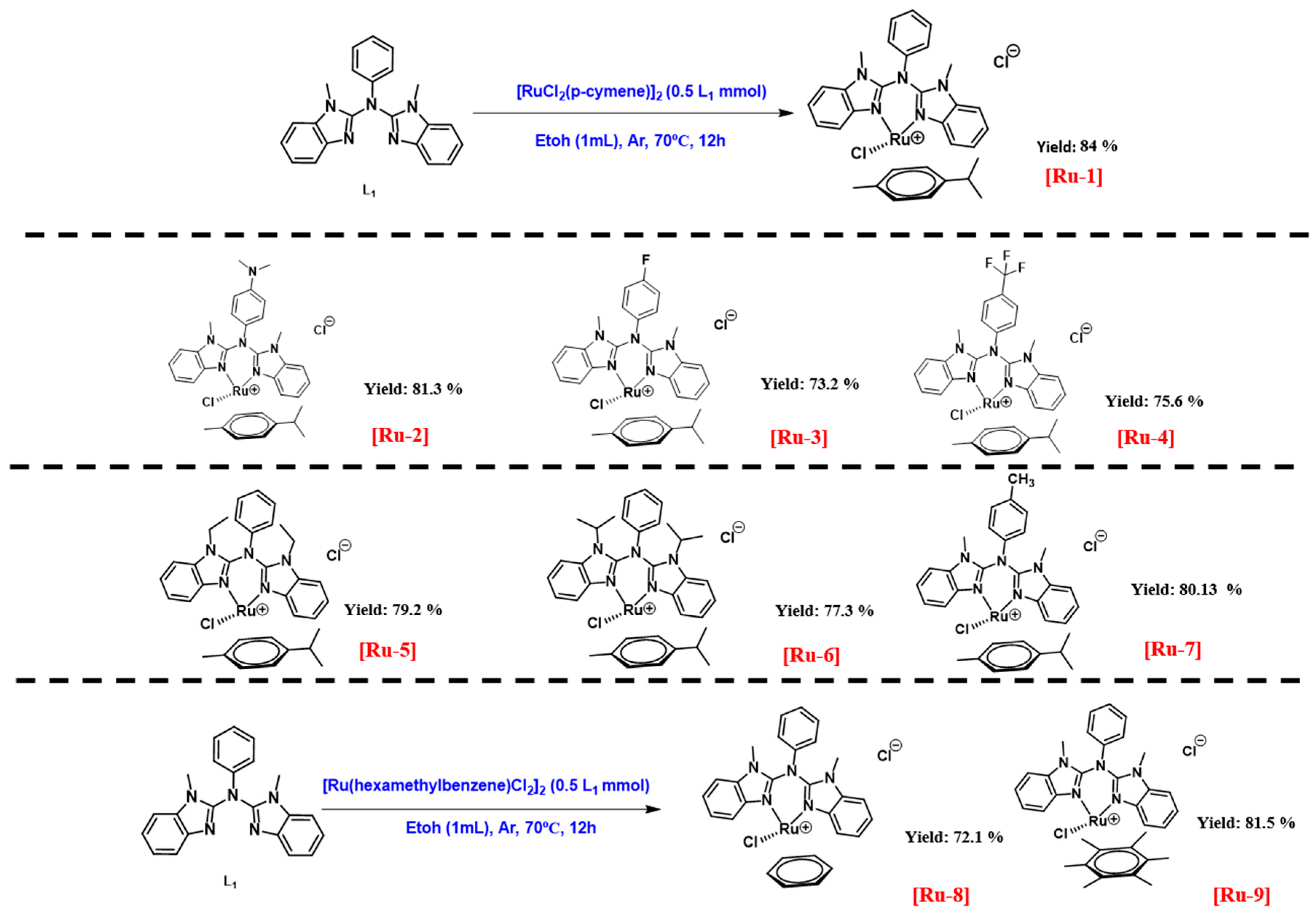
2.1. Characterization of Ruthenium Complexes
2.2. Exploration of Catalytic Performance
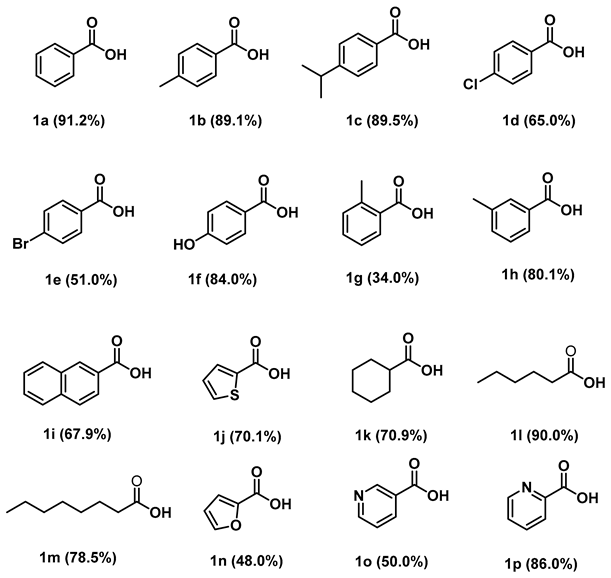
2.3. Substrate Range for [Ru-2] Catalyzed Catalytic Dehydrogenation
2.4. Catalytic Mechanism
3. Experiment
3.1. Generality
3.2. Synthesis
3.2.1. General Synthesis Procedure for Dibenzimidazole Ligands
Synthesis of Ligand (L1)
Synthesis of Ligands (L2, L3, L4, L7)
Synthesis of Ligands (L5, L6)
3.2.2. General Procedure for Synthesizing [Ru-1], [Ru-2], [Ru-3], [Ru-4], [Ru-5], [Ru-6], and [Ru-7]
3.2.3. General Procedure for Synthesis of [Ru-8] and [Ru-9]
3.3. X-Ray Crystallography
3.4. Catalytic Reaction Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, H.; Zhang, W.; Fan, Z.; Chen, Z. Recent Advances in Furfural Reduction via Electro-and Photocatalysis: From Mechanism to Catalyst Design. ACS Catal. 2023, 13, 15263–15289. [Google Scholar]
- Ma, J.; Yu, W.; Wang, M.; Jia, X.; Lu, F.; Xu, J. Advances in Selective Catalytic Transformation of Ployols to Value-Added Chemicals. Cuihua Xuebao/Chin. J. Catal. 2013, 34, 492–507. [Google Scholar]
- He, L.; Zhou, B.C.; Sun, D.H.; Li, W.C.; Lv, W.L.; Wang, J.; Liang, Y.Q.; Lu, A.H. Catalytic Conversion of Ethanol to Oxygen-Containing Value-Added Chemicals. ACS Catal. 2023, 13, 11291–11304. [Google Scholar] [CrossRef]
- Malineni, J.; Keul, H.; Möller, M. A Green and Sustainable Phosphine-Free NHC-Ruthenium Catalyst for Selective Oxidation of Alcohols to Carboxylic Acids in Water. Dalton Trans. 2015, 44, 17409–17414. [Google Scholar] [CrossRef]
- Nguyen, Q.P.; Chau, H.K.; Lobban, L.; Crossley, S.; Wang, B. Mechanistic Insights into the Conversion of Polyalcohols over Brønsted Acid Sites. Catal. Sci. Technol. 2023, 13, 4477–4488. [Google Scholar] [CrossRef]
- Zhan, Y.; Hou, W.; Li, G.; Shen, Y.; Zhang, Y.; Tang, Y. Oxidant-Free Transformation of Ethylene Glycol toward Glycolic Acid in Water. ACS Sustain. Chem. Eng. 2019, 7, 17559–17564. [Google Scholar] [CrossRef]
- Werner, A.Z.; Clare, R.; Mand, T.D.; Pardo, I.; Ramirez, K.J.; Haugen, S.J.; Bratti, F.; Dexter, G.N.; Elmore, J.R.; Huenemann, J.D.; et al. Tandem Chemical Deconstruction and Biological Upcycling of Poly(Ethylene Terephthalate) to β-Ketoadipic Acid by Pseudomonas Putida KT2440. Metab. Eng. 2021, 67, 250–261. [Google Scholar] [CrossRef]
- Sarbajna, A.; Dutta, I.; Daw, P.; Dinda, S.; Rahaman, S.M.W.; Sarkar, A.; Bera, J.K. Catalytic Conversion of Alcohols to Carboxylic Acid Salts and Hydrogen with Alkaline Water. ACS Catal. 2017, 7, 2786–2790. [Google Scholar] [CrossRef]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of Fuels and Chemicals from Biomass: Condensation Reactions and Beyond. Chem 2016, 1, 32–58. [Google Scholar]
- Jiang, X.; Zhang, J.; Ma, S. Iron Catalysis for Room-Temperature Aerobic Oxidation of Alcohols to Carboxylic Acids. J. Am. Chem. Soc. 2016, 138, 8344–8347. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Dutta, I.; Bera, J.K. Acceptorless Alcohol Dehydrogenation: A Mechanistic Perspective. Proc. Natl. Acad. Sci. India Sect. A-Phys. Sci. 2016, 86, 561–579. [Google Scholar] [CrossRef]
- Chakraborty, S.; Brennessel, W.W.; Jones, W.D. A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-Heterocycles. J. Am. Chem. Soc. 2014, 136, 8564–8567. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; You, H.; Dong, B.; He, Y.; Li, F. Selective Conversion of Glycerol to Lactic Acid in Water via Acceptorless Dehydrogenation Catalyzed by a Water-Soluble Metal-Ligand Bifunctional Iridium Catalyst. Inorg. Chem. 2024, 63, 12929–12934. [Google Scholar] [CrossRef]
- Nicolau, G.; Tarantino, G.; Hammond, C. Acceptorless Alcohol Dehydrogenation Catalysed by Pd/C. ChemSusChem 2019, 12, 4953–4961. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, E.; Khaskin, E.; Leitus, G.; Milstein, D. Catalytic Transformation of Alcohols to Carboxylic Acid Salts and H 2 Using Water as the Oxygen Atom Source. Nat. Chem. 2013, 5, 122–125. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Kang, M.J.; Lee, H.; Kim, H.S. Tunable Selectivity of Photocatalytic Benzyl Alcohol Transformation over Ag-Ion-Exchanged CdS Nanowires. ACS Sustain. Chem. Eng. 2023, 11, 4364–4373. [Google Scholar] [CrossRef]
- Monda, F.; Madsen, R. Zinc Oxide-Catalyzed Dehydrogenation of Primary Alcohols into Carboxylic Acids. Chem.-A Eur. J. 2018, 24, 17832–17837. [Google Scholar] [CrossRef]
- Li, H.; Cao, L.; Yang, C.; Zhang, Z.; Zhang, B.; Deng, K. Selective Oxidation of Benzyl Alcohols to Benzoic Acid Catalyzed by Eco-Friendly Cobalt Thioporphyrazine Catalyst Supported on Silica-Coated Magnetic Nanospheres. J. Environ. Sci. 2017, 60, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, E.; Greco, R.; Mon, M.; Ballesteros-Soberanas, J.; Ferrando-Soria, J.; López-Haro, M.; Hernández-Garrido, J.C.; Oliver-Meseguer, J.; Marini, C.; Boronat, M.; et al. Soluble/MOF-Supported Palladium Single Atoms Catalyze the Ligand-, Additive-, and Solvent-Free Aerobic Oxidation of Benzyl Alcohols to Benzoic Acids. J. Am. Chem. Soc. 2021, 143, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Awasthi, S.K. Bimetallic Oxide Catalyst for the Dehydrogenative Oxidation Reaction of Alcohols: Practical Application in the Synthesis of Value-Added Chemicals. ACS Sustain. Chem. Eng. 2022, 10, 1702–1713. [Google Scholar] [CrossRef]
- Pal, P.; Saravanamurugan, S. Heterostructured Manganese Catalysts for the Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran. ChemCatChem 2020, 12, 2324–2332. [Google Scholar] [CrossRef]
- Zhou, T.; Utecht-Jarzyńska, G.; Szostak, M. Ring-Expanded N-Heterocyclic Carbene (ReNHC) Complexes: Applications in Transition Metal Catalysis. Coord. Chem. Rev. 2024, 512, 215867. [Google Scholar] [CrossRef]
- Crudden, C.M.; Allen, D.P. Stability and Reactivity of N-Heterocyclic Carbene Complexes. Coord. Chem. Rev. 2004, 248, 2247–2273. [Google Scholar] [CrossRef]
- Bera, S.S.; Szostak, M. Cobalt-N-Heterocyclic Carbene Complexes in Catalysis. ACS Catal. 2022, 12, 3111–3137. [Google Scholar] [CrossRef]
- Trodden, E.C.; Delve, M.P.; Luz, C.; Newland, R.J.; Andresen, J.M.; Mansell, S.M. A Rutheniumcis-Dihydride with 2-Phosphinophosphinine Ligands Catalyses the Acceptorless Dehydrogenation of Benzyl Alcohol. Dalton Trans. 2021, 50, 13407–13411. [Google Scholar] [CrossRef]
- Stubbs, J.M.; Firth, K.F.; Bridge, B.J.; Berger, K.J.; Hazlehurst, R.J.; Boyle, P.D.; Blacquiere, J.M. Phosphine-Imine and-Enamido Ligands for Acceptorless Dehydrogenation Catalysis. Dalton Trans. 2017, 46, 647–650. [Google Scholar] [CrossRef]
- Patra, S.; Deka, H.; Singh, S.K. Bis-Imidazole Methane Ligated Ruthenium(II) Complexes: Synthesis, Characterization, and Catalytic Activity for Hydrogen Production from Formic Acid in Water. Inorg. Chem. 2021, 60, 14275–14285. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, W.; Ma, H.; Obolda, A.; Yan, W.; Dong, S.; Zhang, M.; Li, F. Novel Luminescent Benzimidazole-Substituent Tris(2,4,6-Trichlorophenyl)Methyl Radicals: Photophysics, Stability, and Highly Efficient Red-Orange Electroluminescence. Chem. Mater. 2017, 29, 6733–6739. [Google Scholar] [CrossRef]
- Matveevskaya, V.V.; Pavlov, D.I.; Samsonenko, D.G.; Ermakova, E.A.; Klyushova, L.S.; Baykov, S.V.; Boyarskiy, V.P.; Potapov, A.S. Synthesis and Structural Characterization of Half-Sandwich Arene–Ruthenium(Ii) Complexes with Bis(Imidazol-1-Yl)Methane, Imidazole and Benzimidazole. Inorganics 2021, 9, 34. [Google Scholar] [CrossRef]
- Wang, W.Q.; Cheng, H.; Yuan, Y.; He, Y.Q.; Wang, H.J.; Wang, Z.Q.; Sang, W.; Chen, C.; Verpoort, F. Highly Efficient N-Heterocyclic Carbene/Ruthenium Catalytic Systems for the Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids: Effects of Ancillary and Additional Ligands. Catalysts 2020, 10, 10. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Tang, X.S.; Yang, Z.Q.; Yu, B.Y.; Wang, H.J.; Sang, W.; Yuan, Y.; Chen, C.; Verpoort, F. Highly Active Bidentate N-Heterocyclic Carbene/Ruthenium Complexes Performing Dehydrogenative Coupling of Alcohols and Hydroxides in Open Air. Chem. Commun. 2019, 55, 8591–8594. [Google Scholar] [CrossRef]
- Santilli, C.; Makarov, I.S.; Fristrup, P.; Madsen, R. Dehydrogenative Synthesis of Carboxylic Acids from Primary Alcohols and Hydroxide Catalyzed by a Ruthenium N-Heterocyclic Carbene Complex. J. Org. Chem. 2016, 81, 9931–9938. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Gavi, A.J.; Yu, B.Y.; Cheng, H.; Yuan, Y.; Wu, Y.; Lommens, P.; Chen, C.; Verpoort, F. Palladium-Catalyzed Ligand-Free C-N Coupling Reactions: Selective Diheteroarylation of Amines with 2-Halobenzimidazoles. Chem. Asian J. 2020, 15, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Gong, Y.Y.; Cheng, H.; Zhang, R.; Yuan, Y.; Fan, G.G.; Wang, Z.Q.; Chen, C.; Verpoort, F. Transition-Metal-Free Base-Controlled C−N Coupling Reactions: Selective Mono Versus Diarylation of Primary Amines with 2-Chlorobenzimidazoles. Adv. Synth. Catal. 2021, 363, 1408–1416. [Google Scholar] [CrossRef]
- Chen, C.; Sang, W.; Chaemchuen, S.; Fan, G.G.; Jiang, B.W.; Chen, Y.X.; Jiang, M. A Metal-Organic Complex Containing a Benzimidazole Skeleton and its Preparation Method and Application. China Patent CN114213469A, 22 March 2022. [Google Scholar]
- Yin, S.; Zheng, Q.; Chen, J.; Tu, T. Acceptorless Dehydrogenation of Primary Alcohols to Carboxylic Acids by Self-Supported NHC-Ru Single-Site Catalysts. J. Catal. 2022, 408, 165–172. [Google Scholar] [CrossRef]
- Chen, Z.W.; Ma, F.; Liu, Y.; Mo, X.F.; Chen, G.; Peng, X.; Yi, X.Y. Geometrical Isomerization and Acceptorless Dehydrogenative Alcohol Oxidation Based on Pyrrole-Based Ru(II) Complexes. Inorganica Chim. Acta 2022, 541, 121034. [Google Scholar] [CrossRef]
- Choi, J.H.; Heim, L.E.; Ahrens, M.; Prechtl, M.H.G. Selective Conversion of Alcohols in Water to Carboxylic Acids by in Situ Generated Ruthenium Trans Dihydrido Carbonyl PNP Complexes. Dalton Trans. 2014, 43, 17248–17254. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.K.; Saini, A.K.; Mobin, S.M.; Mathur, P. Facile Oxidation of Alcohols to Carboxylic Acids in Basic Water Medium by Employing Ruthenium Picolinate Cluster as an Efficient Catalyst. Appl. Organomet Chem. 2018, 32, e4574. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Singh, S.K. Ruthenium Catalyzed Dehydrogenation of Alcohols and Mechanistic Study. Inorg. Chem. 2019, 58, 14912–14923. [Google Scholar] [CrossRef] [PubMed]
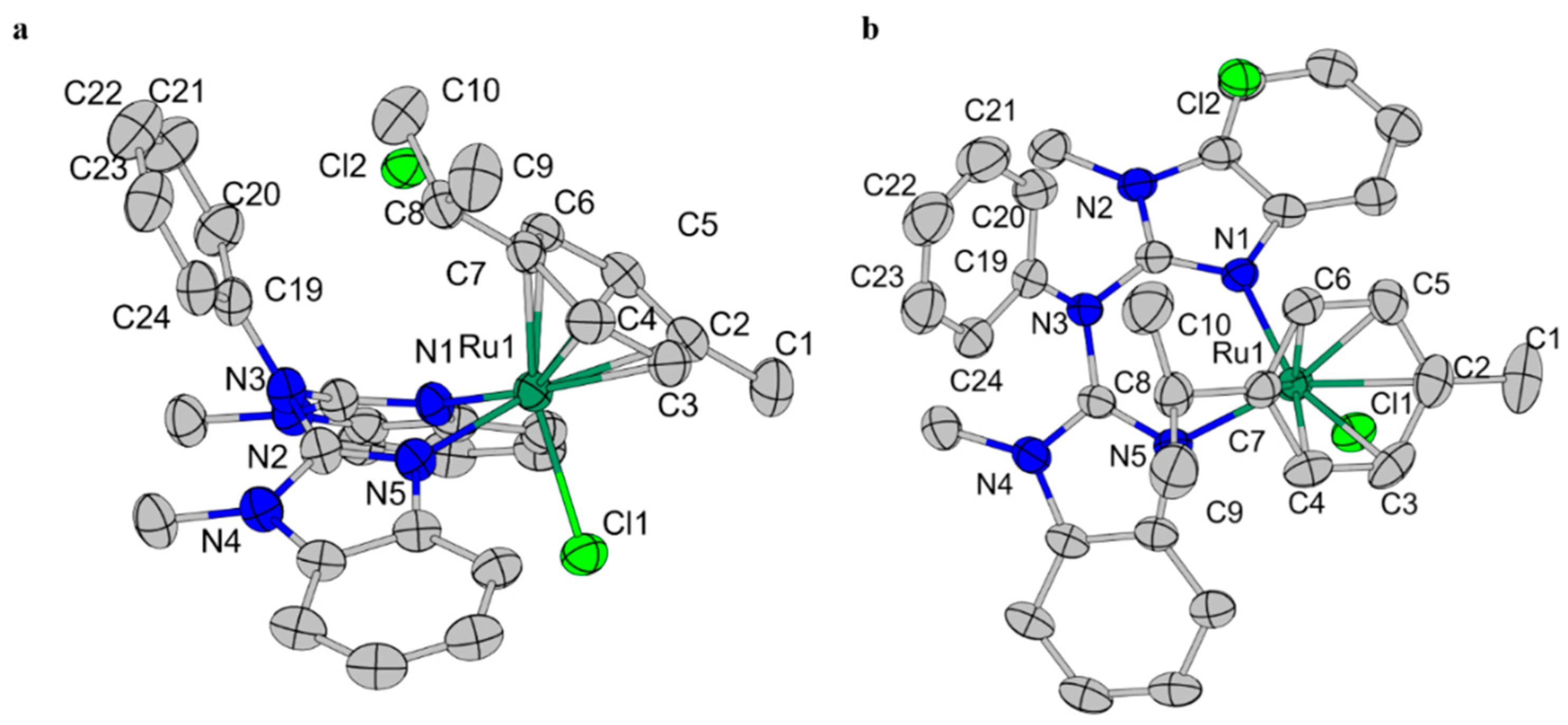
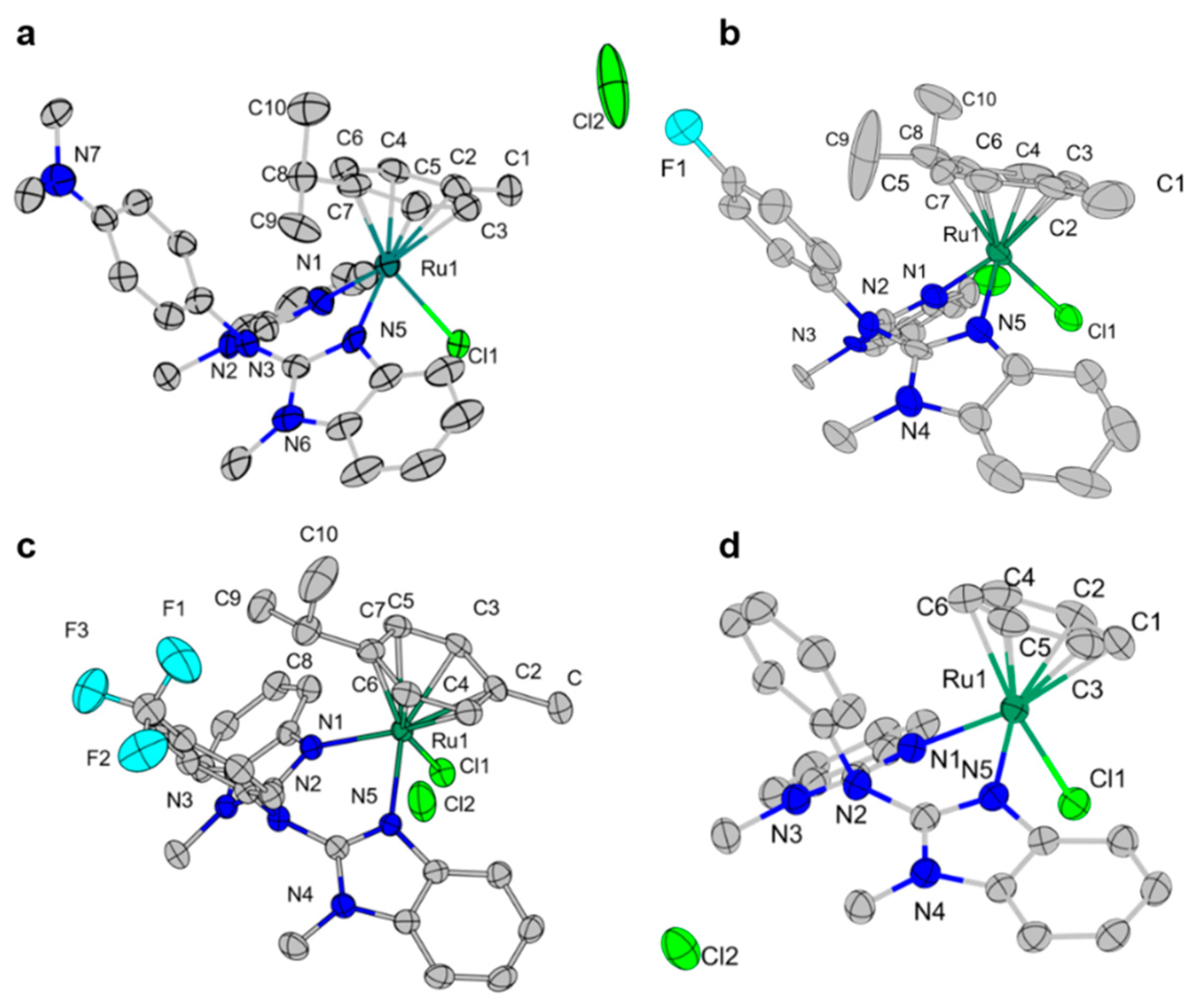


| [Ru] | Angle(N1-Ru-N5) (°) | r(Ru-CAromatic) (Å) | r(Ru-N1) (Å) | r(Ru-N5) (Å) |
|---|---|---|---|---|
| [Ru-1] | 81.52 | 2.210 | 2.124 | 2.121 |
| [Ru-2] | 82.7 | 2.20 | 2.117 | 2.082 |
| [Ru-3] | 80.85 | 2.193 | 2.111 | 2.104 |
| [Ru-4] | 81.30 | 2.196 | 2.123 | 2.111 |
| [Ru-9] | 82.36 | 2.181 | 2.100 | 2.103 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Y (mmol) | Solvents | Base | Z (mmol) | X (mL) | 1a |
| 1 | 0.002 | toluene | KOH | 1.00 | 1.0 | 53.0% |
| 2 | 0.002 | m-xylene | KOH | 1.00 | 1.0 | 39.3% |
| 3 | 0.002 | mesitylene | KOH | 1.00 | 1.0 | 51.4% |
| 4 | 0.002 | toluene | NaOH | 1.00 | 1.0 | 33.0% |
| 5 | 0.002 | toluene | LiOH | 1.00 | 1.0 | 16.6% |
| 6 | 0 | toluene | KOH | 1.00 | 1.0 | 0.00% |
| 7 | 0.001 | toluene | KOH | 1.00 | 1.0 | 36.9% |
| 8 | 0.003 | toluene | KOH | 1.00 | 1.0 | 64.0% |
| 9 | 0.004 | toluene | KOH | 1.00 | 1.0 | 63.1% |
| 10 | 0.003 | toluene | KOH | 1.50 | 1.0 | 81.0% |
| 11 | 0.003 | toluene | KOH | 0 | 0.4 | 0.0% |
| 12 | 0.003 | toluene | KOH | 1.50 | 0.4 | 85.8% |
 | |||
|---|---|---|---|
| Entry | [Ru] | Base | 1a |
| 1 | [Ru-1] | KOH | 85.8% |
| 2 | [Ru-2] | KOH | 91.2% |
| 3 | [Ru-3] | KOH | 78.6% |
| 4 | [Ru-4] | KOH | 83.3% |
| 5 | [Ru-5] | KOH | 74.2% |
| 6 | [Ru-6] | KOH | 78.3% |
| 7 | [Ru-7] | KOH | 86.1% |
| 8 | [Ru-8] | KOH | 79.2% |
| 9 | [Ru-9] | KOH | 83.6% |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Jiang, M.; Dekyvere, S.; Wang, P.; Chen, C.; Verpoort, F. Exploration of Regulation of Alcohol Dehydrogenation Reaction of Dibenzimidazole-Based Ruthenium Complexes. Molecules 2025, 30, 842. https://doi.org/10.3390/molecules30040842
Li S, Jiang M, Dekyvere S, Wang P, Chen C, Verpoort F. Exploration of Regulation of Alcohol Dehydrogenation Reaction of Dibenzimidazole-Based Ruthenium Complexes. Molecules. 2025; 30(4):842. https://doi.org/10.3390/molecules30040842
Chicago/Turabian StyleLi, Shuai, Min Jiang, Sander Dekyvere, Peng Wang, Cheng Chen, and Francis Verpoort. 2025. "Exploration of Regulation of Alcohol Dehydrogenation Reaction of Dibenzimidazole-Based Ruthenium Complexes" Molecules 30, no. 4: 842. https://doi.org/10.3390/molecules30040842
APA StyleLi, S., Jiang, M., Dekyvere, S., Wang, P., Chen, C., & Verpoort, F. (2025). Exploration of Regulation of Alcohol Dehydrogenation Reaction of Dibenzimidazole-Based Ruthenium Complexes. Molecules, 30(4), 842. https://doi.org/10.3390/molecules30040842








