Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications
Abstract
1. Introduction
2. Results
2.1. Qualitative and Semi-Quantitative Analysis of Bufadienolides Content
| Name | Formula | RT [min] | Expected [M+H]+ m/z | Measurement Error [mDa] | Isotopic Fit (mσ) | Identification Conf. Level | K. daigremontiana | K. pinnata | |
|---|---|---|---|---|---|---|---|---|---|
| A | 3β -(O-α-L-rhamnopyranosyl)-5 β,11α,14 β,19-tetrahydroxybufa-20,22-diene (ref. cmpd.) | C30H44O11 | 2.1 | 581.2956 | 0.7 | 59.2 | 1 | trace | n.d. |
| B | bryophyllin B | C26H34O9 | 2.7 | 491.2276 | 0.2 | 10.4 | 2 | trace | trace |
| C | tetrahydroxy-bufadiene-O-dHex (an isomer of 1) | C30H44O11 | 2.9 | 581.2956 | −0.1 | 24.5 | 2 | trace | n.d. |
| D | bersaldegenin-acetate isomer 1 | C26H34O8 | 3.7 | 475.2326 | 0.5 | 78.5 | 2 | trace | 2.6 ± 0.7 |
| E | bryotoxin-B | C26H32O9 | 4.8 | 489.2119 | 0.2 | 5.3 | 3 | trace | n.d. |
| F | bryophyllin A/bryotoxin C (ref.cmpd.) | C26H32O8 | 4.9 | 473.2174 | −0.2 | 21.0 | 1 | 5.9 ± 0.5 | n.d. |
| G | bersaldegenin-acetate isomer 2 | C26H34O8 | 5.6 | 475.2326 | −0.1 | 8.5 | 3 | trace | trace |
| H | bryophyllin A/bryotoxin C isomer 2 | C26H32O8 | 5.7 | 473.2176 | −0.8 | 22.4 | 3 | trace | n.d. |
| I | diagremontianin isomer | C26H30O9 | 6.2 | 487.1963 | −0.9 | 24.5 | 3 | 1.9 ± 0.4 | n.d. |
| J | bryophyllin -C | C26H34O8 | 6.9 | 475.2326 | −0.7 | 7.2 | 2 | 3.0 ± 0.4 | trace |
| K | diagremontianin (ref. cmpd.) | C26H30O9 | 8.3 | 487.1963 | −0.5 | 9.2 | 1 | 4.6 ± 0.4 | n.d. |
| L | 1,3,5-bersaldegenin-orthoacetate (ref. cmpd.) | C26H32O7 | 13.9 | 457.2221 | 0.7 | 4.2 | 1 | 80.4 ± 1.0 | 7.7 ± 0.6 |
2.2. Bufadienolides’ Permeability Through the Strat-M Membrane
2.3. Antioxidant Activity
2.4. Enzyme Inhibition
2.5. The Sun Protection Factor In Vitro of the Kalanchoe spp. Juices
- No protection SPF in vitro ≤ 5.9;
- Low protection 6.0 ≤ SPF in vitro ≤ 14.9;
- Medium protection 15.0 ≤ SPF in vitro ≤ 29.9;
- High protection 30.0 ≤ SPF in vitro ≤ 59.9 [31].
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material
4.3. Qualitative and Semi-Quantitative Analyses of Bufadienolides
4.4. Bufadienolides’ Permeability Through the Strat-M Membrane
4.5. Antioxidant and Reduction Power Assays
4.5.1. DPPH Assays
4.5.2. ABTS Assays
4.5.3. FRAP Assay
4.5.4. Molybdenum Reducing Power
4.6. Enzyme Inhibition
4.6.1. Tyrosinase Assay
4.6.2. Hyaluronidase Assay
4.7. SPF Factor Determination
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Intern. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Kumar, V. Perspective of Natural Products in Skincare. Pharm. Pharmacol. Int. J. 2016, 4, 1–3. [Google Scholar] [CrossRef]
- Liang, W. Toxicity and Effect of Chemicals in Skin Care Products on Human Health. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012081. [Google Scholar] [CrossRef]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the Use of Kojic Acid—A Skin-Lightening Ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Alves, W.d.S.; Nascimento, L.F.d.; Santos, M.A.d.; Júnior, G.R.L.M.; Neto, F.A.d.S.; Batista, D.R.d.S.S.; Araújo, G.S.; Batista, D.E.d.S.; Uchôa, V.T.; Carvalho, F.A.S.d. Uso de plantas do gênero Kalanchoe como potencial tratamento de doenças inflamatórias. Seven Ed. 2024. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R. Biological Activities of Leaf Extracts from Selected Kalanchoe Species and Their Relationship with Bufadienolides Content. Pharm. Biol. 2020, 58, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Sztormowska-Achranowicz, K.; Kowalczyk, M.; Soluch, A.; Ochocka, J.R. An In Vitro Anticancer, Antioxidant, and Phytochemical Study on Water Extract of Kalanchoe Daigremontiana Raym.-Hamet and H. Perrier. Molecules 2022, 27, 2280. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Kowalczyk, M.; Hałasa, R.; Gucwa, M.; Ochocka, J.R. Kalanchoe Sp. Extracts—Phytochemistry, Cytotoxic, and Antimicrobial Activities. Plants 2023, 12, 2268. [Google Scholar] [CrossRef] [PubMed]
- Deolindo, V.D.S.; Gomes, R.M.S.; Filho, P.A.L.G.; Barros, F.U.; Martins, M.D.C.D.C.E.; Magalhães, M.M.; Moura, S.D.O.; Netto, N.A.D.B.; Rodrigues, M.O.A.; Melo, V.V.C.; et al. Effect of Aqueous Extract of Leaves of Kalanchoe Pinnata in the Healing of Skin Wounds in Wistar Rats. J. Health Sci. 2022, 2, 2–12. [Google Scholar] [CrossRef]
- Araújo, E.R.D.; Xavier-Santos, J.B.; Da Silva, V.C.; De Lima, J.B.F.; Schlamb, J.; Fernandes-Pedrosa, M.D.F.; Da Silva Júnior, A.A.; De Araújo Júnior, R.F.; Rathinasabapathy, T.; Moncada, M.; et al. Gel Formulated with Bryophyllum Pinnatum Leaf Extract Promotes Skin Wound Healing in Vivo by Increasing VEGF Expression: A Novel Potential Active Ingredient for Pharmaceuticals. Front. Pharmacol. 2023, 13, 1104705. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S.; Van Heerden, F.R. Bufadienolides of Plant and Animal Origin. Nat. Prod. Rep. 1998, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.d.S.; Casanova, L.M.; Costa, S.S. Bioactive Compounds from Kalanchoe Genus Potentially Useful for the Development of New Drugs. Life 2023, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Hering, A.; Ochocka, J.R.; Baranska, H.; Cal, K.; Stefanowicz-Hajduk, J. Mangiferin and Hesperidin Transdermal Distribution and Permeability through the Skin from Solutions and Honeybush Extracts (Cyclopia sp.)—A Comparison Ex Vivo Study. Molecules 2021, 26, 6547. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Guidance Notes on Dermal Absorption; OECD: Paris, France, 2022. [Google Scholar]
- European Medicines Agency. Concept Paper on Development of a Guideline on Quality and Equivalence of Topical Products; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Grillo, R.; Dias, F.V.; Querobino, S.M.; Alberto-Silva, C.; Fraceto, L.F.; De Paula, E.; De Araujo, D.R. Influence of Hybrid Polymeric Nanoparticle/Thermosensitive Hydrogels Systems on Formulation Tracking and in Vitro Artificial Membrane Permeation: A Promising System for Skin Drug-Delivery. Colloids Surf. B Biointerfaces 2019, 174, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Carrer, V.; Guzmán, B.; Martí, M.; Alonso, C.; Coderch, L. Lanolin-Based Synthetic Membranes as Percutaneous Absorption Models for Transdermal Drug Delivery. Pharmaceutics 2018, 10, 73. [Google Scholar] [CrossRef]
- Karadzovska, D.; Riviere, J.E. Assessing Vehicle Effects on Skin Absorption Using Artificial Membrane Assays. Eur. J. Pharm. Sci. 2013, 50, 569–576. [Google Scholar] [CrossRef]
- Assis de Andrade, E.; Machinski, I.; Terso Ventura, A.C.; Barr, S.A.; Pereira, A.V.; Beltrame, F.L.; Strangman, W.K.; Williamson, R.T. A Review of the Popular Uses, Anatomical, Chemical, and Biological Aspects of Kalanchoe (Crassulaceae): A Genus of Plants Known as “Miracle Leaf”. Molecules 2023, 28, 5574. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko-Szajwaj, B.; Pecio, Ł.; Kowalczyk, M.; Stochmal, A. New Bufadienolides Isolated from the Roots of Kalanchoe Daigremontiana (Crassulaceae). Molecules 2016, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Oufir, M.; Seiler, C.; Gerodetti, M.; Gerber, J.; Fürer, K.; Eiff, M.M.; Elsas, S.-M.; Brenneisen, R.; Mandach, U.v.; Hamburger, M.; et al. Quantification of Bufadienolides in Bryophyllum Pinnatum Leaves and Manufactured Products by UHPLC-ESIMS/MS. Planta Med. 2015, 81, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and Lipofuscin as Hallmarks of Skin Aging. Postępy Dermatol. Alergol. 2017, 2, 97–103. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.-P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From Clinical Applications to Molecular and Cellular Mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Derm. -Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S. Do the Polyphenolic Compounds from Natural Products Can Protect the Skin from Ultraviolet Rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Lionetti, N.; Rigano, L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics 2017, 4, 15. [Google Scholar] [CrossRef]
- Costa, S.S.; Muzitano, M.F.; Camargo, L.M.M.; Coutinho, M.A.S. Therapeutic Potential of Kalanchoe Species: Flavonoids and Other Secondary Metabolites. Nat. Prod. Commun. 2008, 3, 1934578X0800301. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Nowak, A.; Hering, A.; Kucharski, Ł.; Graczyk, P.; Kowalczyk, M.; Sulikowski, T.; Muzykiewicz-Szymańska, A. Antiaging Properties of Kalanchoe Blossfeldiana Ethanol Extract—Ex Vivo and In Vitro Studies. Molecules 2024, 29, 5548. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) Fragments Induce Chemokine Gene Expression in Alveolar Macrophages. The Role of HA Size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Stapelberg, J.; Nqephe, M.; Lambrechts, I.; Crampton, B.; Lall, N. Selected South African Plants with Tyrosinase Enzyme Inhibition and Their Effect on Gene Expression. S. Afr. J. Bot. 2019, 120, 280–285. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic Acid Content, Antioxidant and Cytotoxic Activities of Four Kalanchoë Species. Saudi J. Biol. Sci. 2018, 25, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Puschett, J.B.; Agunanne, E.; Uddin, M.N. Emerging Role of the Bufadienolides in Cardiovascular and Kidney Diseases. Am. J. Kidney Dis. 2010, 56, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, K.; Bonikowski, R. Bufadienolides—Natural, Biologically Active Compounds for Medicines and Cosmetics. A Review. Biotechnol. Food Sci. 2023, 85, 3–15. [Google Scholar] [CrossRef]
- Cunha-Filho, G.A.; Resck, I.S.; Cavalcanti, B.C.; Pessoa, C.Ó.; Moraes, M.O.; Ferreira, J.R.O.; Rodrigues, F.A.R.; Dos Santos, M.L. Cytotoxic Profile of Natural and Some Modified Bufadienolides from Toad Rhinella schneideri Parotoid Gland Secretion. Toxicon 2010, 56, 339–348. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; De Andrade, H.F.; Antoniazzi, M.M.; Jared, C. Antileishmanial and Antitrypanosomal Activity of Bufadienolides Isolated from the Toad Rhinella jimi Parotoid Macrogland Secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef]
- Gao, H.; Zehl, M.; Leitner, A.; Wu, X.; Wang, Z.; Kopp, B. Comparison of Toad Venoms from Different Bufo Species by HPLC and LC-DAD-MS/MS. J. Ethnopharmacol. 2010, 131, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Stochmal, A. Bufadienolides of Kalanchoe Species: An Overview of Chemical Structure, Biological Activity and Prospects for Pharmacological Use. Phytochem. Rev. 2017, 16, 1155–1171. [Google Scholar] [CrossRef]
- Koorbanally, N.A.; Koorbanally, C.; Harilal, A.; Mulholland, D.A.; Crouch, N.R. Bufadienolides from Drimia Robusta and Urginea Epigea (Hyacinthaceae). Phytochemistry 2004, 65, 3069–3073. [Google Scholar] [CrossRef]
- Moodley, N.; Crouch, N.R.; Mulholland, D.A. Bufadienolides from Drimia macrocentra and Urginea riparia (Hyacinthaceae: Urgineoideae). Phytochemistry 2007, 68, 2415–2419. [Google Scholar] [CrossRef]
- Crouch, N.R.; Toit, K.D.; Mulholland, D.A.; Drewes, S.E. Bufadienolides from Bulbs of Urginea lydenburgensis (Hyacinthaceae: Urgineoideae). Phytochemistry 2006, 67, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Puschett, J.B. Vascular Effects of the Bufodienolides. Trans. Am. Clin. Climatol. Assoc. 2008, 119, 103–112; discussion 112. [Google Scholar] [PubMed]
- Finger Banfi, F.; Camila Krombauer, G.; Luisa Da Fonseca, A.; Rachide Nunes, R.; Nunes Andrade, S.; Alves De Rezende, M.; Helena Chaves, M.; Dos Santos Monção Filho, E.; Guterres Taranto, A.; De Jesus Rodrigues, D.; et al. Dehydrobufotenin Extracted from the Amazonian Toad Rhinella marina (Anura: Bufonidae) as a Prototype Molecule for the Development of Antiplasmodial Drugs. J. Venom. Anim. Toxins incl. Trop. Dis 2021, 27, e20200073. [Google Scholar] [CrossRef] [PubMed]
- Laird, G.M.; Eisele, E.E.; Rabi, S.A.; Nikolaeva, D.; Siliciano, R.F. A Novel Cell-Based High-Throughput Screen for Inhibitors of HIV-1 Gene Expression and Budding Identifies the Cardiac Glycosides. J. Antimicrob. Chemother. 2014, 69, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Botha, C. Potential Health Risks Posed by Plant-Derived Cumulative Neurotoxic Bufadienolides in South Africa. Molecules 2016, 21, 348. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Mersni, D.; Lourith, N. Plant-Derived Saponins and Their Prospective for Cosmetic and Personal Care Products. Bot. Stud. 2024, 65, 32. [Google Scholar] [CrossRef] [PubMed]
- Schafer, N.; Balwierz, R.; Biernat, P.; Ochędzan-Siodłak, W.; Lipok, J. Natural Ingredients of Transdermal Drug Delivery Systems as Permeation Enhancers of Active Substances through the Stratum Corneum. Mol. Pharm. 2023, 20, 3278–3297. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Ochocka, R.; Hering, A.; Stefanowicz–Hajduk, J.; Cal, K.; Barańska, H. The Effect of Mangiferin on Skin: Penetration, Permeation and Inhibition of ECM Enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef] [PubMed]
- Arce, F.J.; Asano, N.; See, G.L.; Itakura, S.; Todo, H.; Sugibayashi, K. Usefulness of Artificial Membrane, Strat-M®, in the Assessment of Drug Permeation from Complex Vehicles in Finite Dose Conditions. Pharmaceutics 2020, 12, 173. [Google Scholar] [CrossRef]
- Kichou, H.; Bonnier, F.; Dancik, Y.; Bakar, J.; Michael-Jubeli, R.; Caritá, A.C.; Perse, X.; Soucé, M.; Rapetti, L.; Tfayli, A.; et al. Strat-M® Positioning for Skin Permeation Studies: A Comparative Study Including EpiSkin® RHE, and Human Skin. Int. J. Pharm. 2023, 647, 123488. [Google Scholar] [CrossRef]
- Wang, Z.; Man, M.-Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-Associated Alterations in Epidermal Function and Their Clinical Significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Wang, Z.; Hwang, S.H.; Kang, Y.-H.; Lee, J.-Y.; Lim, S.S. Comprehensive Evaluation of the Antioxidant Capacity of Perilla Frutescens Leaves Extract and Isolation of Free Radical Scavengers Using Step-Wise HSCCC Guided by DPPH-HPLC. Int. J. Food Prop. 2017, 20, 921–934. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Yagi, A.; Kanbara, T.; Morinobu, N. Inhibition of Mushroom-Tyrosinase by Aloe Extract. Planta Med. 1987, 53, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Hering, A.; Stefanowicz-Hajduk, J.; Gucwa, M.; Wielgomas, B.; Ochocka, J.R. Photoprotection and Antiaging Activity of Extracts from Honeybush (Cyclopia Sp.)—In Vitro Wound Healing and Inhibition of the Skin Extracellular Matrix Enzymes: Tyrosinase, Collagenase, Elastase and Hyaluronidase. Pharmaceutics 2023, 15, 1542. [Google Scholar] [CrossRef] [PubMed]
- Kaessler, A.; Nourrisson, M.-R.; Duflos, M.; Jose, J. Indole Carboxamides Inhibit Bovine Testes Hyaluronidase at pH 7.0 and Indole Acetamides Activate the Enzyme at pH 3.5 by Different Mechanisms. J. Enzym. Inhib. Med. Chem. 2008, 23, 719–727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of In Vivo and In Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinacao Do Fator de Protecao Solar Por Espectrofotometria. An. Bras. Dermatol. Rio Jan. 1986, 61, 121–124. [Google Scholar]

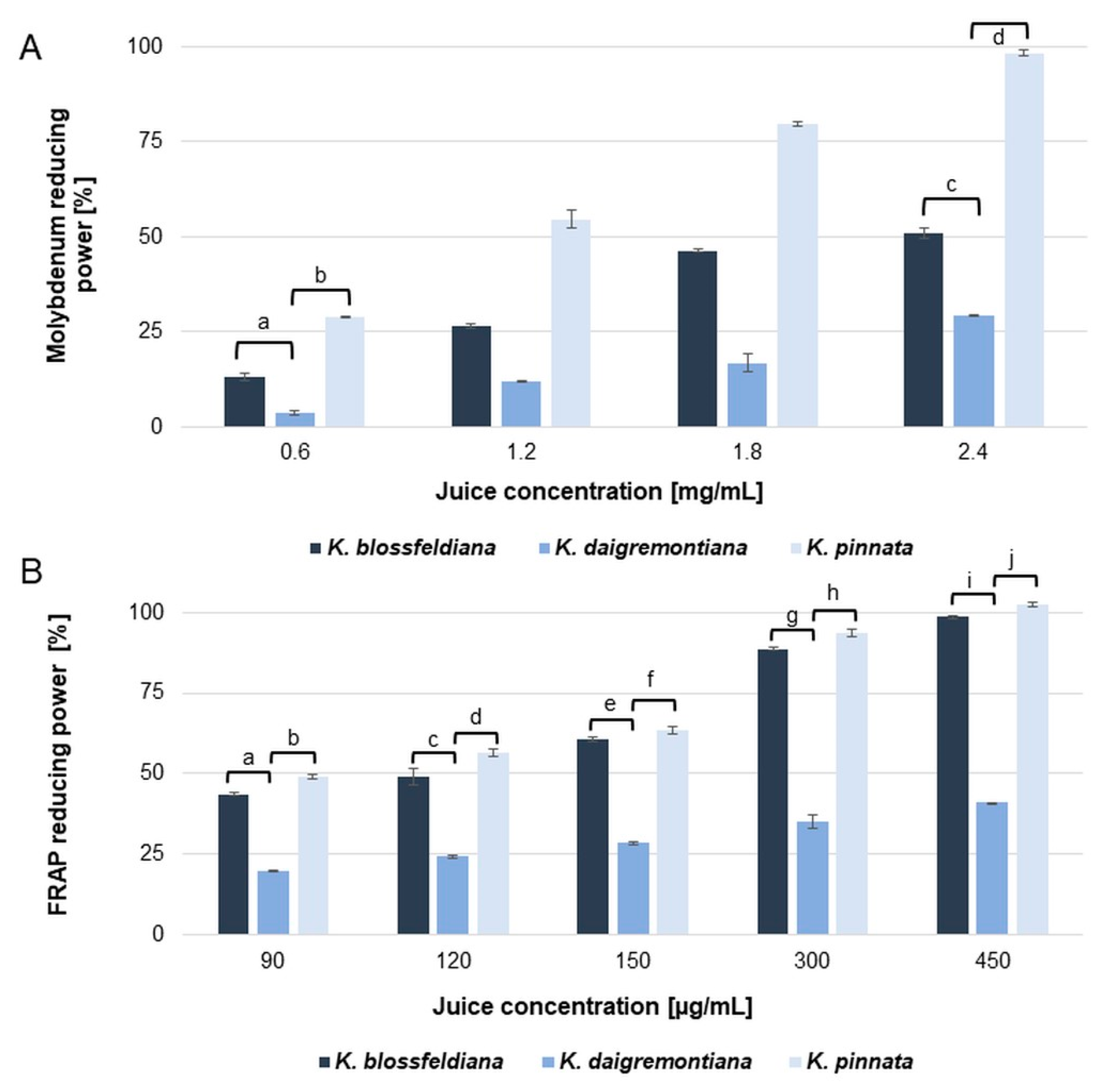
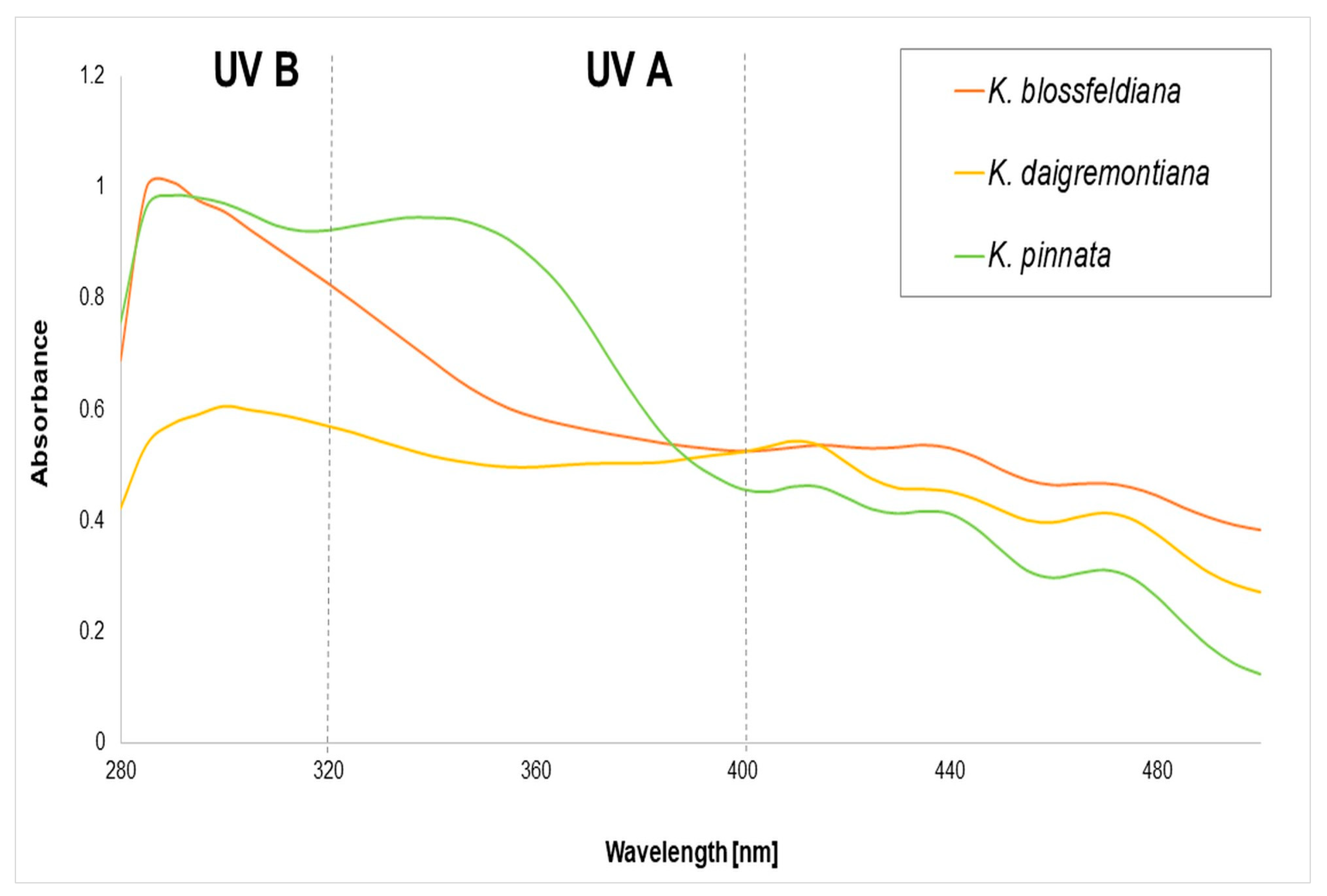
| IC50 µg/mL | ||||
|---|---|---|---|---|
| K. blossfeldiana | K. daigremontiana | K. pinnata | Ascorbic Acid | |
| DPPH | 50.7 ± 0.77 * | 496.13 ± 1.74 * | 61.1 ± 0.89 * | 12.12 ± 0.18 |
| ABTS | 18.8 ± 0.21 * | 559.75 ± 5.75 * | 28.27 ± 0.73 * | 3.25 ± 0.15 |
| Ferric reduction power | 123.19 ± 3.72 * | 561.36 ± 2.91 * | 99.03 ± 1.75 * | 5.75 ± 0.55 |
| Molybdenum reduction power | 2183.42 ± 12.52 * | NR | 1148.86 ± 15.46 * | 22.12 ± 0.35 |
| IC50 [µg/mL] | |||||
|---|---|---|---|---|---|
| K. blossfeldiana | K. daigremontiana | K. pinnata | Kojic Acid | Oleanolic Acid | |
| Tyrosinase | 317.17 ± 6.94 * | 457.12 ± 5.33 * | 206.13 ± 7.4 * | 22.75 ± 0.15 | n.t. |
| Hyaluronidase | 251.5 ± 4.28 * | 413.43 ± 5.71 * | 116.15 ± 5.15 * | n.t. | 50.12 ± 2.15 |
| K. blossfeldiana | K. daigremontiana | K. pinnata | |
|---|---|---|---|
| SPF in vitro | 9.2 ± 0.61 | 6.0 ± 0.77 | 9.6 ± 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hering, A.; Cal, K.; Kowalczyk, M.; Kastsevich, A.; Ivashchanka, Y.; Ochocka, J.R.; Stefanowicz-Hajduk, J. Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications. Molecules 2025, 30, 802. https://doi.org/10.3390/molecules30040802
Hering A, Cal K, Kowalczyk M, Kastsevich A, Ivashchanka Y, Ochocka JR, Stefanowicz-Hajduk J. Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications. Molecules. 2025; 30(4):802. https://doi.org/10.3390/molecules30040802
Chicago/Turabian StyleHering, Anna, Krzysztof Cal, Mariusz Kowalczyk, Alina Kastsevich, Yahor Ivashchanka, J. Renata Ochocka, and Justyna Stefanowicz-Hajduk. 2025. "Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications" Molecules 30, no. 4: 802. https://doi.org/10.3390/molecules30040802
APA StyleHering, A., Cal, K., Kowalczyk, M., Kastsevich, A., Ivashchanka, Y., Ochocka, J. R., & Stefanowicz-Hajduk, J. (2025). Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications. Molecules, 30(4), 802. https://doi.org/10.3390/molecules30040802








