1. Introduction
Chicken coccidiosis, a parasitic disease caused by protozoa of the
Eimeria genus, poses a significant challenge to the global poultry industry. This disease inflicts heavy economic losses, estimated at around USD 3 billion annually, primarily due to decreased growth rates, impaired feed efficiency, and elevated mortality in infected flocks [
1,
2]. Apart from these financial implications, coccidiosis adversely affects food security and animal welfare on a global scale. Historically, the treatment of coccidiosis has relied heavily on anticoccidial drugs, including polyether antibiotics (such as monensin and salinomycin) and synthetic compounds (such as amprolium and diclazuril) [
3,
4,
5]. These drugs have played a key role in containing the disease and ensuring productivity in the poultry industry. Nevertheless, the often excessive use of anticoccidial agents has led to the rapid emergence and spread of drug-resistant
Eimeria strains [
6,
7]. Consequently, this phenomenon has severely undermined the effectiveness of existing treatments, highlighting the urgent need to formulate novel therapeutic strategies.
Halofuginone (HFG), a halogenated product derived from the natural compound febrifugine, has shown outstanding effectiveness among the anticoccidial agents against apicomplexan parasites, including
Eimeria spp. [
8,
9]. Its high efficacy has made HFG a valuable tool in combating coccidiosis. Moreover, recent research has revealed crucial insights into its mechanism of action, paving new pathways for advanced drug development and resistance management. The identification of prolyl-tRNA synthetase (PRS) as a molecular target of HFG in
Plasmodium falciparum, another apicomplexan parasite, marks a breakthrough in elucidating its mode of action [
10,
11]. PRS, a vital member of the aminoacyl-tRNA synthetase (AARS) family, is essential for protein synthesis as it catalyzes the covalent attachment of proline to its corresponding transfer RNA (tRNA) [
12]. This process is vital for accurate genetic translation and parasite survival. Its high sequence conservation among apicomplexan parasites also underscores the significance of PRS as a potent drug target [
13] and its potential use as a reliable molecular marker for early detection of HFG resistance in
Eimeria spp., along with the management of resistant strains [
14]. Additionally, the conserved nature of PRS across species offers a promising approach for the development of broad-spectrum antiparasitic agents.
Structural studies have revealed that PRS consists of two key domains: a catalytic domain (CD) and an insertion (INS) domain [
15]. The former is directly involved in the aminoacylation reaction, while the latter is essential in substrate binding and activation [
16]. The active site of PRS encompasses three distinct pockets that bind ATP, proline, and the 3′-terminal adenosine residue of tRNAPro (A76), respectively [
17], thereby providing numerous potential binding sites for inhibitors. Furthermore, HFG has been recognized as an effective PRS inhibitor, attributed to its distinctive structural properties that allow it to mimic both adenine and L-proline, thus enabling dual-site inhibition of PRS [
18]. This dual-targeting mechanism enhances HFG’s potency and minimizes the risk of resistance arising through single-point mutations. Despite these advantages, emerging HFG resistance highlights the need to formulate new inhibitors that target the same or alternative active sites on PRS.
Computational approaches provide substantial benefits in the initial stages of drug discovery, facilitating rapid large-scale screening of compound libraries and elucidating protein–ligand interactions. Molecular docking is one of the vital computational techniques that enable the virtual screening of millions of compounds against a target protein structure, which identifies potential inhibitors according to their predicted binding affinities and interactions [
19]. Complementing this, molecular dynamics (MD) simulations offer a dynamic perspective on protein–ligand complexes, revealing details about the conformational changes, binding stability, and potential allosteric effects that static structures fail to recognize [
20]. These structure-based simulations yield crucial insights into the mechanism of action of potential inhibitors and help anticipate their efficacy and mode of resistance. In addition, the incorporation of ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) predictions into drug discovery research has become increasingly vital [
21]. In particular, ADMET analysis evaluates the drug-likeness and toxicity profiles of promising compounds in the early stages of the development process, minimizing the risk of late-stage failures and expediting the selection of viable drug candidates.
This study employs a comprehensive computational technique to identify and validate potential inhibitors targeting PRS in Eimeria species. The proposed methodology integrates: (1) virtual screening via two-stage molecular docking to identify novel inhibitors of E. tenella (EtPRS) from a compound library; (2) ADMET predictions to examine the drug-likeness and toxic profile of promising compounds; and (3) MD simulations to assess the binding stability and conformational dynamics of potential inhibitors with EtPRS. The combination of these computational methods is aimed to accelerate the discovery of novel and effective anticoccidial agents targeting PRS in E. tenella.
By advancing the understanding of drug–parasite interactions at the molecular level, this study lays a pathway for developing novel therapeutic strategies to manage the threat of coccidiosis in the poultry industry. This study also emphasizes the importance of applying combined computational approaches with experimental validation to reveal the molecular mechanisms underlying drug resistance and develop effective strategies for addressing resistance in other parasites.
2. Results and Discussion
2.1. Sequence and Model Reliability Analysis
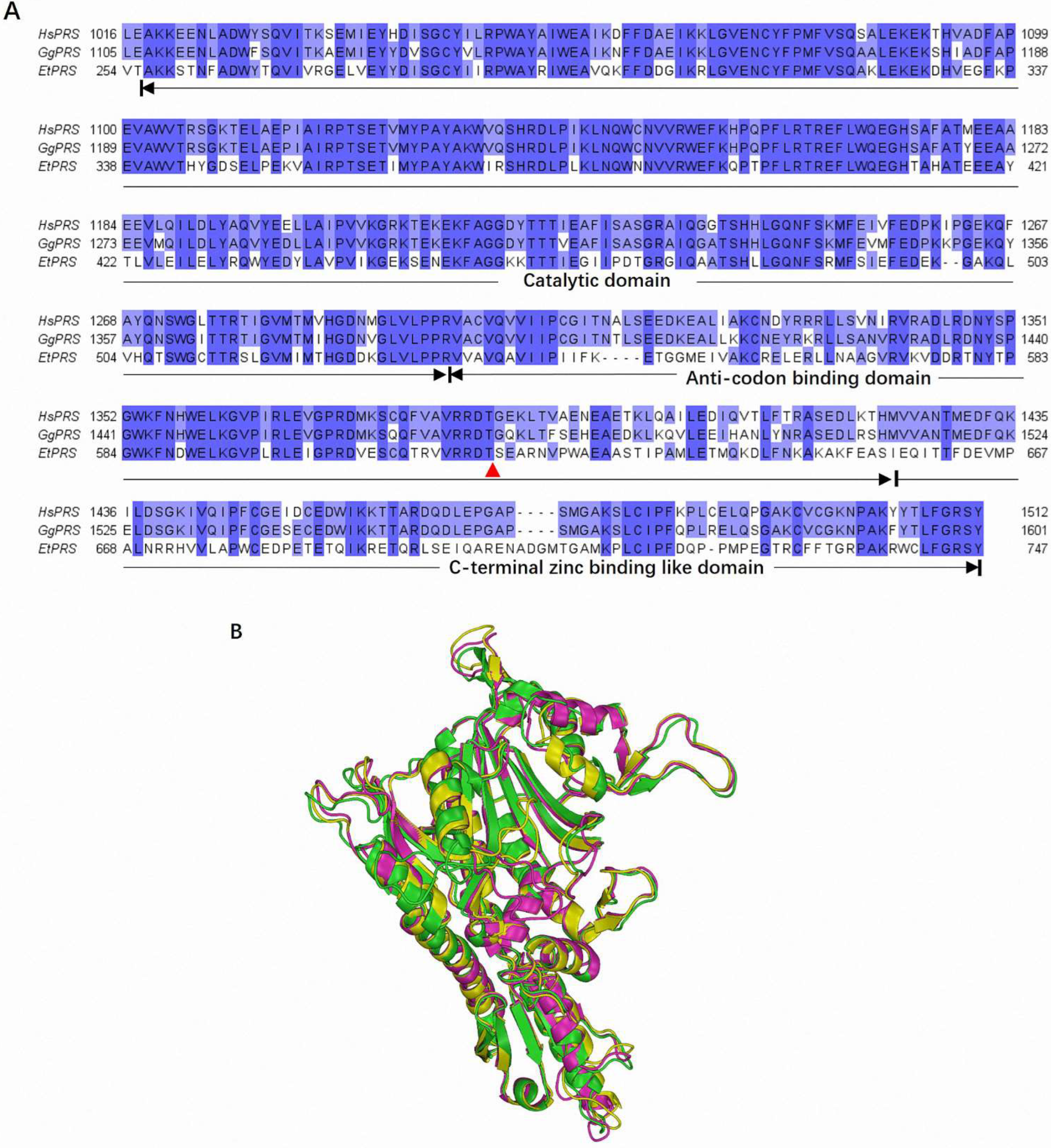
The multiple sequence alignment of HsPRS, GgPRS, and EtPRS revealed significant conservation within the catalytic domain (CD), anticodon binding domain (ABD), and C-terminal zinc-binding-like domain (Z-domain). These regions are essential for enzymatic activity and structural stability, displaying key sequence similarity across the three species, as depicted in
Figure 1A. Interestingly, this conservation aligns with prior studies emphasizing the evolutionary pressure to preserve critical enzymatic functions across diverse organisms [
22]. The prominent conserved motifs in the CD underscores their essential role in the enzymatic activity of PRS enzymes, as highlighted in earlier research [
23]. Notably, specific mutations linked to drug resistance in EtPRS were mapped onto the sequence alignment and were absent in HsPRS and GgPRS [
24], suggesting potential targets for selective drug design, as discussed in previous studies [
25]. The unique variations in EtPRS highlight its potential as a therapeutic target for parasitic infections, thereby providing a promising solution for drug development.
Structural validation analyses verified the high reliability of all three PRS models. Ramachandran plots detected the majority of residues within favored regions of EtPRS (85.9%), HsPRS (91.9%), and GgPRS (94.2%), with minimal outliers (
Figure S1A,C,E). These findings are consistent with those previously determined in experimental structural studies [
14,
26]. The ERRAT scores for all models, which represent the overall quality of protein structures, were within the 86.4–96.4% range, indicating their acceptable quality (
Table S1). Similarly, the ProSA-web Z-scores for all models (EtPRS: −8.92, HsPRS: −10, GgPRS: −9.87) fell within the typical range for high-resolution protein structures (
Figure S1B,D,F). These results further validate the accuracy of the predicted and experimentally derived structures, aligning with previous studies by Kalman et al. [
27].
Structural alignment of the three PRS enzymes demonstrated significant spatial overlap, specifically in the CD and ABDs, which are crucial for their enzymatic function (
Figure 1B). Minor fluctuations were found in the loop regions and C-terminal regions, which may contribute to species-specific interactions or enzymatic efficiencies, potentially affecting enzyme–substrate interactions [
28]. The predicted GgPRS structure from AlphaFold correlates closely with the experimentally determined HsPRS and EtPRS structures, with minimal variations in vital functional regions. This significant structural conservation underscores the functional similarity of PRS enzymes across species and validates the use of computational modeling for studying the PRS structure [
29].
The combined sequence alignment and structural analyses emphasize the evolutionary conservation of PRS enzymes across species, specifically within key functional domains, such as the CD and ABD regions. These conserved motifs underscore their essential role in PRS activity, while unique mutations in EtPRS offer a promising option for species-specific drug targeting. Structural validation further confirmed the suitability of the PRS models for functional and comparative analyses. The AlphaFold-predicted structure of GgPRS displayed high consistency with experimentally determined structures, highlighting the accuracy of advanced computational modeling in structural prediction. However, the minor structural variations in loop regions suggest potential adaptations influencing enzyme–substrate interactions or specificity, warranting further assessment.
In short, the high-quality PRS models and the conserved sequence features identified in this study established a solid framework for future studies into PRS function, evolution, and its viability as a promising therapeutic target. The structural and functional insights revealed in this study will facilitate the practical design of selective inhibitors that target PRS in parasitic organisms, such as E. tenella, with minimal impact on host enzymes.
2.2. Docking Parameter Reliability Analysis
The molecular docking parameters and findings were thoroughly assessed to validate the accuracy and reliability of the docking simulations performed on EtPRS, HsPRS, and GgPRS. The analysis examined the grid box configurations, ligand preparation procedures, and docking outputs to ensure consistency and predictive reliability. The grid box parameters were specifically developed to include the active sites of each PRS enzyme, precisely targeting critical regions for ligand binding. As portrayed in
Figure S2, the grid boxes were accurately centered and sized, with a 54–70 Å dimension range across the x-, y-, and z-axes (
Table S2). These dimensions were appropriate to accommodate the docking of small molecules, such as ATP and HFG, aligning with past studies that emphasize the significant impact of precise grid box configurations in enhancing docking accuracy [
30].
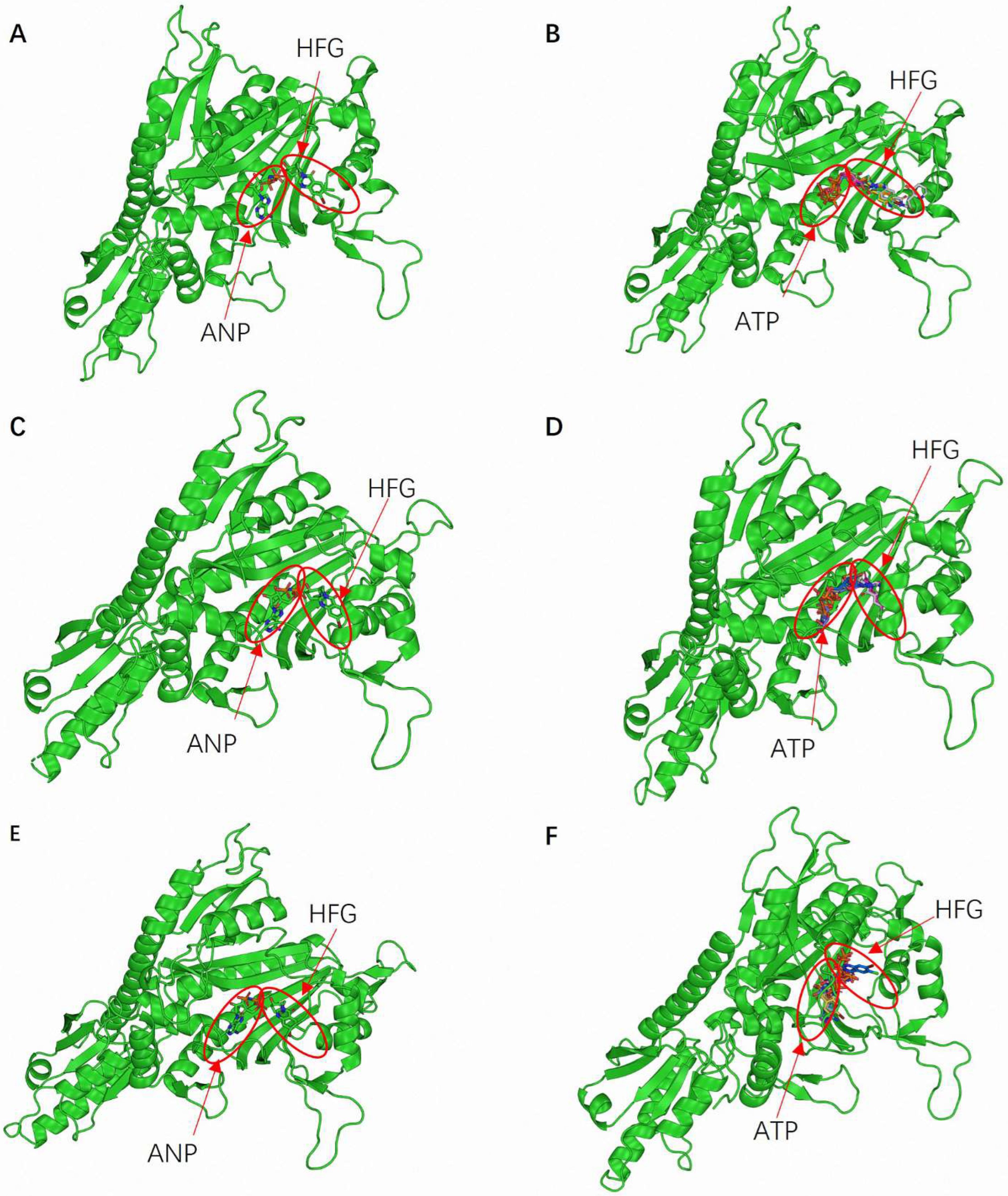
The selected docking parameters were validated through the accurate binding of ATP and HFG at known or predicted active sites during the simulations. This validation process confirmed the precise definition of binding sites, ensuring that the docking simulations targeted the most relevant enzyme regions. Moreover, the binding orientations of ATP and HFG were consistent across the three PRS enzymes.
Figure 2A,C,E illustrates the predicted binding conformations of ATP and HFG within EtPRS, HsPRS, and GgPRS, respectively. Both ligands primarily interacted with the CD, and the complexes were stabilized by hydrogen bonds and hydrophobic interactions. These observed binding conformations were comparable to the experimental data for EtPRS and HsPRS, corroborating the accuracy of the docking procedure. Likewise, the predicted GgPRS model displayed similar binding poses, reinforcing the robustness of the proposed docking approach.
Docking simulations were performed using the four docking tools—QVina2, iDock, Smina, and Vina—to evaluate the reliability of the results further. A comparative analysis of the binding poses revealed a high consistency across the four pieces of software (
Figure 2B,D,F). This inter-software agreement boosted confidence in the predicted binding modes and validated the proposed docking protocols. The alignment between experimental data and docking-based results verified the accuracy of the grid box configurations and the effectiveness of the docking methodologies. Moreover, the stereochemical integrity of the protein models was validated through energy minimization and specific validation tools, significantly enhancing the reliability of the docking simulations. Energy minimization, an essential step prior to docking, ensured that the structural models accurately represented the biologically relevant conformations, as highlighted by Pierce et al. [
31].
Overall, the thorough analysis of docking parameters and binding interaction validation underscores the robustness of the docking simulations, offering a solid reference for future research into the interactions between PRS enzymes and potential therapeutic ligands. This study not only deepens the present understanding of PRS functionality but also facilitates the rational advancement of selective inhibitors targeting PRS in parasitic organisms, thereby paving the way for developing novel therapeutic strategies against parasitic infections.
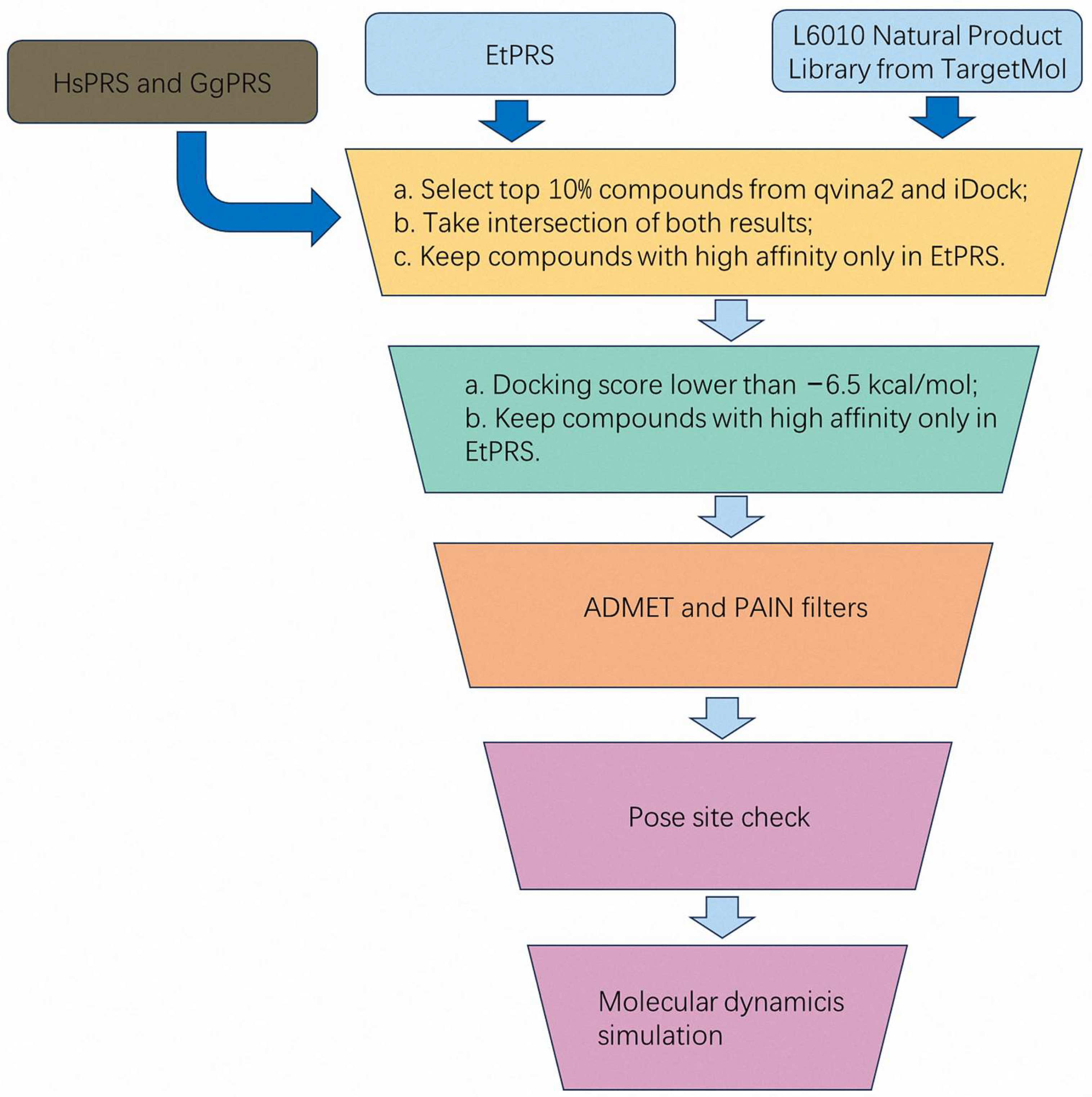
2.3. Two-Stages Virtual Screening for EtPRS Inhibitors
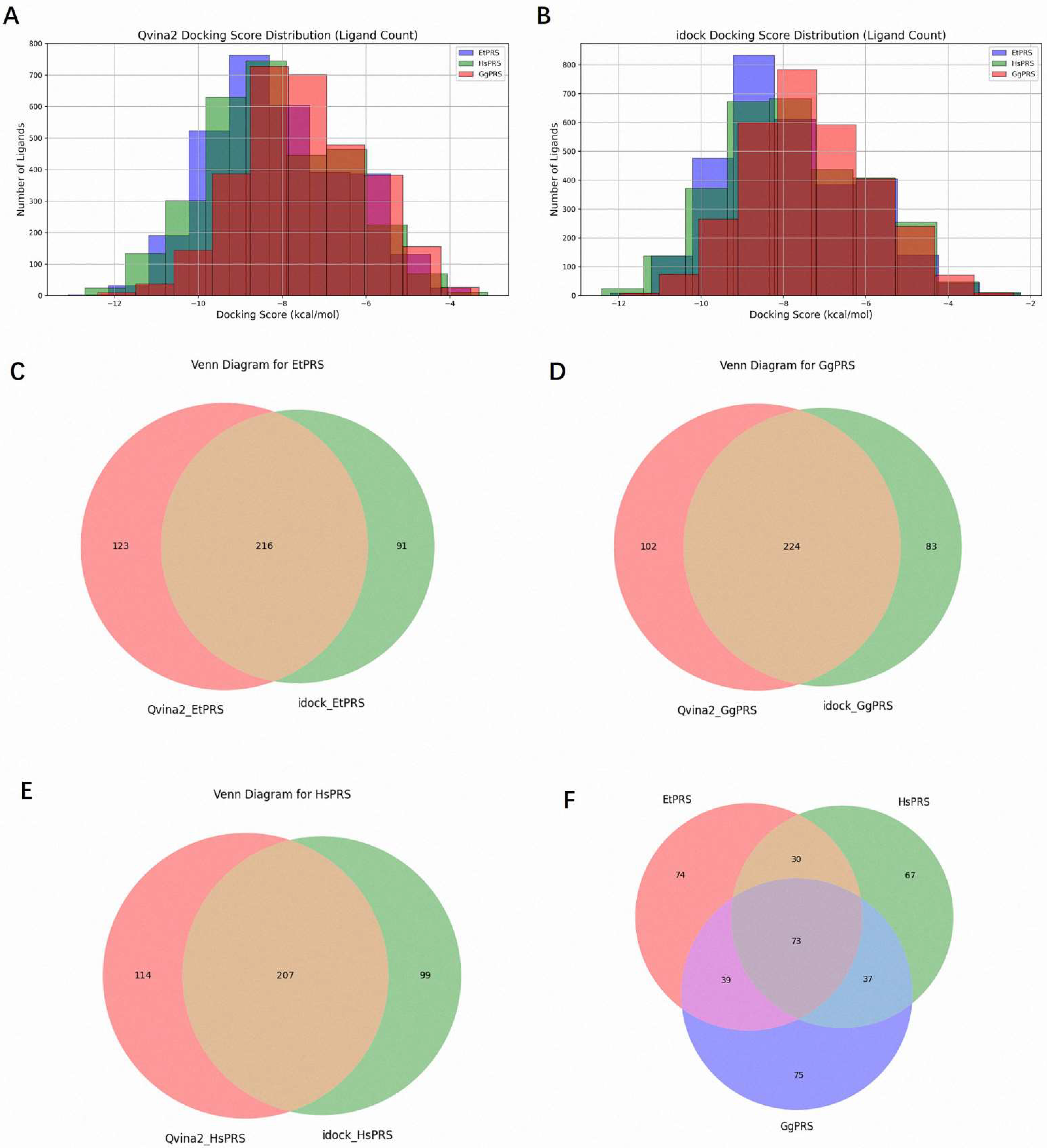
A comprehensive virtual screening of 3045 natural compounds was performed using QVina2 and iDock to identify potential ligands for EtPRS. The top 10% of compounds with the lowest docking scores, indicative of higher binding affinity, were selected for each enzyme (EtPRS, HsPRS, and GgPRS). The distribution of docking scores is shown in
Figure 3A (QVina2) and
Figure 3B (iDock). Notably, compounds with lower docking scores exhibited consistent trends across both tools. Comparatively, EtPRS recorded the largest subset of high-affinity compounds, followed by GgPRS and HsPRS. This finding suggests a potential specificity for EtPRS that aligns with past studies and highlights the significance of target specificity in drug design [
32].
Overlap analyses were conducted to refine the selection further. In total, 216 compounds for EtPRS were identified by both QVina2 and iDock (
Figure 3C), while 224 and 207 compounds were confirmed for GgPRS and HsPRS, respectively (
Figure 3D,E). Further analysis among the top compounds for all three enzymes revealed 74 compounds unique to EtPRS (
Figure 3F). This step was critical in removing compounds with potential binding affinity to HsPRS or GgPRS, thereby ensuring high specificity for EtPRS. Following this rigorous process, 74 initial hits were selected for further evaluation as potential EtPRS inhibitors (
Table S3).
The 74 compounds identified in stage 1 were then refined to improve the selection accuracy and ensure high specificity for EtPRS. The compounds were initially filtered according to their docking scores, with only those scoring lower than −6.5 kcal/mol in both Smina and Vina retained. This threshold, reflecting a strong binding affinity, was established in accordance with previous studies linking lower docking scores to a higher likelihood of effective binding [
33]. Finally, compounds were filtered as per their specificity for EtPRS, ensuring that those with better docking scores for EtPRS compared to HsPRS and GgPRS were retained. This filtering step effectively removed non-specific inhibitors, ensuring the final selection included compounds with both strong binding affinity and high selectivity for EtPRS. Eventually, 42 high-confidence inhibitors were identified, as listed in
Table S4.
From an initial library of 3045 natural compounds, the two-stage virtual screening process effectively integrated multiple docking tools (QVina2, iDock, Smina, and Vina) along with intersection and overlap analyses, resulting in a robust selection of 42 high-confidence inhibitors for EtPRS. The comprehensive methodology combined docking score thresholds, ligand pose consistency checks, and specificity filtering to identify potential inhibitors, with the final 42 compounds representing a promising pool of candidates for further experimental validation. Future studies will focus on evaluating the inhibitory activity and pharmacological potential of these compounds via in vitro and in vivo analysis, advancing the discovery and development of selective inhibitors targeting EtPRS. This systematic approach also lays a reliable foundation for the development of therapeutic strategies against parasitic infections.
2.4. Prediction of Pharmacokinetic Properties
The results of the ADMET analysis are shown in
Table S5. Accordingly, the pharmacokinetic evaluation of the selected compounds (T5S0055, T2850, and T5574) complied favorably with Lipinski’s Rule of Five, implying their potential suitability for oral administration. These compounds demonstrated acceptable molecular weights, lipophilicity, and hydrogen bond characteristics, which are essential for efficient absorption and distribution [
34]. Nevertheless, none of the compounds fulfilled the more stringent criteria set by Pfizer’s 3/75 rule or GSK’s 4/400 rule, indicating potential issues regarding oral bioavailability, necessitating further assessment in future development stages [
35].
Metabolic predictions revealed that the selected compounds interacted with key cytochrome P450 (CYP) isoenzymes, including CYP2C9, CYP3A4, and CYP2D6. These interactions could substantially affect the metabolic clearance of the compounds and potentially impede drug–drug interactions, which are critical considerations during early drug development [
36]. Understanding these metabolic pathways is crucial for predicting the pharmacokinetic behavior of the compounds and mitigating potential adverse effects. Notably, the compounds recorded a low likelihood of hERG channel blockage, a vital factor for evaluating cardiac safety. Additionally, the predictions indicated no mutagenic, tumorigenic, reproductive, or irritant toxicities, further supporting their overall safety profiles [
37].
Although these findings underscore the potential of T5S0055, T2850, and T5574 as promising drug candidates, further structural optimization may be required to improve bioavailability and reduce metabolic liabilities. The pharmacokinetic profiles of these compounds could be enhanced for practical therapeutic applications by considering several strategies, such as modifying their chemical structures to improve solubility or mitigate CYP-mediated metabolism [
38]. In summary, while the initial pharmacokinetic assessments suggest promising attributes for oral administration and safety, additional optimization and thorough in vitro and in vivo studies are crucial to characterize the pharmacokinetic profiles and therapeutic potential of these compounds. This systematic approach will facilitate the development of effective and safe inhibitors targeting EtPRS.
2.5. Binding Interaction and Interpretation of Selected Compounds
The T5S0055 (Chelidonine), T2850 (Bicuculline), and T5574 (Guggulsterone) protein–ligand complexes were assessed to observe their binding interactions with EtPRS. The binding affinities and non-bonding interactions of the compounds were examined through 2D interaction profiles and spatial conformations, which were generated using Discovery Studio version 24.1.0.23298 and PyMOL visualization tools version 3.1.1 (
Table 1;
Figure 4). These analyses emphasize the compounds’ ability to effectively stabilize within the EtPRS binding site, supporting their potential as appropriate EtPRS inhibitors.
Chelidonine demonstrated an array of interactions that facilitate its strong anchoring at the active site, including pi–pi interactions with Phe335 and pi–sigma/amide interactions with Thr359. These interactions indicate a high affinity for the binding site. Furthermore, the stability of the complex was enhanced through van der Waals forces with several residues, such as Glu326, Lys327, and Arg390. Alkyl interactions with Leu325 and Pro358 also supported Chelidonine’s stable conformation within the binding pocket. The diverse interactions suggest a multifaceted binding mode that may significantly contribute to its high binding affinity and potential efficacy as an EtPRS inhibitor.
Meanwhile, Bicuculline exhibited effective binding properties through strong hydrogen bonding with Thr359, as well as pi–pi stacking interactions with Trp407 and His480. The van der Waals interactions with multiple residues, including Glu361, Arg390, and Ala476, further strengthened its binding affinity. Pi–sigma interactions with Thr512 and carbon–hydrogen bonding with Thr478 also contributed to its stability within the active site. These interactions suggest that Bicuculline possesses a dual affinity for both hydrophilic and hydrophobic residues, positioning it as a promising candidate for EtPRS inhibition [
39].
Conversely, Guggulsterone depends mainly on van der Waals forces for stability, interacting with key residues, such as Glu326, Glu333, Val339, and Thr359. It also forms alkyl interactions with Phe335 and Leu325, compensating for its limited hydrogen bond formation. This reliance on hydrophobic interactions indicates that Guggulsterone may adopt a distinct binding mechanism compared to Chelidonine and Bicuculline, highlighting the essential role of non-polar interactions in stabilizing its position within the EtPRS binding site.
The findings of this study demonstrate that all three compounds form stable and diverse interactions with EtPRS, involving a mixture of hydrogen bonds, pi–pi stacking, van der Waals forces, and alkyl interactions. In comparison, Chelidonine exhibited the broadest range of interactions, which supports its strong binding affinity, while Bicuculline possessed specific interactions with both hydrophilic and hydrophobic residues, reflecting its dual affinity for the binding site. Contrarily, Guggulsterone relied primarily on non-covalent forces, particularly van der Waals and alkyl interactions, for stability. Overall, the binding profiles highlight the effective interaction between these compounds and the EtPRS active site, reinforcing their potential as appropriate inhibitors. Further experimental studies are needed to validate these interactions and optimize their binding affinities for therapeutic use.
2.6. Stability, Compactness, and Structural Flexibility of the EtPRS Complex
MD simulations were performed for 100 ns in triplicate on both the apo form of EtPRS and its complexes with three potential inhibitors (T5S0055, T2850, and T5574). The simulations aimed to investigate the structural stability, compactness, and flexibility of the systems, revealing key structural and conformational differences. These insights are crucial for understanding the dynamic behavior of EtPRS and its interactions with inhibitors.
The structural stability of the EtPRS backbone was analyzed based on the RMSD values over the simulation period. According to
Figure 5A, the apo protein and the three complexes recorded average RMSD values of 0.14–0.40 nm, with all systems stabilizing within the initial 40–60 ns. In comparison, EtPRS/T5574 displayed a slightly higher RMSD during the early simulation phase, implying transient structural rearrangements that stabilized over the latter half of the trajectory. This finding aligns with recent studies indicating that ligand binding can induce conformational changes in target proteins, influencing their dynamic behavior [
40,
41]. The apo form of EtPRS maintained consistently lower RMSD values, suggesting stable dynamics without ligands, which corresponds with findings from similar unbound protein systems [
42]. Furthermore, the ligand-specific RMSD analysis revealed distinct binding stability patterns among the three inhibitors throughout the simulation (
Figure 5B). T2850 exhibited remarkably stable binding dynamics with consistently low RMSD values, while T5574 demonstrated more dynamic behavior, characterized by higher fluctuations and a distinctive peak reaching approximately 1.0 nm around 45–50 ns. Notably, T5S0055 maintained intermediate stability throughout the trajectory. These differential binding dynamics, particularly the pronounced conformational flexibility of T5574, suggest its potential for inducing significant structural adaptations in EtPRS, which may contribute to its inhibitory efficacy. These findings provide valuable insights into the molecular basis of inhibitor–target interactions and further validate T5574 as a promising EtPRS inhibitor candidate.
Structural flexibility was evaluated using RMSF values for each EtPRS backbone residue, as depicted in
Figure 5C. The analysis revealed remarkably similar flexibility patterns across all systems, including both the apo form and inhibitor-bound complexes. Major fluctuations were consistently observed around residues 300–350 and near residue 700, with these regions showing comparable mobility patterns regardless of inhibitor binding. This stabilization is particularly crucial, given its enhancement of the binding affinity by mitigating the entropic penalty linked to the ligand binding [
43]. The conservation of flexibility patterns across all conditions indicates that while these inhibitors effectively bind to EtPRS, they do so without dramatically altering the protein’s inherent dynamic behavior. This consistency in structural flexibility suggests that the inhibitors’ effectiveness may be more related to their specific binding interactions rather than through induced changes in protein dynamics.
The Rg was calculated to assess the system compactness during the simulations.
Figure 5D shows the Rg values for the apo protein and the complexes, ranging from 2.6 to 2.65 nm. The apo form of EtPRS maintained a slightly more compact structure throughout the simulation, while EtPRS/T5574 exhibited marginal Rg variations, indicating minor conformational changes induced by its binding. This behavior suggests the ligand’s potential to regulate the protein’s structural dynamics, as previously associated with alterations in enzymatic activity [
44]. In contrast, both EtPRS/T5S0055 and EtPRS/T2850 displayed stable Rg trajectories similar to the apo protein, reflecting their minimal impact on the overall protein compactness. These findings demonstrate that while both inhibitors effectively bind to EtPRS, they do so without inducing substantial changes in the protein’s overall dimensions or compactness, suggesting that their inhibitory mechanisms likely involve local interactions rather than global conformational changes.
Figure S3 illustrates the secondary structural elements of EtPRS observed throughout the simulation period. Comparatively, the apo form retained a highly stable secondary structure across the trajectory, while the inhibitor-bound systems showed subtle yet substantial changes, especially in the helical and loop regions near the binding pocket. Among the inhibitors, T5S0055 most effectively preserved the protein’s secondary structure, highlighting its stabilizing effect on the EtPRS complex. This observation is essential, as maintaining secondary structure integrity is frequently associated with the functional stability of enzymes.
Overall, the RMSD, Rg, and RMSF analyses collectively highlight that T5S0055 forms the most stable complex with EtPRS, effectively preserving its structural integrity and compactness. T2850 also displayed strong stabilizing effects, although to a slightly lesser extent, while T5574 induced mild structural variations. These findings offer valuable insights into the dynamic behavior and binding properties of EtPRS and its inhibitors, establishing a foundation for future drug development efforts. The study underscores the significance of considering structural dynamics when designing potent and selective EtPRS-targeting inhibitors, contributing to the advancement of therapeutic strategies against parasitic infections. Further experimental validation is needed to confirm the impact of these dynamic interactions in biological systems, paving the way for efficient drug design.
2.7. Principal Component and Free Energy Analyses of the EtPRS Complex
PCA was conducted to assess the combined atomic motions in the apo form of EtPRS and its complexes with T5S0055, T2850, and T5574 during the 100 ns molecular dynamics simulation. The analysis emphasized eigenvectors that corresponded to the largest eigenvalues, which represented the dominant motions within the systems. Stable PCA clusters were derived from the equilibrated and stable time frames of the simulation trajectories for all four systems, as presented in
Figure 6.
The computed trace values of the covariance matrices denoted the degree of motion in the systems for the EtPRS apo protein, EtPRS/T5S0055, EtPRS/T2850, and EtPRS/T5574, respectively. The apo protein recorded the smallest trace value, indicating a more compact and stable structural configuration compared to the inhibitor-bound complexes. This aligns with past studies suggesting the often reduced conformational flexibility of unbound proteins, essential for maintaining functional integrity [
45]. Among the inhibitor-bound complexes, T5S0055 demonstrated a lower trace value, signifying its stabilizing effect on the protein dynamics. Conversely, T2850 and T5574 triggered more substantial collective motions, evidenced by their higher trace values, implying a more dynamic binding environment.
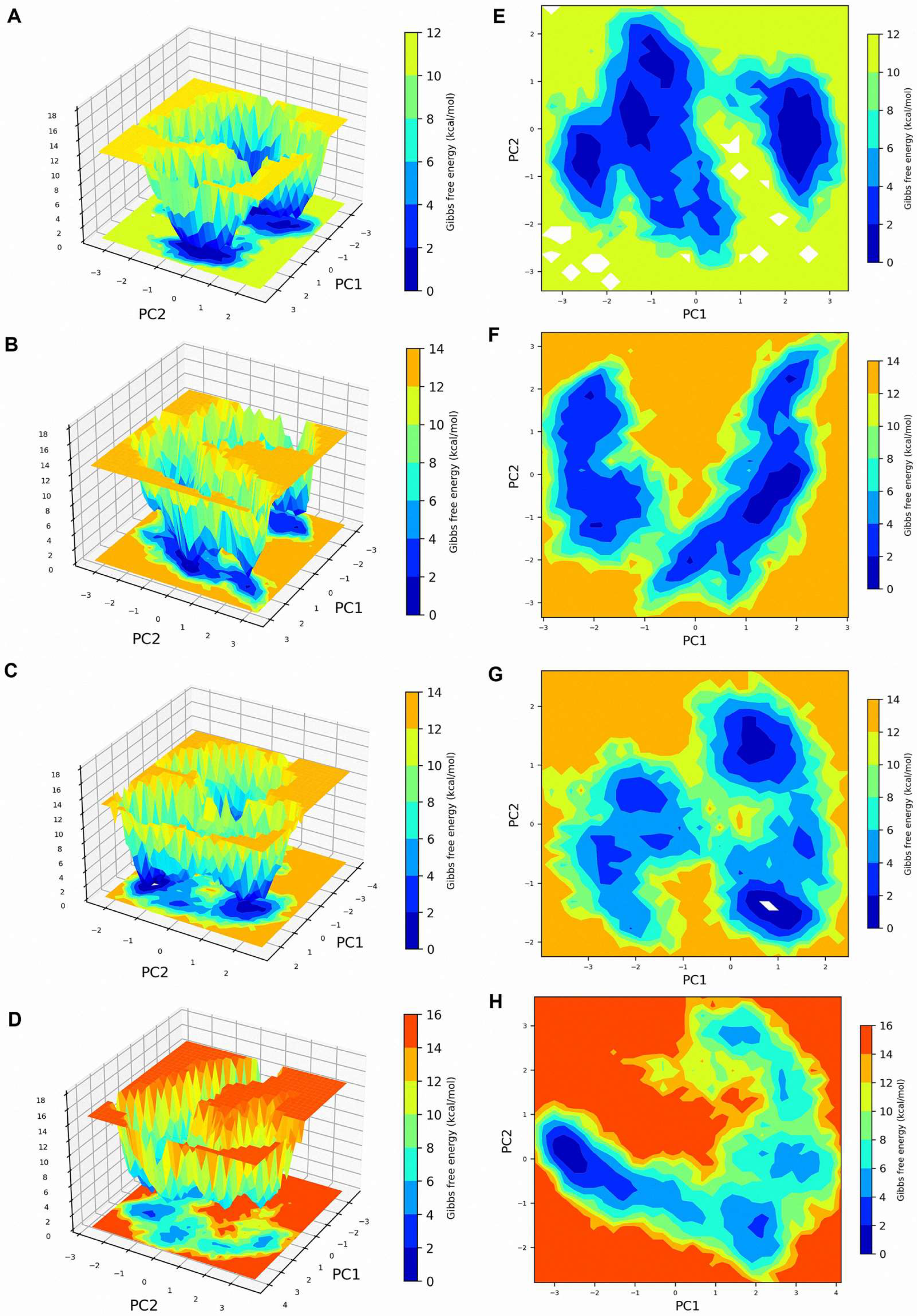
The system dynamics were further analyzed by generating Gibbs FEL using the first two principal components (PC1 and PC2) for all systems (
Figure 6A–H). The apo protein exhibited two well-defined low-energy basins (0–12 kcal/mol), indicating two major stable conformational states (deep blue regions in
Figure 6A,E). This stability indicates a well-defined conformational state frequently reported in unbound proteins [
46]. Contrarily, multiple low-energy states were observed in the presence of inhibitors, suggesting conformational diversity induced by ligand binding. EtPRS/T5S0055 maintained a similar dual-basin pattern but with altered energy well shapes, suggesting moderate conformational changes (
Figure 6B,F). EtPRS/T2850 displayed multiple scattered low-energy regions, indicating increased conformational flexibility (
Figure 6C,G). This finding aligns with the notion that effective inhibitors induce favorable conformational changes that enhance binding affinity [
47].
On the contrary, T5574 showed the most dramatic changes, with extensive high-energy regions (up to 16 kcal/mol) and a significantly altered energy landscape, implying substantial conformational rearrangements upon binding (
Figure 6D,H). The observed multiple shallow energy basins suggest a higher effectiveness of T5574 in the stabilization of the protein compared to T5S0055 and T2850, potentially leading to increased binding efficacy and specificity. Such conformational state variations could affect their pharmacological profiles, as it is well-established that conformational stability can impact the binding kinetics and overall effectiveness of drug candidates [
48,
49].
The PCA and FEL analyses reveal marked differences in the dynamic behavior of EtPRS in its apo form and when complexed with inhibitors. Notably, T5S0055 and T2850 effectively stabilized the protein, while T5574 induced more significant conformational variations, potentially influencing their binding characteristics and efficacy. These findings align with the results from structural stability and compactness analyses, offering a comprehensive understanding of system dynamics and inhibitor interactions. These insights are valuable for guiding future drug design efforts to develop potent and selective EtPRS-targeting inhibitors, thereby advancing therapeutic strategies against parasitic infections. Additional experimental validation is essential to investigate the biological relevance of these dynamic interactions and their effects on the functional outcomes of EtPRS inhibition.
2.8. Binding Free Energy Analysis of the EtPRS Complexes with Potential Inhibitors
The binding affinities and energetic contributions of T5S0055, T2850, and T5574 to EtPRS were determined using the MM/GBSA method. The binding free energy was calculated based on the final 30 ns of the MD simulation trajectories, with the contributions of numerous energetic components detailed in
Table 2. This analysis presents a quantitative understanding of the interactions between the inhibitors and EtPRS and their relative binding strengths, which is critical for rational drug design.
T5574 recorded the highest binding affinity, with a total binding free energy (ΔG) of −24.60 ± 2.42 kcal/mol, signifying its strong interaction with EtPRS. These results align with recent studies identifying Guggulsterone as a potent inhibitor of multiple biological targets, highlighting its potential as a lead compound for further development [
50]. In comparison, T5S0055 and T2850 showed comparable binding affinities (ΔG of −14.42 ± 3.32 kcal/mol and −14.23 ± 2.51 kcal/mol, respectively). The differences in binding affinities among the inhibitors can be linked to their unique structural features and interaction profiles within the EtPRS active site.
The ΔVDWAALS interactions induced significant contributions to the binding free energy of all three inhibitors, ranging from −37.21 to −40.88 kcal/mol. These robust van der Waals interactions are critical for stabilizing the inhibitor–protein complex and have been shown to substantially influence the binding of small molecules to their targets [
51]. The ΔEEL varied among the inhibitors, with T5S0055 showing moderate contributions (−11.35 ± 3.40 kcal/mol) compared to T2850 (−15.67 ± 5.51 kcal/mol) and T5574 (−5.85 ± 2.72 kcal/mol). This variation in electrostatic interactions highlights the importance of charge complementarity in ligand binding, as favorable electrostatic interactions can improve binding affinity and specificity [
52].
The ΔEGB showed a positive contribution for T5S0055 (28.83 ± 3.70 kcal/mol), T2850 (32.55 ± 6.67 kcal/mol), and T5574 (18.62 ± 2.52 kcal/mol), indicating destabilizing effects. While the inhibitors interact favorably with the protein, the solvation environment may impose energetic penalties that impact the overall binding. The ΔESURF values were relatively consistent across the inhibitors, contributing minor favorable effects to binding. The balance between polar and non-polar solvation energies is vital, given its significant impact on the thermodynamic landscape of ligand binding [
53].
The ΔGGAS and ΔGSOLV emerged as the key factors influencing binding affinity. T5574 demonstrated the most favorable balance between van der Waals interactions and reduced solvation penalties, resulting in its superior binding affinity. The results suggest that T5574 forms the most stable complex with EtPRS, while T5S0055 and T2850 exhibit weaker yet comparable binding. These findings offer valuable insights into the inhibitor’s binding mechanisms and provide the framework for further optimization of potential EtPRS-targeted therapeutics. The results also underscore the need for additional structural and functional studies to validate these computational predictions and assess the therapeutic potential of these inhibitors for combating parasitic infections. Future research should focus on synthesizing analogs and exploring their biological activities to boost the efficacy and selectivity of EtPRS inhibitors.
2.9. Study Limitations and Future Directions
While this study provides valuable insights into potential EtPRS inhibitors through molecular docking and molecular dynamics simulations, several limitations should be addressed in future research. First, our virtual screening was conducted using only the wild-type EtPRS structure, without considering known resistance mutations, particularly the L482F mutations [
24] associated with halofuginone resistance. Future work should examine how these mutations impact inhibitor binding by performing comparative virtual screening against both wild-type and mutant EtPRS structures, which would help identify compounds that maintain efficacy against resistant strains.
Our analysis identified three promising compounds, Chelidonine, Bicuculline, and Guggulsterone, each exhibiting distinct binding characteristics with EtPRS. Chelidonine forms multiple hydrogen bonds and van der Waals interactions with residues such as Phe335, Glu326, and Arg390. Bicuculline demonstrates strong hydrogen bonds with Thr359 and π–π stacking interactions with Trp407 and His480. Guggulsterone, while showing the highest binding affinity through van der Waals interactions with Glu326, Val339, and Thr359, still has room for optimization.
The structural optimization of these compounds presents several opportunities for improvement. For Chelidonine, introducing additional hydrogen bond donors/acceptors and optimizing hydrophobic interactions could enhance its binding affinity. Bicuculline could benefit from enhanced π-π stacking and electrostatic interactions. Guggulsterone’s binding affinity could be further improved by introducing hydrogen bonds and optimizing hydrophobic interactions.
All three compounds face challenges with metabolic stability and bioavailability. These issues could be addressed by introducing metabolically stable groups, such as fluorine atoms or methyl groups, to reduce cytochrome P450 enzyme-mediated metabolism. Additionally, adjusting molecular properties including molecular weight, LogP values, and solubility could improve their oral bioavailability. To minimize off-target effects, structural modifications should focus on enhancing selectivity for EtPRS, particularly targeting unique residues identified in sequence alignment.
Moving forward, experimental validation through site-directed mutagenesis and binding assays will be crucial to confirm the predicted interactions. Implementation of these structural optimizations could significantly enhance the binding affinity, bioavailability, and therapeutic potential of these compounds, ultimately advancing the development of effective EtPRS-targeted anticoccidial drugs.
3. Materials and Methods
3.1. Sequence Alignment and Protein Preparation
The amino acid sequences of prolyl-tRNA synthetase (PRS) were retrieved from the National Center for Biotechnology Information (NCBI) database. The analysis included PRS sequences from
Gallus (GgPRS, Accession number: NP_001006398.2),
Homo sapiens (HsPRS, Accession number: NP_004437.2), and
E. tenella (EtPRS, Accession number: CDJ42472.1). The proS_fam_I, a critical enzymatic region for PRS activity, was identified across all sequences. The sequences were aligned using Clustal Omega version 1.2.4 to examine similarities and conserved regions [
54]. The alignment results were then visualized using Jalview version 2.11.4.1 [
55] to identify the conserved motifs and functional regions vital to enzyme activity.
The EtPRS and HsPRS crystal structures were obtained from the RCSB Protein Data Bank, with the corresponding PDB IDs (EtPRS: 5XIP) and (HsPRS: 4HVC). Non-relevant ligands and water molecules were removed, and a single chain was retained at the beginning of each structure. Modeller version 10.5 was applied to perform local reconstruction for regions with missing loops [
56]. Meanwhile, the GgPRS structure was predicted using AlphaFold version 2.3.1 [
57] with default parameters. Both the predicted GgPRS model and the experimental crystal structures underwent energy minimization using Swiss Protein Data Bank Viewer (SPDBV) version 4.10 [
58] to achieve optimal geometry and decrease steric clashes.
The resulting structures were assessed using the Structure Analysis and Verification Server (SAVES) version 6.0, comprising several analytical tools, such as PROCHECK [
59] and ERRAT [
60], to determine stereochemical quality and overall structural validity. ProSA-web (
https://prosa.services.came.sbg.ac.at/prosa.php) [
61] was also utilized to detect errors in the three-dimensional (3D) structures. The model with the best scores and acceptable stereochemical parameters was selected for further processing. Subsequently, protein preparation was conducted using AutoDockTools in MGLTools version 1.5.6 [
62]. Water molecules, ligands, and ions were removed from the structure, followed by the addition of polar hydrogens and labeling Kollman charges to all atoms. Protonation states of histidine residues were determined at pH 7.4 using PROPKA version 3.1 [
63], and the final structure was saved in PDBQT format for further docking studies.
3.2. Ligand Preparation
This study employed the L6010 Natural Product Library (TargetMol, version 2023.1), which constitutes 3045 compounds. Ligand preparation was performed using OpenBabel version 2.4.1 [
64], where 3D conformers were generated with the command—gen3d to select the best conformer. Next, the ligands underwent energy minimization using the MMFF94 force field (—ff MMFF94—steps 5000—sd) to achieve optimal geometry. The protonation states of the ligands were adjusted to pH 7.4 (-p 7.4), followed by tautomer generation (—tautomer) to consider diverse chemical forms. Further preparation of the ligands was carried out using prepare_ligand4.py in AutoDockTools in MGLTools version 1.5.6, and the ligands were saved in PDBQT, MOL2, and SDF formats to ensure compatibility with various docking software, facilitating smoother integration into the docking workflow.
3.3. Preparation of Docking Parameters for Virtual Screening
Potential ligand–protein interactions were investigated by preparing docking parameters using four molecular docking tools: QuickVina version 2.1 (QVina2) [
65], iDock version 1.5 [
66], Smina version 1.1.2 [
67], and AutoDock Vina version 1.1.2 [
68]. The primary objective of the docking study was to establish reliable parameters for identifying binding sites on target proteins and evaluate the docking efficiency. Either experimentally determined crystal structures or homology models of each target protein were utilized as templates during the analysis. Furthermore, complexes of ATP and HFG, derived from experimental data or in silico models, served as essential reference points during parameter setup to identify relevant binding sites and ensure accurate docking outcomes. Grid box parameters were configured using AutoDockTools to specify regions around the active sites of target proteins, enabling docking simulations to concentrate on relevant protein regions while maintaining computational efficiency.
A test docking procedure was conducted using ATP and HFG as ligands to validate the reliability and accuracy of the docking parameters. These ligands were docked into the pre-defined grid boxes using the four docking tools, QVina2, iDock, Smina, and Vina. The docking results were compared against experimental data and predicted binding modes to evaluate the consistency and accuracy of the parameters. Only parameters that demonstrated reliable results, with precise binding orientations and robust correlations to known binding poses, were selected for subsequent virtual screening of the ligand library.
3.4. Two-Stage Virtual Screening for the Identification of Potential Ligands for EtPRS
3.4.1. Stage 1: Initial Selection and Intersection of Docking Results
Docking simulations for EtPRS, HsPRS, and GgPRS were performed using both QVina2 and iDock. The top 10% of compounds with the lowest binding energies (highest affinity) for each protein were selected from both docking tools [
69] and then compared through Venn analysis to identify the overlap of top compounds for each protein. A second Venn analysis was performed to identify compounds unique to EtPRS across all three proteins (EtPRS, HsPRS, and GgPRS). This step ensured the selection of compounds with the highest binding potential to EtPRS for further analysis. The intersections and final selections were visualized and documented using Venn diagrams.
3.4.2. Stage 2: Refining the Results Based on Docking Scores and Ligand Poses
From stage 1, the identified compounds were further refined based on their docking scores and consistency in ligand poses. Only compounds with docking scores lower than −6.5 kcal/mol in both Smina and Vina, indicating strong binding affinity, were retained [
33,
70]. For further refinement, compounds targeting EtPRS were required to exhibit higher docking scores (in both Smina and Vina) compared to those for HsPRS and GgPRS, ensuring that the final compound selection was specific to EtPRS and demonstrated strong binding affinity and consistent poses across various docking tools.
3.5. Pharmacokinetic Properties Predictions
ADMETlab version 3.0 [
71] was employed to predict the ADMET properties of the selected compounds, with an emphasis on critical pharmacokinetic and drug-likeness parameters. The evaluation was based on the following criteria: (1) adherence to Lipinski’s Rule of Five; (2) Pfizer’s 3/75 rule for oral bioavailability; (3) GSK’s 4/400 rule for drug-likeness; and (4) the Pan-Assay Interference Compounds (PAINS) filter to examine potential assay interference. All compounds adhered to Lipinski’s Rule of Five [
72], indicating favorable drug-likeness for oral administration. Nevertheless, the compounds exhibited varying compliance with the Pfizer [
73] and GSK [
74] criteria, suggesting potential limitations in oral bioavailability and overall drug-likeness.
3.6. Molecular Dynamics Simulations
Molecular dynamics simulations were performed using GROMACS 2022 [
75], with the AMBER99SB-ILDN force field for the protein. The ligands were parameterized using acpype.py, with Antechamber and ACPYPE programs in Ambertools version 23.6 [
76] to define relevant ligand parameters. The simulation protocol involved several steps: primarily, each protein–ligand complex was solvated in a dodecahedral box of TIP3P water, with a minimum solute-to-box edge distance of 10 Å, and Na
+ or Cl
− ions were added for neutralization. Energy minimization was conducted using the steepest descent algorithm with a convergence criterion of Fmax < 1000 kJ/mol/nm, followed by a 100 ps NVT equilibration with the V-rescale thermostat (τ = 0.1 ps) at 300 K and a 100 ps NPT equilibration with the Parrinello–Rahman barostat (τ = 2.0 ps) at 1 bar. The production MD run was carried out for 100 ns with a 2 fs time step, applying the LINCS algorithm for bond constraints.
3.7. Trajectory Analysis and Calculations of Binding Free Energies
Post-simulation analysis involved calculating the Root Mean Square Deviation (RMSD), Root Mean Square Fluctuation (RMSF), and radius of gyration (Rg) using GROMACS 2022 and MDAnalysis 2.0. Principal Component Analysis (PCA) was then conducted to assess the dominant collective motions of the EtPRS–inhibitor complex. Eigenvectors and eigenvalues were also calculated through essential dynamics by constructing a variance-covariance matrix using the gmx covar and gmx anaeig modules. The projections of the first two principal components were analyzed, while the Free Energy Landscape (FEL) of the EtPRS-inhibitor complex was mapped using the gmx sham module. The corresponding 3D and 2D contour plots were generated based on the variations in the principal components.
The binding free energy of the EtPRS-inhibitor complex was computed using gmx_MMPBSA version 1.6.1 [
77], a specific tool for end-state free energy calculations. This method integrates molecular mechanics (MM) to assess the potential energy of molecular structures, and the solvation term is defined as the total of polar and non-polar contributions. The gmx_MMPBSA script was employed during the calculations with several key input files, including the trajectory file, topology file, and an index file that classified EtPRS and the inhibitor into distinct groups. Additional input files included GROMACS portable topology files (.itp), protein structure files (.pdb or .psf), trajectory files (.xtc), protein topology files (.gro), and run topology files (.tpr).
The binding free energy was computed by executing the final gmx_MMPBSA command on the last 10 ns of the simulation trajectory. The output provided a detailed breakdown of the energy contributions to the total energies of the receptor, ligand, and receptor–ligand complex for each frame, as well as an average value over all frames. Subsequently, the energetic contributions were grouped into GGAS (interaction energy) and GSOLV (solvation energy). GGAS was computed as the total internal (bonded) energy components (BOND, ANGLE, DIHED) and non-bonded components (VDWAALS, EEL). In contrast, GSOLV was calculated by determining polar (EGB or EPB) and non-polar (ESURF or ENPOLAR + EDISPER) contributions using Generalized Born (GB) or Poisson–Boltzmann (PB) solvation models, respectively.
3.8. Data Analysis and Visualization
Data analysis was carried out using Python version 3.8, utilizing specific libraries, including NumPy version 1.21.0 for numerical computations and Pandas version 1.3.0 for data manipulation and analysis. Molecular visualizations were generated using PyMOL version 2.5.0, while graphs and plots were constructed using Matplotlib version 3.4.2. Lastly, two-dimensional (2D) residual interaction diagrams were created with Discovery Studio Visualizer version 21.1.0.20298 (BIOVIA, Dassault Systèmes, San Diego, CA, USA).