Structural Diversity and Anti-Diabetic Potential of Flavonoids and Phenolic Compounds in Eriobotrya japonica Leaves
Abstract
1. Introduction
2. Results and Discussion
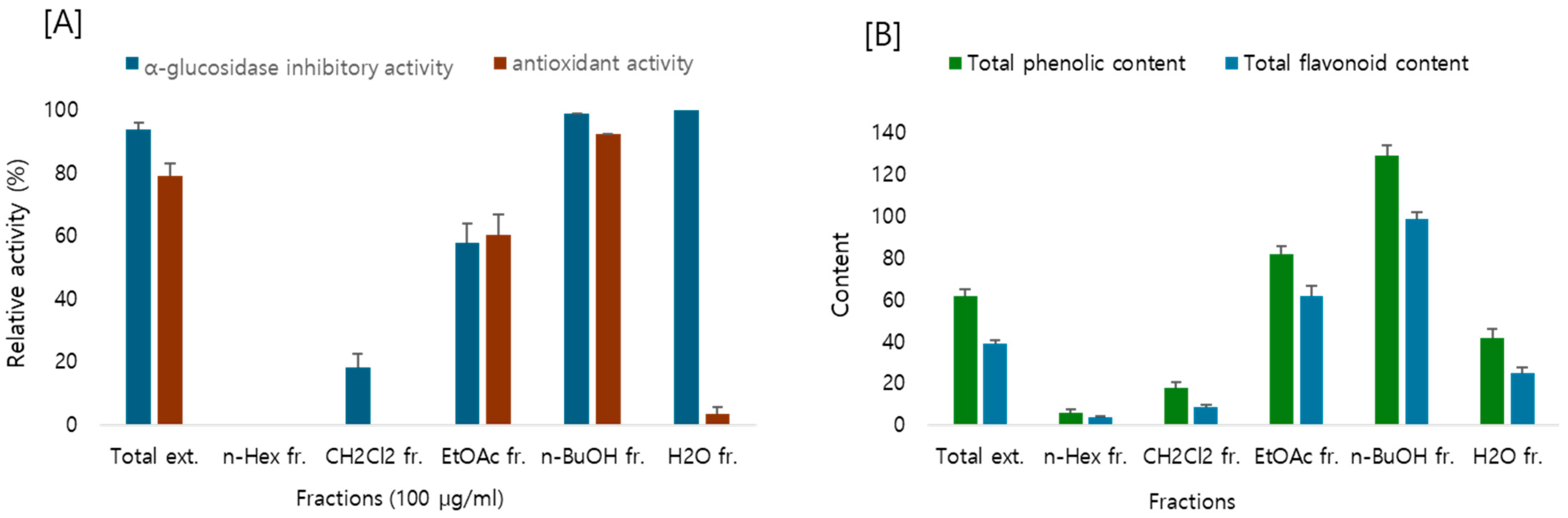
2.1. Biological Activity and Contents of Flavonoids and Phenolic Compounds
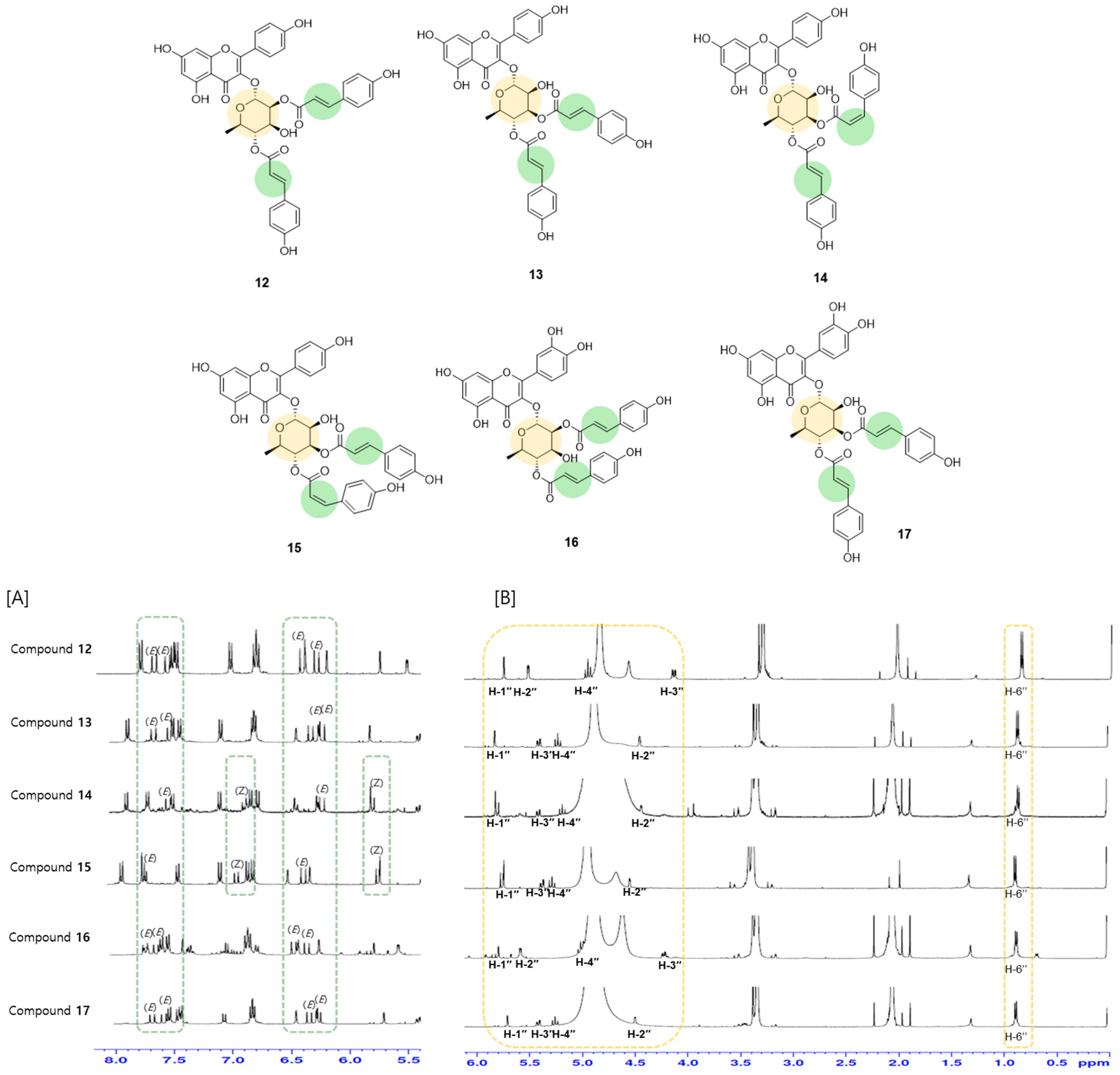
2.2. Isolation and Characterization of the Constituents of E. japonica
2.3. Evaluation of Antioxidant and α-Glucosidase Inhibitory Activity
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedure
3.3. Measurement of Antioxidant and α-Glucosidase Activity
3.4. Quantitation of Phenolic and Flavonoid Contents
3.5. Extraction and Isolation
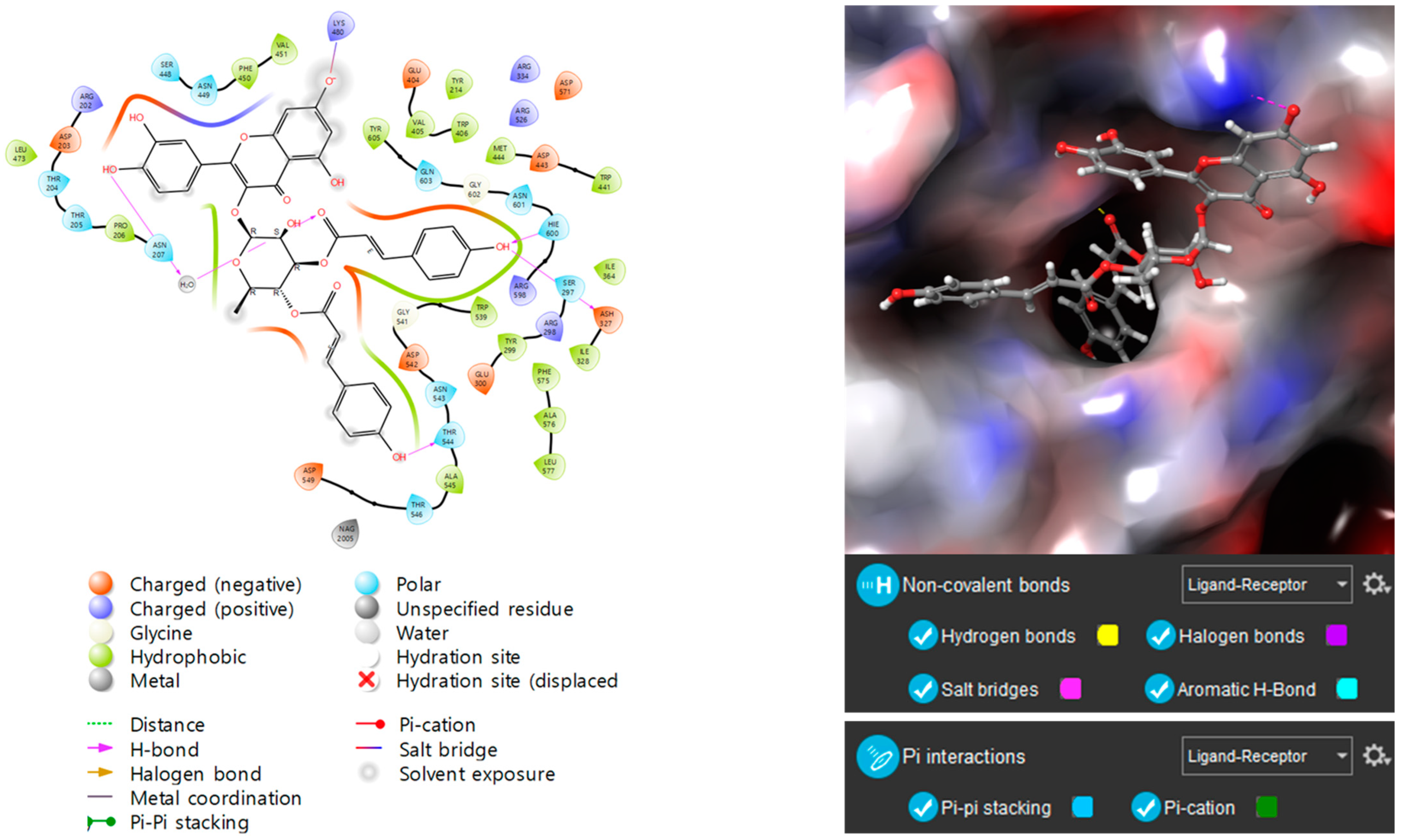
3.6. Molecular Docking Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawłowska, A.M.; Żurek, N.; Kapusta, I.; De Leo, M.; Braca, A. Antioxidant and antiproliferative activities of phenolic extracts of Eriobotrya japonica (Thunb.) Lindl. fruits and leaves. Plants 2023, 12, 3221. [Google Scholar] [CrossRef]
- Yan, Q.J.; Chen, Y.Y.; Wu, M.X.; Yang, H.; Cao, J.P.; Sun, C.D.; Wang, Y. Phenolics and terpenoids profiling in diverse Loquat fruit varieties and systematic assessment of their mitigation of alcohol-induced oxidative stress. Antioxidants 2023, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, I.; Mokhtari, C.; Moumou, M.; Harnafi, M.; Milenkovic, D.; Amrani, S.; Hakmaoui, A.; Harnafi, H. Polyphenol-rich extract from loquat fruit peel prevents hyperlipidemia and hepato-nephrotoxicity in mice: In vivo study and in silico prediction of possible mechanisms involving identified polyphenols and/or their circulating metabolites. Food Funct. 2023, 14, 7489–7505. [Google Scholar] [CrossRef]
- Jian, T.; Ao, X.; Wu, Y.; Lv, H.; Ma, L.; Zhao, L.; Tong, B.; Ren, B.; Chen, J.; Li, W. Total sesquiterpene glycosides from Loquat (Eriobotrya japonica) leaf alleviate high-fat diet induced non-alcoholic fatty liver disease through cytochrome P450 2E1 inhibition. Biomed. Pharmacother. 2017, 91, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, I.; Moumou, M.; Mokhtari, C.; Harnafi, M.; Milenkovic, D.; Amrani, S.; Harnaf, I.H. Traditional uses, phytochemistry, pharmacology, and toxicity of Eriobotrya japonica leaves: A summary. J. Ethnopharmacol. 2022, 298, 115566. [Google Scholar]
- Zhang, L.; Saber, F.R.; Rocchetti, G.; Zengin, G.; Hashem, M.M.; Lucini, L. UHPLC-QTOF-MS based metabolomics and biological activities of different parts of Eriobotrya japonica. Food Res. Int. 2021, 143, 110242. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications a unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2018, 224, 242–253. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the management of diabetes: A review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Lee, S.; Yeon, S.W.; Turk, A.; Lee, J.H.; Hong, S.M.; Han, Y.K.; Lee, K.Y.; Hwang, B.Y.; Kim, S.Y.; et al. Anti-diabetic potential of Masclura tricuspidata leaves: Prenylated isoflavonoids with α-glucosidase inhibitory and anti-glycation activity. Bioorg. Chem. 2021, 114, 105098. [Google Scholar] [CrossRef]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Malazy, O.; Peimani, M.; Mohseni, S.; Nikfar, S.; Abdollahi, M.; Larijani, B. Therapeutic effects of dietary antioxidative supplements on the management of type 2 diabetes and its complications; umbrella review of observational/trials meta-analysis studies. J. Diabetes Metab. Disord. 2022, 21, 1833–1859. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.G.; Ha, M.T.; Le, T.T.; Kim, J.A.; Min, B.S. Inhibitory Activity of PTP1B and α-Glucosidase by compounds from whole plants of Houttuynia cordata Thunb. Nat. Prod. Sci. 2023, 29, 206–216. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 30, 708. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hou, J.; Fang, Q.; Sun, H.; Liu, X.; Zhang, L.; George, P. Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconjug. J. 2012, 29, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M.; Rezali, M.F.; Ujang, Z. Isolation and identification of radical scavenging and tyrosinase inhibition of polyphenols from Tibouchina semidecandra L. J. Agric. Food Chem. 2010, 58, 10404–10409. [Google Scholar] [CrossRef] [PubMed]
- Poaty, B.; Dumarçay, S.; Perrin, D. New lipophilic catechin derivatives by oxa-Pictet-Spengler reaction. Eur. Food Res. Technol. 2009, 230, 111–117. [Google Scholar] [CrossRef]
- Chao, P.M.; Kuo, Y.H.; Lin, Y.S.; Chen, C.H.; Chen, S.W.; Kuo, Y.H. The metabolic benefits of polygonum hypoleucum Ohwi in HepG2 cells and wistar rats under lipogenic stress. J. Agric. Food Chem. 2010, 58, 5174–5180. [Google Scholar] [CrossRef]
- Wan, C.; Yuan, T.; Cirello, A.L.; Seeram, N.P. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012, 135, 1929–1937. [Google Scholar] [CrossRef]
- Utari, F.; Itam, A.; Syafrizayanti, S.; Putri, W.H.; Ninomiya, M.; Koketsu, M.; Tanaka, K.; Efdi, M. Isolation of flavonol rhamnosides from Pometia pinnata leaves and investigation of α-glucosidase inhibitory activity of flavonol derivatives. J. Appl. Pharmaceut. Sci. 2019, 9, 53–65. [Google Scholar]
- Schliemann, W.; Schneider, B.; Wray, V.; Schmidt, J.; Nimtz, M.; Porzel, A.; Bohm, H. Flavonols and an indole alkaloid skeleton bearing identical acylated glycosidic groups from yellow petals of Papaver nudicaule. Phytochemistry 2006, 67, 191–201. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Wang, Y.; Berhow, M.A.; Black, M.; Jeffery, E.H. A comparison of the absorption and metabolism of the major quercetin in brassica, quercetin-3-O-sophoroside, to that of quercetin aglycone, in rats. Food Chem. 2020, 311, 125880. [Google Scholar] [CrossRef]
- Lee, S.S.; Lin, H.C.; Chen, C.K. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochemistry 2008, 69, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Tao, W.W.; Duan, J.A. Antithrombotic flavonoids from the faeces of Trogopterus xanthipes. Nat. Prod. Res. 2010, 24, 1843–1849. [Google Scholar] [CrossRef]
- Jagan, M.; Rao, L.; Yada, H.; Ono, H.; Yoshida, M. Acylated and non-acylated flavonol monoglycosides from the indian minor spice Nagkesar (Mammea longifolia). J. Agric. Food Chem. 2002, 50, 3143–3146. [Google Scholar] [CrossRef]
- Pabst, A.; Barron, D.; Semon, E.; Schreier, P. Two diastereomeric 3-oxo-α-ionol β-d-glucosides from raspberry fruit. Phytochemistry 1992, 31, 1649–1652. [Google Scholar] [CrossRef]
- Yajima, A.; Oono, Y.; Nakagawa, R.; Nukada, T.; Yabuta, G. A simple synthesis of four stereoisomers of roseoside and their inhibitory activity on leukotriene release from mice bone marrow-derived cultured mast cells. Bioorg. Med. Chem. 2009, 17, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Feng, B.M.; Shi, L.Y.; Wang, H.G.; Tang, L.; Wang, Y.Q. Two new 3-oxo-α-ionol glucosides from Urtica laetevirens Maxim. Nat. Prod. Res. 2011, 25, 1219–1223. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Kim, C.M.; Lee, S.H.; Kim, W.S.; Jeon, T.I.; Park, K.H.; Moon, J.H. Coumaroyl quinic acid derivatives and flavonoids from immature pear (Pyrus pyrifolia Nakai) fruit. Food Sci. Biotechnol. 2013, 22, 803–810. [Google Scholar] [CrossRef]
- Ozawa, T.; Imagawa, H. Polyphenolic compounds from female flowers of Typha latifolia L. Agric. Biol. Chem. 1988, 52, 595–957. [Google Scholar] [CrossRef][Green Version]
- Saito, T.; Yamane, H.; Murofushi, N.; Takahashi, N.; Phinney, B.O. 4-O-Caffeoylshikimic and 4-O-(p-coumaroyl)shikimic acids from the dwarf tree fern, Dicksonia antarctica. Biosci. Biotechnol. Biochem. 1997, 61, 1397–1398. [Google Scholar] [CrossRef]
- Ina, H.; Komakid, K.; Iida, H. Hydroxycinnamylglucoses from Spiraea thunbergii. Planta Med. 1987, 53, 502. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Chae, S.H.; Kim, S.H.; Kim, K.H. Phenolic compounds isolated from Juncus decipiens and their effects on osteoblast differentiation in the mouse mesenchymal stem cell line C3H10T1/2. Nat. Prod. Sci. 2024, 30, 135–142. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, K.J.; Htwe, K.H.; Yoon, K.D. A New vomifoliol derivative and flavonoids from the aerial parts of Orthosiphon aristatus. Nat. Prod. Sci. 2024, 30, 72–79. [Google Scholar] [CrossRef]
- Ahn, J.H.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Turk, A.; Han, Y.K.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Aromatic constituents from the leaves of Actinidia arguta with antioxidant and α-glucosidase inhibitory activity. Antioxidants 2021, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Khouya, T.; Ramchoun, M.; Elbouny, H.; Hmidani, A.; Bouhlali, E.D.T.; Alem, C. Loquat (Eriobotrya japonica (Thunb) Lindl.): Evaluation of nutritional value, polyphenol composition, antidiabetic effect, and toxicity of leaf aqueous extract. J. Ethnopharmacol. 2022, 296, 115473. [Google Scholar]
- Mogole, L.; Omwoyo, W.; Mtunzi, F. Phytochemical screening, anti-oxidant activity and alpha-amylase inhibition study using different extracts of loquat (Eriobotrya japonica) leaves. Heliyon 2020, 6, e04736. [Google Scholar] [CrossRef]
- Xu, S.; Wang, G.; Peng, W.; Xu, Y.; Zhang, Y.; Ge, Y.; Jing, Y.; Gong, Z. Corosolic acid isolated from Eriobotrya japonica leaves reduces glucose level in human hepatocellular carcinoma cells, zebrafish and rats. Sci. Rep. 2019, 9, 4388. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.Y.; Xiang, X.R.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.Y.; et al. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int. J. Biol. Macromol. 2020, 146, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.L.; Wu, J.L.; Ren, B.R.; Zhang, H.Q. Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl.). Phytomedicine 2008, 15, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Indrianingsih, A.W.; Tachibana, S.; Itoh, K. In vitro evaluation of antioxidant and α-glucosidase inhibitory assay of several tropical and subtropical plants. Proc. Environ. Sci. 2015, 28, 639. [Google Scholar] [CrossRef]
- An, H.; Thanh, L.N.; Khanh, L.Q.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Lee, H.H.; Turk, A.; Lee, K.Y.; Hwang, B.Y.; et al. Characterization of antioxidant and α-glucosidase inhibitory compounds of Cratoxylum formosum ssp. pruniflorum and optimization of extraction condition. Antioxidants 2023, 12, 511. [Google Scholar] [PubMed]
- Ahn, J.H.; Park, Y.; Yeon, S.W.; Jo, Y.H.; Han, Y.K.; Turk, A.; Ryu, S.H.; Hwang, B.Y.; Lee, K.Y.; Lee, M.K. Phenylpropanoid-conjugated triterpenoids from the leaves of Actinidia arguta and their inhibitory activity on α-glucosidase. J. Nat. Prod. 2020, 83, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
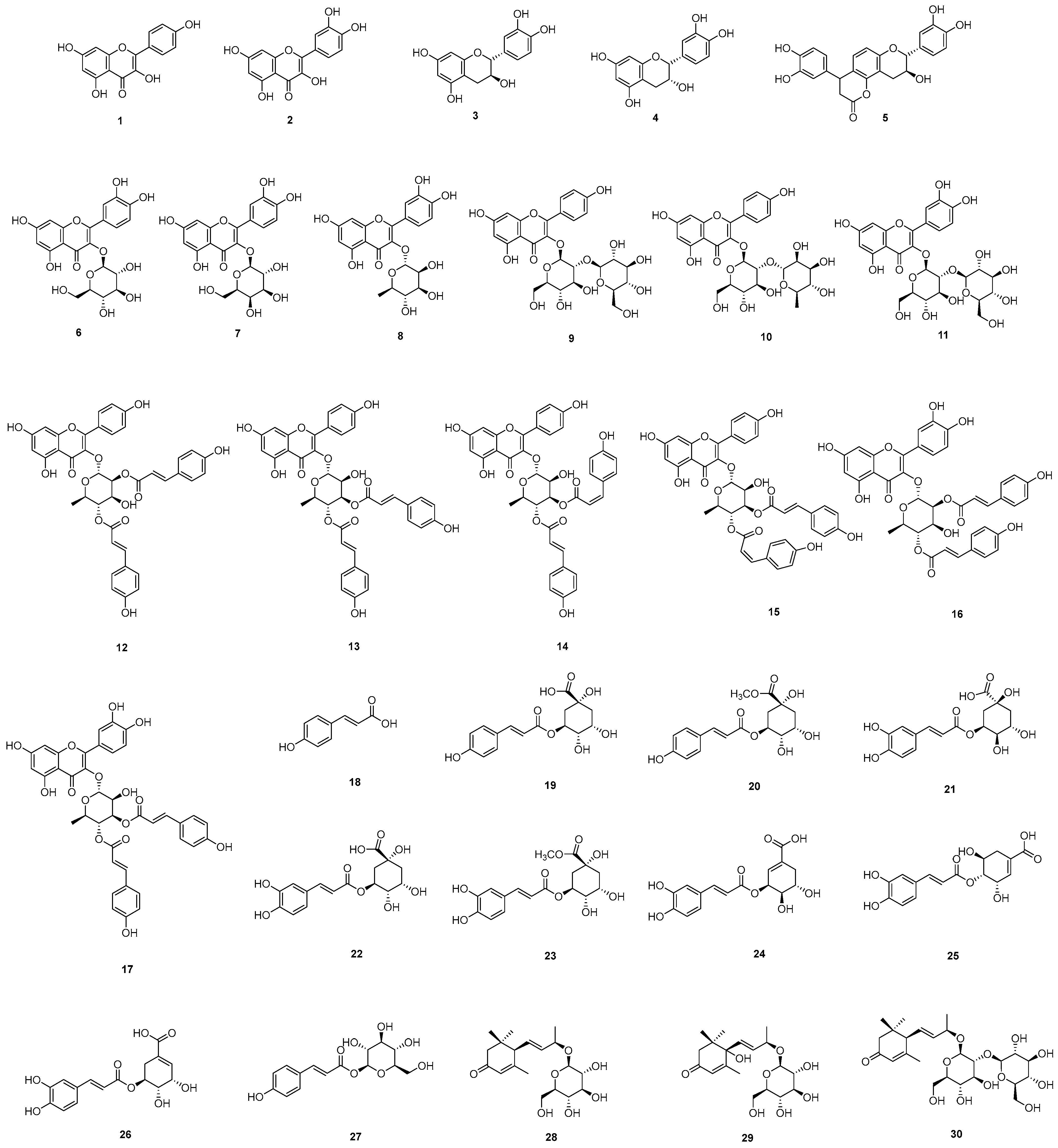
| Compound No. | α-Glucosidase Inhibitory Activity (%) | Antioxidant Activity (%) | Compound No. | α-Glucosidase Inhibitory Activity (%) | Antioxidant Activity (%) |
|---|---|---|---|---|---|
| 1 | 90.5 ± 4.8 | 92.3 ± 1.5 | 16 | 85.2 ± 8.0 | 84.4 ± 1.4 |
| 2 | 88.9 ± 3.3 | 93.3 ± 1.6 | 17 | 90.9 ± 8.7 | 69.6 ± 2.2 |
| 3 | 87.0 ± 9.8 | 88.4 ± 2.8 | 18 | 11.3 ± 2.6 | 14.4 ± 2.8 |
| 4 | 36.4 ± 9.5 | 89.4 ± 2.6 | 19 | 80.7 ± 4.4 | 25.0 ± 2.1 |
| 5 | 10.7 ± 7.8 | 83.6 ± 2.8 | 20 | 88.0 ± 5.8 | 21.5 ± 3.9 |
| 6 | 59.0 ± 3.1 | 88.6 ± 4.2 | 21 | 59.0 ± 2.7 | 81.0 ± 4.8 |
| 7 | 78.9 ± 3.0 | 47.3 ± 1.9 | 22 | 76.5 ± 4.3 | 67.4 ± 2.7 |
| 8 | 35.1 ± 5.2 | 60.6 ± 3.4 | 23 | 47.4 ± 2.5 | 57.2 ± 4.9 |
| 9 | 22.6 ± 8.2 | 11.6 ± 3.7 | 24 | 62.2 ± 3.0 | 86.0 ± 6.1 |
| 10 | 30.4 ± 3.4 | 17.9 ± 1.8 | 25 | 63.6 ± 3.1 | 87.6 ± 3.1 |
| 11 | 58.1 ± 5.6 | 86.2 ± 2.4 | 26 | 75.7 ± 9.7 | 86.0 ± 2.8 |
| 12 | 72.7 ± 2.8 | 54.4 ± 6.3 | 27 | 51.5 ± 8.6 | 19.2 ± 5.1 |
| 13 | 87.2 ± 9.6 | 68.2 ± 5.7 | 28 | 2.7 ± 7.8 | 4.8 ± 3.4 |
| 14 | 84.5 ± 2.2 | 42.6 ± 2.0 | 29 | 4.0 ± 4.8 | 15.5 ± 3.9 |
| 15 | 88.9 ± 8.8 | 45.1 ± 5.3 | 30 | 4.5 ± 2.6 | 4.7 ± 3.6 |
| PC | 53.7 ± 5.9 | 94.5 ± 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Yeon, S.W.; Ryu, S.H.; Lee, H.H.; Turk, A.; Jeong, S.Y.; Kim, Y.J.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Structural Diversity and Anti-Diabetic Potential of Flavonoids and Phenolic Compounds in Eriobotrya japonica Leaves. Molecules 2025, 30, 736. https://doi.org/10.3390/molecules30030736
Kim MH, Yeon SW, Ryu SH, Lee HH, Turk A, Jeong SY, Kim YJ, Lee KY, Hwang BY, Lee MK. Structural Diversity and Anti-Diabetic Potential of Flavonoids and Phenolic Compounds in Eriobotrya japonica Leaves. Molecules. 2025; 30(3):736. https://doi.org/10.3390/molecules30030736
Chicago/Turabian StyleKim, Min Hee, Sang Won Yeon, Se Hwan Ryu, Hak Hyun Lee, Ayman Turk, So Yeong Jeong, Young Jun Kim, Ki Yong Lee, Bang Yeon Hwang, and Mi Kyeong Lee. 2025. "Structural Diversity and Anti-Diabetic Potential of Flavonoids and Phenolic Compounds in Eriobotrya japonica Leaves" Molecules 30, no. 3: 736. https://doi.org/10.3390/molecules30030736
APA StyleKim, M. H., Yeon, S. W., Ryu, S. H., Lee, H. H., Turk, A., Jeong, S. Y., Kim, Y. J., Lee, K. Y., Hwang, B. Y., & Lee, M. K. (2025). Structural Diversity and Anti-Diabetic Potential of Flavonoids and Phenolic Compounds in Eriobotrya japonica Leaves. Molecules, 30(3), 736. https://doi.org/10.3390/molecules30030736








