Tocochromanols in the Leaves of Plants in the Hypericum and Clusia Genera
Abstract
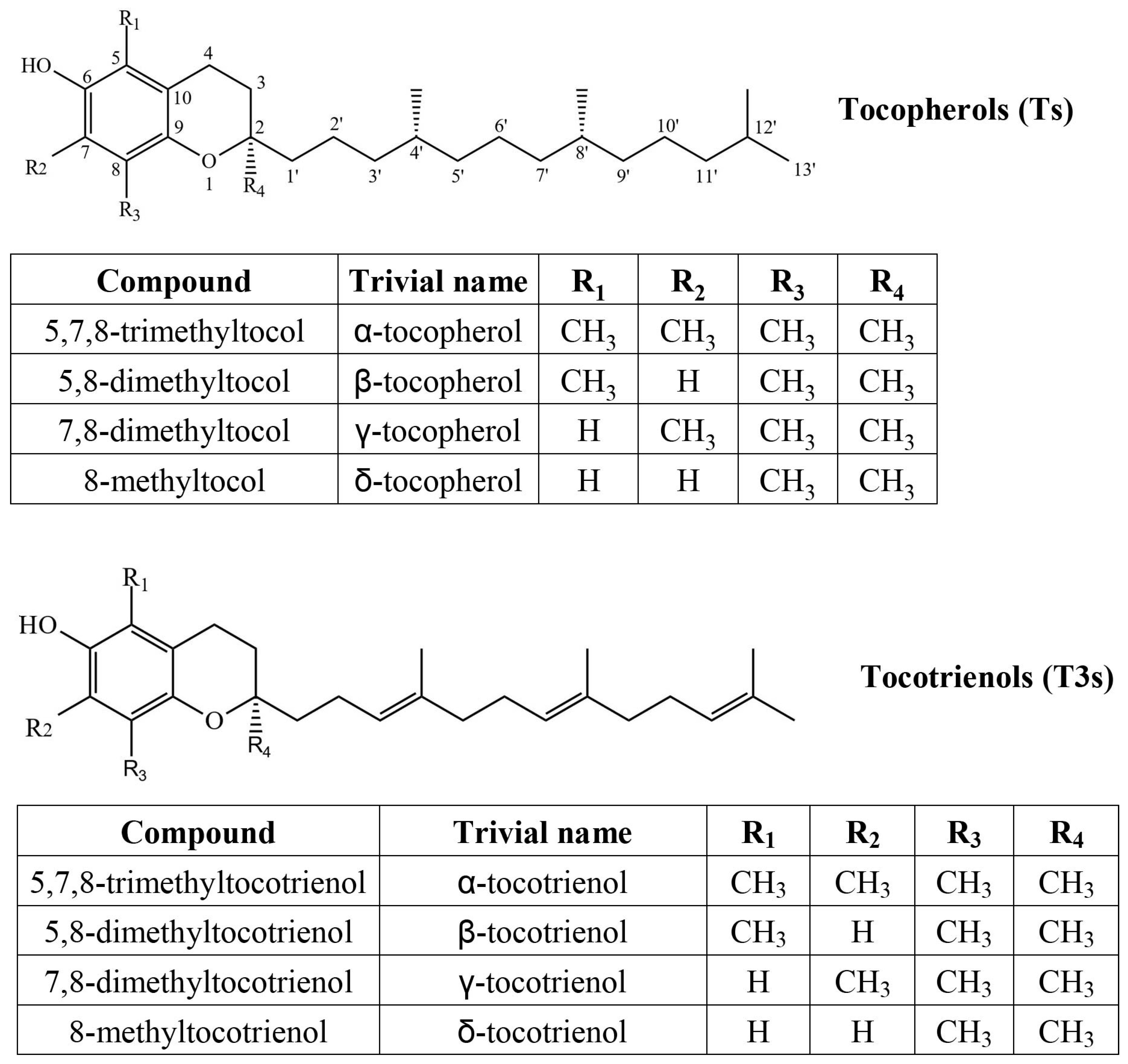
1. Introduction
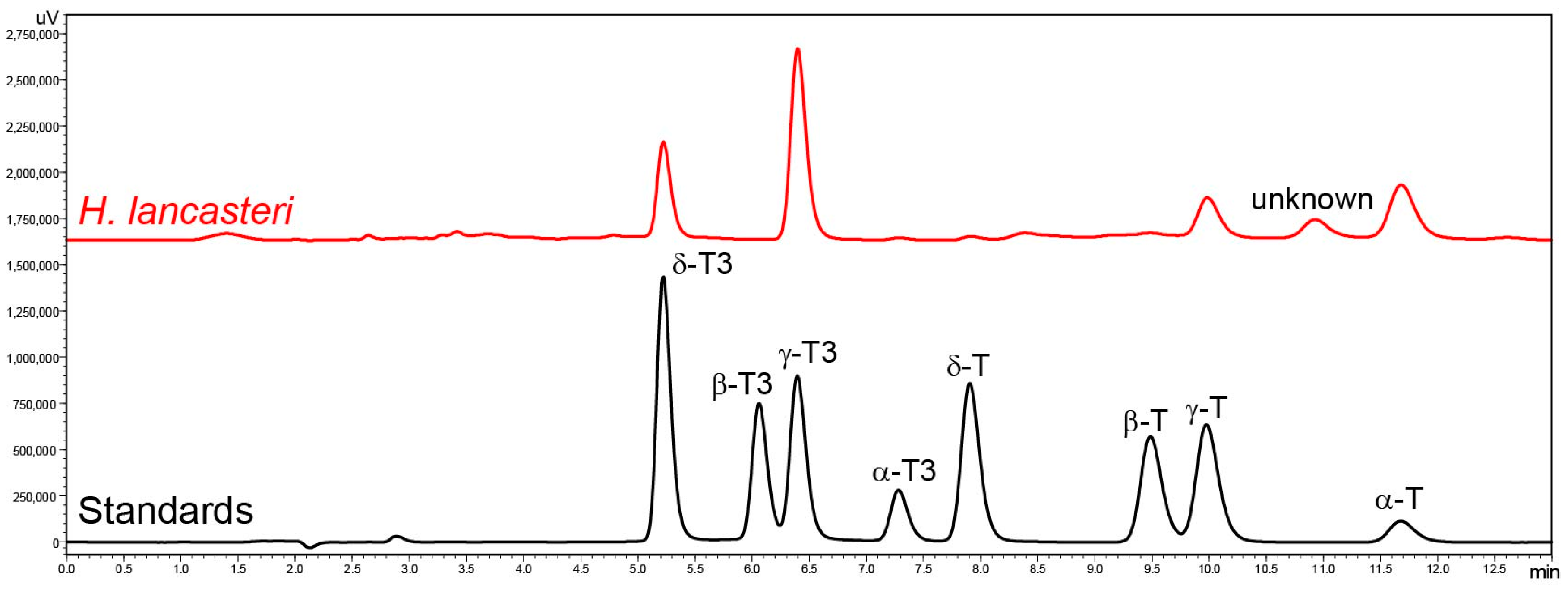
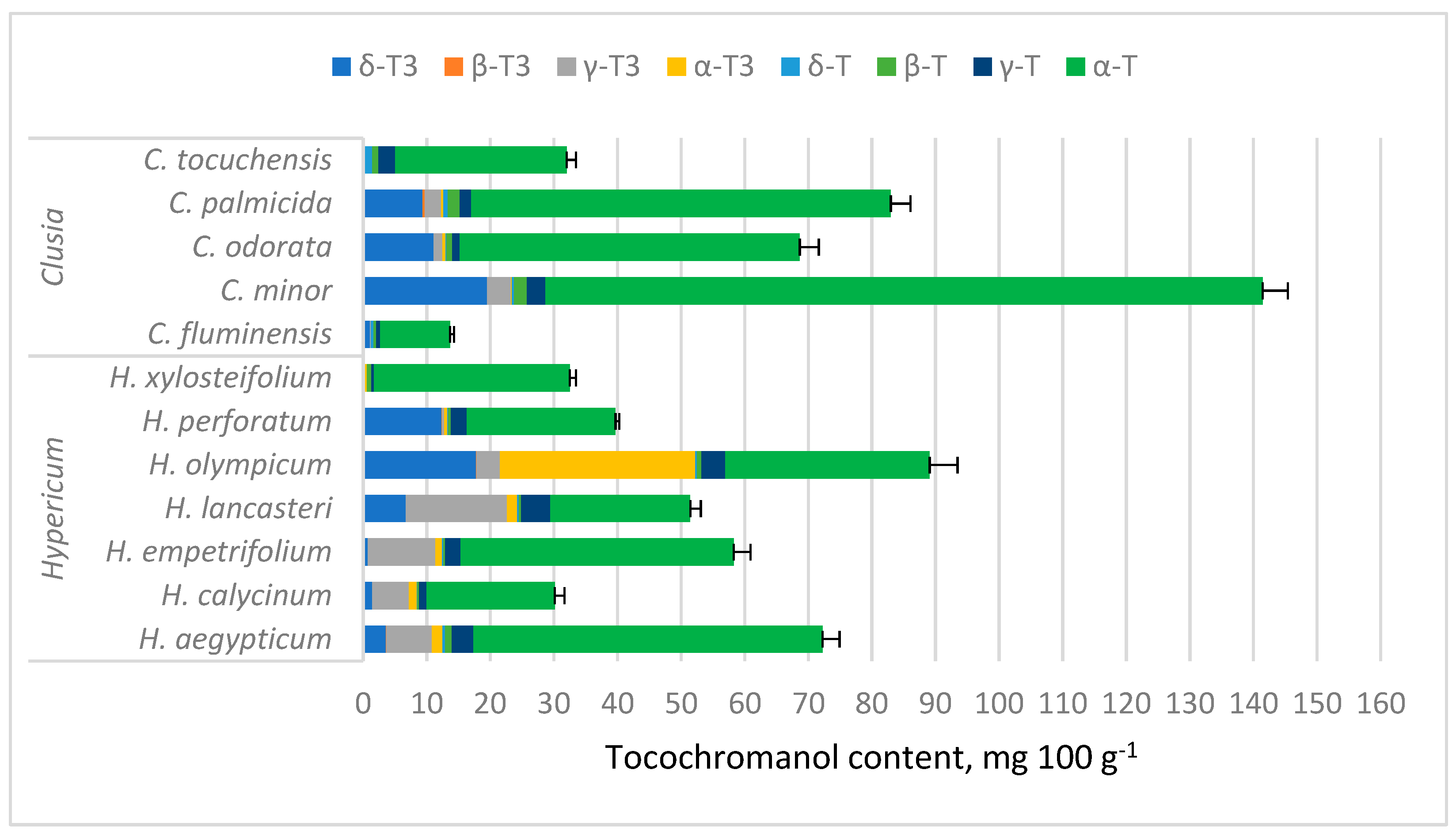
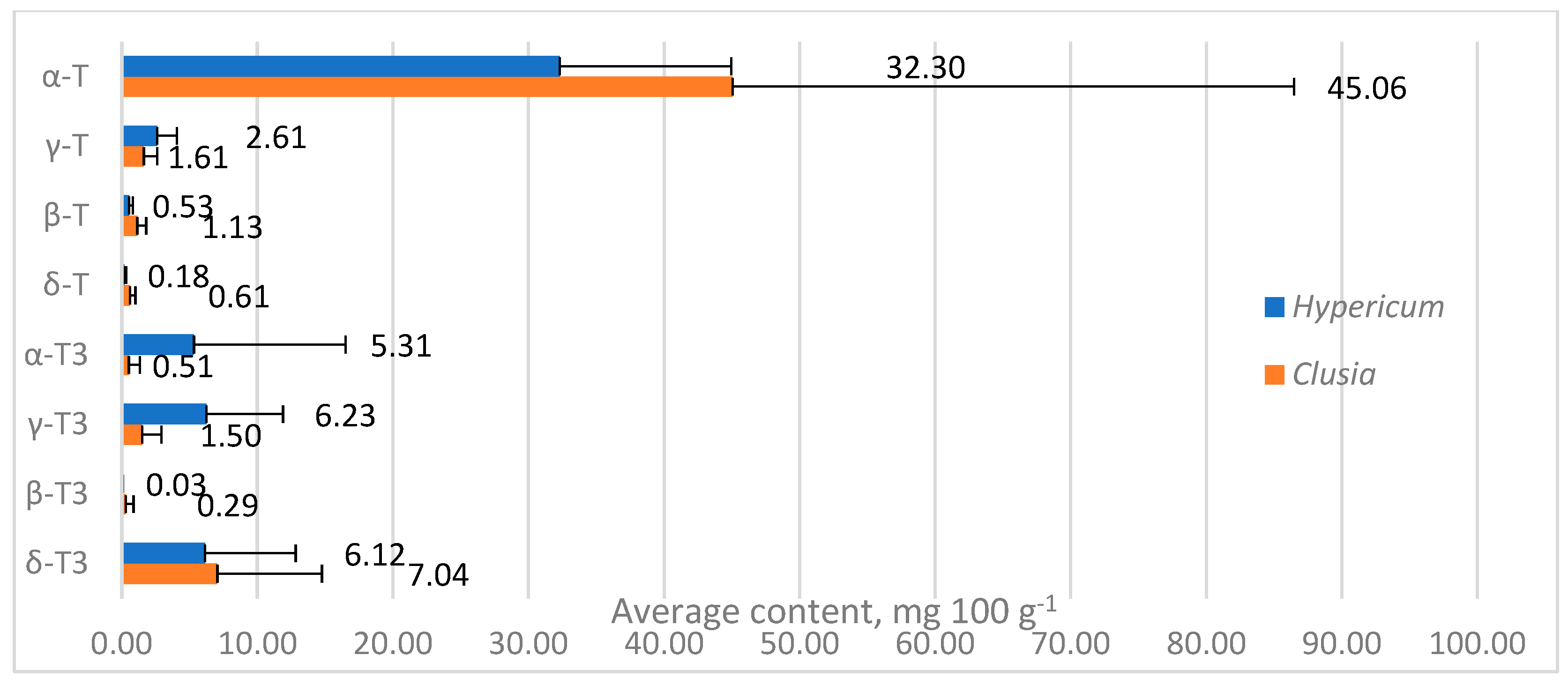
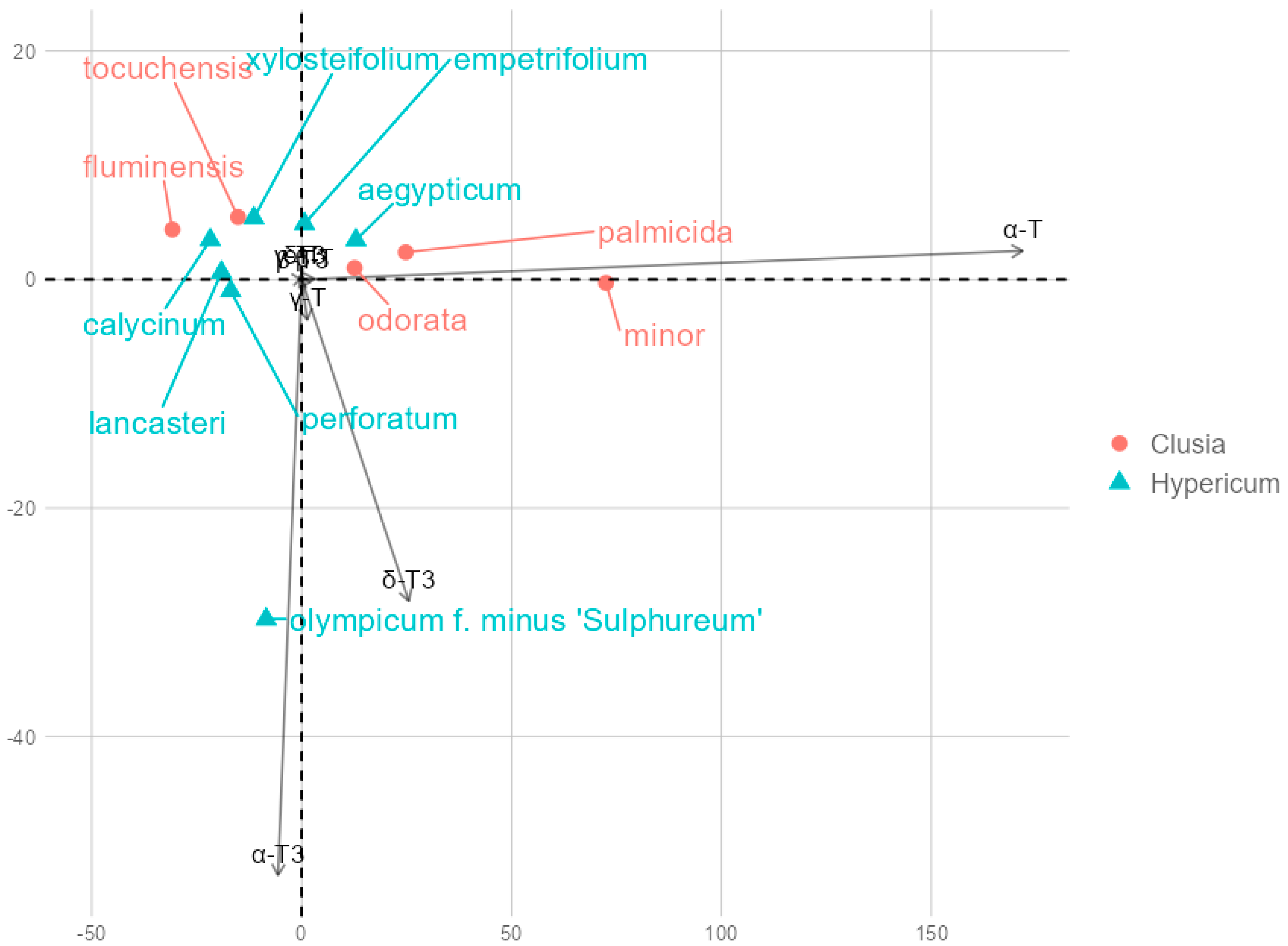
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Saponification and n-Hexane–Ethyl Acetate Extraction Protocol
3.4. Tocopherol and Tocotrienol Determination by RP-HPLC-FLD
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2018, 7, 2. [Google Scholar] [CrossRef]
- Sun, T.; Tadmor, Y.; Li, L. Pathways for carotenoid biosynthesis, degradation, and storage. Methods Mol. Biol. 2020, 2083, 3–23. [Google Scholar]
- Perez-Gil, J.; Behrendorff, J.; Douw, A.; Vickers, C.E. The methylerythritol phosphate pathway as an oxidative stress sense and response system. Nat. Commun. 2024, 15, 5303. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Xiang, X.; Fu, J.; Wang, X.; Zhou, Y.; Xing, W. Biosynthesis and extraction of chlorophyll, carotenoids, anthocyanins, and betalaine in vivo and in vitro. Curr. Issues Mol. Biol. 2024, 46, 10662–10676. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Nor Azman, N.H.E.; Goon, J.A.; Abdul Ghani, S.M.; Hamid, Z.; Wan Ngah, W.Z. Comparing palm oil, tocotrienol-rich fraction and α-tocopherol supplementation on the antioxidant levels of older adults. Antioxidants 2018, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, C.M.; Song, Q.; Saari Csallany, A. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Shahidi, F.; De Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef]
- Di Vincenzo, A.; Tana, C.; El Hadi, H.; Pagano, C.; Vettor, R.; Rossato, M. Antioxidant, anti-inflammatory, and metabolic properties of tocopherols and tocotrienols: Clinical implications for vitamin E supplementation in diabetic kidney disease. Int. J. Mol. Sci. 2019, 20, 5101. [Google Scholar] [CrossRef]
- Ng, Y.T.; Phang, S.C.W.; Tan, G.C.J.; Ng, E.Y.; Botross Henien, N.P.; Palanisamy, U.D.M.; Ahmad, B.; Abdul Kadir, K. The effects of tocotrienol-rich vitamin E (Tocovid) on diabetic neuropathy: A phase II randomized controlled trial. Nutrients 2020, 12, 1522. [Google Scholar] [CrossRef]
- Nair, A.B.; Gorain, B.; Pandey, M.; Jacob, S.; Shinu, P.; Aldhubiab, B.; Almuqbil, R.M.; Elsewedy, H.S.; Morsy, M.A. Tocotrienol in the treatment of topical wounds: Recent updates. Pharmaceutics 2022, 14, 2479. [Google Scholar] [CrossRef]
- He, X.; Wang, D.; Yi, Y.; Tan, Y.; Wu, M.; Wang, H.; Hu, W.; Chen, H.; Zhang, Q.; Wu, Y. δ-Tocotrienol preconditioning improves the capability of bone marrow-derived mesenchymal stem cells in promoting wound healing by inhibiting BACH1-related ferroptosis. Cell Death Discov. 2023, 9, 349. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.-M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Siger, A.; Górnaś, P. Free tocopherols and tocotrienols in 82 plant species’ oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar] [CrossRef]
- Sookwong, P.; Nakagawa, K.; Yamaguchi, Y.; Miyazawa, T.; Kato, S.; Kimura, F.; Miyazawa, T. Tocotrienol distribution in foods: Estimation of daily tocotrienol intake of Japanese population. J. Agric. Food Chem. 2010, 58, 3350–3355. [Google Scholar] [CrossRef]
- Casadesus, A.; Arabia, A.; Pujolriu, R.; Munné-Bosch, S. Differential accumulation of tocochromanols in photosynthetic and non-photosynthetic tissues of strawberry plants subjected to reiterated water deficit. Plant Physiol. Biochem. 2020, 155, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef]
- Knecht, K.; Sandfuchs, K.; Kulling, S.E.; Bunzel, D. Tocopherol and tocotrienol analysis in raw and cooked vegetables: A validated method with emphasis on sample preparation. Food Chem. 2015, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Soba, D.; Müller, M.; Aranjuelo, I.; Munné-Bosch, S. Vitamin E in legume nodules: Occurrence and antioxidant function. Phytochemistry 2020, 172, 112261. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. 2014, 1, 14015. [Google Scholar]
- Muñoz, P.; Briones, M.; Munné-Bosch, S. Photoinhibition and photoprotection during flower opening in lilies. Plant Sci. 2018, 272, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Arroyo, A.; Toro-González, C.; Sus, N.; Warner, J.; Esquivel, P.; Jiménez, V.M.; Frank, J. Vitamin E and carotenoid profiles in leaves, stems, petioles and flowers of stinging nettle (Urtica leptophylla Kunth) from Costa Rica. J. Sci. Food Agric. 2022, 102, 6340–6348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef] [PubMed]
- Sõukand, R.; Kalle, R. Where does the border lie: Locally grown plants used for making tea for recreation and/or healing, 1970s–1990s Estonia. J. Ethnopharmacol. 2013, 150, 162–174. [Google Scholar] [CrossRef]
- Kasper, S.; Caraci, F.; Forti, B.; Drago, F.; Aguglia, E. Efficacy and tolerability of Hypericum extract for the treatment of mild to moderate depression. Eur. Neuropsychopharmacol. 2010, 20, 747–765. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Bhattacharya, S.K.; Wonnemann, M.; Singer, A.; Müller, W.E. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 1998, 63, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Laakmann, G.; Schüle, C.; Baghai, T.; Kieser, M. St. John’s wort in mild to moderate depression: The relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry 1998, 31, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef]
- Cervo, L.; Rozio, M.; Ekalle-Soppo, C.; Guiso, G.; Morazzoni, P.; Caccia, S. Role of hyperforin in the antidepressant-like activity of Hypericum perforatum extracts. Psychopharmacology 2002, 164, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol. Immunother. 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A.M. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xiao, R.; Fu, H.; Yuan, Z.; Zhang, W.; Yin, L.; He, C.; Li, C.; Zhou, J.; Liu, G. Hypericin-loaded graphene oxide protects ducks against a novel duck reovirus. Mater. Sci. Eng. 2019, 105, 110052. [Google Scholar] [CrossRef]
- Prince, A.M.; Pascual, D.; Meruelo, D.; Liebes, L.; Mazur, Y.; Dubovi, E.; Mandel, M.; Lavie, G. Strategies for evaluation of enveloped virus inactivation in red cell concentrates using hypericin. Photochem. Photobiol. 2000, 71, 188–195. [Google Scholar] [CrossRef]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antiviral Res. 1990, 13, 313–325. [Google Scholar] [CrossRef]
- Ferreira, R.O.; Da Silva, T.M.S.; De Carvalho, M.G. New Polyprenylated phloroglucinol and other compounds isolated from the fruits of Clusia nemorosa (Clusiaceae). Molecules 2015, 20, 14326–14333. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhong, F.; Teng, H.; Li, Q.; Li, Y.; Mei, Z.; Chen, Y.; Yang, G. Acylphloroglucinol and tocotrienol derivatives from the fruits of Garcinia paucinervis. Fitoterapia 2020, 146, 104688. [Google Scholar] [CrossRef]
- Liu, H.; Gan, F.; Jin, S.; Li, J.; Chen, Y.; Yang, G. Acylphloroglucinol and tocotrienol derivatives from the fruits of Garcinia multiflora. RSC Adv. 2017, 7, 29295–29301. [Google Scholar] [CrossRef]
- Farasati Far, B.; Gouranmohit, G.; Naimi-jamal, M.R.; Neysani, E.; El-Nashar, H.A.; El-Shazly, M.; Khoshnevisan, K. The potential role of Hypericum perforatum in wound healing: A literature review on the phytochemicals, pharmacological approaches, and mechanistic perspectives. Phytother. Res. 2024, 38, 3271–3295. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yan, F.; Gao, L.H.; Ma, Q.H.; Wang, J. Hypericin as a potential drug for treating Alzheimer’s disease and type 2 diabetes with a view to drug repositioning. CNS Neurosci. Ther. 2023, 29, 3307–3321. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.W.; Regner, L.; Mett, J.; Stahlmann, C.P.; Schorr, P.; Nelke, C.; Streidenberger, O.; Stoetzel, H.; Winkler, J.; Zaidan, S.R. Tocotrienol affects oxidative stress, cholesterol homeostasis and the amyloidogenic pathway in neuroblastoma cells: Consequences for Alzheimer’s disease. Int. J. Mol. Sci. 2016, 17, 1809. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. Novel and rare prenyllipids—Occurrence and biological activity. Plant Physiol. Biochem. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Crane, S.; Aurore, G.; Joseph, H.; Mouloungui, Z.; Bourgeois, P. Composition of fatty acids triacylglycerols and unsaponifiable matter in Calophyllum calaba L. oil from Guadeloupe. Phytochemistry 2005, 66, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Hellio, C.; Patel, A.V.; Abbas, G. Phytochemical profile, antioxidant and antibacterial activity of four Hypericum species from the UK. S. Afr. J. Bot. 2020, 133, 45–53. [Google Scholar] [CrossRef]
- Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical characterization and antioxidant activity of nine Hypericum species from Greece. Antioxidants 2023, 12, 899. [Google Scholar] [CrossRef]
- Hosni, K.; Msaâda, K.; Taârit, M.B.; Marzouk, B. Fatty acid composition and tocopherol content in four Tunisian Hypericum species: Hypericum perforatum, Hypericum tomentosum, Hypericum perfoliatum and Hypericum ericoides Ssp. Roberti. Arabian J. Chem. 2017, 10, S2736–S2741. [Google Scholar] [CrossRef]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The Identification of Vitamin E Homologues in Medicinal Plant Samples Using ESI (+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Tocopherol and tocotrienol homologue recovery from Hypericum perforatum L. and extraction residues after hydroethanolic extraction. Ind. Crops Prod. 2025, 224, 120321. [Google Scholar] [CrossRef]
- Górnaś, P.; Symoniuk, E.; Soliven, A. Reversed phase HPLC with UHPLC benefits for the determination of tocochromanols in the seeds of edible fruits in the Rosaceae family. Food Chem. 2024, 460, 140789. [Google Scholar] [CrossRef] [PubMed]
- Mangas Marín, R.; Montes de Oca Porto, R.; Herrera Paredes, M.E.; Bello Alarcón, A.; Hernández Balmaseda, I.; Menéndez Soto del Valle, R.; Lopes, M.T.P.; Rodeiro Guerra, I. GC/MS analysis and bioactive properties of extracts obtained from Clusia minor L. leaves. J. Mex. Chem. Soc. 2018, 62, 177–188. [Google Scholar] [CrossRef]
- Marques, E.d.J.; Ferraz, C.G.; dos Santos, I.B.F.; dos Santos, I.I.P.; El-Bachá, R.S.; Ribeiro, P.R.; Cruz, F.G. Chemical constituents isolated from Clusia criuva subsp. Criuva and their chemophenetics significance. Biochem. Syst. Ecol. 2021, 97, 104293. [Google Scholar]
- Teixeira, J.S.; Moreira, L.d.M.; Guedes, M.L.d.S.; Cruz, F.G. A new biphenyl from Clusia melchiorii and a new tocotrienol from C. obdeltifolia. J. Braz. Chem. Soc. 2006, 17, 812–815. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Ferraz, C.G.; Guedes, M.L.S.; Martins, D.; Cruz, F.G. A new biphenyl and antimicrobial activity of extracts and compounds from Clusia burlemarxii. Fitoterapia 2011, 82, 1237–1240. [Google Scholar] [CrossRef]
- Raksat, A.; Maneerat, W.; Andersen, R.J.; Pyne, S.G.; Laphookhieo, S. A tocotrienol quinone dimer and xanthones from the leaf extract of Garcinia nigrolineata. Fitoterapia 2019, 136, 104175. [Google Scholar] [CrossRef]
- Popova, M.P.; Trusheva, B.S.; Nedialkov, P.T.; Tsvetkova, I.; Pardo-Mora, D.P.; Najdenski, H.; Torres-García, O.A.; Sforcin, J.M.; Bankova, V.S. New Δ-tocotrienol derivatives from Colombian propolis. Nat. Prod. Res. 2020, 34, 2779–2786. [Google Scholar] [CrossRef]
- Morcillo, F.; Vaissayre, V.; Serret, J.; Avallone, S.; Domonhédo, H.; Jacob, F.; Dussert, S. Natural diversity in the carotene, tocochromanol and fatty acid composition of crude palm oil. Food Chem. 2021, 365, 130638. [Google Scholar] [CrossRef]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef] [PubMed]
- Krauß, S.; Darwisch, V.; Vetter, W. Occurrence of tocopheryl fatty acid esters in vegetables and their non-digestibility by artificial digestion juices. Sci. Rep. 2018, 8, 7657. [Google Scholar] [CrossRef]
- Zou, L.; Akoh, C.C. Identification of tocopherols, tocotrienols, and their fatty acid esters in residues and distillates of structured lipids purified by short-path distillation. J. Agric. Food Chem. 2013, 61, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Klink, G.; Buchs, A.; Gülacar, F.O. Tocopheryl esters from Nymphea alba and Nuphar luteum. Phytochemistry 1994, 36, 813–814. [Google Scholar] [CrossRef]
- Rosado, M.J.; Marques, G.; Rencoret, J.; Gutiérrez, A.; Bausch, F.; Rosenau, T.; Potthast, A.; Del Río, J.C. Chemical composition of the lipophilic compounds from the rind and pith of papyrus (Cyperus papyrus L.) stems. Front. Plant Sci. 2022, 13, 1097866. [Google Scholar] [CrossRef]
- Noleto-Dias, C.; Farag, M.A.; Porzel, A.; Tavares, J.F.; Wessjohann, L.A. A multiplex approach of MS, 1D-, and 2D-NMR metabolomics in plant ontogeny: A case study on Clusia minor L. organs (leaf, flower, fruit, and seed). Phytochem. Anal. 2024, 35, 445–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Park, H.M.; Lee, C.K.; Kim, S.L.; Hwang, T.-Y.; Choi, M.S.; Kwon, Y.-U.; Kim, W.H.; Kim, S.J.; Lee, S.C. Comparing extraction methods for the determination of tocopherols and tocotrienols in seeds and germinating seeds of soybean transformed with OsHGGT. J. Food Compos. Anal. 2012, 27, 70–80. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Waśkiewicz, A.; Perkons, I.; Pugajeva, I.; Segliņa, D. Simultaneous extraction of tocochromanols and flavan-3-ols from the grape seeds: Analytical and industrial aspects. Food Chem. 2025, 462, 140913. [Google Scholar] [CrossRef] [PubMed]
- Krauß, S.; Hermann-Ene, V.; Vetter, W. Fate of free and bound phytol and tocopherols during fruit ripening of two Capsicum cultivars. Sci. Rep. 2020, 10, 17310. [Google Scholar] [CrossRef] [PubMed]
- Tir, R.; Dutta, P.C.; Badjah-Hadj-Ahmed, A.Y. Effect of the extraction solvent polarity on the sesame seeds oil composition. Eur. J. Lipid Sci. Technol. 2012, 114, 1427–1438. [Google Scholar] [CrossRef]
- Ko, E.-Y.; Lee, J.-H.; Sivanesan, I.; Choi, M.-J.; Keum, Y.-S.; Saini, R.K. Carotenoid and tocopherol profiling in 18 Korean traditional green leafy vegetables by LC-SIM-MS. Foods 2023, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Bi, X.; Henry, C.J. Carotenoids, tocopherols and phylloquinone content of 26 green leafy vegetables commonly consumed in Southeast Asia. Food Chem. 2022, 385, 132729. [Google Scholar]
- Wojdyło, A.; Turkiewicz, I.P.; Tkacz, K.; Hernandez, F. Fruit tree leaves as valuable new source of tocopherol and tocotrienol compounds. J. Sci. Food Agric. 2022, 102, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Ergönül, P.G.; Köseoğlu, O. Changes in α-, β-, γ-and δ-tocopherol contents of mostly consumed vegetable oils during refining process. CyTA-J. Food 2014, 12, 199–202. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. Tocopherol content and isomers’ composition in selected plant species. Plant Physiol. Biochem. 2008, 46, 29–33. [Google Scholar] [CrossRef]
- Morales, M.; Garcia, Q.S.; Siqueira-Silva, A.I.; Silva, M.C.; Munné-Bosch, S. Tocotrienols in Vellozia gigantea leaves: Occurrence and modulation by seasonal and plant size effects. Planta 2014, 240, 437–446. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.C.; Johner, J.C.F.; Scopel, E.; Pontes, P.V.A.; Ribeiro, A.P.B.; Zabot, G.L.; Batista, E.A.C.; Meireles, M.A.A.; Martinez, J. Integrated supercritical CO2 extraction and fractionation of passion fruit (Passiflora edulis Sims) by-products. J. Supercrit. Fluids 2021, 168, 105093. [Google Scholar] [CrossRef]
- Barrales, F.M.; Rezende, C.A.; Martínez, J. Supercritical CO2 extraction of passion fruit (Passiflora edulis sp.) seed oil assisted by ultrasound. J. Supercrit. Fluids 2015, 104, 183–192. [Google Scholar] [CrossRef]
- Cárdenas-Toro, F.P.; Meza-Coaquira, J.H.; Nakama-Hokamura, G.K.; Zabot, G.L. Obtaining bixin-and tocotrienol-rich extracts from Peruvian annatto seeds using supercritical CO2 extraction: Experimental and economic evaluation. Foods 2024, 13, 1549. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hejtmánková, A.; Orsák, M.; Popov, M.; Martinek, P. Tocotrienols and tocopherols in colored-grain wheat, tritordeum and barley. Food Chem. 2018, 240, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: A comparative study between two European countries. Eur. J. Lipid Sci. Technol. 2014, 116, 895–903. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mišina, I.; Lazdiņa, D.; Górnaś, P. Tocochromanols in the Leaves of Plants in the Hypericum and Clusia Genera. Molecules 2025, 30, 709. https://doi.org/10.3390/molecules30030709
Mišina I, Lazdiņa D, Górnaś P. Tocochromanols in the Leaves of Plants in the Hypericum and Clusia Genera. Molecules. 2025; 30(3):709. https://doi.org/10.3390/molecules30030709
Chicago/Turabian StyleMišina, Inga, Danija Lazdiņa, and Paweł Górnaś. 2025. "Tocochromanols in the Leaves of Plants in the Hypericum and Clusia Genera" Molecules 30, no. 3: 709. https://doi.org/10.3390/molecules30030709
APA StyleMišina, I., Lazdiņa, D., & Górnaś, P. (2025). Tocochromanols in the Leaves of Plants in the Hypericum and Clusia Genera. Molecules, 30(3), 709. https://doi.org/10.3390/molecules30030709








