Abstract
The fundamental principle of Fourier Transform Infrared (FTIR) spectroscopy is based on the vibration and rotation of atoms, and it has become a universal and widely used spectral methodology for the detection of internal molecular structures in a diverse range of fields. A considerable number of review articles pertaining to the applications of FTIR spectroscopy have been published in recent years. Nevertheless, a comprehensive summary of the application of FTIR spectroscopy in nanoparticles’ (NPs’) green synthesis has yet to be presented. In the present paper, we propose a series of case studies that demonstrate the application of FTIR spectroscopy in the analysis of metal and metal oxide NPs that have been synthesized using green synthesis processes. Furthermore, a summary is presented of the position of functional group bands in FTIR spectra that are responsible for the reduction, capping and stabilization of NPs. In this review, we explore the advantages and limitations of FTIR and propose methodologies for overcoming these challenges. We also present potential solutions for the analysis of complex FTIR spectra. The present summary is intended to serve as a compendium of information for researchers engaged in the field of green synthesis of NPs, utilizing FTIR spectroscopy as a research tool.
1. Introduction
Nanotechnology represents one of the most significant and active areas of investigation within the broader field of modern materials science. Nanoparticles exhibit novel or modified properties that are attributable to specific characteristics, including size, morphology and distribution. In recent years, there have been significant advances in the synthesis of nanoparticles (NPs) of defined size and morphology for specific applications [1]. The extensive practical utilization of nanoparticles (particles of small size falling in the nanoscale range of 1–100 nm) is attributed to their numerous rare, unique and fascinating characteristics, which are valued more than their bulk counterparts [1,2]. The primary objective is to refine the methodologies employed in the synthesis of NPs with a specific size, shape, composition and ordered dispersion, which influence their physical, chemical, catalytic, optical, magnetic, electronic and electrical properties [3,4,5]. This renders them ideal candidates for environmental, biomedical and biotechnological applications.
In order to meet the growing demand for environmentally friendly nanoparticles, researchers have employed diverse strategies to synthesize a variety of metal and metal oxide nanoparticles. Physical and chemical techniques are typically employed in the synthesis of nanoparticles, although the former are often prohibitively expensive. However, the latter are known to have adverse effects on the environment and living organisms. The development of these synthesis techniques for large–scale production is constrained by the high costs associated with high energy consumption; the use of hazardous chemicals and harmful organic solvents; the generation of unreliable intermediates; and the production of harmful, toxic by–products, which contribute to environmental pollution and numerous biohazards. Conversely, green synthesis methods present a viable solution, employing bio–based materials such as plants, microorganisms (bacteria, algae, fungi, yeast) and their enzymes and extracts, isolated sugars and agricultural waste as environmentally friendly sources for nanoparticle synthesis [6,7,8,9]. The concept of green synthesis is predicated on the following principles: the implementation of a reliable and eco–friendly pathway with mild reaction conditions, the utilization of non–toxic precursors and the production of a lower amount of waste, with the objective of ensuring a sustainable environment. Consequently, the design and synthesis of novel products that are safe, reusable and biodegradable is now possible. A substantial body of evidence demonstrates that green synthesis, usually single–step methods, effectively produce nanoparticles with desirable properties. The synthesis of well–defined metal and metal oxide nanoparticles requires precise control of several key variables, including concentration of extract/biomass and metal salt, pH of the reaction mixture, incubation time and temperature. The synthesized nanoparticles can be characterized by a number of analytical techniques, including UV–visible spectrophotometry (UV–Vis), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy–dispersive X–ray spectroscopy (EDX), X–ray diffraction (XRD), X–ray photoelectron spectroscopy (XPS), dynamic light scattering (DLS), Raman spectroscopy and Fourier–transform infrared spectroscopy (FTIR). UV–Vis provides information about nanoparticles based on their optical properties. The magnitude, peak wavelength and spectral bandwidth associated with NPs are contingent on the particle’s size, shape and material composition [10]. However, it does not offer insights into the chemical structure or functional groups present in the NPs. TEM is utilized to obtain high–resolution images of NPs, allowing for the determination of their size, shape and distribution. SEM complements TEM by providing detailed surface morphology images of nanoparticles. EDX is often coupled with SEM to provide elemental analysis of NPs. XRD is employed to determine the crystalline structure and phase purity of NPs. XPS survey spectra give information regarding qualitative and quantitative surface composition, typically of 3–5 nm depth, while high–resolution spectra offer insights into functional groups present on NPs surfaces. DLS measures the size distribution and stability of colloidal suspensions by analyzing fluctuations in scattered light intensity due to Brownian motion. This technique is particularly useful for determining the hydrodynamic diameter of nanoparticles in solution, providing insights into their stability over time. In some cases, all previously mentioned techniques are exclusively employed for the study of a particular property, whereas in others, they are utilized in combination. A comprehensive characterization of the NPs under investigation can only be accomplished by combining the information obtained from these various techniques [11,12]. This multifaceted approach has the potential to elucidate the relationship between the chemical composition of bio–based extracts and the resulting NPs characteristics.
Raman and FTIR spectroscopy are techniques employed for the identification of molecular structures and the characterization of chemical bonding in synthesized nanoparticles. Both techniques provide insights into the vibrational modes of molecules, which can indicate the presence of specific functional groups and the chemical environment surrounding NPs. Raman spectroscopy detects subtle molecular vibrations of molecules with polarizability, while FTIR spectroscopy provides detailed information about molecular bonds and functional groups that have a permanent dipole moment. However, Raman spectroscopy can sometimes induce fluorescence or damage samples due to high–energy laser excitation, particularly with sensitive biological samples. In comparison, FTIR employs longer wavelengths, which are generally less harmful to the samples. This characteristic enables the execution of repeated analyses without compromising the integrity of the samples. The intensity of Raman peaks is typically weaker than that of FTIR because it relies on changes in polarizability rather than direct absorption. This results in generally lower signal strength, requiring longer acquisition times or higher sample concentrations to achieve comparable peak intensities. FTIR spectroscopy proved advantageous in the analysis of bioorganic surface–associated components, whereas Raman spectroscopy exhibited heightened sensitivity to variations in the allotropic modifications and crystalline structure of NPs [13].
FTIR spectroscopy is exceptionally versatile, with applications spanning multiple domains, including materials science, biology and environmental studies. Its ability to analyze complex mixtures without extensive sample preparation makes it particularly valuable for studying nanoparticles and their interactions with biological systems. Key applications include identification of functional groups and molecular structures in nanoparticles, aiding in the understanding of their chemical properties and potential applications in fields like drug delivery (including characterization of drug–polymer interactions [14,15], monitoring of drug loading and release, interaction of nanoparticles with biological tissues and cells [14,16,17] and quality control and process validation [18]), environmental remediation and degradation of pollutants [9,19,20,21,22] and interactions between nanoparticles and biological entities, such as bacteria [23,24]. FTIR spectroscopy has emerged as a pivotal technique in the field of nanoparticle science, providing detailed insights into the molecular composition and interactions of materials at the nanoscale. The employment of FTIR in the analysis of green synthesized NPs is driven by numerous crucial factors associated with their synthesis, stabilization and application. FTIR analysis has the capability to discern specific absorption peaks corresponding to various functional groups, thereby signifying their involvement in the reduction of metal ions, or the formation of metal oxide NPs and their subsequent stabilization. The presence of characteristic peaks in the FTIR spectrum serves to substantiate the efficacious synthesis of nanoparticles by indicating the existence of biomolecules that contribute to nanoparticle formation. Furthermore, the interactions between the synthesized nanoparticles and the capping agents can be studied through FTIR, which provides insights into how these interactions influence particle stability. This understanding is essential for ensuring that the NPs maintain their desired properties over time, making them suitable for various applications such as drug delivery and bioremediation.
The objective of this review is to collate and organize the latest information on the application of FTIR spectroscopy in the analysis of nanoparticles produced via green synthesis, as well as to establish a systematic framework for the spectral information on the functional groups that may be responsible for the reduction, capping and stabilization of nanoparticles. This review explores the diverse applications of FTIR spectroscopy in the green synthesis of metal and metal oxide nanoparticles, highlighting difficulties encountered by researchers when analyzing the FTIR spectra of NPs.
2. FTIR Spectroscopy—Fundamentals, Techniques and Limitations
Fourier–transform infrared spectroscopy operates on the principle that molecules absorb infrared light at specific frequencies, which correspond to the vibrational modes of their chemical bonds. The resulting absorption spectrum serves as a molecular fingerprint, enabling identification and characterization of various organic and inorganic substances. The parts of molecules that determine the main chemical properties of compounds, i.e., functional groups, absorb IR radiation in the same wavenumbers range even though they are part of different compounds [25]. This means that there is a clear relationship between the wavenumber at which a molecule absorbs IR radiation and the structure of the material being tested, so it is possible to identify the substance by recognizing the bands in the IR spectrum that correspond to the functional groups in tested material. This makes infrared spectroscopy a useful tool in chemical analysis. The most commonly used spectral region for analytical purposes is the mid–infrared (mid–IR), covering the range 4000–400 cm−1. A detailed description of the theory and working principles of FTIR is provided by Smith [25].
FTIR spectroscopy relies on the interaction of infrared radiation with molecular vibrations that create a dipole moment. Pure metals, being composed of metallic bonds, do not have the necessary molecular structure to produce significant dipole moments. As a result, they do not absorb infrared radiation effectively, leading to a lack of informative spectral data. However, FTIR spectroscopy can be used for the characterization of molecular adsorbates on metal particles.
Improvements in FTIR instrumentation have led to the development of a wide range of new sensitive techniques to examine samples that were previously difficult to analyze. Among various FTIR spectroscopy techniques transmission spectroscopy (TS), attenuated total reflectance (ATR) and photoacoustic spectroscopy (PAS) are the most commonly used. Each technique is designed to study different types of materials and has its own advantages and disadvantages, while the quality of the spectrum depends, among other things, on the appropriate choice of technique, light source, detector, etc. It is therefore extremely important to determine the suitability and adapt the method of analysis to the specific case. The development of diverse FTIR techniques has significantly diminished the time required for sample preparation, rendering them a compelling option for laboratories and other practical applications.
2.1. Transmission Technique (TS)
The transmission technique (TS) is one of the most commonly employed IR spectroscopy techniques. The TS technique offers high–quality spectra with good signal–to–noise (S/N) ratio and good reproducibility, and is considered satisfactory for qualitative and semi–quantitative analysis. In this regard, it should be noted that the TS technique fails to measure the precise quantity of a substance; rather, it expresses the results as an estimate of the presence of a detected substance.
The main drawbacks of the results obtained by using the transmission technique primarily lie in the sample preparation procedure. Thus, one of primary TS limitations is the necessity to prepare pellets from a mixture of the studied material and compound transparent to infrared radiation (simultaneously serving as a matrix; usually potassium bromide, KBr) within the spectral range employed. The analysis of opaque, plant–derived or carbonaceous materials using FTIR transmission spectroscopy is challenging due to the fact that such materials, being optically opaque, may absorb incident IR radiation to a significant extent. The preparation of samples for analysis by grinding and diluting with KBr can result in the destruction of their structure. Furthermore, potassium bromide is a hygroscopic compound, which means that the prepared pellets may contain some moisture. Water is visible in the IR spectrum as a broad band with maxima at ~3500 and ~1630 cm−1, and these bands are sometimes intense enough to overlap with the bands originating from the sample. This is particularly important when hydroxyl groups are the subject of study. Preparation of the KBr pellets for TS measurements is laborious, time consuming and suffers from risk of samples contamination. Therefore, in some cases, it may be preferable to select an alternative available technique.
2.2. Attenuated Total Reflectance (ATR)
The ATR technique, also called internal reflection spectroscopy (IRS), enables the examination of solid samples (i.e., powders, soft solids) with smooth, flat surfaces and liquids. It is a versatile, non–destructive, high–sensitivity and broad–applicability technique that allows for the examination of samples that strongly absorb IR radiation and which would not be analysable using the TS technique. ATR measures the absorption of infrared light by a sample placed in close contact with a high refractive index (HRI) crystal (i.e., diamond, germanium, silicon or zinc selenide). When a beam of IR radiation traverses HRI material, the light is repeatedly internally reflected, resulting in the generation of an evanescent wave. Upon contact between the sample and the evanescent wave at the sample/crystal interface, the wave is absorbed by the sample, leading to a reduction in its intensity. The attenuated beam then reflects off the HRI material and is subsequently directed towards the detector. The depth of penetration of IR beam into the material under investigation is contingent upon the refractive index at the sample/crystal interface, which is in turn dependent upon the material of the crystal, the angle of incidence and the wavelength of the IR radiation [25]. ATR spectra demonstrate a relative shift in the intensity of the bands, with those at low wavenumbers exhibiting greater intensity than those at high wavenumbers. This can be corrected relatively easily mathematically (ATR correction). A more challenging issue is the shift in vibrational frequencies, which results in a shift of the peak maximum. This may result in an ambiguous interpretation of the spectrum obtained through the ATR technique. Furthermore, the manner in which the measurement is conducted can result in inadequate contact between the sample and the refractive crystal, potentially leading to inaccuracies in the obtained results [26]. It is essential to ensure that the contact between the reflecting surface and the sample is optimized to ensure the efficacy of the technique, e.g., to achieve good reproducibility of the spectra. ATR is generally much more straightforward than the TS technique, as it only requires good contact with the ATR element to produce a reliable measurement. However, there are some differences: the strength of the ATR effect is dependent on wavelength, whereas the transmission measurement is not [25], and reproducibility is better achieved in TS mode compared to ATR mode. The ATR technique is employed in two main configurations, and each of which offers distinct advantages. In Single Reflection ATR, the incident infrared light undergoes a single reflection at the crystal–sample interface (Figure 1), facilitating a straightforward and uncomplicated ATR measurement. This configuration is well–suited for routine analysis and samples with relatively high refractive indices. Conversely, in the Multiple Reflection ATR configuration, infrared light undergoes repeated internal reflections within the crystal prior to exiting, thereby increasing the effective path length and enhancing the sensitivity, particularly in cases where the sample has a low concentration or where absorptions are weak (e.g., thin–film samples).
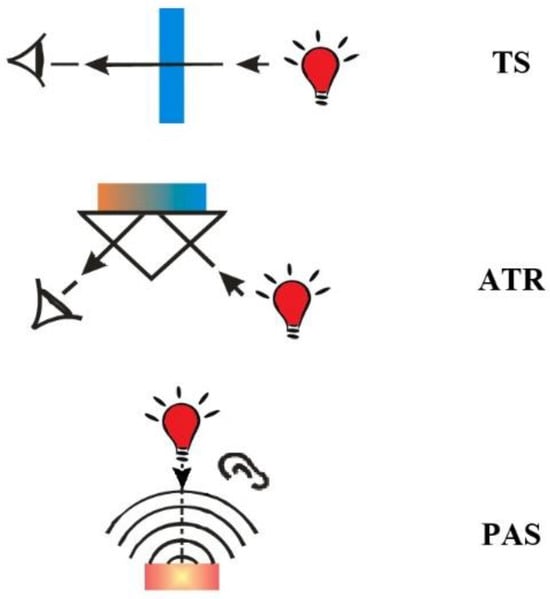
Figure 1.
Schematic representation of FTIR techniques used for nanoparticles analysis: TS, ATR and PAS.
ATR is a technique that provides information mainly from the surface of the sample being tested and limited penetration depth is also a consideration in FTIR/ATR spectroscopy. If the region of interest is located deeper within the sample, the ATR measurement may not capture the desired molecular information accurately. In such cases, alternative sampling techniques such as TS may be more suitable.
2.3. Photoacoustic FTIR Spectroscopy (FTIR/PAS)
Photoacoustic spectroscopy (PAS) detects light absorption (photo) by using sound (acoustic). It is founded upon the phenomenon of the photoacoustic effect, which entails the generation of an acoustic wave at the surface of the tested material as a consequence of the absorption of modulated electromagnetic radiation by this material. Sampling depth is dependent on the wavenumber of infrared radiation and decreases as wavenumber increases. In this regard, PAS is analogous to ATR, wherein the sampling depth is also contingent upon the wavenumber. In the conventional photoacoustic spectroscopy technique, the sample to be investigated is placed inside a closed chamber (the photoacoustic cell) filled with a suitable gas [27] (usually helium). While a modulated radiation source is directed towards the sample surface, the generated photoacoustic signal is detected by a sensitive microphone attached to the photoacoustic cell. Photoacoustic FTIR (FTIR/PAS) spectroscopy has been described as a non–destructive method for the sensitive analysis of solid–phase materials. As only the absorbed radiation is measured, PAS is not affected by the effects of light scattering and reflection. The principal benefit of photoacoustic spectroscopy is that it necessitates no complex sample preparation. This technique can be used in the analysis of materials that are difficult to homogenize or for which the structure or chemical composition may change during grinding. Even samples with rough surfaces and strongly scattering infrared light can be readily measured, as the photoacoustic signal is proportional to the absorbed energy, in contrast to the reflected energy observed in reflectance techniques. However, the PAS method exhibits a relatively poor S/N ratio, necessitating longer data acquisition times than those required for TS and ATR techniques [28]. It is of the utmost importance that the tested samples are kept in a dry state, as moisture could potentially cause damage to the sensitive microphone. Literature reports describing the use of PAS technique in the infrared range in nanoparticle research are still scarce [29,30,31].
PAS and to some extent ATR are not affected by the presence of water. PAS and ATR are surface techniques that allow the bulk of the sample to be distinguished from that of the sample surface [32,33]. In PAS and ATR techniques, the signal is generated at a maximum of a few micrometres from the sample surface and provides information about the chemical structure of the surface, while in the TS technique signal is derived from the entire bulk of the sample. The surface sensitivity is ~1 μm for PAS and monolayer for ATR [34]. All the FTIR techniques mentioned appear to be suitable for the analysis of nanoparticles; however, the choice is typically determined by the availability of specific accessories (ATR, PAS) rather than the suitability of the technique. Transmission spectroscopy, which does not require any additional accessories, is most frequently used to obtain IR spectra of NPs. Figure 1 depicts the schematic representation of FTIR techniques employed for the analysis of nanoparticles, namely, transmission spectroscopy (TS), attenuated total reflection (ATR) and photoacoustic spectroscopy (PAS). The advantages and limitations of each technique are summarized in Table 1.

Table 1.
Advantages and limitations of TS, ATR and PAS techniques.
2.4. Some FTIR Limitations and Potential Improvements
Notwithstanding its undisputed advantages, FTIR is not without its limitations and disadvantages in assessing green synthesized NPs. As it was mentioned, FTIR analysis is highly sensitive to the preparation of samples. Variations in sample handling, such as drying methods, the use of different matrices (like KBr) or the performance of washing steps after NPs synthesis, can lead to inconsistent results. For instance, grinding and pressing samples (using TS technique) can alter the spectral characteristics, potentially masking important information about the functional groups in tested biological samples [35,36]. As demonstrated by Kamnev et al. [35], grinding of the Azospirillum brasilense Sp7 and Azospirillum baldaniorum Sp245 biomass samples with KBr resulted in a shift towards lower wavenumbers of bands of inter– and intramolecular hydrogen bonds between the ester carbonyl group related to cellular poly–3–hydroxybutyrate (PHB) present in the samples. This finding indicated that the grinding step induced a partial transition from the metastable and more amorphous state of the intracellular PHB to its more ordered state. In order to circumvent this issue, it would be advisable to employ a methodology that does not necessitate sample preparation, such as ATR or PAS technique.
As previously mentioned, the presence of water in a sample can alter the measured FTIR spectrum. Furthermore, the H–O–H bending vibration of water (maximum at ~1630 cm−1) falls within the amide I region of proteins (1700–1600 cm−1), which underscores the necessity to dry the samples prior to FTIR analysis. Research has demonstrated [35] that the drying temperature, with a maximum limit of 45 °C in order to prevent protein denaturation, exerts a greater influence on the resulting IR spectrum than the drying duration.
In the context of the green synthesis of NPs, the washing steps have been demonstrated to be instrumental in ensuring the desired properties, stability and ultimately purity of the resulting product [36]. The process of washing serves to remove unreacted precursors, by–products and other contaminants that may interfere with the properties of the NPs. This is important for achieving high purity levels, which in turn directly influence the efficacy and safety of the nanoparticles in applications such as drug delivery or environmental remediation. The process of washing of the green synthesized NPs has the potential to remove the bioorganic capping layer. This has the consequence of hindering accurate analysis and interpretation of the resulting spectroscopic data. Research has demonstrated that the initial washing step is of particular significance, as it facilitates the removal of molecules that are only weakly bound to the surface of NPs. In contrast, the remainder of the biomacromolecular shell remains stable even after repeated washing [35]. Incomplete removal of precursors, especially metal ion precursors (e.g., acetates, nitrates) can also affect the quality of the FTIR spectrum as their residues may be visible in the FTIR spectra.
FTIR spectra of complex biological materials or mixtures often exhibit overlapping peaks. This complicates the identification of specific functional groups and can lead to misinterpretation of the data. The use of second derivatives of the spectra can be a fruitful approach for the purpose of revealing unresolved bands, particularly in the context of complex spectra derived from biological samples [35,37,38]. Implementing machine learning algorithms to analyze FTIR spectra can help in deconvoluting complex spectra and identifying overlapping peaks that may obscure important information about the NPs functional groups [39,40].
The utilization of chemometric techniques such as principal component analysis (PCA) and partial least squares (PLS) modelling has improved the interpretation of complex FTIR spectra. These methods facilitate enhanced classification and quantification of spectral data, thereby simplifying the analysis of the chemical composition of nanoparticles. The utilization of PCA and linear discriminant analysis (LDA) enables the clear revelation of discrepancies between the obtained spectra [41]. Nevertheless, the development of standardized methodologies for the preparation of biological samples, as well as the mathematical methods for the analysis of the resulting complex spectra, remains ongoing [35].
The employment of novel approaches and techniques has enabled researchers to circumvent the limitations associated with FTIR in the assessment of green nanoparticles. This has resulted in more accurate characterization and enhanced understanding of their properties and potential applications in fields such as medicine and environmental science. As previously stated, combining FTIR with other techniques such as UV–Vis spectrophotometry, DLS, TEM, EDX and XRD can provide a more comprehensive understanding of NPs properties. Combining FTIR with Raman spectroscopy can provide complementary information about molecular vibrations and chemical bonding [36]. This dual approach can help mitigate some limitations of FTIR, such as its inability to analyze non–polar compounds effectively [42,43]. The nano–FTIR spectroscopy technique combines FTIR with Atomic Force Microscopy (AFM) to achieve nanoscale resolution. It enables the characterization of materials at the molecular level, providing detailed information about the chemical composition and structure of NPs [18]. It is also important to consider other spectroscopic techniques that are useful for the analysis of green synthesized nanoparticles: near–infrared spectroscopy (NIR) and far–infrared spectroscopy (FIR).
In comparison to FTIR (operating within the mid–IR range), the NIR spectroscopy is more beneficial for a quantitative analysis of aqueous samples due to the significantly weaker nature of O–H absorption bands. The absorption bands in the near–infrared (wavenumber range from ~13,000 to 4000 cm−1) are frequently overtones and combination bands of group frequencies (4000–1500 cm−1 within mid–IR range, i.e., O–H, C–H and N–H group vibrations). NIR spectroscopy may be useful for the investigation of nanostructured materials such as nanoporous silica particles, dendrimers, nanocoated capillaries and carbon nanomaterials [44,45]. FIR spectroscopy is a technique that involves the absorption of infrared radiation in the far–infrared region, typically between 400 and 10 cm−1. It is used for studying lattice vibrations in samples and finds applications in the analysis of a variety of materials, including metals, metal oxides, metal sulphides and metal–ligand complexes [46].
3. The Application of FTIR Spectroscopy to Green Synthesized Nanoparticles Characterization
The distinctive characteristics of NPs are typically determined by the synthesis method employed. Even minor alterations in the technological process can result in significant variations in their intrinsic properties. Successful green synthesis of nanoparticles relies on a combination of biological agents (microorganisms or plant extracts), metal precursors, optimized reaction conditions (pH, temperature) and process optimization strategies. The morphology of the NPs formed is dependent on the type of plant extract and its concentration. The temperature and pH of the extract medium exert control over the growth and size of the NPs [47]. The most important role of biological agent is to act as reducing, capping and stabilizing factor. Reducing agents are substances that facilitate the reduction of metal ions to their elemental form, which is essential for the formation of nanoparticles. The primary functional groups engaged in the reduction of metal ions are the hydroxyl, carbonyl, amino and methoxide groups, which interact with the metal ions via electrostatic forces, resulting in their reduction [48]. In green synthesis, natural materials such as plant extracts or biological molecules, such as polyphenols [49], glycosides [50], carbohydrates [51], amino acids [52], terpenoids, carboxylic acids and flavonoids [53], polyols [54], enzymes [55], alkaloids [56], thiamine [57], ascorbic acid [58], having in their molecular structure the mentioned functional groups, often serve as reducing agents [59] (Figure 2). For example, phenolic compounds in plant extracts can reduce silver ions (Ag+) to silver nanoparticles (Ag0) while also providing some stabilization [59,60,61]. Enzymes produced by microorganisms can also function as bioreductants [55,62], as well as polysaccharides isolated from agricultural waste [63] and extracellular polymeric substances (EPS) produced by bacteria [64]. Reducing agents and capping agents may be added separately during physical and chemical methods of NPs synthesis. Capping agents are used to coat the surface of nanoparticles after they have formed. Their primary role is to prevent agglomeration and to stabilize the nanoparticles in a colloidal solution. Capping agents can influence the size, shape and overall properties of the nanoparticles, forming mono– or multi–layered protective coatings and providing long–term stability for the NPs. They often contain functional groups that bind to the nanoparticle surface, providing steric hindrance that prevents the particles from coming into close proximity and aggregating. They can also be used for biofunctionalization, i.e., to provide the presence of functional groups for attaching other biomolecules or drugs [65,66,67]. Capping agents can be broadly categorized into the following groups: organic ligands, surfactants, polysaccharides, proteins (i.a., bovine serum albumin, BSA), amino acids (i.a., ethylenediaminetetraacetic acid, EDTA, synthetic amino acid), lipids, polymers (i.a., chitosan, polyethylene glycol, PEG, polyvinylpyrrolidone, PVP), dendrimers, cyclodextrins and bio–extracts [65]. The differences between the aforementioned entities can be attributed to their functional groups and surface charge. The selection of capping agents is dependent on the targeted size and morphology of NPs, in addition to their potential applicability. Other factors are also taken into consideration, including the type of functional groups, the hydrophobic or hydrophilic nature of the functional groups and the charge on capping agents, the length of carbon chains/linkers and so forth. A slight modification in any particular parameter can have a considerable impact on the overall NPs properties.
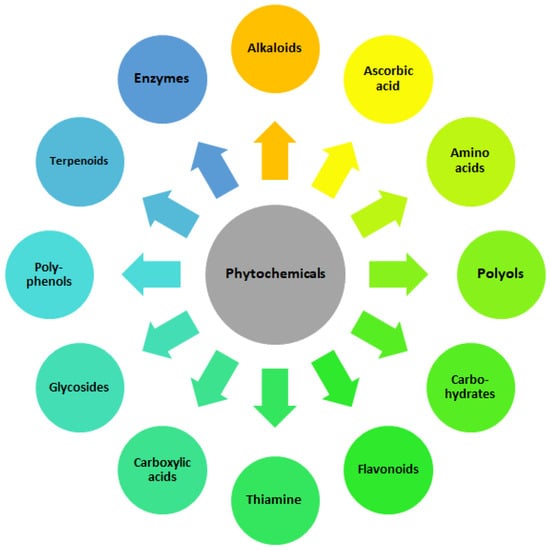
Figure 2.
Important bioreductants found in plant extracts.
While sometimes used interchangeably with capping agents, stabilizing agents specifically refer to substances that maintain the stability of nanoparticles in suspension over time. This includes preventing sedimentation and ensuring that the nanoparticles do not aggregate during storage or application. Stabilizing agents can be capping agents but may also include other types of molecules that do not directly bind to the nanoparticle surface [68,69]. In the context of green synthesis of nanoparticles, reducing agents, capping agents and stabilizing agents are not the same, although they can sometimes overlap in function. The main mechanism of green biosynthesis through plants is illustrated in Figure 3.
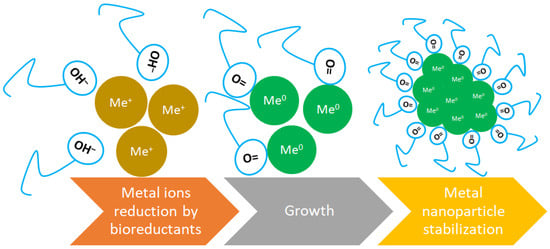
Figure 3.
Green synthesis mechanism (based on [70]).
FTIR analysis can be employed to identify the functional groups of diverse active substances or plant secondary metabolites that are accountable for the reduction, capping and stabilization of NPs. FTIR can be therefore successfully used in the analysis by (1) assessing the chemical identity of nanoparticles synthesized through various methods, including green synthesis techniques that utilize bio–based materials, (2) identification of functional groups accountable for the reduction of metal ions to their elemental forms, (3) identification of functional groups, thus revealing the presence of biomolecules that act as capping agents, which are crucial for stabilizing the nanoparticles by keeping them separated, eluding the aggregation and coalescence and (4) optimization of synthesis conditions by analyzing the FTIR spectra at different time intervals, which can allow one to determine how reaction conditions affect the formation and characteristics of nanoparticles, such as size and stability [71,72].
In the synthesis of green nanoparticles, various functional groups of secondary plants metabolites play a role in the reduction and stabilization of NPs. These include, among others, phenolic compounds (i.e., phenolic acids), terpenoids, saponins, flavonoids, steroids, alkaloids, tannins, proteins and peptides, sugars and polysaccharides. A number of these metabolites are capable of acting as both reducing and stabilizing agents, thereby inhibiting the aggregation and agglomeration processes. The reduction potential of ions and the reducing capacity of plants are influenced by the presence of polyphenols, enzymes and other chelating agents, which are intrinsic to plants. Phenolic –OH groups are prevalent in polyphenols and are known for their strong reducing capabilities. Phenolic compounds, possessing hydroxyl and carboxyl groups, are one of the best candidates for nanoparticle synthesis, and their antioxidant action is due to their high tendency to chelate metals [73], facilitating their reduction to nanoparticles. The phenolic compounds, especially those from the polyphenol family, often act as electron donors in the reduction process, making them vital in the synthesis of nanoparticles [8,12]. The nucleophilic aromatic rings of such compounds provide substantial support for antioxidant activity and metal chelation. In the presence of elevated levels of polyphenols, a protective coating was observed around the nascent nanoparticles, which inhibited their coalescence and aggregation. Terpenoids, which include many essential oils, can stabilize nanoparticles and enhance their formation through redox reactions, contributing to the overall efficiency of the synthesis process [73]. Based on the FTIR spectroscopy data, it was suggested that dissociation of a proton of the eugenol –OH groups results in the formation of resonance structures capable of further oxidation. This process is accompanied by the active reduction of metal ions, followed by nanoparticle formation [74]. Alkaloids, nitrogen–containing compounds, can also participate in the reduction of metal ions. Proteins found in plant extracts can reduce metal ions through mechanisms involving electron transfer. Amino acids, i.e., tryptophan and tyrosine, enhance the reduction process due to their specific side chains that can donate electrons [75,76]. Amino groups in proteins and amino acids can bind to metal surfaces, providing steric hindrance that stabilizes the nanoparticles and prevents their aggregation [67]. Carboxyl groups (–COOH) found in organic acids and amino acids, can interact with metal ions, enhancing stabilization through electrostatic repulsion and forming complexes that prevent particle aggregation [77]. Carbohydrates can stabilize nanoparticles during synthesis by providing steric stabilization through their large molecular structures, which create a physical barrier around the nanoparticles and may also contribute to the reduction of metal ions by acting as reducing agents themselves [78].
In FTIR spectra, the position of functional groups involved in reduction, capping and stabilization of metallic nanoparticles produced with plant extracts, exhibit a high degree of consistency, irrespective of the metal ions employed as the precursor for the NPs. Therefore, this review will focus on the most frequently described metal and metal oxide nanoparticles.
3.1. Metal Nanoparticles
As previously stated, metal nanoparticles themselves have no infrared absorption properties, thus direct FTIR analysis of pure nanoparticles is not possible. However, FTIR spectroscopy allows the identification of functional groups in the molecules under study, which in turn allows one to recognize the groups responsible for the reduction, capping and stabilizing of nanoparticles. A comparison of the spectrum of the pure extract used to obtain the NPs with that of the NPs synthesized using this extract may reveal the disappearance, reduction in intensity or shift of several characteristic peaks. This may indicate the involvement of these groups in the reduction process. Conversely, if the FTIR peaks remain unchanged, it suggests that these specific functional groups are responsible for the stabilization of the nanoparticles.
Given the importance of NPs shape and size in biomedical applications and toxicology, precise and controlled synthesis is essential. In order to enhance the biocompatibility of NPs, it is advisable to utilize non–toxic, green reagents. Literature reports indicate that the vast majority of antioxidants in bio–based extracts can serve as reducing, capping and stabilizing agents. Green synthesis was applied, i.a., to obtain metallic Au, Ag, Pt, Pd, Cu, Ti nanoparticles [79,80,81], flexible nanostructures with controllable shape, composition, size, structure and optical properties.
3.1.1. Gold Nanoparticles
Gold nanoparticles (AuNPs) have garnered considerable interest across a multitude of disciplines, particularly in the biomedical realm. Their distinctive physical and chemical attributes, including their nanoscale dimensions, extensive surface area and tunable optical properties, render them well–suited for a wide range of applications, including diagnostics, therapy and drug delivery. AuNPs are extensively used in biosensors for detecting biomarkers associated with diseases such as cancer and cardiovascular disorders [82,83,84]. Commonly seen in home pregnancy tests, AuNPs facilitate rapid diagnostic tests by providing visual signals when they bind to target molecules [85]. Their optical properties enable colorimetric detection methods, which can indicate the presence of specific analytes. Additionally, AuNPs enhance imaging techniques like electron microscopy and dark–field microscopy, providing high contrast for biological samples [86]. In medical imaging, AuNPs serve as effective contrast agents in computed tomography (CT) scans [87]. They have a longer circulation time in the bloodstream compared to traditional iodine–based contrast agents, improving imaging quality and allowing better delineation of blood vessels [88]. AuNPs can be functionalized with drugs or therapeutic agents, allowing for targeted delivery to specific cells or tissues. Their high surface area allows for the attachment of multiple molecules, enhancing the efficacy of drug delivery systems [89]. AuNPs exhibit antibacterial properties and are being explored for use in coatings for medical devices to reduce infection rates [87,90,91]. In addition to their use in the field of medicine AuNPs are employed in environmental sensing applications to detect pollutants. This is due to their sensitivity to alterations in their optical properties when they interact with a range of substances [92]. The conventional method for synthesizing AuNPs involves two steps: firstly, a chemical reduction process is employed to extract the AuNPs, and secondly, stabilization methods are used to prevent further aggregation [93]. It is possible to synthesize AuNPs of varying shapes and sizes by modifying the synthesis methods and routes employed.
In a study conducted by Elia et al. [94], four distinct plant extracts of sage, lemon verbena, rose geranium and pomegranate (Salvia officinalis, Lippia citriodora, Pelargonium graveolens and Punica granatum, respectively) were employed as reducing and stabilizing agents in the preparation of AuNPs. The pure extracts and the resulting colloidal solutions of AuNPs were subjected to FTIR spectroscopy. The analysis of the spectra of the pure extracts enabled the identification of functional groups, and a change in the position of previously identified bands was observed in the AuNPs spectra. The band of hydroxyl groups (alcohols, phenols) and N–H (primary (1°) amines, amides) was identified at approximately 3330 cm−1, the band of P–H groups (phosphines) at 2332 cm−1, and the band of C=O groups at 1848 cm−1 and 1714 cm−1 was attributed to anhydrides and aldehydes/ketones, respectively. The N–H group bands were identified at approximately 1613 cm−1 (1°, secondary (2°) amines) and in the range 1606–1574 cm−1 (1° amines). The band of C=C groups in aromatic compounds was observed at 1420 cm−1. The band at ~1270 cm−1 was attributed to the vibrations of the S=O groups (sulfones, sulfonates), C–N groups (aromatic amines) and C–O groups (alcohols, carboxylic acids, esters, ethers). The band at approximately 1109 cm−1 was attributed to the vibrations of the C–N groups (aliphatic amines). In the AuNPs spectra, these bands were shifted towards higher or lower wavenumbers or completely disappeared. Authors hypothesized that these shifts in peaks positions were attributable to the adsorption of extract constituents onto the AuNPs surface.
The bioactive compounds present in the leaf extract of olive (Olea europaea) were engaged in the synthesis of AuNPs, which were subsequently identified through FTIR spectroscopy [95]. FTIR results demonstrated the presence of O–H (3409 cm−1) and C=O (1733 cm−1) stretching possibly of oleuropein, apigenin–7–glucoside and/or luteolin–7–glucoside, C–C stretching vibrations and –C–O–H bands at ~800 cm−1 and 1261 cm−1, respectively, in AuNPs. Additionally, a sharp, strong band observed at 1077 cm−1 was assigned to the C–OH groups of the proteins in Olea europaea extract. The shift of C=O stretching to 1721 cm−1 suggested that the biomolecules were bound to the AuNPs through this group. The band at 1624 cm−1, which has been assigned to amide I, has become more prominent in the spectrum of AuNPs and split into two bands at 1622 and 1648 cm−1. The observed split is likely attributable to alterations in the secondary protein structure [96,97], which may arise from either processing–related modifications or interactions with the NPs. The presence of IR bands resulting from C=O stretching vibrations in AuNPs spectrum and the emergence of amide I bands with a shift from that of the plain leaf suggested that proteins and antioxidant molecules bind to gold nanoparticles via free amine, C=O and O–H groups. This confirmed that the phytochemicals present in the extract acted as a reducing and stabilizing agent. Suriyakala et al. [98] performed FTIR spectra which suggested that phenolic compounds from peregrina (Jatropha integerrima Jacq.) flower extract were likely the agents responsible for the reduction of gold ions and formation of AuNPs. The presence of phenolic compounds was indicated by the peaks at 3237 cm−1 (phenolic O–H stretching) and 760 cm−1 (out of plane deformation of O–H group). Other bands indicate mainly protein content: 2920 cm−1 (C–H stretching), 1613 cm−1 (amine groups or carboxylate ion of the amino acid residues) and a band of C–N stretching in proteins at 1317 cm−1, demonstrating that protein acts as a ligand for AuNPs.
Ethanolic extract from gotu cola (Centella asiatica) leaves was used for the synthesis of AuNPs [99]. High content of phenolic compounds with strong antioxidant activity in the leaf extract was instrumental in reducing Au3+ to Au0 and the presence of phenolic groups was confirmed while analyzing FTIR spectrum of C. asiatica extract. The spectrum of extract revealed bands characteristic for alcohol (3380 cm−1 and 911 cm−1), phenolic groups (1130 cm−1), C–N stretching vibration of aromatic and aliphatic amines (1376 cm−1 and 1050 cm−1, respectively), methyl group deformation vibrations (1432 cm−1), C=C in aromatic structures (1607 cm−1), carbonyl (1695 cm−1) and C–H groups stretching (2850 cm−1). Additionally, FTIR spectrum of AuNPs exhibited bands, indicating that some phenolic compounds were bound to the surfaces of AuNPs, which remained despite repeated washing. These bands originated from the functional groups (alcohol, amines, phenols, carbonyl) present in the various plant metabolites of the leaf extract. Spectroscopic results revealed that high stability of nanoparticles was achieved due to the presence of amino (–NH2) or carboxylic (–COOH) groups.
Babu et al. [100] synthesized AuNPs using water hyssop (Bacopa monnieri) ethanolic extract. The authors assigned the bands in the FTIR spectrum of AuNPs to O–H hydroxyl groups (3394 cm−1), amide I in proteins (1632 cm−1), C–H groups vibration (1377 cm−1) and C–N stretching in amide groups (1085 cm−1), and indicated that these functional groups facilitated the reduction of the Au3+ ions to Au0 and capped the AuNPs during the particle growth termination process. AuNPs were synthesized using dragonhead (Dracocephalum kotschyi) leaf extract [101]. FTIR analysis identified the biomolecules which were involved in nanoparticles synthesis. IR spectrum of aqueous leaf extract revealed broad band at 3387 cm−1 (hydroxyl groups of alcohols or N–H of amines), a band at 2923 cm−1 (stretching of C–H groups), a band at 1416 cm−1 (C–N stretching vibration of aromatic amines) and a band at 1603 cm−1 (carbonyl group of carboxylic acid or amides). In turn, the FTIR spectrum of AuNPs revealed a slight shift in the N–H and/or OH stretching band from 3387 cm−1 to 3385 cm−1, C–H band from 2927 cm−1 to 2919 cm−1, C=O stretching from 1603 cm−1 to 1650 cm−1, C–N stretching from 1417 to 1441 cm−1 and –C–O–C stretching from 1078 to 1026 cm−1. Bands of C–O–H bending and C–C stretching at 1261 and 800 cm−1, respectively, had higher intensity in the spectrum of AuNPs. FTIR spectra analysis confirmed the presence of groups, which may be accountable for the bioreduction of Au3+ ions, leading to the synthesis, stabilization and avoiding agglomeration of Au0 nanoparticles. Therefore, the components of D. kotschyi act as both bioreductants and surfactants. FTIR measurement was also carried out to study the interaction of the nanoparticles and to identify the possible biomolecules in mango (Mangifera indica) leaf water extract, which can be responsible for capping and stabilization of the AuNPs [102]. It was assumed that water soluble compounds such as flavonoids, terpenoids and thiamine are the capping ligands of the AuNPs. This was evidenced by the following bands in the IR spectra: stretching vibrations of the N–H and O–H groups (3273 cm−1), C=O and C=C (1737 and 1624 cm−1, respectively), –C–O and –C–O–C stretching (1369 and 1020 cm−1, respectively), C–N stretching of aromatic amine groups (1444 cm−1) and –C–O–H bending (1216 cm−1).
FTIR spectroscopy was employed for identification the reducing and capping agents of date palm (Phoenix dactylifera) water extract and to understand the reaction mechanism between the components of the extract and gold salt [103]. Analysis of FTIR spectra revealed the presence of an intensive band at 3415 cm−1 (–OH groups), which shifted to 3398 cm−1 and became broader upon interaction with gold salt. Stretching vibrations at 1632 cm−1 of the C=O groups (most probably amide I band), visible in the extract spectrum, split into two peaks at 1651 and 1613 cm−1 in the AuNPs spectrum, which indicate a change in the secondary structure of proteins [98,99]. Thus, –OH groups and/or C=O groups present in carbohydrates, flavonoids, tannins, proteins and phenolic acids in P. dactylifera extract were accountable for the formation of AuNPs (coordination between Au3+ ions and the oxygen atoms of –OH and/or C=O).
AuNPs were prepared using the extract of lemon juice at different molar ratios as a reducing and capping agent [104]. Peaks of the –OH groups stretching vibrations were identified in FTIR spectra at 3433 cm−1, as well as C–H asymmetric and symmetric stretching vibration (2924 cm−1 and 2840 cm−1), C=O stretching of amide I at 1633 cm−1 and C–C and/or C–O stretching vibration at 1047 cm−1. The band at ~612 cm−1 was attributed to Au–O stretching vibrations. The theoretical calculation indicating the position of the Au–O bond vibration peak in the IR spectrum was 720 cm−1, but the actual position in the spectrum at 612 cm−1 authors interpreted as a consequence of the electrostatic coordination of gold nanoparticles with an oxygen atom in the C=O group and with other functional groups in the lemon extract.
Banana fruit waste (BFW) as multi–functional reducing, capping and stabilizing agent was used for the synthesis of the AuNPs of diverse shapes [105]. FTIR spectroscopy was used for identification of the functional groups causing reduction, capping and stabilization of nanoparticles. Authors observed shift of bands in the spectrum of BWF extract in relation to bands in the AuNPs spectrum. The band of hydroxyl groups shifted from 3117 cm−1 to 3126 cm−1, the band of asymmetric stretching of C–H groups shifted from 2938 cm−1 to 2942 cm−1 and new peak of symmetric stretching of C–H groups at 2890 cm−1 appeared, while the one at 1341 cm−1 vanished (C–H deformation vibration). The peak of C=O stretching vibrations of amide I in proteins at 1678 cm−1 shifted to 1684 cm−1 and its intensity decreased. Authors suggested that the proteins were engaged in the active reduction of metal ions by oxidizing aldehyde to carboxylic acid. Another change was observed for C–O and C–C band, which shifted from 1056 cm−1 to 1063 cm−1. The modified peaks in AuNPs spectrum indicated that the nanoparticles were formed as a result of the BFW extract application. A comparison of the absorption bands of AuNPs revealed an increase in wavenumbers, which might be attributed to the formation of coordination bonds between AuNPs and functional groups (O–H, C=O) of carbohydrates, which are predominantly present in the BFW extract. Thus, it was demonstrated that BFW extract was capable of acting as a reducer, stabilizer and capping agent.
3.1.2. Silver Nanoparticles
In recent years, silver nanoparticles (AgNPs) have become one of the most studied and explored nanostructures derived from nanotechnology, as nanosilver–based materials have shown interesting, challenging and promising properties suitable for various biomedical applications. AgNPs, exhibiting broad–spectrum antimicrobial activity against bacteria, viruses and fungi, are mainly used for antimicrobial and anticancer therapy, as well as to promote wound repair and bone healing, or as vaccine adjuvants, antidiabetic agents and biosensors [106,107]. They serve as carriers for drugs, enhancing bioavailability and targeting specific tissues or cells. This targeted delivery system is particularly beneficial in cancer therapy, where AgNPs can deliver anticancer drugs directly to tumour cells [108]. AgNPs are also employed, i.a., in the field of imaging and diagnostics, catalysis and sensing, environmental remediation, electronics and optoelectronics and food industry [109].
As it was mentioned, FTIR spectroscopy may be used to probe the chemical composition of the surface of nanoparticles. FTIR spectroscopy was employed to investigate the chemical composition of the surface of the AgNPs prepared using aqueous seed extract of horsegram (Macrotyloma uniflorum) [110]. The strong and intense band of C=O stretching in –COOH groups of reducing agent was observed at 1721 cm−1. The band at 1614 cm−1 was attributed to the amide I band, while the amide II band manifested as a shoulder at 1574 cm−1. The occurrence of the amide bands was due to the carbonyl stretch (amide I) and C–N stretching and N–H bending (amide II) [111] vibrations in the amide linkage of proteins present in the sample. The C–O–H groups vibrations was visible at 1443 cm−1 and the band at 1222 cm−1 was assigned to polyphenols, whereas the band at 1074 cm−1 corresponded to C–N stretching vibrations of aliphatic amines. The presence of compounds having these functional groups indicated a dual function of reduction and stabilization of AgNPs, namely the involvement of proteins in capping and polyphenols in reduction of Ag+ ions. Proteins may therefore play a role in the biosynthesis and stability of metallic nanoparticles.
The green biosynthesis of AgNPs using the plant extract of sage (Salvia spinosa) demonstrated that compounds containing –OH and C=O groups played a role in the reduction and stabilization of AgNPs [112]. Analysis of FTIR spectra revealed the presence of phenols and alcohols with free OH group and/or N–H stretching vibrations (~3423 cm−1), both in extract and AgNPs spectra. The band observed at ~2359 cm−1 indicated the presence of symmetric stretching of COO–; however, this was only evident in the spectrum of AgNPs. Analysis of spectra also confirmed the presence of compounds with: C≡C groups (2240 cm−1), N–C and N=C groups in R–N=C=S structure (2138 cm−1), C=C in aromatic structures and/or amide I (1630 cm−1), amide II in proteins (~1520 cm−1), S=O (sulphate ester) groups (1407 cm−1), C–O in COOH (~1150 cm−1), =CH in aromatic structures (821 cm−1, 675 cm−1), C–Cl (alkyl halides) (603 cm−1). The authors attributed slight shifts in peak positions to the involvement of plant extract compounds in the biosynthesis of AgNPs.
The –C=O groups of amino acids often function as capping ligands for NPs, and FTIR spectra allow one to confirm the involvement of diverse C=O groups on the NP surface, which prevents their aggregation and maintains their stability in the aqueous phase. Proteins can bind to AgNPs through free amine groups in the proteins [113] and stabilize them by surface–bound proteins [114]. Cell free, rich in proteins and enzymes extract of fungus Candida albicans was used to prepare gold and silver nanoparticles [115]. The presence of proteins was confirmed by the bands at 3225 cm−1 (–NH2 in primary amides), ∼1656 cm−1 and 1520 cm−1 (C=O stretching and N–H bending of amide groups in proteins, respectively), bands at 1371 cm−1 (AuNPs) and 1380 cm−1 (AgNPs) due to C–O stretching of the carboxylate ions in amino acids. Other bands were an indication of the presence of the nitrogen compounds like nitriles (–C≡N) and cyanates (–O–CN) (2355 cm−1), and C–H stretching at 2850 cm−1. The peak at ∼509 cm−1 was attributed to the metal–ligand stretching resulting from the interaction of biomolecules with the nanoparticles surfaces. The findings of the FTIR investigation have validated the hypothesis that the carbonyl group present in amino acid residues and peptides of proteins has the capacity to form stronger binding interactions with metallic elements. The resulting complexes were observed to encapsulate AuNPs and AgNPs, effectively preventing agglomeration and stabilizing the nanoparticles in their surrounding medium.
Wan Mat Khalir et al. [116] biosynthesized AgNPs mediated by aqueous stem extract of Entada spiralis, containing terpenoid saponins and glycosides, which are able to act as a reducing agent due to the presence of carboxyl and hydroxyl groups. When analyzing the spectra of the E. spiralis extract and synthesized with the extract AgNPs, some shifts in the bands and increase/decrease in peaks intensities were noticeable, suggesting that following functional groups were involved in the binding mechanism on the AgNPs: the peaks observed at 3399 cm−1 (hydroxyl O–H and/or amine –NH2), 2932 cm−1 (C–H stretching), 1522 cm−1 (aromatic ring of the terpenoid saponin structure) and 530 cm−1 (bonding of oxygen from the hydroxyl groups) exhibited a shift to 3402, 2925 cm−1, 1516 cm−1 and 521 cm−1 (Ag–O), respectively. The emergence of a new peak at 1829 cm−1 indicated the presence of carboxylate and C=C in the aromatic groups derived from the terpenoid saponin structure. This observation substantiated the hypothesis that the glucose moiety attached to the terpenoid saponin as an aldehyde underwent oxidation to gluconic acid. Additionally, the new peak at 823 cm−1 appeared, implying the potential bonding of C–H groups with the AgNPs. The authors also observed disappearance of bands at 1445 cm−1 and 1258 cm−1, which they attributed to vibrations of carboxylate groups and C–C(=O)–O stretching of the ester, respectively. The involvement of N–H groups of glycoside in the synthesis and stabilization of nanoparticles was confirmed by the decrease in the intensity of the peak at 1621 cm−1 in the spectrum of AgNPs. The band at 1072 cm−1 (C–O stretching) remained unchanged.
In a subsequent study, FTIR spectroscopy was used to identify compounds present in monarch redstem (Ammannia baccifera) leaf extract possibly responsible for reducing Ag+ into silver nanoparticles (Ag0). Based on the analysis of the spectra, Alam et al. [117] concluded that shifts or decreases in intensity of the specific peaks indicated the involvement of the following functional groups in the synthesis of AgNPs: –OH groups in alcohols and phenolic compounds (shift from 3266 cm−1 to 3323 cm−1 and from 1046 cm−1 to 993 cm−1 in AgNPs spectrum) and C=O stretching at ~1600 cm−1 (decrease in intensity). The alternation of these peaks indicated that water–soluble plant metabolites, including quinones, flavonoids and polyphenols, were involved in the synthesis of AgNPs.
The synthesis and characterization of green tea leaf extract–mediated AgNPs were conducted with a view to evaluating their biological activity and potential chemotherapeutic properties [118]. The objective of FTIR analysis was to investigate the functional groups in aqueous plant extracts in order to ascertain their roles, to determine how these groups facilitate the reduction of metal precursors into nanostructures and furthermore, to identify how these groups act as capping and stabilizing agents for the final end products. It appeared that the spectra of the extract and AgNPs exhibited a consistent pattern, with only minor variations in peak position and intensity, what indicated that these biomolecules were involved in the reduction of Ag+ and the stabilization of AgNPs. Some peaks in FTIR spectra shifted or their intensity changed, namely 3310 cm−1 in the extract spectrum (stretching vibration of the –OH group in the flavonoid ring’s phenolic component) shifted to 3315 cm−1 in AgNPs spectrum, intensity of peaks at 2920 and 2852 cm−1 (stretching vibrations of the C–H groups of aliphatic hydrocarbons) decreased, and those observed at 1145 cm−1 (C–O–C in carbohydrates), at 1029 cm−1 (C–N stretching) and in the 822–722 cm−1 range (C–H and/or C–S) shifted to 1183, 1092 and 789–787 cm−1 in the green synthesized AgNPs, respectively. The other peaks indicated the presence of the following functional groups: C=O stretching of polysaccharide components and proteins’ primary and secondary amide bonds (1624 cm−1), =NH (1440 cm−1), C–O–H bending of alcohol (1368 and 1250 cm−1) and C–N stretching (1029 cm−1). The peak at ~600 cm−1 pointed to the formation of Ag–O. Other authors also indicate the presence of Ag–O–Ag stretching vibrations and the appearance of this peak while formation of silver nanostructures [119,120]. FTIR analysis revealed that the diverse range of compounds present in the aqueous green tea leaf extract, including proteins, polysaccharides, flavonoids and primary and secondary amines, facilitated the reduction of Ag+ to generate AgNPs. The presence of functional groups, namely free amine groups from proteins and carbonyl groups from flavonoids, played a crucial role in stabilizing and capping the resulting silver nanoparticles.
FTIR spectroscopy was used to reveal the functional groups involved in the surface coating and stabilization of the AgNPs synthesized with aqueous and hydroalcoholic extracts of tansy flowers and leaves (Tanaceti flos and Tanaceti folium, respectively) [121]. Extracts of tansy contain essential oils with varying compositions, including beta–thujone, isomers, terpenes and camphene. Additionally, the essential oils contain various other compounds, including monoterpenes (such as alpha–pinene, beta–pinene and myrcene), polyphenols, amino acids, proteins, flavonoids (derivatives of quercetin and luteolin), oxygenated monoterpenes, monoterpene hydrocarbons, alcohols, carbohydrates, enzymes and mineral compounds. The observed shift of some peaks and the emergence of new peaks in the spectra of AgNPs substantiate the bioreduction of Ag+ to Ag0 by specific functional groups of plant extract: a shift of the phenolic groups bands from ~1395 cm−1 to 1441 cm−1 and 1258 to ~1240 cm−1, a shift of the ether groups bands from 1060 cm−1 to 1025 cm−1 (Tanaceti flos extract) and from 1045 cm−1 to 1029 cm−1 (Tanaceti folium) and disappearance of C–CO–C and C–CO in–plane deformation vibrations in ketone groups (~598 cm−1 and ~510 cm−1). The spectra of AgNPs exhibited the presence of COOH carboxyl groups bands (~3280 cm−1 and 1731 cm−1). These bands were a consequence of the oxidation of phenolic groups. A slight shift or decrease in the intensity of the aforementioned bands, accompanied by an increase in the intensity of the amide II band at ~1520 cm−1 and the amine C–N stretching vibrations at 1027 cm−1 in the AgNPs spectra, suggested that proteins extracted from tansy coated the silver nanoparticles and stabilized them. High intensity of the amide groups band at 1516 cm−1 also indicated the coverage of the AgNPs with those groups and their stabilization. The analysis of the spectra also allowed the presence of ketones, aldehydes, quinones and esters (peaks between 1700 and 1600 cm−1) assigned to the C=O vibration of carbonyl groups and/or C=C vibrations of aromatic structures to be confirmed. The peaks at ~1595 cm−1 and ~1392 cm−1 in the extracts spectra also pointed to COO– asymmetric and symmetric stretching, respectively.
Madaniyah et al. [122] explored the biosynthesis of silver nanoparticles using the medicinal plant Indian copperleaf (Acalypha indica Linn) as a mediator under sunlight and at 60 °C. The synthesized AgNPs were further coated by water–soluble chitosan to stabilize the nanoparticles in order to improve their anticancer efficacy. Authors observed shifts in FTIR spectra, which they attributed to the reduction and adsorption of bioactive functional groups from the extract onto the surface of AgNPs. Additionally, the presence of amide bands at 1556 cm−1 (N–H bending of the chitosan amide) in AgNPs with chitosan suggested that the interaction between AgNPs and chitosan did not disrupt the secondary structure during the synthesis process. The presence of strong peaks at ~470 cm−1 (C–O–Ag) suggested the formation of AgNPs via carbonyl bonds from flavonoids or alkaloids in Acalypha indica L.
AgNPs are the most widely used nanomaterial as a broad–spectrum antimicrobial agent, due to the release of silver ions from the crystalline core of these particles, which contributes to their antimicrobial toxicity [123]. However, bacterial adaptation and their defence mechanisms have the potential to compromise the efficacy of disinfection processes or mitigate unintended consequences, such as alterations to the microbial ecosystem services associated with silver release into the environment. Extracellular polymeric substances (EPSs), composed mostly of polysaccharides and proteins, secreted by bacteria could play a role in protecting cells against environmental stresses. As demonstrated by Kang et al. [64], Escherichia coli–secreted EPSs can reduce Ag+ and entrap it as AgNPs, thereby acting as a permeability barrier to impede intracellular penetration by silver. Authors have compared the FTIR spectra of an EPS before and after reaction with Ag+ to prove the involvement of reducing sugar components of EPS in Ag+ reduction. Following the reaction with Ag+, the bands of rhamnose (988 cm−1) and pyranose (924 cm−1), structures in FTIR spectra exhibited a significant decrease in intensity, while the band of carboxyl groups (1385 cm−1) demonstrated a notable enhancement in intensity and clarity. This observation allowed us to conclude that the aldehyde groups present in the reducing sugars had undergone oxidation to form carboxyl groups by Ag+. These results were confirmed by 13C nuclear magnetic resonance (13C NMR) analysis.
3.1.3. Platinum Nanoparticles
Platinum nanoparticles (PtNPs) have garnered significant attention across various fields and their applications span diagnostics, catalysis and biomedical uses, showcasing their potential in both industrial and medical settings. PtNPs can be utilized in catalytic processes, including automotive catalytic converters, oxidation and hydrogenation processes, hydrogen fuel cells but also in electrochemical reactions and in sensors for detecting various substances [124,125]. The biomedical field has seen innovative uses of platinum nanoparticles due to their biocompatibility and reactivity. Key applications include cancer treatment, photothermal therapy, drug delivery systems, diagnostics and antibacterial agents [126].
Halfa grass (Desmostachya bipinnata) extract was used for preparation of platinum nanoparticles incorporated into mouthwash formulations [127]. The objective of FTIR study was to identify the functional groups present and to examine the interactions between the nanoparticles and biomolecules. Alkyl halides, visible in the spectrum as a peak at 688 cm−1, were responsible for the stability of PtNPs and preventing their aggregation. The peak of O–H stretching vibrations at ~3435 cm−1 indicated the presence of hydroxyl groups, which are essential for bioreduction as they donate electrons to reduce Pt4+ to Pt0, thereby initiating the formation of nanoparticles. The carbonyl C=O functional groups of amide I, identified at ~1637 cm−1, were also crucial for the reduction of platinum ions. They contributed to the stabilization of the reduced metal atoms and the promotion of nanoparticle growth through their electron–donating capacity. Similar findings were reported by Kora and Rastogi [128], who synthesized PtNPs using renewable, biodegradable plant exudate gum and gum olibanum (Boswellia serrata) as a reducing and stabilizing agent. Based on the shifts in the peaks in FTIR spectra of gum and nanoparticles, the authors assumed that the reduction of platinum ions occurs due to the presence of hydroxyl and carboxylate groups of the gum (shift of O–H groups band from 3395 to 3464 cm−1; shift of carboxylate groups band from 1420 to 1433 cm−1 and from 1385 to 1368 cm−1; shift of C−O band of carboxylic acids from 1261 to 1219 cm−1, respectively). The stabilization of the PtNPs was assured by the functional groups of proteins (disappearance of amide I band at 1651 cm−1, appearance of C=O band at 1740 cm−1 and shift of amide II band from 1605 to 1535 cm−1).
Green synthesis of biogenic PtNPs from Prunella vulgaris (herbaceous plant in the mint family) leaf extract was performed in order to achieve synthetic enzyme–mimics (artificial nanozymes) [129]. Synthetic enzyme–mimics offer a number of advantages over their natural counterparts. These include greater stability against deactivation in a variety of chemical conditions, ease of surface modification, lower synthesis costs, less sophisticated purification steps and a longer shelf life. FTIR spectroscopy was used to demonstrate the effectiveness of the P. vulgaris extract characterized by a high concentration of active compounds, including flavonoids, alkaloids, anthraquinones, terpenoids and polyphenolics, as an effective reducing and stabilizing agents. Spectroscopic analysis revealed that the intensities and peaks positions of all identified functional groups in the extract exhibited a reduction and shift at the following wavenumbers: 3520 cm−1 (O–H stretching of phenolic compounds and N–H stretching), 2936 cm−1 and 1399 cm−1 (C–H), 1710 cm−1 (C=O), 1580 cm−1 (C=C), 1081 cm−1 (C–O–C in flavonoids) and 1037 cm−1 (C–OH). The bands observed at 823 cm−1 and 680 cm−1 are indicative of the presence of =CH in aromatic compounds and the peak at 774 cm−1 peak is suggestive of the presence of alkyl halides in the extract. The latter peak was also observed by Hosny et al. [130].
Persimmon (Diospyros kaki) leaf extract was used as a reducing agent in the eco–friendly extracellular synthesis of PtNPs [131] and FTIR spectroscopy was utilized to identify the biomolecules responsible for reducing platinum ions and stabilizing the platinum nanoparticles formed, but only the spectrum of PtNPs was made. The spectrum revealed the presence of intense bands at 1692 cm−1 (C=O), 1613 cm−1 (C=C in aromatic rings), 1410 cm−1 (methyl groups), 1325 cm−1 (C–N stretching in aromatic amines), 1195 cm−1 (phenolic groups), 1033 (C–N stretching in aliphatic amines) and 924 cm−1 (alcohols). No peaks of amide I (~1640 cm−1) or amide II (~1540 cm−1) characteristic of proteins were found, what indicated that PtNPs synthesized with D. kaki extract were surrounded by plant metabolites, such as terpenoids, which possess functional groups of amines, alcohols, ketones, aldehydes and carboxylic acids.
Platinum nanoparticles can also be synthesized using fungal species such as Neurospora crassa [132] and Fusarium oxysporum [133], where proteins, amides, long chain fatty acids and polysaccharides were pointed as biological molecules responsible for capping and stabilizing agents of PtNPs along with bioreduction of platinum ions. Penicillium pinophilum cell–free filtrate was used as reducing and stabilizing agent for PtNPs production [134]. The PtNPs synthesized using the fungal filtrate were observed to remain non–aggregated, indicating that a capping agent was responsible for their stabilization. This hypothesis was subsequently confirmed by FTIR analysis. The primary peaks observed in the fungal cell–free filtrate spectrum were also evident in the spectra of biogenic PtNPs, although with diminished intensities and minor shifts. The peaks observed at ~3313 cm−1 in fungal filtrate spectrum and attributed to N–H stretching (1°, 2° amines or amides) shifted to 3381 cm−1 in PtNPs spectrum. The peak at 1602 cm−1 (N–H bending in amines) shifted to 1621 cm−1, respectively. The peaks assigned to C–N stretching in amines at 1022 cm−1 shifted to 1056 cm−1. Therefore, the change in intensity and peak shifts indicated the presence of compounds that could serve as the reducing, capping and stabilizing agents. Thirumurugan et al. [135] have attempted to synthesize and characterize platinum nanoparticles using neem leaf (Azadirachta indica) water extracts. FTIR spectrum revealed the presence of following functional groups: C=O (1728 cm−1, 1643 cm−1), C–N (1527 cm−1, 1219 cm−1) and C–H (1365 cm−1). FTIR analysis confirmed the presence of functional groups such as carbonyls, alkanes and aliphatic amines.
3.2. Metal Oxide Nanoparticles
As previously stated, the bands of organic functional groups that play a reducing, capping or stabilizing role in metal nanoparticles synthesis exhibit a similar position in the IR spectra, irrespective of the metallic surface of the NPs to which they are bound. However, the analysis of FTIR spectra of metal oxide nanoparticles presents a certain degree of difficulty, given that the metal–oxygen bond, in contrast to the metallic bond, is discernible in the infrared region. Bands of metal–oxygen vibrations can overlap with bands of organic functional groups derived from extracts. Usually metal–oxygen bond peaks reside in the 800–400 cm−1 range, but broad peaks of hydroxyl group –OH vibrational stretching within 3700–3100 cm−1 range (maximum at ~3450 cm−1) as well as –OH bending of water (moisture) at ~1640 cm−1 may also appear [136,137]. In the infrared spectra of metal oxide nanoparticles, bands originating from metal precursors, i.e., nitrates, may also be discernible. The nitrates may be visible in the spectra as bands at ~1760, 1620, 1430, 1390, 1290, 1050, 830 cm−1 [138]. Therefore, these should also be taken into account when interpreting the FTIR spectra of metal oxide NPs. Table 2 provides a summary of the spectral positions of the infrared absorption bands exhibited by the metal oxides that constitute the nanoparticles.

Table 2.
Spectral positions of infrared absorption bands of metal oxides.
There is a rising trend in the number of scientific articles being published that address the biosynthesis of metal oxide nanoparticles as well as their various applications, usually as photocatalysts. Cobalt oxide plant–based nanoparticles (CoONPs) were synthesized using the grape Jumbo Muscadine (Vitis rotundifolia) [142]. ZnO nanoparticles were biosynthesized with Hibiscus sabdariffa extract [154]. Magnetic iron oxide nanoparticles (Fe2O3NPs) were synthesized using the fruit extract of Cynometra ramiflora, a by–product of the fruit’s natural ripening process [157]. Iron oxide FeONPs nanoparticles were synthesized using leaf extract of minnieroot (Ruellia tuberosa) [144]. Cupric oxide nanoparticles (CuONPs) were prepared via plant mediated green synthesis with butter tree (Madhuca longifolia) extract [143]. Nickel oxide (NiONPs) nanoparticles were prepared using chia seeds (Salvia hispanica L.) extract [148]. Biosynthesis of tin dioxide nanoparticles (SnO2NPs) was achieved using extracts of tea (Camellia sinensis) [149]. Zinc oxide nanoparticles (ZnONPs) were successfully synthesized via chemical and green, environmentally benign method from banana peel waste extract [158]. All of the aforementioned nanoparticles demonstrated activity in photocatalytic reactions.
Calcium oxide nanoparticles CaONPs have been synthesized utilizing a green precursor Lala clam seashells [140] and tested as catalyst material for developing the modified electrode sensor in electroanalytical platform for urea detection in milk. Moringa oleifera extract was used for the green synthesis of magnetic iron oxide (Fe3O4NPs) nanoparticles, potential candidates for being integrated in photovoltaic devices [159]. Consequently, the robust magnetic properties of iron oxide nanoparticles (FeONPs) render them valuable in a number of medical imaging techniques. Potential applications of FeONPs include magnetic resonance imaging (MRI), targeted drug delivery systems and environmental remediation for the removal of contaminants [160].
Titanium dioxide nanoparticles (TiO2NPs) are among the most extensively researched materials, due to their photocatalytic properties and chemical and thermal stability, which make them suitable for use in a wide range of industrial applications [146]. These nanoparticles can be engineered to deliver drugs, including anticancer agents and antibiotics, directly to target cells. This targeted delivery is facilitated by their small size and ability to be functionalised with specific biomolecules [161]. TiO2NPs function as photosensitizers, generating reactive oxygen species (ROS) upon exposure to UV light. This property is exploited in the treatment of malignant tumours and the inactivation of antibiotic–resistant bacteria [161]. Due to their photocatalytic properties, TiO2NPs can be used to coat medical devices and implants, providing antimicrobial surfaces that reduce infection rates associated with these devices [162]. They are effective in the photocatalytic degradation of organic pollutants in wastewater.
The synthesis of TiO2NPs has typically been conducted using chemical routes. However, there is a growing trend towards the utilization of environmentally friendly, green synthesis methods. Neem (Azadirachta indica) leaf extract has been used for the synthesis of TiO2NPs [153]. FTIR study revealed the presence of terpenoids, flavonoids and proteins considered responsible for the formation and stabilization of titanium dioxide nanoparticles. Analysis of the TiO2NPs spectrum confirmed the presence of –OH groups in alcohols and phenolic compounds, C=C groups of aromatic rings, C=O vibrations of carboxylic acid and Ti–O–Ti stretching vibration of titanium dioxide nanoparticles at ~710 cm−1. In turn, Dobrucka [150] identifies the presence of TiO2 at 431 cm−1. The available literature data indicate that TiO2 exhibits strong absorption bands within the 700–600 cm−1 and 525–460 cm−1 ranges [136,152,163]. Similar environmentally benign methodology in the TiO2NPs synthesis has been implemented utilizing the plant species Sesbania grandiflora [164]. FTIR spectra of TiO2NPs revealed the presence of bands, which the authors attributed to –NH stretching of amide II (3403 cm−1), C–H stretch of the flavonoids (2914 cm−1), Si–H stretch of organosilicon (2266 cm−1), amide groups (1626 cm−1), C–N stretching of the aromatic amide group (1389 cm−1) and C–OH stretching in secondary alcohols (1047 cm−1). Thus, the presence of flavonoids, organosilicon compounds, aromatic amine groups and secondary alcohols, which were present in the S. grandiflora leaf extract, could act as capping ligands for the TiO2NPs.
Zinc oxide nanoparticles (ZnONPs) possess distinctive semiconducting, optical and piezoelectric properties. Their applications extend across a range of sectors, including industry, biomedicine and cosmetics, thereby demonstrating their multifunctionality [165,166]. ZnONPs have seen a surge in use in the rubber, paint, coatings and cosmetics industries and have attracted considerable attention in biological applications due to their remarkable biocompatibility, economic viability and minimal toxicity. ZnONPs have demonstrated significant promise within the field of biomedicine, particularly in the context of anticancer and antibacterial applications. This is attributable to their ability to induce elevated levels of reactive oxygen species (ROS), the release of zinc ions and the subsequent initiation of cell apoptosis [167]. ZnONPs have been demonstrated to enhance the mechanical strength and antibacterial properties of dental composites and have been shown to minimize bacterial adhesion and enamel demineralisation when used to coat orthodontic appliances [168].
FTIR spectroscopy was used to determine the functional groups of compounds present in the Deverra tortuosa extract involved in the formation and stabilization of green synthesized ZnONPs [155]. Analysis of peaks in FTIR spectrum revealed the presence of 1° and 2° amines, characteristic of proteins/enzymes, which have been shown to stabilize ZnONPs by forming a coating, covering the metal nanoparticles and preventing the nanoparticles from agglomerating. The presence of flavonoids and other phenolics, confirmed by the presence of O–H and C–OH stretching vibrations (3434 cm−1 and 1117 cm−1, respectively) have been shown to assist in the formation of ZnONPs. The presence of additional peaks indicated the hexagonal structure of the synthesized ZnO, with the peaks at 878 cm−1 and 442 cm−1. In the study of Uysal et al. [156] polyphenolic compounds were recognized as substances supporting formation of ZnONPs. Additionally, the formation of ZnONPs was confirmed by the peaks at 889 cm−1 and 554 cm−1.
ZnNPs were synthesized using onion waste peel extract (Allium cepa L.) via the green synthesis approach [169]. The FTIR data demonstrated the presence of metabolites, including flavonoids, alkaloids, carboxylic acids and polyphenols, which were found to be linked to ZnONPs. It was observed that these substances, particularly flavonoids and phenolics, played a crucial role in the transformation of Zn2+ into ZnONPs. The presence of carboxylic groups was found to contribute to the stabilization of ZnONPs through interaction with the NP surface.
Iron can form several types of nanoparticles, primarily categorized as iron oxide nanoparticles (IONPs) and zero–valent iron nanoparticles (nZVI). Each type exhibits distinct properties and applications. Recent research has focused intensely on investigating the potential of nZVI for the removal of organic and inorganic pollutants. It has been demonstrated that both nZVI and IONPs function through a Fenton–like mechanism, generating reactive oxygen species (ROS) that facilitate the degradation of pollutants. The efficacy of these materials in the removal of organic contaminants has been well–documented [170,171,172]. The results have demonstrated that nZVI can be effective for the treatment of contaminated soil and groundwater due to its elevated surface area, elevated reaction rate and large number of active sites [173,174,175]. Nevertheless, owing to their high reactivity, nZVI are susceptible to rapid oxidation when exposed to air. This oxidation results in the formation of iron oxides, thereby significantly reducing the reactivity of NPs and limiting their effectiveness in remediation applications. The immediate formation of oxide layers upon exposure to oxygen can hinder their intended reactions with contaminants [176]. IONPs maintain their reactivity over time better than nZVI, which can suffer from rapid passivation due to oxidation. This stability enhances their effectiveness in long–term remediation strategies [177]. Green synthesized nZVI, differing in shape, morphology and size, reveal a complete coating of the iron surfaces by, e.g., proteins and polyphenols. This coating has been found to inhibit the oxidation of the nanoparticles. Furthermore, the NPs have been observed to be uniformly dispersed throughout the reaction medium, exhibiting no signs of agglomeration [178,179]. Thus, the use of biogenic materials not only reduces reliance on harmful chemicals but also enhances the stability of the nanoparticles due to the capping effect of phytochemicals, like polyphenols and flavonoids, present in the extracts [180]. This leads to better dispersion and diminished aggregation compared to chemically synthesized nZVI.
It has been established that both IONPs and nZVI fulfil key roles in a range of applications, yet it is widely accepted that the former are considered more favourable in biomedical contexts on account of their superior biocompatibility and multifunctionality. IONPs, particularly magnetite (Fe3O4), maghemite (γ–Fe2O3) and hematite (α–Fe2O3), are known for their biocompatibility and low toxicity, making them suitable for medical applications [181]. In addition to this, in environmental settings, IONPs performance in terms of pollutant degradation and stability is also enhanced relative to that of nZVI nanoparticles [182].
As mentioned, the potential applications of IONPs are manifold, extending across a number of disciplines. This demonstrates their potential as multifunctional agents in biomedical fields such as diagnostics and therapeutics. The distinctive magnetic properties, biocompatibility and ease of surface modification render them suitable for a wide range of innovative uses [181]. The green synthesis of IONPs generally yields amorphous iron oxide [178], which is considered to be the most unstable form among its diverse types [183]. However, it is important to form stable and crystalline IONPs for precise characterization and the potential utilization in a range of applications. The process of calcination may be a strategy to enhance the performance of iron oxide. The synthesis of IONPs with magnetic core followed by calcination was achieved through the utilization of clove and green coffee extracts [182]. Eugenol, as identified through FTIR analysis, functioned as a reducing and stabilizing agent for clove extract–assisted NPs, and polyphenolic acids (chlorogenic acids) and caffeine for coffee extract–assisted NPs were responsible for the reduction of Fe3+ to Fe. Following the annealing of IONPs at 550 °C, the authors identified bands at 597 and 462 cm−1 in the FTIR spectrum of clove extract–assisted NPs, and 549 and 460 cm−1 in the spectrum of coffee extract–assisted IONPs, which they attributed to Fe–O bonds. XRD was employed to confirm magnetite and magnetite/hematite formation (clove extract–assisted NPs and coffee extract–assisted NPs, respectively). It is noteworthy that the position of the Fe–O bands in the FTIR spectrum of the Fe2O3 is contingent on parameters such as particle size, shape and structure [184]. Consequently, the observed variation in the position of the bands may be attributed to these factors.
In the study of Abdullah et al. [185], IONPs in the form of magnetite and maghemite were synthesized using date palm (Pheonix dactylifera) leaf extract. The analysis of FTIR spectra indicated that phenolic groups were responsible for the reduction of Fe3+ ions. The band of phenolic O–H stretching shifted from 3416 cm−1 in P. dactylifera leaf extract spectrum to 3375 cm−1 in IONPs spectrum. Additionally, the reduction of Fe3+ into IONPs caused the 1643 cm−1 IR band of P. dactylifera to be split into multiple peaks at 1653, 1633 and 1623 cm−1. The authors hypothesized that this was due to the coordination of Fe3+, Fe2+ ions and the oxygen atoms of –OH and/or C=C groups during the formation of IONPs. The authors also employed FTIR to evaluate the proportion of magnetite and maghemite in the NPs formed, although they noted that FTIR was unable to accurately quantify the proportion of each phase. FTIR spectra of the synthesized IONPs exhibited peaks that were characteristic of both magnetite (a broad band with a maximum at 573 cm−1, accompanied by a shoulder at ~700 cm−1) and maghemite (six bands within the 800–500 cm−1 range, with the strongest band occurring at 638 cm−1).
To summarize, the principal wavenumber ranges of functional groups engaged in the reduction, capping and stabilization of nanoparticles, as documented in the literature, are presented in Table 3.

Table 3.
Wavenumber ranges of vibrations of functional groups, involved in reduction, capping and stabilization of nanoparticles [64,74,94,95,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,127,128,129,130,131,132,133,134,135,140,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,164,166,173,174,175,176,177,178,179,182,185,186].
The analysis of peaks in FTIR spectra, their spectral shifts and intensities variations can provide insights into the reduction of metal ions and other structural alterations that are associated with the synthesis and stabilization of nanoparticles. The reduction of metal ions to NPs during the synthesis process and their further stabilization is indicated by changes, i.a., in O–H, C=O, –COOH, C–O–C, N–H, C–N bands position and intensity. The bands of O–H groups shift towards lower or higher wavenumbers, depending on the presence of hydrogen bonding. If a hydrogen bonding is formed, the band shifts to lower wavenumbers [186]. When the O–H group is isolated, e.g., because of steric hindrance effects, the band is narrower and observed at higher wavenumbers. Additionally, the loss of hydroxyl groups, e.g., the consumption of groups for the reduction of metal ions and their oxidation to aldehydes, ketones, quinones, carboxylic acids, is associated with a concomitant decrease in hydrogen–bonded groups, leading to a shift towards higher wavenumbers. Consequently, additional bands of C=O groups may appear in the spectra of NPs in comparison to those of pure extracts, and/or the intensity of these bands may increase. Aldehyde C=O groups present, e.g., in reducing sugars may be oxidized to carboxyl groups by metal ions, resulting in the appearance of carboxyl group bands in the spectra of NPs [64]. In turn, the shift of C=O stretching may suggest that the biomolecules bind to NPs through this group, providing electrostatic stabilization of NPs. Analysis of the FTIR spectra allow us to demonstrate the involvement of diverse C=O groups on the surface of NPs, thereby preventing their aggregation and maintaining their stability during the aqueous phase. For instance, the splitting of a band of amide I into two bands indicate a change in the secondary structure of proteins, i.e., the involvement of these groups in the NPs’ formation (reduction and/or stabilization). The shift or disappearance of C–O–C, C–N, N–H and C–H bands also confirms the involvement of these functional groups in NPs’ formation and stabilization. In turn, the increase in amide II band intensity, may indicate the coverage of the NPs with those groups and their stabilization. Any changes in molecular geometry may lead to variations in vibrational frequencies due to differences in steric interactions and electronic environments surrounding the bonds. The molecule’s stiffening, a consequence of its stabilizing role in the NPs’ formation, may result in the disappearance of deformation vibrations or a shift of the stretching vibration bands of the C–H groups.
4. Challenges in the Interpretation of FTIR Spectra of Nanoparticles
There is a certain degree of subjectivity and interpretative flexibility inherent to the interpretation of FTIR spectra of NPs within the literature. A review of the literature reveals numerous examples where the same peak with a comparable wavenumber has been attributed to disparate functional groups, dependent on the author’s perspective. This phenomenon persists even when the chemical composition of the compounds under examination is analogous. While a comprehensive and precise interpretation of IR spectra of complex mixtures or complex compounds is unfeasible due to the presence of multiple functional groups in the IR spectra vibrating at similar wavenumbers, overly flexible interpretation can lead to confusion and errors in assigning peaks to individual functional groups, i.e., in the study of groups responsible for bio–reduction, capping and stabilization of nanoparticles. There are many publications where FTIR spectroscopy is employed for valid purposes, yet the spectra are misinterpreted. This primarily pertains to the assignment of bands to N–H and C–N groups, whose intensities in the spectra are typically of medium or low intensity [163]. The bands of N–H groups are visible in IR spectra within 3550–3250 cm−1 (aliphatic amines) and 3420–3340 cm−1 (aromatic amines) and in these ranges they may overlap with the bands of O–H stretching of –COOH groups in carboxylic acids (3300–2500 cm−1), O–H of alcohols (3670–3230 cm−1) and O–H in phenolic compounds (unassociated within 3620–3590 cm−1; associated within 3250–3000 cm−1). Medium to strong intensity vibrations of N–H groups vibration in 1° amines and amides in the 1650–1580 cm−1 range, medium–weak vibrations in 2° amines within 1580–1490 cm−1 and may be masked by aromatic ring C=C vibrations within 1625–1575 cm−1, while in the range 1295–1080 cm−1 (NH2 rocking/twisting in amines or amides) can be mistaken with C–O deformation and O–H stretching combination vibrations in phenols (1410–1310 cm−1; 1260–1180 cm−1) or C–H symmetrical deformation vibrations (1390–1340 cm−1). Similar situation occurs in the case of medium intensity C–N bond vibrations (1240–1020 cm−1, band position contingent upon the existence of neighbouring groups).
Assigning the vibrations of aromatic structures to specific bands in IR spectrum is also a challenge. Usually, the strongest absorptions for aromatic compounds occur within 3080–3010 cm−1 (stretching vibrations of the ring C–H bonds) and within 900–650 cm−1 (out of plane C–H vibrations of aromatic ring). Ring C=C stretching vibrations occur within 1625–1430 cm−1, but a weak band ~1000 cm−1 may also be observed, as well as weak combination and overtone bands within 2000–1650 cm−1. When an aromatic ring has a substituent, the bond between the carbon and the substituent is affected by the mass of this substituent and, consequently, a shift in the IR spectrum will be observed. In the case of phenolic compounds, the presence of additional bands originating from O–H and C–O bonds vibrations becomes evident. The position of these bands is also contingent upon the existence of substituents on the aromatic ring or the amount of phenolic moieties. Furthermore, if strong intramolecular hydrogen bonding occurs (e.g., in the presence of C=O groups), the band at ~1200 cm−1 may be found; however, this is dependent upon the presence and position of substituents in the aromatic ring (e.g., in ortho position). Consequently, the position of the O–H group band may change [163].
In light of the intricate composition of the extracts, the interpretation of their FTIR spectra represents a challenging endeavour, hindering the ability to draw definitive conclusions regarding the chemical nature and interactions occurring within the nanoparticles. Consequently, it is essential to approach the interpretation of the results with a meticulous attention. Overlapping peaks complicate the identification of specific functional groups in FTIR spectra and can lead to misinterpretation of the data. As it was mentioned, the use of second derivatives of the spectra can be a fruitful approach for the purpose of revealing unresolved bands [35,37]. Additionally, the implementation of machine learning algorithms to analyze FTIR spectra can assist in deconvoluting complex spectra and identifying overlapping peaks [39,40]. Nevertheless, it is always advisable to correlate the results obtained from the use of FTIR spectroscopy with those obtained by alternative techniques in order to ensure a comprehensive and informed understanding of the experimental findings.
5. Summary
The green synthesis of metal nanoparticles employs a variety of chemical functional groups that act as reducing, capping and stabilizing agents. Hydroxyl groups (–OH) can donate electrons to metal ions, reducing them to their elemental forms, and may act as both a reducing and stabilizing agent (hydrogen bonding). Carboxyl groups (–COOH) present in many organic acids and amino acids participate in the reduction process, enhance the solubility of nanoparticles in aqueous solutions and prevent agglomeration by providing steric hindrance. Amine groups (–NH2) facilitate electron transfer during the reduction of metal ions and this makes them effective reducing agents in nanoparticle synthesis also providing stability to the NPs surface. Aldehyde or ketone functionalities can effectively reduce metal ions and help in stabilizing formed NPs by creating a protective layer around them. Carbonyl groups (C=O) of ketones and aldehydes, present in various organic compounds and proteins, can facilitate complex formation with metal ions which is essential for controlling NPs size and morphology during synthesis. The analysis of these and a multitude of other functional groups can be effectively conducted through the utilization of FTIR spectroscopy.
This review presented information regarding application of FTIR spectroscopy in analysis of green synthesized nanoparticles and interpretation of infrared spectra with particular emphasis on the assignments of individual functional groups. Considering the potential of nanotechnology applications in everyday life and its dynamic development, it is necessary to assure detailed information about the chemical composition, functional groups involved in synthesis and molecular interactions in nanomaterials chemistry. FTIR spectroscopy offers a suite of advantages for nanoparticle analysis, including non–destructive testing, detailed surface characterization, high sensitivity and resolution, versatility across materials and the ability to monitor synthesis processes. These features make it an invaluable tool in nanotechnology research and development.
Author Contributions
All authors have contributed to this paper. S.P.-P. is the primary author, responsible for drafting, literature review and concept development. M.C. and J.F. are responsible for proofreading, peer–reviewing the draft and preparing the article for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AFM | Atomic force microscopy |
| ATR | Attenuated total reflectance |
| DLS | Dynamic light scattering |
| EDX | Energy–dispersive X–ray spectroscopy |
| FIR | Far–infrared spectroscopy |
| FTIR | Fourier Transform Infrared spectroscopy |
| IR | Infrared |
| HRI | High refractive index |
| NMR | Nuclear magnetic resonance |
| NIR | Near–infrared spectroscopy |
| NPs | Nanoparticles |
| PAS | Photoacoustic spectroscopy |
| PCA | Principal Component Analysis |
| PLS | Partial Least Squares |
| ROS | reactive oxygen species |
| SEM | Scanning electron microscopy |
| S/N | signal–to–noise ratio |
| TEM | Transmission electron microscopy |
| UV–Vis | Ultraviolet–visible spectrophotometry |
| XPS | X–ray photoelectron spectroscopy |
| XRD | X–ray diffraction |
References
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 17, 1155622. [Google Scholar] [CrossRef]
- Hasan, S. A review on nanoparticles: Their synthesis and types. Res. J. Recent Sci. 2015, 4, 9–11. [Google Scholar]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on green Synthesis of Zinc Oxide Nanoparticles—An Eco–Friendly Approach. Resour.–Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.S.Y.; Chin, J.E. Biogenic Synthesis of Iron Nanoparticles from Apple Peel Extracts for Decolorization of Malachite green Dye. Water Air Soil Pollut. 2020, 231, 278. [Google Scholar] [CrossRef]
- Mochochoko, T.; Oluwafemi, O.S.; Jumbam, D.N.; Songca, S.P. Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohydr. Polym. 2013, 98, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Marjani, A.; Mousavi, Z. Agricultural waste biomass–assisted nanostructures: Synthesis and application. Green Process. Synth. 2019, 8, 421–429. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Selvaraj, R.; Nagendran, V.; Varadavenkatesan, T.; Goveas, L.C.; Vinayagam, R. Stable silver nanoparticles synthesis using Tabebuia aurea leaf extract for efficient water treatment: A sustainable approach to environmental remediation. Chem. Eng. Res. Des. 2024, 208, 456–463. [Google Scholar] [CrossRef]
- George, G.; Wilson, R.; Joy, J. Ultraviolet Spectroscopy: A Facile Approach for the Characterization of Nanomaterials. In Micro and Nano Technologies, Spectroscopic Methods for Nanomaterials Characterization; Sabu, T., Raju, T., Ajesh, K.Z., Raghvendra, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 55–72. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim. Acta Part A 2018, 192, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cong, Y.; Wang, B.; Zhang, N. Applications of Fourier transform infrared spectroscopy to pharmaceutical preparations. Expert Opin. Drug Deliv. 2020, 17, 551–571. [Google Scholar] [CrossRef]
- Kamaraj, N.; Rajaguru, P.Y.; Issac, P.K.; Sundaresan, S. Fabrication, characterization, in vitro drug release and glucose uptake activity of 14–deoxy,11,12–didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J. Pharm. Sci. 2017, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, G.; Montalbán, M.G.; Víllora, G.; Barth, A. Direct Quantification of Drug Loading Content in Polymeric Nanoparticles by Infrared Spectroscopy. Pharmaceutics 2020, 12, 912. [Google Scholar] [CrossRef]
- Galiyeva, A.; Daribay, A.; Zhumagaliyeva, T.; Zhaparova, L.; Sadyrbekov, D.; Tazhbayev, Y. Human Serum Albumin Nanoparticles: Synthesis, Optimization and Immobilization with Antituberculosis Drugs. Polymers 2023, 15, 2774. [Google Scholar] [CrossRef]
- Eid, M.M. Characterization of Nanoparticles by FTIR and FTIR–Microscopy. In Handbook of Consumer Nanoproducts; Mallakpour, S., Hussain, C.M., Eds.; Springer: Singapore, 2022; pp. 645–673. [Google Scholar]
- Rónavári, A.; Balázs, M.; Szilágyi, Á.; Molnár, C.; Kotormán, M.; Ilisz, I.; Kiricsi, M.; Kónya, Z. Multi–round recycling of green waste for the production of iron nanoparticles: Synthesis, characterization, and prospects in remediation. Discov. Nano 2023, 18, 8. [Google Scholar] [CrossRef]
- Akhil, T.; Bhavana, V.; Ann Maria, C.G.; Nidhin, M. Role of biosynthesized silver nanoparticles in environmental remediation: A review. Nanotechnol. Environ. Eng. 2023, 8, 829–843. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella-Pajulas, L.L.; Alemaida, I.M.; Grilli, M.L.; Mikhaylov, A.; Senjyu, T. Green Synthesis of Silver Oxide Nanoparticles for Photocatalytic Environmental Remediation and Biomedical Applications. Metals 2022, 12, 769. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Pal, D.; Thakur, C.; Kumar, S.; Devnani, G.L. Bio–synthesis of iron nanoparticles for environmental remediation: Status till date. Mater. Today Proc. 2021, 44, 3150–3155. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Anaya, N.M.; Schifman, L.A.; Oyanedel-Craveret, V. Fourier transform infrared spectroscopy to assess molecular–level changes in microorganisms exposed to nanoparticles. Nanotechnol. Environ. Eng. 2016, 1, 1. [Google Scholar] [CrossRef]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Pokhrel, K.; Li, X.; Tiquia-Arashiro, S. Applications of Fourier Transform–Infrared spectroscopy in microbial cell biology and environmental microbiology: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral–enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Avelino, S.R.; Oliveira, F.M.L.; Oliveira, A.C.; Morais, P.C. Use of the photoacoustic spectroscopy in the investigation of the dilution process in surface–coated nanoparticles. J. Non–Cryst. Solids 2006, 352, 3692–3696. [Google Scholar] [CrossRef]
- Michaelian, K.H. Photoacoustic IR Spectroscopy, Instrumentation, Applications and Data Analysis, 2nd ed.; Wiley–VCH: Weinheim, Germany, 2010. [Google Scholar]
- Kumar, K.; Singh, A.K.; Pandey, A.C. Photoacoustic Spectroscopy and Its Applications in Characterization of Nanomaterials. In Nanomaterials: Processing and Characterization with Lasers; Singh, S.C., Zeng, H., Guo, C., Cai, W., Eds.; Wiley–VCH: Weinheim, Germany, 2012; pp. 621–649. [Google Scholar]
- Lumen, D.; Wang, S.; Mäkilä, E.; Imlimthan, S.; Sarparanta, M.; Correia, A.; Haug, C.W.; Hirvonen, J.; Santos, H.A.; Airaksinen, A.J.; et al. Investigation of silicon nanoparticles produced by centrifuge chemical vapor deposition for applications in therapy and diagnostics. Eur. J. Pharm. Biopharm. 2021, 158, 254–265. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Pasieczna-Patkowska, S.; Schroeder, G. Photoacoustic Spectroscopy of Surface–Functionalized Fe3O4–SiO2 Nanoparticles. Appl. Spectrosc. 2020, 74, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Simms, J.R. Comparison of photoacoustic, diffuse reflectance and transmission infrared spectroscopy for the study of carbon fibers. Fuel 1995, 74, 543–548. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Piriz-Almeida, F.; Duran-Valle, C.J.; Pastor-Villegas, J. Formation of oxygen structures by air activation. A study by FT–IR spectroscopy. Carbon 1999, 37, 1517–1528. [Google Scholar] [CrossRef]
- Yarwood, J. Fourier Transform Infrared Reflection Spectroscopy for surface analysis. Anal. Proc. Surf. Anal. 1993, 30, 13–18. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Dyatlova, Y.A.; Kenzhegulov, O.A.; Vladimirova, A.A.; Mamchenkova, P.V.; Tugarova, A.V. Fourier Transform Infrared (FTIR) Spectroscopic Analyses of Microbiological Samples and Biogenic Selenium Nanoparticles of Microbial Origin: Sample Preparation Effects. Molecules 2021, 26, 1146. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Tkachenko, Y.; Niedzielski, P. FTIR as a Method for Qualitative Assessment of Solid Samples in Geochemical Research: A Review. Molecules 2022, 27, 8846. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Scheludko, A.V.; Dyatlova, Y.A.; Filip’echeva, Y.A.; Kamnev, A.A. FTIR spectroscopic study of biofilms formed by the rhizobacterium Azospirillum brasilense Sp245 and its mutant Azospirillum brasilense Sp245.1610. J. Mol. Struct. 2017, 1140, 142–147. [Google Scholar] [CrossRef]
- Candan, F.; Markushin, Y.; Ozbay, G. Uptake and Presence Evaluation of Nanoparticles in Cicer arietinum L. by Infrared Spectroscopy and Machine Learning Techniques. Plants 2022, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Candan, F.; Markushin, Y.; Ozbay, G. Nanoparticle Uptake and Bioaccumulation in Pisum sativum L. (Green Pea) Analyzed via Dark–Field Microscopy, Infrared Spectroscopy, and Principal Component Analysis Combined with Machine Learning. Agronomy 2024, 14, 1473. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Mizaikoff, B. Recent advances on the characterization of nanoparticles using infrared spectroscopy. TrAC Trends Anal. Chem. 2016, 84, 97–106. [Google Scholar] [CrossRef]
- Álvarez, R.A.B.; Cortez-Valadez, M.; Britto-Hurtado, R.; Oscar Neira Bueno, L.; Flores-Lopez, N.S.; Hernández-Martínez, A.R.; Gámez-Corrales, R.; Vargas-Ortiz, R.; Bocarando-Chacon, J.-G.; Arizpe-Chavez, H.; et al. Raman scattering and optical properties of lithium nanoparticles obtained by green synthesis. Vib. Spectrosc. 2015, 77, 5–9. [Google Scholar] [CrossRef]
- Sivan, S.K.; Shankar, S.S.; Sajina, N.; Padinjareveetil, A.K.; Pilankatta, R.; Kumar, V.B.S.; Beena, M.; Bini, G.; Makvandi, P.; Černík, M.; et al. Fabrication of a Greener TiO2@Gum Arabic–Carbon Paste Electrode for the Electrochemical Detection of Pb2+ Ions in Plastic Toys. ACS Omega 2020, 5, 25390–25399. [Google Scholar] [CrossRef] [PubMed]
- Heigl, N.; Petter, C.H.; Najam–Ul-Haq, M.; Rainer, M.; Vallant, R.M.; Bonn, G.K.; Huck, C.W. When Size Matters—Near Infrared Reflection Spectroscopy of Nanostructured Materials. J. Near Infrared Spectrosc. 2008, 16, 211–221. [Google Scholar] [CrossRef]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid–response analytical tool. TrAC Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Trajić, J.; Romčević, M.; Romčević, N.; Babić, B.; Matović, B.; Baláž, P. Far–infrared spectra of mesoporous ZnS nanoparticles. Opt. Mater. 2016, 57, 225–230. [Google Scholar] [CrossRef]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T.; Shivashangari, K.S.; Ravikumar, V. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta Part A 2014, 121, 746–750. [Google Scholar] [CrossRef]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesis of nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 411–446. [Google Scholar]
- Jiménez-Rosado, M.; Gomez–Zavaglia, A.; Guerrero, A.; Romero, A. Green synthesis of ZnO nanoparticles using polyphenol extracts from pepper waste (Capsicum annuum). J. Clean. Prod. 2022, 350, 131541. [Google Scholar] [CrossRef]
- Majlesi, Z.; Ramezani, M.; Gerami, M. Investigation on some main glycosides content of Stevia rebaudian B. under different concentrations of commercial and synthesized silver nanoparticles. Pharm. Biomed. Res. 2018, 4, 8–14. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, G. Review on marine carbohydrate–based gold nanoparticles represented by alginate and chitosan for biomedical application. Carbohydr. Polym. 2020, 244, 116311. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; Khalifa, S.A.M.; Guo, Z.; Xu, B.; Zou, X.; El-Seedi, H.R. Marine organisms: Pioneer natural sources of polysaccharides/proteins for green synthesis of nanoparticles and their potential applications. Int. J. Biol. Macromol. 2021, 193, 1767–1798. [Google Scholar] [CrossRef]
- Mao, X.; Wu, L.-F.; Guo, H.-L.; Chen, W.-J.; Cui, Y.-P.; Qi, Q.; Li, S.; Liang, W.-Y.; Yang, G.-H.; Shao, Y.-Y.; et al. The Genus Phyllanthus: An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evid. Based Complement. Altern. Med. 2016, 2016, 7584952. [Google Scholar] [CrossRef]
- Pechyen, C.; Tangnorawich, B.; Toommee, S.; Marks, R.; Parcharoen, Y. Green synthesis of metal nanoparticles, characterization, and biosensing applications. Sens. Int. 2024, 5, 100287. [Google Scholar] [CrossRef]
- Seth, R.; Meena, A. Enzymes–based nanomaterial synthesis: An eco-friendly and green synthesis approach. Clean Technol. Environ. Policy 2024. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.-L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in Phytonanotechnology: A Plant–Mediated Green Synthesis of Metal Nanoparticles Using Phyllanthus Plant Extracts and Their Antimicrobial and Anticancer Applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef]
- Ungor, D.; Gombár, G.; Juhász, Á.; Samu, G.F.; Csapó, E. Promising Bioactivity of Vitamin B1–Au Nanocluster: Structure, Enhanced Antioxidant Behavior, and Serum Protein Interaction. Antioxidants 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Hao, W.; Kong, L.; Zhao, K.; Bai, H.; Liu, Z. A simple method for the synthesis of copper nanoparticles from metastable intermediates. RSC Adv. 2023, 13, 14361–14369. [Google Scholar] [CrossRef]
- Amini, S.M.; Akbari, A. Metal nanoparticles synthesis through natural phenolic acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Bahari, N.; Hashim, N.; Abdan, K.; Md Akim, A.; Maringgal, B.; Al-Shdifat, L. Role of Honey as a Bifunctional Reducing and Capping/Stabilizing Agent: Application for Silver and Zinc Oxide Nanoparticles. Nanomaterials 2023, 13, 1244. [Google Scholar] [CrossRef] [PubMed]
- Villagrán, Z.; Anaya-Esparza, L.M.; Velázquez-Carriles, C.A.; Silva-Jara, J.M.; Ruvalcaba-Gómez, J.M.; Aurora-Vigo, E.F.; Rodríguez-Lafitte, E.; Rodríguez-Barajas, N.; Balderas-León, I.; Martínez-Esquivias, F. Plant–Based Extracts as Reducing, Capping, and Stabilizing Agents for the Green Synthesis of Inorganic Nanoparticles. Resources 2024, 13, 70. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.-O. Microbe–Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; González-González, R.B.; Pablo Pizaña-Aranda, J.J.; Parra-Arroyo, L.; Rodríguez-Aguayo, A.A.; Iñiguez-Moreno, M.; González-Meza, G.M.; Araújo, R.G.; Ramírez-Gamboa, D.; Parra-Saldívar, R.; et al. Agricultural waste as a sustainable source for nanoparticle synthesis and their antimicrobial properties for food preservation. Front. Nanotechnol. 2024, 6, 1346069. [Google Scholar] [CrossRef]
- Kang, F.; Alvarez, P.J.; Zhu, D. Microbial Extracellular Polymeric Substances Reduce Ag+ to Silver Nanoparticles and Antagonize Bactericidal Activity. Environ. Sci. Technol. 2014, 48, 316–322. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2021, 3, 801620. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Sajjad, A.; Naz, S.; Sajjad, H.; Ao, Q. Significance of Capping Agents of Colloidal Nanoparticles from the Perspective of Drug and Gene Delivery, Bioimaging, and Biosensing: An Insight. Int. J. Mol. Sci. 2022, 23, 10521. [Google Scholar] [CrossRef] [PubMed]
- Chokkareddy, R.; Redhi, G.G. Green Synthesis of Metal Nanoparticles and its Reaction Mechanisms. In Green Metal Nanoparticles: Synthesis, Characterization and Their Applications; Kanchi, S., Ahmed, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 120. [Google Scholar]
- Moreno, A.G.; Guerrero, M.M.L.; Alonso, E.V.; de Torres, A.G.; Pavón, J.M.C. Development of a new FT–IR method for the determination of iron oxide. Optimization of the synthesis of suitable magnetic nanoparticles as sorbent in magnetic solid phase extraction. New J. Chem. 2017, 41, 8804. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Strong Antimicrobial Activity of Silver Nanoparticles Obtained by the Green Synthesis in Viridibacillus sp. Extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Singh, A.K.; Talat, M.; Singh, D.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Xiaoyi, L.; Shaoqin, Z.; Horváth, P.G.; Bak, M.; Bejó, L.; Sipos, G.; Alpár, T. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023—A review on game changing materials. Heliyon 2022, 8, 12322. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S336–S343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ambika, S.; Hassani, A.; Nidheesh, P.V. Waste to catalyst: Role of agricultural waste in water and wastewater treatment. Sci. Total Environ. 2023, 858, 159762. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Fan, S.; Ding, S.; Ren, L. Gold Nanoparticles Based Optical Biosensors for Cancer Biomarker Proteins: A Review of the Current Practices. Front. Bioeng. Biotechnol. 2022, 10, 877193. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Cluster Sci. 2022, 33, 1–16. [Google Scholar] [CrossRef]
- Kumalasari, M.R.; Alfanaar, R.; Andreani, A.S. Gold nanoparticles (AuNPs): A versatile material for biosensor application. Talanta Open 2024, 9, 100327. [Google Scholar] [CrossRef]
- Kakkar, S.; Gupta, P.; Yadav, S.P.S.; Raj, D.; Singh, G.; Chauhan, S.; Mishra, M.K.; Martín-Ortega, E.; Chiussi, S.; Kant, K. Lateral flow assays: Progress and evolution of recent trends in point–of–care applications. Mater. Today Bio 2024, 28, 101188. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Select 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Lusic, K.; Grinstaff, M.W. X–ray–Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Rajeshkumar, R.; Kumar, S.V. Phyto–assisted synthesis, characterization and applications of gold nanoparticles—A review. Biochem. Biophys. Rep. 2017, 11, 46–57. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar]
- Khalil, M.M.H.; Ismail, E.H.; El-Magdoub, F. Biosynthesis of Au nanoparticles using olive leaf extract: 1st Nano Updates. Arab. J. Chem. 2012, 5, 431–437. [Google Scholar] [CrossRef]
- Kumosinski, T.F.; Farrell, H.M. Determination of the global secondary structure of proteins by Fourier transform infrared (FTIR) spectroscopy. Trends Food Sci. Technol. 1993, 4, 169–175. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud Univ. Sci. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Das, R.K.; Borthakur, B.B.; Bora, U. Green synthesis of gold nanoparticles using ethanolic leaf extract of Centella asiatica. Mater. Lett. 2010, 64, 1445–1447. [Google Scholar] [CrossRef]
- Babu, P.J.; Sharma, P.; Saranya, S.; Bora, U. Synthesis of gold nanoparticles using ethonolic leaf extract of Bacopa monnieri and UV irradiation. Mater. Lett. 2013, 93, 431–434. [Google Scholar] [CrossRef]
- Dorosti, N.; Jamshidi, F. Plant–mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: Synthesis, characterization, and evaluation of anticancer and antibacterial activity. J. Appl. Biomed. 2016, 14, 235–245. [Google Scholar] [CrossRef]
- Philip, D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta Part A 2010, 77, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Eisa, W.H. Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity. Spectrochim. Acta Part A 2014, 121, 238–244. [Google Scholar] [CrossRef]
- Barnawi, N.; Allehyani, S.; Seoudi, R. Biosynthesis and characterization of gold nanoparticles and its application in eliminating nickel from water. J. Mater. Res. Technol. 2022, 17, 537–545. [Google Scholar] [CrossRef]
- Deokar, G.K.; Ingale, A.G. Green synthesis of gold nanoparticles (Elixir of Life) from banana fruit waste extract—An efficient multifunctional agent. RSC Adv. 2016, 6, 74620–74629. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.H. Green synthesis of silver and gold nanoparticles and their potential applications as therapeutics in cancer therapy; a review. Inorg. Chem. Commun. 2022, 143, 109610. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Aromal, S.A.; Philip, D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta Part A 2011, 83, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Ramachandran, V.; Mandale, A.B.; Sainkar, S.R.; Rao, M.; Sastry, M. Pepsin−Gold Colloid Conjugates: Preparation, Characterization, and Enzymatic Activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Balaji, D.S.; Basavaraja, S.; Deshpandeb, R.; Maheshb, D.B.; Prabhakara, B.K.; Venkataraman, A. Extracellular Biosynthesis of Functionalized Silver Nanoparticles by Strains of Cladosporium cladosporioides Fungus. Colloids Surf. B 2009, 68, 88–92. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Manzoor, N.; Ahmed, J.; Asiri, A.M. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B 2013, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wan Mat Khalir, W.K.A.; Shameli, K.; Jazayeri, S.D.; Othman, N.A.; Che Jusoh, N.W.; Hassan, N.M. Biosynthesized Silver Nanoparticles by Aqueous Stem Extract of Entada spiralis and Screening of Their Biomedical Activity. Front. Chem. 2020, 8, 620. [Google Scholar] [CrossRef]
- Alam, S.; Aziz, M.A.; Karim, M.R.; Rahman, M.H.; Khatun, M.; Rabbi, M.A.; Habib, M.R. Green biosynthesis of silver nanoparticles using leaf extract of Ammannia baccifera for evaluating their antioxidant, antibacterial and catalytic potentials. J. Mol. Struct. 2025, 1321, 140264. [Google Scholar] [CrossRef]
- Sahu, S.K.; Mansoori, A.; Jana, S.K.; Kumar, A.; Ghorai, T.K. Biosynthesis of silver nanoparticles using green tea aqueous leaf extract and their biological and chemotherapeutic activity. J. Mol. Struct. 2025, 1320, 139690. [Google Scholar] [CrossRef]
- Kayed, K.; Issa, M.; Al-ourabi, H. The FTIR spectra of Ag/Ag2O composites doped with silver nanoparticles. J. Exp. Nanosci. 2024, 19, 2336227. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Asiri, A.M. Fabrication of highly sensitive acetone sensor based on sonochemically prepared as–grown Ag2O nanostructures. Chem. Eng. J. 2012, 192, 122–128. [Google Scholar] [CrossRef]
- Radzikowska-Büchner, E.; Flieger, W.; Pasieczna-Patkowska, S.; Franus, W.; Panek, R.; Korona-Głowniak, I.; Suśniak, K.; Rajtar, B.; Świątek, Ł.; Żuk, N.; et al. Antimicrobial and Apoptotic Efficacy of Plant–Mediated Silver Nanoparticles. Molecules 2023, 28, 5519. [Google Scholar] [CrossRef] [PubMed]
- Madaniyah, L.; Fiddaroini, S.; Hayati, E.K.; Rahman, M.F.; Sabarudin, A. Biosynthesis, characterization, and in–vitro anticancer effect of plant–mediated silver nanoparticles using Acalypha indica Linn: In–silico approach. OpenNano 2025, 21, 100220. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle–Specific Antibacterial Activity of Silver Nanoparticles. Nano. Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Shape–dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Application of Platinum Nanoparticles Decorating Mesoporous Carbon Derived from Sustainable Source for Hydrogen Evolution Reaction. Catalysts 2024, 14, 423. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Krishnasamy, N.; Ramadoss, R.; Vemuri, S.; Sujai, G.V.N.S. Optimizing Desmostachya bipinnata–derived platinum nanoparticles for enhanced antibacterial and biofilm reduction. Microb. Pathogen. 2024, 196, 107004. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Marvi, P.K.; Ahmed, S.R.; Das, P.; Ghosh, R.; Srinivasan, S.; Rajabzadeh, A.R. Prunella vulgaris–phytosynthesized platinum nanoparticles: Insights into nanozymatic activity for H2O2 and glutamate detection and antioxidant capacity. Talanta 2024, 274, 125998. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M.; El–Fakharany, E.M.; Omer, A.M.; Abd El-Monaem, E.M.; Khalifa, R.E.; Eltaweil, A.S. Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J. Environ. Chem. Eng. 2022, 10, 106806. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess. Biosyst. Eng. 2010, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Castro-Longoria, E.; Moreno-Velasquez, S.D.; Vilchis-Nestor, A.R.; Arenas-Berumen, E.; Avalos-Borja, M. Production of Platinum Nanoparticles and Nanoaggregates Using Neurospora Crassa. J. Microbiol. Biotechnol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Chundawat, T.S. Bio–inspired synthesis of platinum nanoparticles from fungus Fusarium oxysporum: Its characteristics, potential antimicrobial, antioxidant and photocatalytic activities. Mater. Res. Exp. 2019, 6, 1050d6. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Sotoodehnejadnematalahi, F.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, A.E.M. Platinum Nanoparticles as Potent Anticancer and Antimicrobial Agent: Green Synthesis, Physical Characterization, and In–Vitro Biological Activity. J. Cluster Sci. 2023, 34, 501–516. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Christy, A.N. Green synthesis of platinum nanoparticles using Azadirachta indica—An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Kholief, M.G.; Hesham, A.E.-L.; Hashem, F.S.; Mohamed, F.M. Synthesis and utilization of titanium dioxide nano particle (TiO2NPs) for photocatalytic degradation of organics. Sci. Rep. 2024, 14, 11327. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, Y.; Aguirre, M.E.; Brusa, M.A.; Grela, M.A. Surface Chemistry Determines Electron Storage Capabilities in Alcoholic Sols of Titanium Dioxide Nanoparticles. A Combined FTIR and Room Temperature EPR Investigation. J. Phys. Chem. C 2012, 116, 9646–9652. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Ryczkowski, J. Spectroscopic studies of alumina–supported nickel catalysts precursors: Part I. Catalysts prepared from acidic solutions. Appl. Surf. Sci. 2007, 253, 5910–5913. [Google Scholar] [CrossRef]
- Kamamori, O.; Yamaguchi, N.; Sato, K. Infrared absorption spectra of metal oxides. Bunseki Kakagu 1967, 16, 1050–1055. [Google Scholar] [CrossRef][Green Version]
- Naik, T.S.S.K.; Singh, S.; Narasimhappa, P.; Varshney, R.; Singh, J.; Khan, N.A.; Zahmatkesh, S.; Ramamurthy, P.C.; Shehata, N.; Kiran, G.N.; et al. Green and sustainable synthesis of CaO nanoparticles: Its solicitation as a sensor material and electrochemical detection of urea. Sci. Rep. 2023, 13, 19995. [Google Scholar] [CrossRef]
- Saravan, R.S.; Muthukumaran, M.; Mubashera, S.; Abinaya, M.; Prasath, P.V.; Parthiban, R.; Mohammad, F.; Oh, W.C.; Sagadevan, S. Evaluation of the photocatalytic efficiency of cobalt oxide nanoparticles towards the degradation of crystal violet and methylene violet dyes. Optik 2020, 207, 164428. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Mathimani, T.; Santhanam, N.; Phuong, T.N.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of cobalt–oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo–catalytic activity of acid Blue–74. J. Photochem. Photobiol. B 2020, 211, 112011. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ghosh, S.; Ghosh, R.; Dam, S.; Baskey, M. Madhuca longifolia plant mediated green synthesis of cupric oxide nanoparticles: A promising environmentally sustainable material for waste water treatment and efficient antibacterial agent. J. Photochem. Photobiol. B 2018, 189, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B 2019, 192, 74–82. [Google Scholar] [CrossRef]
- Maji, B.A.S.K.; Mukherjee, N.; Mondal, A. Synthesis, characterization and photocatalytic activity of α–Fe2O3 nanoparticles. Polyhedron 2012, 33, 145–149. [Google Scholar] [CrossRef]
- Hwang, S.W.; Umar, A.; Dar, G.N.; Kim, S.H.; Badran, R.I. Synthesis and Characterization of Iron Oxide Nanoparticles for Phenyl Hydrazine Sensor Applications. Sens. Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Flieger, J.; Pasieczna-Patkowska, S.; Żuk, N.; Panek, R.; Korona-Głowniak, I.; Suśniak, K.; Pizoń, M.; Franus, W. Characteristics and Antimicrobial Activities of Iron Oxide Nanoparticles Obtained via Mixed–Mode Chemical/Biogenic Synthesis Using Spent Hop (Humulus lupulus L.) Extracts. Antibiotics 2024, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, Z.; Rangrazi, A.; Amiri, M.S.; Khatami, M.; Darroudi, M. Green synthesis of nickel oxide nanoparticles using Salvia hispanica L. (chia) seeds extract and studies of their photocatalytic activity and cytotoxicity effects. Bioprocess. Biosyst. Eng. 2021, 44, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Luque, P.A.; Chinchillas-Chinchillas, M.J.; Nava, O.; Lugo-Medina, E.; Martínez-Rosas, M.E.; Carrillo-Castillo, A.; Vilchis-Nestor, A.R.; Madrigal-Muñoz, L.E.; Garrafa-Gálvez, H.E. Green synthesis of tin dioxide nanoparticles using Camellia sinensis and its application in photocatalytic degradation of textile dyes. Optik 2021, 229, 166259. [Google Scholar] [CrossRef]
- Dobrucka, R. Synthesis of Titanium Dioxide Nanoparticles Using Echinacea purpurea Herba. Iran. J. Pharm. Res. 2017, 16, 756–762. [Google Scholar]
- El-Sherbiny, S.; Morsy, F.; Samir, M.; Fuad, O.A. Synthesis, characterization and application of TiO2 nanopowders as special paper coating pigment. Appl. Nanosci. 2014, 4, 305–313. [Google Scholar] [CrossRef]
- Ansari, F.S.; Daneshjou, S. Optimizing the green synthesis of antibacterial TiO2—Anatase phase nanoparticles derived from spinach leaf extract. Sci. Rep. 2024, 14, 22440. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [PubMed]
- Uysal, Y.; Doğaroğlu, Z.G.; Çaylali, Z.; Karakulak, D.S. Rosemary–Mediated Green Synthesis of ZnO Nanoparticles and their Integration into Hydrogel Matrices: Evaluating Effects on Wheat Growth and Antibacterial Properties. Glob. Chall. 2024, 8, 2400120. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Abu Bakar, N.H.H.; Abu Bakar, M. Comparative study of chemically synthesized and low temperature bio-inspired Musa acuminata peel extract mediated zinc oxide nanoparticles for enhanced visible–photocatalytic degradation of organic contaminants in wastewater treatment. J. Hazard. Mater. 2021, 406, 124779. [Google Scholar] [CrossRef] [PubMed]
- Alamu, G.A.; Ayanlola, P.S.; Babalola, K.K.; Adedokun, O.; Sanusi, Y.K.; Fajinmi, G.R. Green synthesis and characterizations of magnetic iron oxide nanoparticles using Moringa oleifera extract for improved performance in dye–sensitized solar cell. Chem. Phys. Impact 2024, 8, 100542. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczyńska-Goślinska, B.; Młynarczyk, D.T.; Głowacka-Sobotta, A.; Stanisz, B.; Goślinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Srinivasan, M.; Venkatesan, M.; Arumugam, V.; Natesan, G.; Saravanan, N.; Murugesan, S.; Ramachandran, S.; Ayyasamy, R.; Pugazhendhi, A. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019, 80, 197–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Gaur, J.; Kumar, S.; Pal, M.; Kaur, H.; Batoo, K.M.; Momoh, J.O.; Supreet. Current trends: Zinc oxide nanoparticles preparation via chemical and green method for the photocatalytic degradation of various organic dyes. Hybrid Adv. 2024, 5, 100128. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 5, 1062562. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.F.; Islam, S.; Miah, M.A.S.; Huq, A.K.O.; Saha, A.K.; Mou, Z.J.; Mondol, M.M.H.; Bhuiyan, M.N.I. Green synthesis of zinc oxide nano particles using Allium cepa L. waste peel extracts and its antioxidant and antibacterial activities. Heliyon 2024, 10, e25430. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ye, Y.; Xu, L.; Gao, T.; Zhong, A.; Song, Z. Recent Advances in Nanoscale Zero–Valent Iron (nZVI)–Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects. Nanomaterials 2023, 13, 2830. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, S.; González-Rodríguez, J.; Fernández, L.; Vázquez-Vázquez, C.; Feijoo, G.; Moreira, M.T. Fenton and Photo–Fenton Nanocatalysts Revisited from the Perspective of Life Cycle Assessment. Catalysts 2020, 10, 23. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Wang, J.L. Fenton/Fenton–like processes with in–situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Samadi, Z.; Yaghmaeian, K.; Mortazavi-Derazkola, S.; Khosravi, R.; Nabizadeh, R.; Alimohammadi, M. Facile green synthesis of zero–valent iron nanoparticles using barberry leaf extract (GnZVI@BLE) for photocatalytic reduction of hexavalent chromium. Bioorg. Chem. 2021, 114, 105051. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, A.M.; Fawzy, M.; Eltaweil, A.S.; El-Khouly, M.E. Green Synthesis of Nano–Zero–Valent Iron Using Ricinus Communis Seeds Extract: Characterization and Application in the Treatment of Methylene Blue–Polluted Water. ACS Omega 2021, 6, 25397–25411. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero–Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Komárek, M. Perspectives of soil nanoremediation: The case of nano zerovalent iron and metal(loid) contaminants. NPJ Mater. Sustain. 2024, 2, 8. [Google Scholar] [CrossRef]
- Namakka, M.; Rahman, M.R.; Said, K.A.B.M.; Muhammad, A. Insights into micro–and nano–zero valent iron materials: Synthesis methods and multifaceted applications. RSC Adv. 2024, 14, 30411–30439. [Google Scholar] [CrossRef]
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron–based nanoparticles by green tea extract and their degradation of malachite. Ind. Crops Prod. 2013, 51, 342–347. [Google Scholar] [CrossRef]
- Pan, Z.; Lin, Y.; Sarkar, B.; Owens, G.; Chen, Z. Green synthesis of iron nanoparticles using red peanut skin extract: Synthesis mechanism, characterization and effect of conditions on chromium removal. J. Colloid Interface Sci. 2020, 558, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rasero, C.; Montes-Jimenez, V.; Alexandre-Franco, M.F.; Fernández-González, C.; Píriz-Tercero, J.; Cuerda-Correa, E.M. Use of Zero–Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review. Water 2024, 16, 1607. [Google Scholar] [CrossRef]
- Hussain, A.; Yasar, M.; Ahmad, G.; Ijaz, M.; Aziz, A.; Nawaz, M.G.; Khan, F.A.; Iqbal, H.; Shakeel, W.; Momand, H.; et al. Synthesis, characterization, and applications of iron oxide nanoparticles. Int. J. Health Sci. 2023, 17, 3–10. [Google Scholar]
- Mohamed, A.; Atta, R.R.; Kotp, A.A.; Abo El-Ela, F.I.; El-Raheem, H.A.; Farghali, A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mahmoud, R. Green synthesis and characterization of iron oxide nanoparticles for the removal of heavy metals (Cd2+ and Ni2+) from aqueous solutions with Antimicrobial Investigation. Sci. Rep. 2023, 13, 7227. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.T.; Zhou, L. Extraction techniques for selective dissolution of amorphous iron oxides from soils and sediments. Soil Sci. Soc. Am. 1983, 47, 225–232. [Google Scholar] [CrossRef]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well–defined α–Fe2O3 particles. Colloids Surf. A 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Eddine, L.S.; Abderrhmane, B.; Alonso-Gonzalez, M.; Guerrero, A.; Romero, A. Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 2020, 17, 100280. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).