Synthesis of Amorphous MnFe@SBA Composites for Efficient Adsorptive Removal of Pb(Ⅱ) and Sb(V) from Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
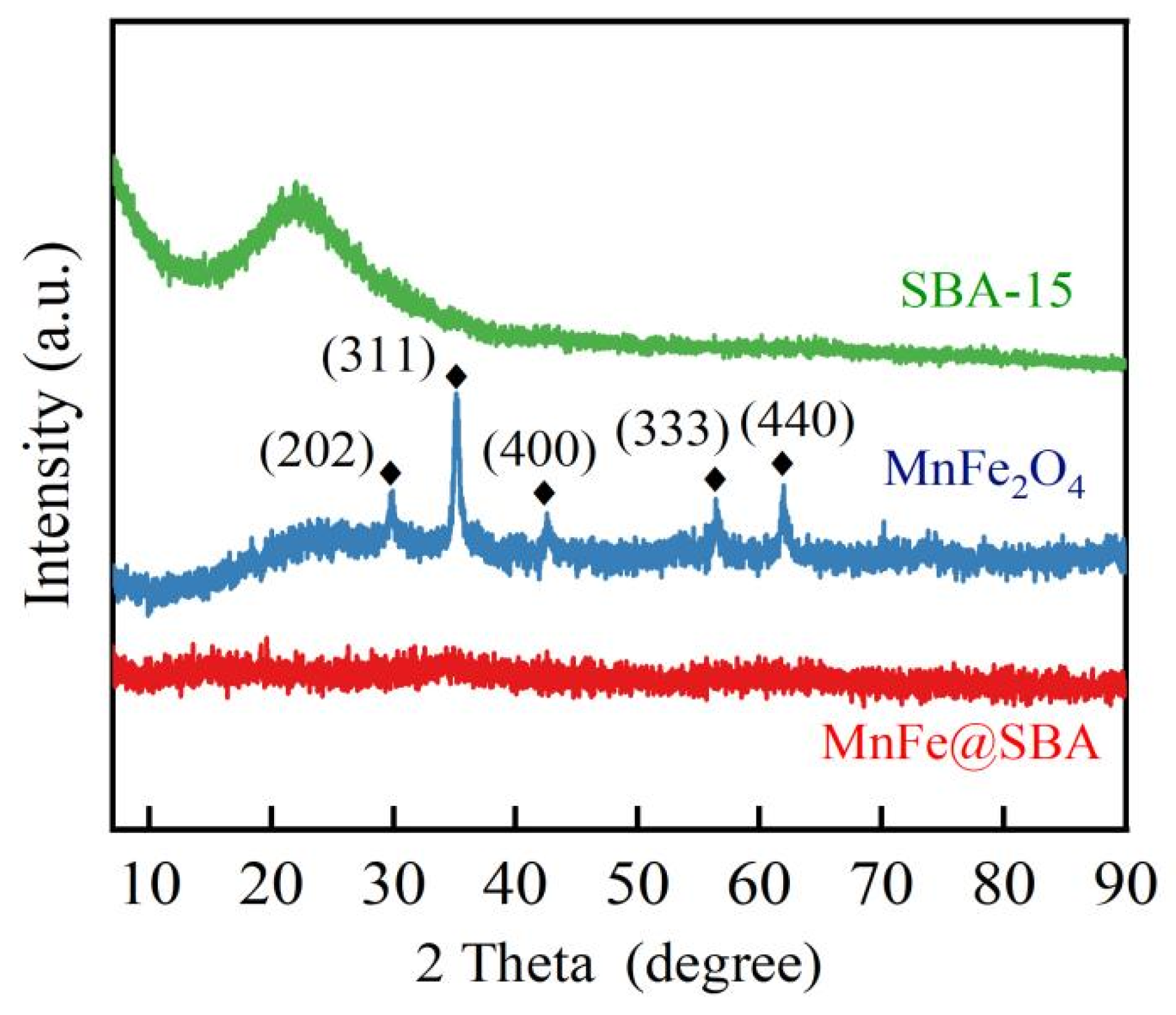
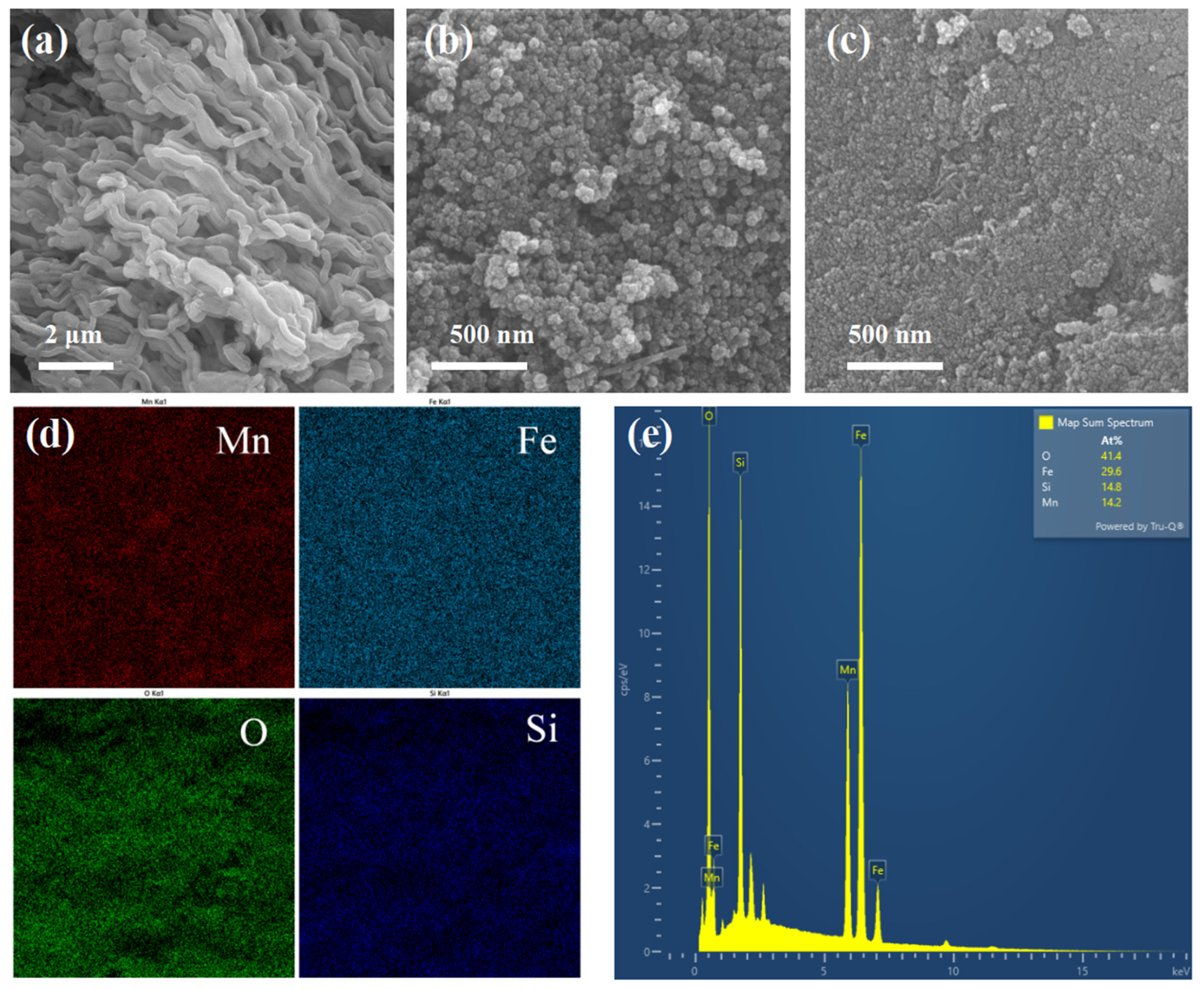
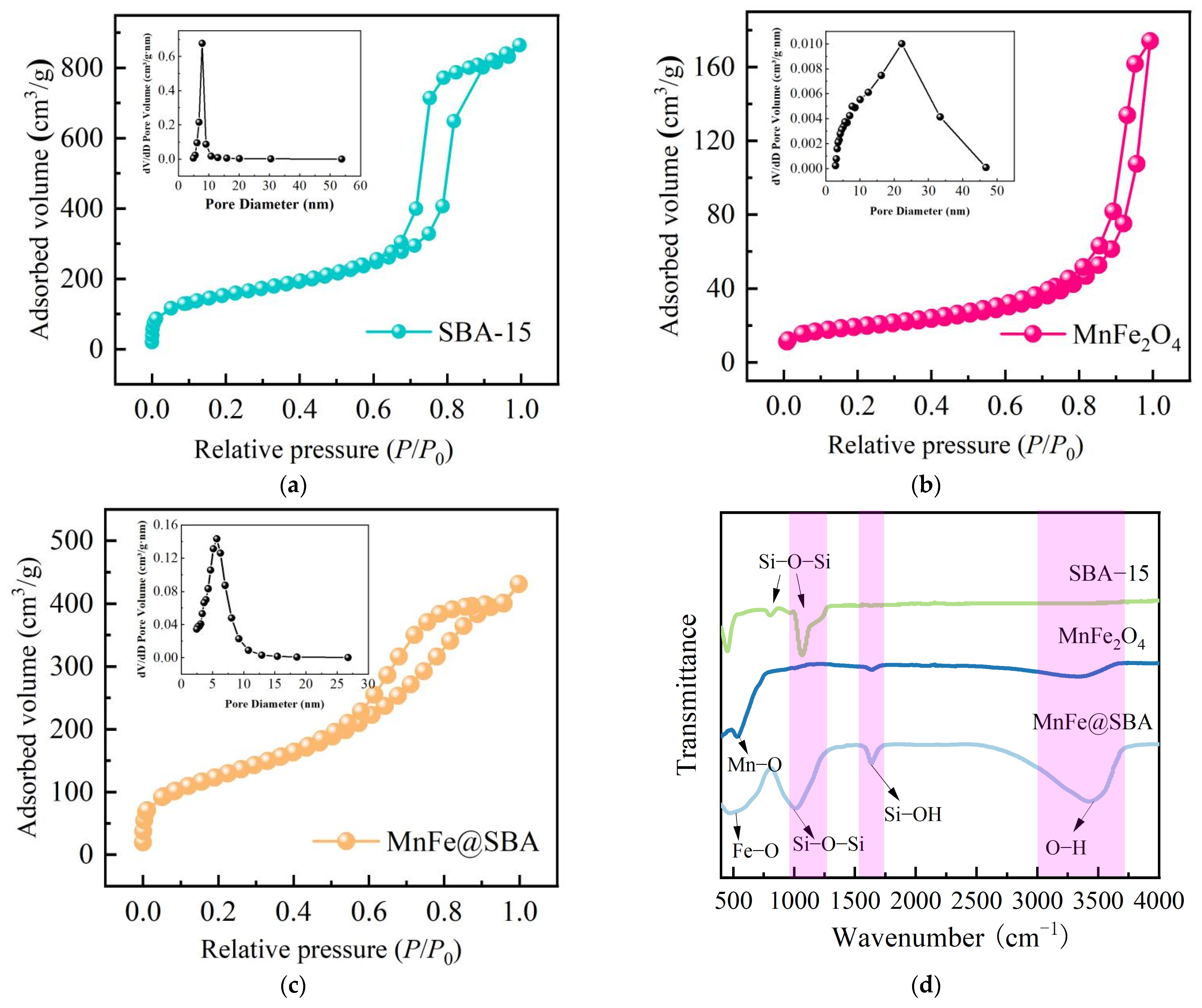
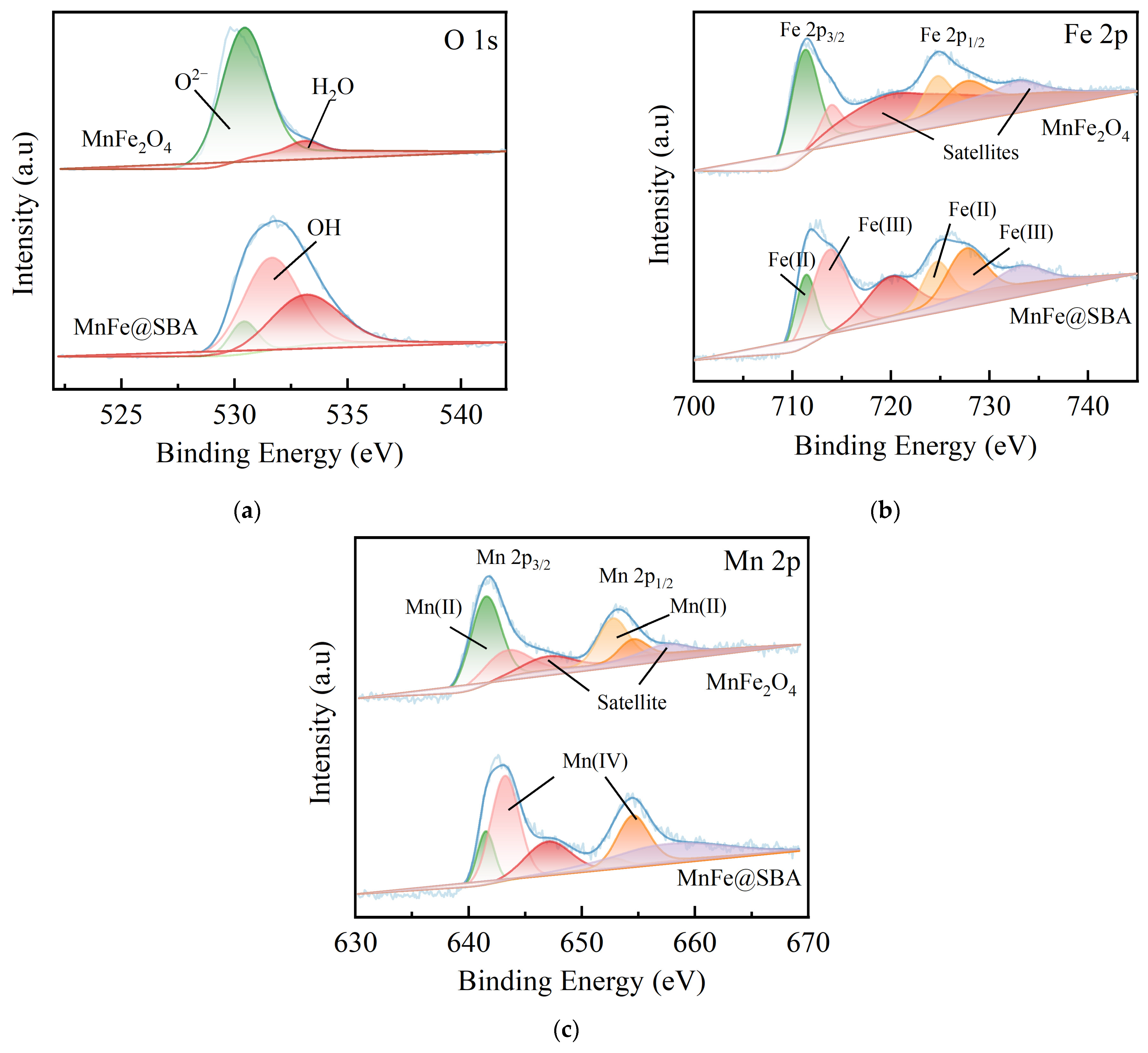
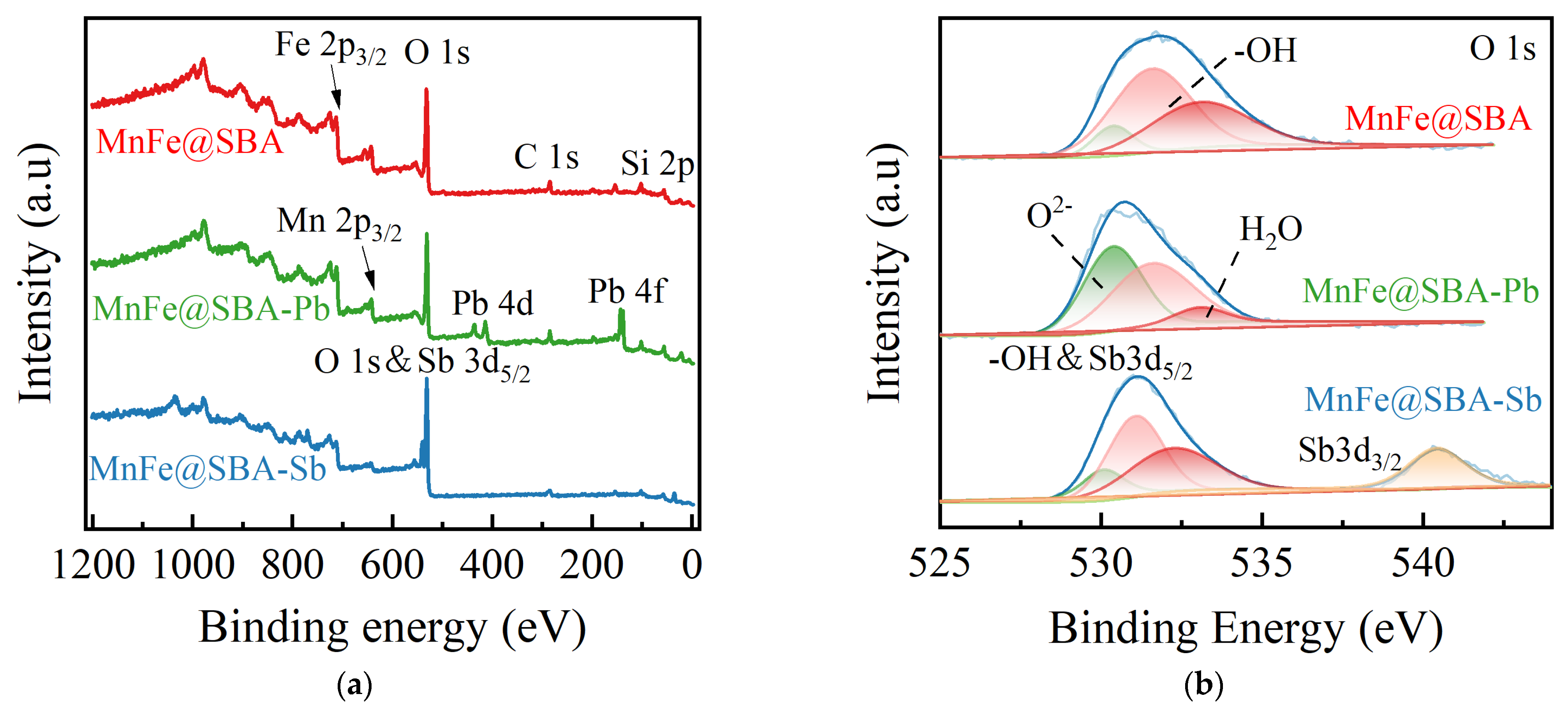
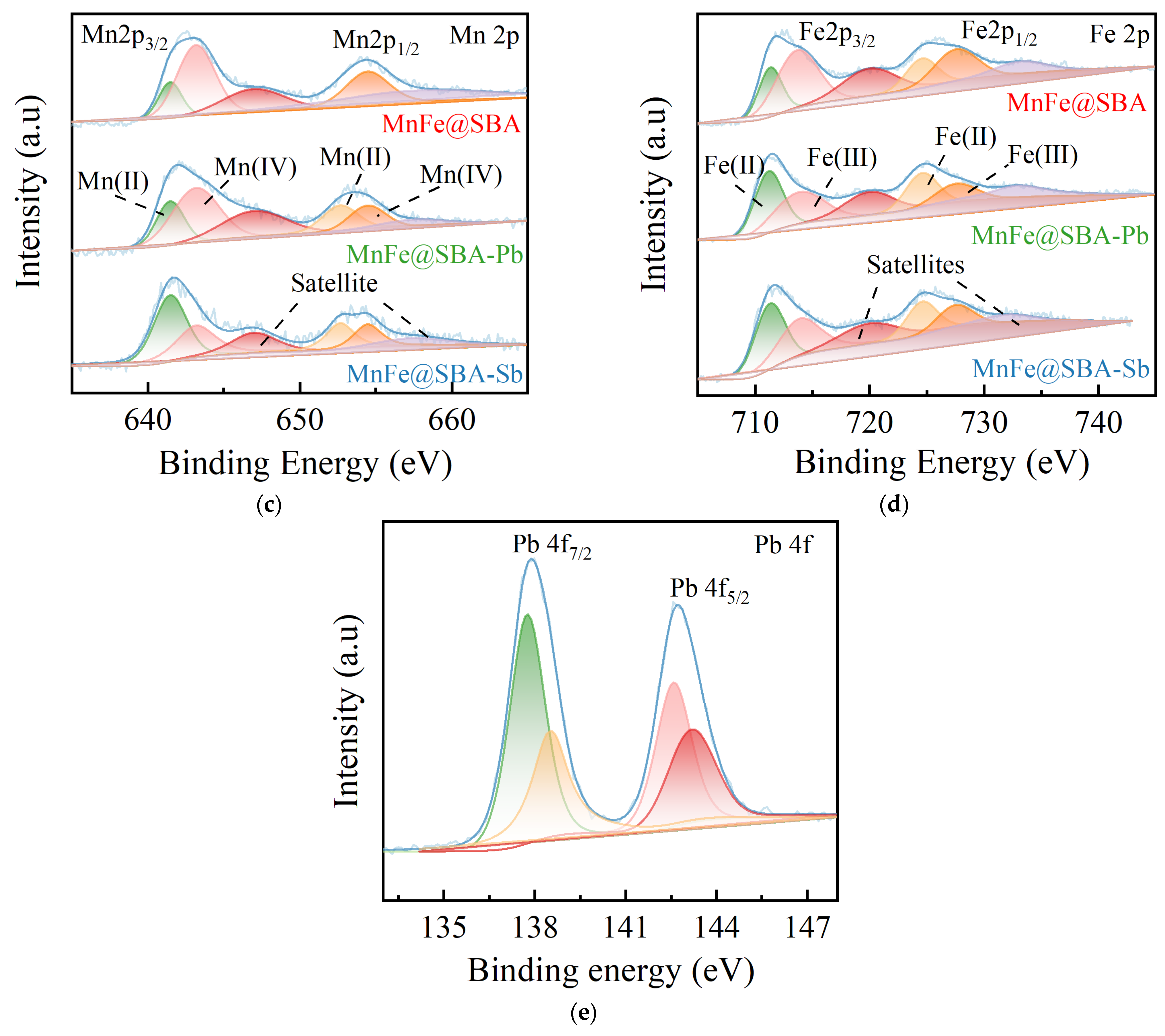
2.1. Characterizations
2.2. Adsorption Performance
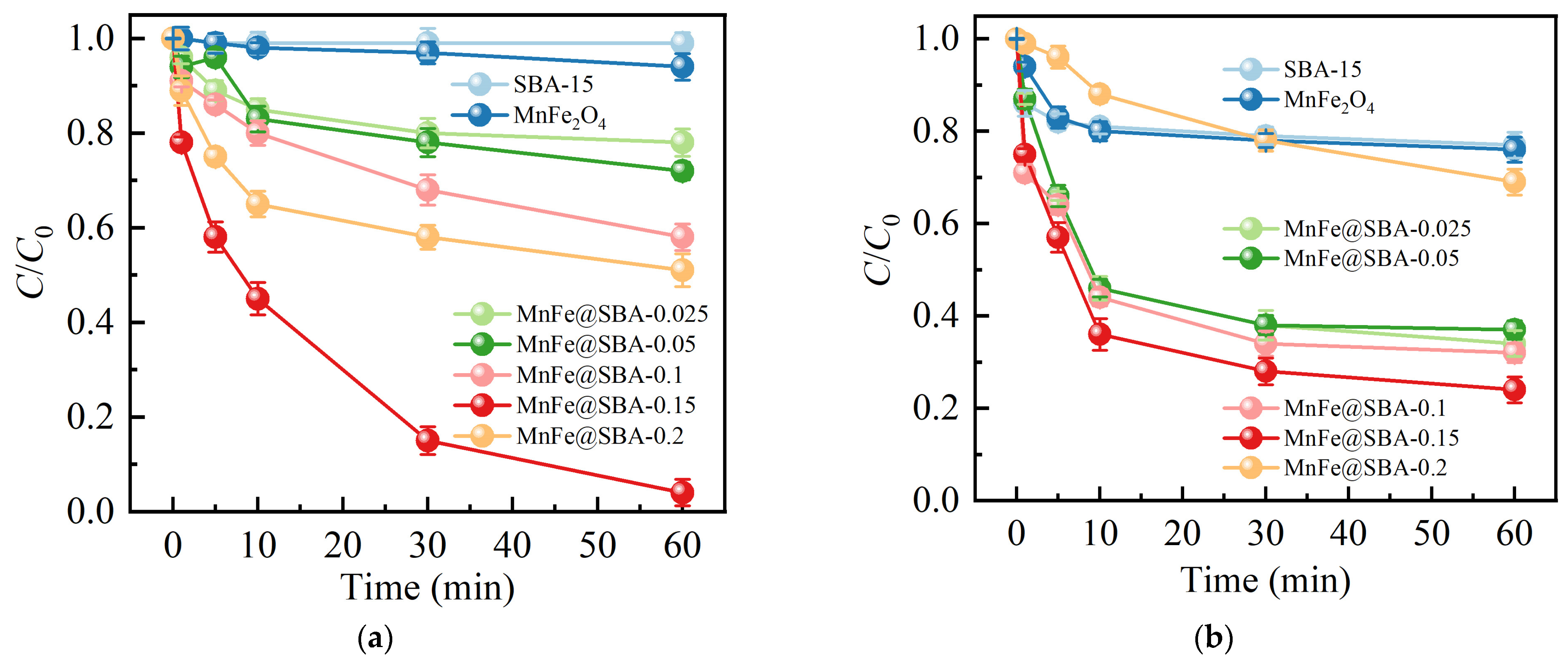
2.2.1. Effect of Doping Amount of SBA-15
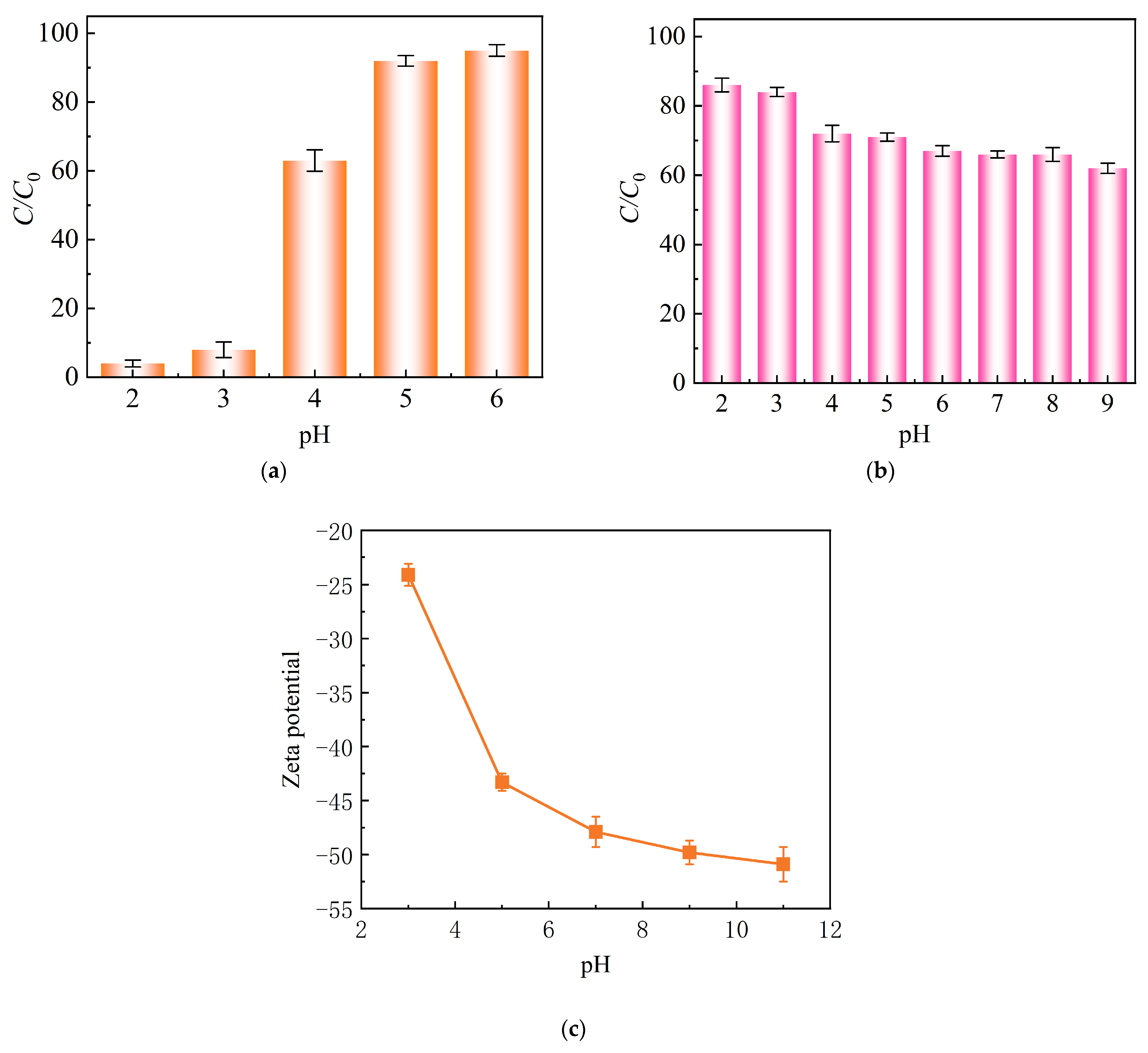
2.2.2. Effect of Solution pH and Coexisting Cations
2.2.3. Adsorption Kinetics
2.2.4. Adsorption Isotherms
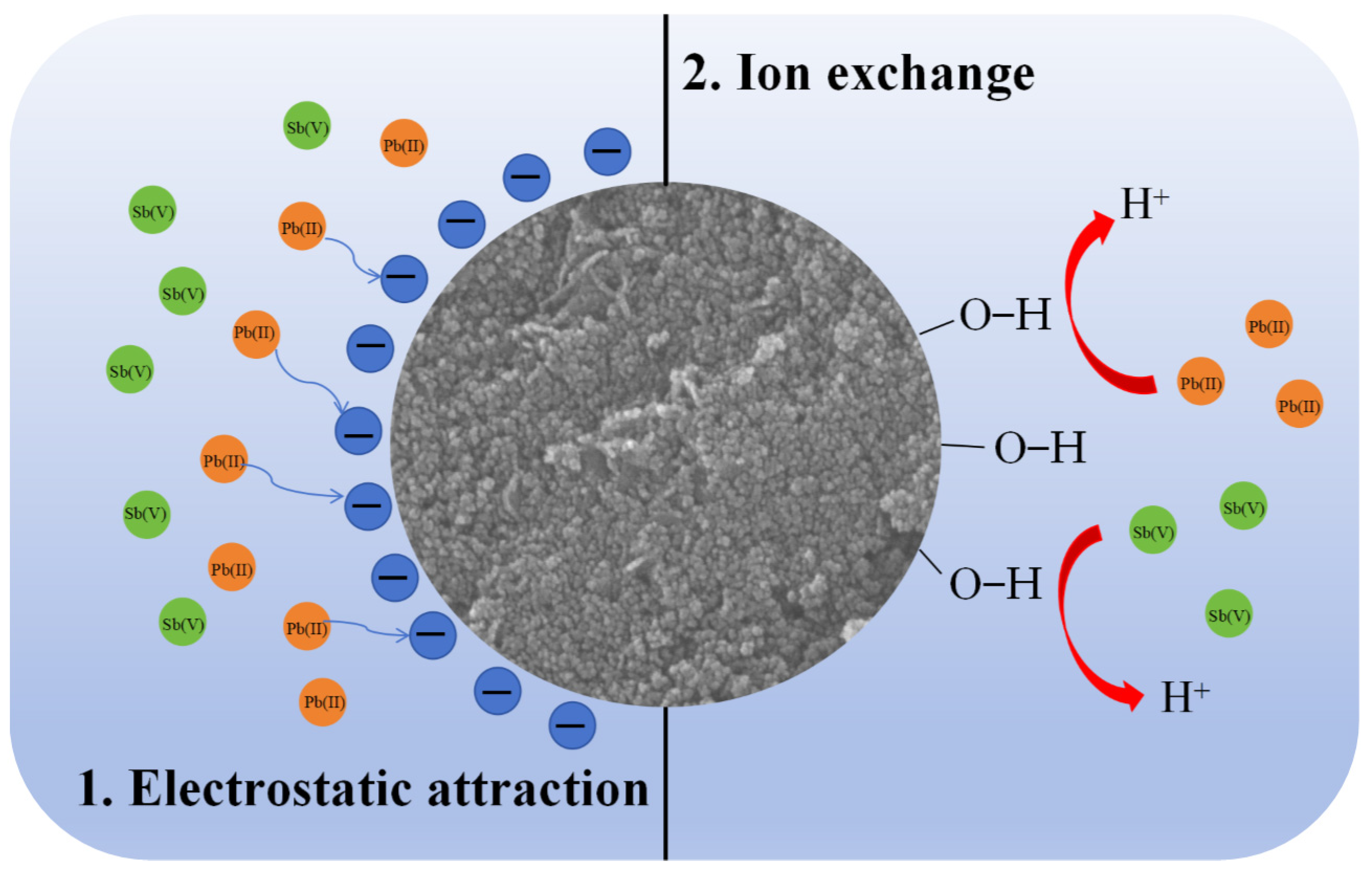
2.2.5. Adsorption Mechanisms Investigation
3. Chemicals and Methods
3.1. Chemicals
3.2. Synthesis of MnFe@SBA
3.3. Characterizations
3.4. Batch Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saif, S.; Zaidi, A.; Khan, M.S.; Rizvi, A. Metal-Legume-Microbe Interactions: Toxicity and Remediation. In Microbes for Legume Improvement; Zaidi, A., Khan, M.S., Musarrat, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 367–385. [Google Scholar]
- Cao, Y.; Hu, X.; Zhu, C.; Zhou, S.; Li, R.; Shi, H.; Miao, S.; Vakili, M.; Wang, W.; Qi, D. Sulfhydryl functionalized covalent organic framework as an efficient adsorbent for selective Pb (II) removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 125004. [Google Scholar] [CrossRef]
- Wajima, T. Preparation of Adsorbent with Lead Removal Ability Using Sulfur-Impregnation. Adv. Mater. Res. 2013, 831, 253–257. [Google Scholar] [CrossRef]
- Hasan, R.; Chong, C.C.; Bukhari, S.N.; Jusoh, R.; Setiabudi, H.D. Effective removal of Pb(II) by low-cost fibrous silica KCC-1 synthesized from silica-rich rice husk ash. J. Ind. Eng. Chem. 2019, 75, 262–270. [Google Scholar] [CrossRef]
- Shukla, V.; Shukla, P.; Tiwari, A. Lead poisoning. Indian J. Med. Spec. 2018, 9, 146–149. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Zhang, Y.; Wang, D.; Shen, M.; Hu, D. Iron-modified rice husk hydrochar and its immobilization effect for Pb and Sb in contaminated soil. J. Hazard. Mater. 2020, 398, 122977. [Google Scholar] [CrossRef]
- Feng, X.; Yan, R.; Zhang, Q.; Wan, Q.; Hagio, T.; Ichino, R.; Kong, L.; Cao, X.; Li, L. Nano ferric oxide adsorbents with self-acidification effect for efficient adsorption of Sb(V). Chemosphere 2021, 272, 129933. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wu, F.C.; Yu, F.; Bai, Y.C.; Fu, Z.Y.; Zhu, Y.R.; Guo, W.J. Fate and removal of antimony in response to stringent control activities after a mine tailing spill. Sci. Total Environ. 2019, 693, 133604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ouyang, S.; Li, P.; Sun, Z.; Ding, N.; Huang, Y. Ultrahigh removal performance of lead from wastewater by tricalcium aluminate via precipitation combining flocculation with amorphous aluminum. J. Clean. Prod. 2020, 246, 118728. [Google Scholar] [CrossRef]
- Choumane, R.; Peulon, S. Development of an efficient electrochemical process for removing and separating soluble Pb(II) in aqueous solutions in presence of other heavy metals: Studies of key parameters. Chem. Eng. J. 2021, 423, 130161. [Google Scholar] [CrossRef]
- Rong, Y.; Yan, W.; Wang, Z.; Hao, X.; Guan, G. Rapid and selective removal of Pb ions by electroactive titanium Dioxide/Polyaniline ion exchange film. Sep. Purif. Technol. 2023, 312, 123386. [Google Scholar] [CrossRef]
- Xu, Z.; Gu, S.; Rana, D.; Matsuura, T.; Lan, C.Q. Chemical precipitation enabled UF and MF filtration for lead removal. J. Water Process Eng. 2021, 41, 101987. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, C.; Ying, L.; Wang, W.; Wang, S.; Ding, J.; Lu, J. A post-functional Ti-based MOFs composite for selective removal of Pb (II) from water. J. Hazard. Mater. 2022, 432, 128700. [Google Scholar] [CrossRef]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.-S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Kumar, R.; Bhattacharya, S.; Sharma, P. Novel insights into adsorption of heavy metal ions using magnetic graphene composites. J. Environ. Chem. Eng. 2021, 9, 106212. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef]
- Abdel-Magied, A.F.; Abdelhamid, H.N.; Ashour, R.M.; Fu, L.; Dowaidar, M.; Xia, W.; Forsberg, K. Magnetic metal-organic frameworks for efficient removal of cadmium(II), and lead(II) from aqueous solution. J. Environ. Chem. Eng. 2022, 10, 107467. [Google Scholar] [CrossRef]
- Abdulkareem, A.S.; Hamzat, W.A.; Tijani, J.O.; Egbosiuba, T.C.; Mustapha, S.; Abubakre, O.K.; Okafor, B.O.; Babayemi, A.K. Isotherm, kinetics, thermodynamics and mechanism of metal ions adsorption from electroplating wastewater using treated and functionalized carbon nanotubes. J. Environ. Chem. Eng. 2023, 11, 109180. [Google Scholar] [CrossRef]
- Eren, Z.S.; Tunçer, S.; Gezer, G.; Yildirim, L.T.; Banerjee, S.; Yilmaz, A. Improved solubility of celecoxib by inclusion in SBA-15 mesoporous silica: Drug loading in different solvents and release. Microporous Mesoporous Mater. 2016, 235, 211–223. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Sun, Q.; Xu, W.Q.; Han, Y. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method. Microporous Mesoporous Mater. 2017, 252, 105–115. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, N.; Cheong, L.Z.; Huang, J.; Chen, H.; Lin, S. Immobilization of Candida antarctica Lipase B onto organically-modified SBA-15 for efficient production of soybean-based mono and diacylglycerols. Int. J. Biol. Macromol. 2018, 120 Pt A, 886–895. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Esmailzadeh, F.; Asgharnasl, S.; Ganjali, F.; Taheri-Ledari, R.; Maleki, A. Efficient removal of Pb(II)/Cu(II) from aqueous samples by a guanidine-functionalized SBA-15/Fe3O4. Sep. Purif. Technol. 2022, 291, 120956. [Google Scholar] [CrossRef]
- Xing, X.; Ren, X.; Alharbi, N.S.; Chen, C. Efficient adsorption and reduction of Cr(VI) from aqueous solution by Santa Barbara Amorphous-15 (SBA-15) supported Fe/Ni bimetallic nanoparticles. J. Colloid. Interface Sci. 2023, 629 Pt A, 744–754. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, T.; Zhang, M.; Hao, L.; Phoutthavong, T.; Liang, P. Cu-Zn oxides nanoparticles supported on SBA-15 zeolite as a novel adsorbent for simultaneous removal of H2S and Hg0 in natural gas. Chem. Eng. J. 2021, 426, 131286. [Google Scholar] [CrossRef]
- Yao, J.; Deng, Y.; Pan, S.; Korna, R.; Wen, J.; Yuan, N.; Wang, K.; Li, H.; Yang, Y. The difference in the adsorption mechanisms of magnetic ferrites modified carbon nanotubes. J. Hazard. Mater. 2021, 415, 125551. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, H.; Wan, B.; Zhang, C.; Yao, S. Effective removal of Cd(II) from aqueous solution by MnFe2O4 composite modified by surfactant. Desalination Water Treat. 2022, 262, 168–179. [Google Scholar] [CrossRef]
- Liu, J.; Ren, S.; Cao, J.; Tsang, D.C.W.; Beiyuan, J.; Peng, Y.; Fang, F.; She, J.; Yin, M.; Shen, N.; et al. Highly efficient removal of thallium in wastewater by MnFe(2)O(4)-biochar composite. J. Hazard. Mater. 2021, 401, 123311. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, N.; Feng, J.; Luan, T.; Wen, Q.; Li, Z.; Zhang, M. Adsorption of Pb(II) and Cu(II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J. Colloid Interface Sci. 2012, 367, 415–421. [Google Scholar] [CrossRef]
- Verma, M.; Kumar, A.; Singh, K.P.; Kumar, R.; Kumar, V.; Srivastava, C.M.; Rawat, V.; Rao, G.; Kumari, S.; Sharma, P.; et al. Graphene oxide-manganese ferrite (GO-MnFe2O4) nanocomposite: One-pot hydrothermal synthesis and its use for adsorptive removal of Pb2+ ions from aqueous medium. J. Mol. Liq. 2020, 315, 113769. [Google Scholar] [CrossRef]
- Yang, K.; Li, C.; Wang, X.; Liu, Y.; Li, Y.; Zhou, C.; Wang, Z.; Zhou, J.; Cao, Z.; Xu, X. Functional adjustment and cyclic application of Fe–Mn bimetal composite for Sb(V) removal: Transformation of iron oxides forms and a stable regeneration method. J. Clean. Prod. 2020, 266, 122007. [Google Scholar] [CrossRef]
- Ding, W.; Zheng, H.; Sun, Y.; Zhao, Z.; Zheng, X.; Wu, Y.; Xiao, W. Activation of MnFe(2)O(4) by sulfite for fast and efficient removal of arsenic(III) at circumneutral pH: Involvement of Mn(III). J. Hazard. Mater. 2021, 403, 123623. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Q.; Gao, J.; Guo, Y.; Cheng, F. The selective adsorption of rare earth elements by modified coal fly ash based SBA-15. Chin. J. Chem. Eng. 2022, 47, 155–164. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, F.; Tang, B. Ethylenediamine-functionalized MnFe2O4 @ferrihydrite as a magnetic adsorbent for removal of Cr(VI): Adsorption and mechanism studies. J. Environ. Chem. Eng. 2023, 11, 110230. [Google Scholar] [CrossRef]
- Betiha, M.A.; Moustafa, Y.M.; El-Shahat, M.F.; Rafik, E. Polyvinylpyrrolidone-Aminopropyl-SBA-15 schiff Base hybrid for efficient removal of divalent heavy metal cations from wastewater. J. Hazard. Mater. 2020, 397, 122675. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xi, J.; Lu, J.; Zhang, Y.; Cheng, G.; Zhang, Y.; Chen, R. Porous biochar-supported MnFe(2)O(4) magnetic nanocomposite as an excellent adsorbent for simultaneous and effective removal of organic/inorganic arsenic from water. J. Hazard. Mater. 2021, 411, 124909. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Yin, W.; Cao, Y.; Hou, J.; Wang, S.; Wang, X. Solvent-induced facile synthesis of MnFe2O4 and the As(V) removal mechanism study. J. Mol. Liq. 2023, 371, 120845. [Google Scholar] [CrossRef]
- Bai, M.; Chai, Y.; Chen, A.; Yuan, J.; Shang, C.; Peng, L.; Peng, C. Enhancing cadmium removal efficiency through spinel ferrites modified biochar derived from agricultural waste straw. J. Environ. Chem. Eng. 2023, 11, 109027. [Google Scholar] [CrossRef]
- Huang, W.-H.; Wu, R.-M.; Chang, J.-S.; Juang, S.-Y.; Lee, D.-J. Manganese ferrite modified agricultural waste-derived biochars for copper ions adsorption. Bioresour. Technol. 2023, 367, 128303. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, H.; Zeng, T.; Chen, J.; Song, S. Synergistically enhanced heterogeneous activation of persulfate for aqueous carbamazepine degradation using Fe3O4@SBA-15. Sci. Total Environ. 2021, 760, 144027. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Deng, L.; Liu, X.; Yang, L.; Yang, X.; Shi, Z.; Pei, Y. Fabrication of magnetic nickel incorporated carbon nanofibers for superfast adsorption of sulfadiazine: Performance and mechanisms exploration. J. Hazard. Mater. 2022, 423 Pt B, 127219. [Google Scholar] [CrossRef]
- Kavand, M.; Eslami, P.; Razeh, L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Z.; Li, H.; Wu, M.; Zhao, Q.; Pan, B. An electron-scale comparative study on the adsorption of six divalent heavy metal cations on MnFe2O4@CAC hybrid: Experimental and DFT investigations. Chem. Eng. J. 2020, 381, 122656. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Latifi, P.; Ahmadi, A.; Alizadeh, M.; Ramavandi, B. Carbon nanotubes/β-cyclodextrin/MnFe2O4 as a magnetic nanocomposite powder for tetracycline antibiotic decontamination from different aqueous environments. J. Environ. Chem. Eng. 2021, 9, 106344. [Google Scholar] [CrossRef]
- Huang, X.; Kong, L.; Huang, S.; Liu, M.; Li, L. Synthesis of novel magnetic sulfur-doped Fe3O4 nanoparticles for efficient removal of Pb(II). Sci. China Chem. 2017, 61, 164–171. [Google Scholar] [CrossRef]
- Medina, R.P.; Nadres, E.T.; Ballesteros, F.C.; Rodrigues, D.F. Incorporation of graphene oxide into a chitosan–poly(acrylic acid) porous polymer nanocomposite for enhanced lead adsorption. Environ. Sci. Nano 2016, 3, 638–646. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Wang, S.; Zhang, L. Ninhydrin-functionalized chitosan for selective removal of Pb(II) ions: Characterization and adsorption performance. Int. J. Biol. Macromol. 2021, 177, 29–39. [Google Scholar] [CrossRef]
- Ivanets, A.; Prozorovich, V.; Kouznetsova, T.; Dontsova, T.; Yanushevska, O.; Hosseini-Bandegharaei, A.; Srivastava, V.; Sillanpaa, M. Effect of Mg(2+) ions on competitive metal ions adsorption/desorption on magnesium ferrite: Mechanism, reusability and stability studies. J. Hazard. Mater. 2021, 411, 124902. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Zhang, C.; Pang, H.; Li, J.; Li, S. Investigation of the adsorption behavior of Pb(II) onto natural-aged microplastics as affected by salt ions. J. Hazard. Mater. 2022, 431, 128643. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fei, Y.; Zhong, L.; Wei, W. Arsenic stabilization performance of a novel starch-modified Fe-Mn binary oxide colloid. Sci. Total Environ. 2020, 707, 136064. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhang, Q.; Li, W.; Ownes, G.; Chen, Z. Removal mechanism of Sb(V) by a hybrid ZIF-8@FeNPs and used for treatment of mining wastewater. Chem. Eng. J. 2023, 451, 138691. [Google Scholar] [CrossRef]
- Xue, Y.; Teng, W.; Chen, Y.; Ma, Q.; Chen, X.; Sun, Y.; Fan, J.; Qiu, Y.; Fu, R. Amorphous Mn-La oxides immobilized on carbon sphere for efficient removal of As(Ⅴ), Cd(Ⅱ), and Pb(Ⅱ): Co-adsorption and roles of Mn species. Chem. Eng. J. 2022, 429, 132262. [Google Scholar] [CrossRef]
- Liu, R.; Xu, W.; He, Z.; Lan, H.; Liu, H.; Qu, J.; Prasai, T. Adsorption of antimony(V) onto Mn(II)-enriched surfaces of manganese-oxide and FeMn binary oxide. Chemosphere 2015, 138, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Ren, B.; Hou, B.; Deng, X.; Deng, R.; Zhu, G.; Cheng, S. Adsorption of Sb(III) and Pb(II) in wastewater by magnetic γ-Fe2O3-loaded sludge biochar: Performance and mechanisms. Chemosphere 2024, 349, 140914. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Hu, J.; Zhou, S. Adsorption properties of graphene oxide@sodium alginate composite for Pb(II). J. Iran. Chem. Soc. 2023, 21, 399–407. [Google Scholar] [CrossRef]

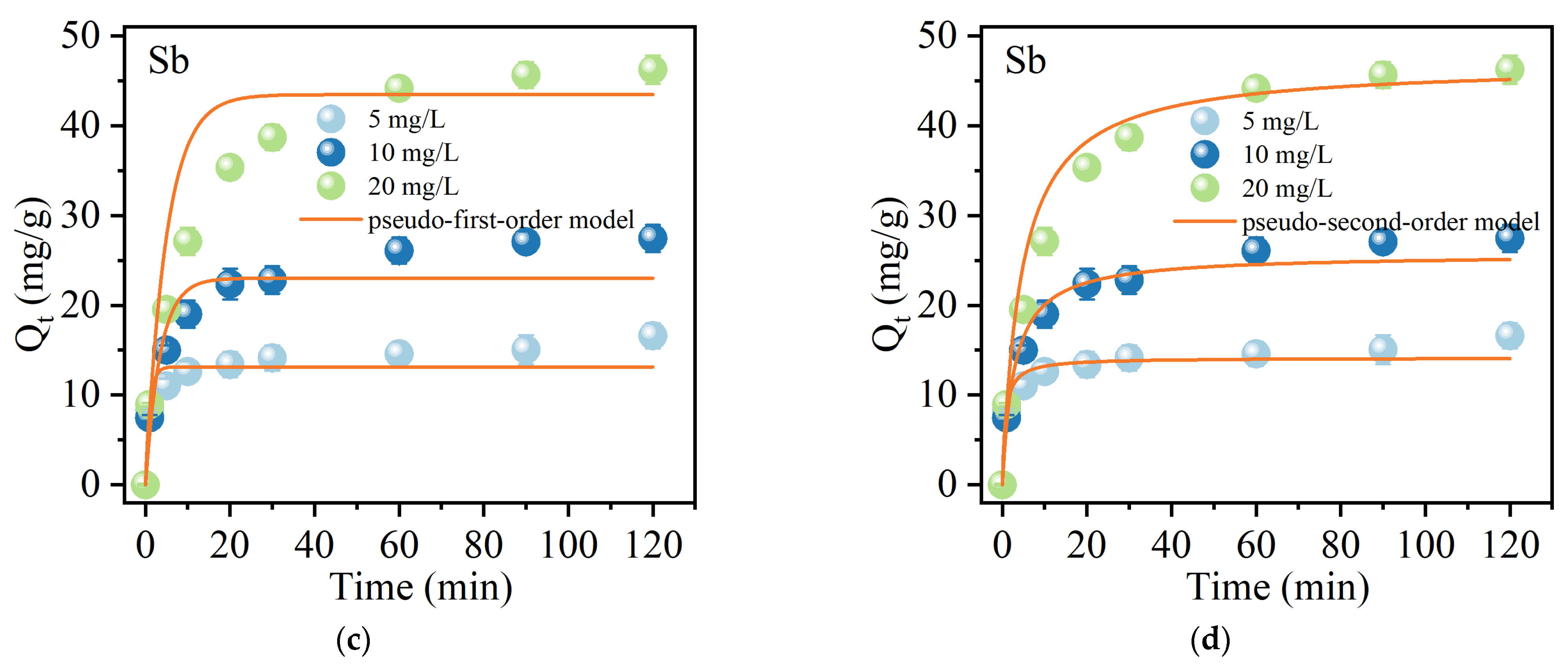
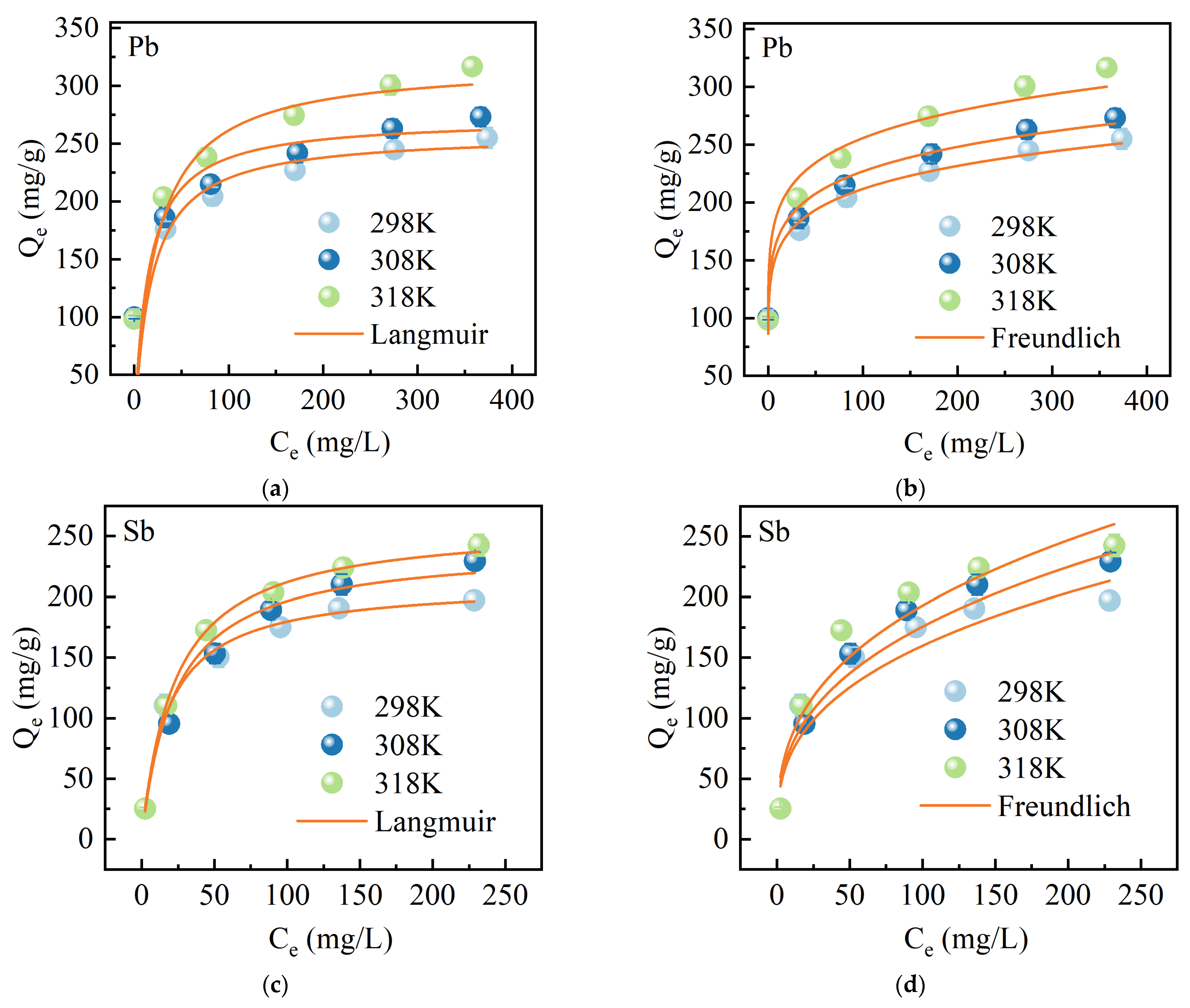
| C0 (mg/L) | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|
| K1 (L/min) | Qe (mg/g) | R2 | K2 (g/(mg min)) | Qe (mg/g) | R2 | ||
| Pb(II) | 5 | 0.22611 | 49.68 | 0.9983 | 0.00663 | 53.07 | 0.9983 |
| 10 | 0.08088 | 89.61 | 0.9983 | 0.00092 | 103.27 | 0.9986 | |
| 20 | 0.07198 | 103.58 | 0.9982 | 0.00069 | 120.08 | 0.9988 | |
| Sb(Ⅴ) | 5 | 1.00037 | 13.15 | 0.9928 | 0.09955 | 14.15 | 0.9969 |
| 10 | 0.28702 | 23.02 | 0.9680 | 0.01387 | 25.69 | 0.9920 | |
| 20 | 0.20328 | 43.47 | 0.9765 | 0.00474 | 46.84 | 0.9937 | |
| T (K) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| KL (L/min) | Qm (mg/g) | R2 | 1/n | KF [(mg/g)(L/mg)1/n] | R2 | ||
| Pb(II) | 298 | 0.0569 | 258.77 | 0.852 | 0.130 | 116.41 | 0.977 |
| 308 | 0.0691 | 271.85 | 0.885 | 0.129 | 125.19 | 0.991 | |
| 318 | 0.0447 | 329.86 | 0.872 | 0.126 | 143.09 | 0.974 | |
| Sb(Ⅴ) | 298 | 0.0562 | 211.49 | 0.998 | 0.349 | 31.95 | 0.863 |
| 308 | 0.0434 | 241.57 | 0.993 | 0.357 | 33.87 | 0.856 | |
| 318 | 0.0438 | 260.40 | 0.999 | 0.356 | 37.45 | 0.823 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Zhu, A.; Chen, F.; Cai, Y.; Deng, L. Synthesis of Amorphous MnFe@SBA Composites for Efficient Adsorptive Removal of Pb(Ⅱ) and Sb(V) from Aqueous Solution. Molecules 2025, 30, 679. https://doi.org/10.3390/molecules30030679
Shi Z, Zhu A, Chen F, Cai Y, Deng L. Synthesis of Amorphous MnFe@SBA Composites for Efficient Adsorptive Removal of Pb(Ⅱ) and Sb(V) from Aqueous Solution. Molecules. 2025; 30(3):679. https://doi.org/10.3390/molecules30030679
Chicago/Turabian StyleShi, Zhou, Aogui Zhu, Fan Chen, Yishu Cai, and Lin Deng. 2025. "Synthesis of Amorphous MnFe@SBA Composites for Efficient Adsorptive Removal of Pb(Ⅱ) and Sb(V) from Aqueous Solution" Molecules 30, no. 3: 679. https://doi.org/10.3390/molecules30030679
APA StyleShi, Z., Zhu, A., Chen, F., Cai, Y., & Deng, L. (2025). Synthesis of Amorphous MnFe@SBA Composites for Efficient Adsorptive Removal of Pb(Ⅱ) and Sb(V) from Aqueous Solution. Molecules, 30(3), 679. https://doi.org/10.3390/molecules30030679





