Pseudomonas aeruginosa Rhamnolipids Produced by Andiroba (Carapa guianensis Aubl.) (Sapindales: Meliaceae) Biomass Waste from Amazon: A Potential Weapon Against Aedes aegypti L. (Diptera: Culicidae)
Abstract
1. Introduction
2. Results
2.1. BSAW Production
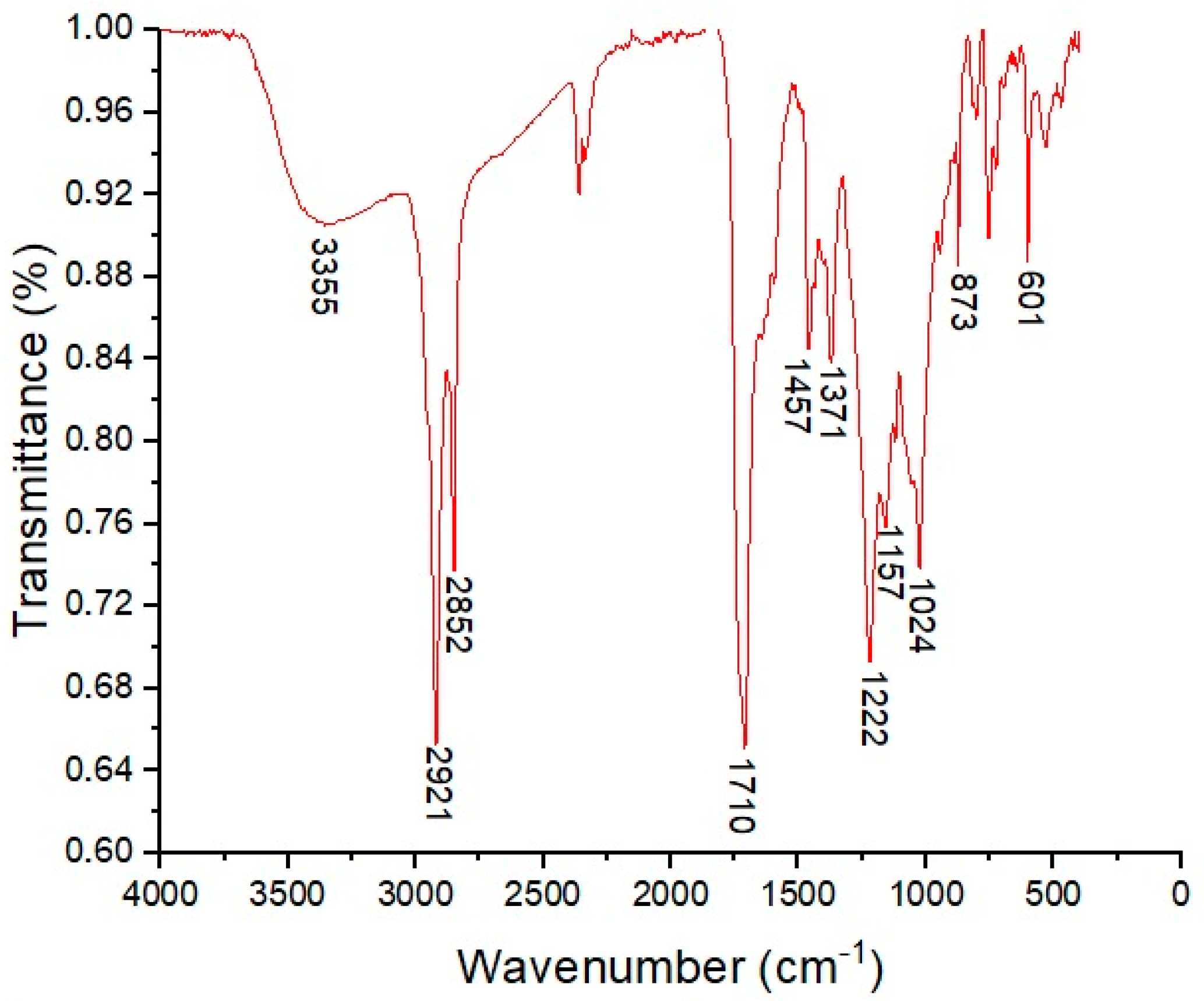
2.2. Structural Characterization of BSAW
2.3. Larvicidal Effects of BSAW
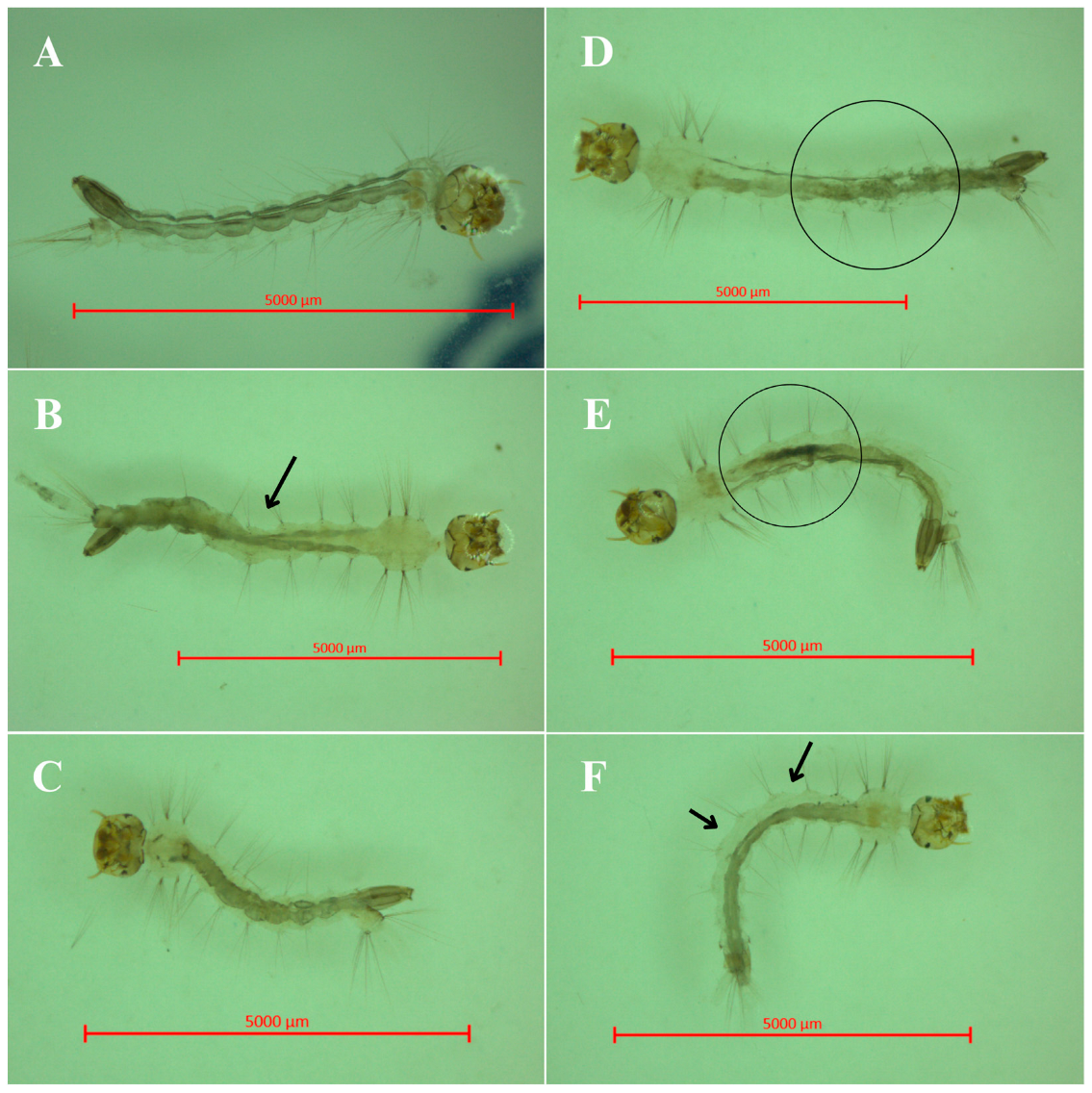
2.4. Impacts of BSAW on the Gut Microbiota Homeostasis
2.5. Effects of BSAW on Oviposition Behavior
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Biological Materials
4.3. Proximate Characterization of Andiroba Biomass Waste
4.4. BSAW Obtaining
4.5. BSAW Characterization
4.6. Insecticide Activity
4.6.1. Larvicidal Trials
4.6.2. Oviposition Deterrence Trials
4.7. Effects of BSAW on the Gut Microbiota Homeostasis
4.7.1. Sample Preparation
4.7.2. Impact of BSAW on Microbiota
4.7.3. Characterization of the Intestinal Microbiota of A. aegypti Larvae
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; de Cienfuegos, L.Á.; Estévez, R.E.; Paradas, M.; Lasanta, A.M.; Oller, J.L.; Rosales, A.; Cuerva, J.M.; Oltra, J.E. Ti-catalyzed transannular cyclization of epoxygermacrolides. Synthesis of antifungal (+)-tuberiferine and (+)-dehydrobrachylaenolide. Tetrahedron 2008, 64, 11938–11943. [Google Scholar] [CrossRef]
- Siqueira, T.P.; Barbosa, W.F.; Rodrigues, E.M.; Miranda, F.R.; Freitas, F.S.; Martins, G.F.; Tótola, M.R. Rhamnolipids on Aedes aegypti larvae: A potential weapon against resistance selection. 3 Biotech. 2021, 11, e172. [Google Scholar] [CrossRef]
- Silva, V.L.; Lovaglio, R.B.; Von Zuben, C.J.; Contiero, J. Rhamnolipids: Solution against Aedes aegypti? Front. Microbiol. 2015, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Silva, M.G.C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, e108377. [Google Scholar] [CrossRef]
- Santos, S.C.; Torquato, C.A.; Santos, D.A.; Orsato, A.; Leite, K.; Serpeloni, J.M.; Losi-Guembarovski, R.; Pereira, E.R.; Dyna, A.L.; Barboza, M.G.L.; et al. Production and characterization of rhamnolipids by Pseudomonas aeruginosa isolated in the Amazon region, and potential antiviral, antitumor, and antimicrobial activity. Sci. Rep. 2024, 14, e4629. [Google Scholar] [CrossRef]
- Dias, K.K.B.; Cardoso, A.L.; Costa, A.A.F.; Passos, M.F.; Costa, C.E.F.; Rocha Filho, G.N.; de Andrade, E.H.A.; Luque, R.; Nascimento, L.A.S.; Noronha, R.C.R. Biological activities from andiroba (Carapa guianensis Aublet.) and its biotechnological applications: A systematic review. Arab. J. Chem. 2023, 16, e104629. [Google Scholar] [CrossRef]
- Barata, L.E.S. A Economia Verde: Amazônia. Cienc. Cult. 2012, 64, 31–35. [Google Scholar] [CrossRef][Green Version]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, e112285. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.C.P.; Santos, A.G.; Pereira, D.S. Saberes Técnico-Científicos Para Extração Artesanal do óleo e Aproveitamento de Resíduos da Andiroba; Embrapa: Boa Vista, RR, Brazil, 2020. [Google Scholar]
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Martins Junior, A.J. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem Inst Oswaldo Cruz 2019, 114, e180544. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef]
- Reed, W.; Carroll, J. The prevention of yellow fever. Public. Health Pap. Rep. 1901, 27, 113–129. [Google Scholar] [PubMed]
- Sá, G.C.S.; Bezerra, P.V.V.; Silva, M.F.A.; Silva, L.B.; Barra, P.B.; Ximenes, M.F.F.M.; Uchôa, A.F. Arbovirus vectors insects: Are botanical insecticides an alternative for its management? J. Pest. Sci. 2023, 96, 1–20. [Google Scholar] [CrossRef]
- Santos, C.R.; Rodovalho, C.M.; Jablonka, W.; Martins, A.J.; Lima, J.B.P.; Dias, L.S.; Neto, M.A.C.S.; Atella, G.C. Insecticide resistance, fitness and susceptibility to zika infection of an interbred Aedes aegypti population from Rio de Janeiro, Brazil. Parasit. Vectors 2020, 13, e293. [Google Scholar] [CrossRef]
- Zuo, Y.; He, C.; Zhang, D.; Zhao, L.; He, X.; Sun, X. Soil variables driven by host plant and growth season affect soil microbial composition and metabolism in extremely arid desert ecosystems. Microbiol. Res. 2023, 269, e127315. [Google Scholar] [CrossRef] [PubMed]
- Dobler, L.; Carvalho, B.R.; Alves, W.S.; Neves, B.C.; Freire, D.M.G.; Almeida, R.V. Enhanced rhamnolipid production by Pseudomonas aeruginosa overexpressing estA in a simple medium. PLoS ONE 2017, 12, e0183857. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Rupani, P.F.; Akor, J.; Zhang, Y.; Zhao, M.; Zhang, C.; Ragauskas, A.J.; Qi, X. Biocatalytic gateway to convert glycerol into 3-hydroxypropionic acid in waste-based biorefineries: Fundamentals, limitations, and potential research strategies. Biotechnol. Adv. 2023, 62, e108075. [Google Scholar] [CrossRef]
- Noordman, W.H.; Janssen, D.B. Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2002, 68, 4502–4508. [Google Scholar] [CrossRef]
- Zhao, K.; Li, J.; Yuan, Y.; Lin, J.; Wang, X.; Guo, Y.; Chu, Y. Nutrient factor-dependent performance of bacterial quorum sensing system during population evolution. Arch. Microbiol. 2020, 202, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Deepika, K.V.; Kalam, S.; Ramu Sridhar, P.; Podile, A.R.; Bramhachari, P.V. Optimization of rhamnolipid biosurfactant production by mangrove sediment bacterium Pseudomonas aeruginosa KVD-HR42 using response surface methodology. Biocatal. Agric. Biotechnol. 2016, 5, 38–47. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Rosenau, F. Heterologous rhamnolipid biosynthesis: Advantages, challenges, and the opportunity to produce tailor-made rhamnolipids. Front. Bioeng. Biotechnol. 2020, 8, e594010. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.C.; Lee, S.; Kim, J.C.; Yun, M.Y.; Kim, I.S. Insecticidal activity of rhamnolipid isolated from Pseudomonas Sp. EP-3 against green peach aphid (Myzus Persicae). J. Agric. Food Chem. 2011, 59, 934–938. [Google Scholar] [CrossRef]
- Kamal, A. Metabolic profiling and biological activities of bioactive compounds produced by Pseudomonas Sp. strain ICTB-745 isolated from Ladakh, India. J. Microbiol. Biotechnol. 2012, 22, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S.R. Aedes aegypti (L.) the Yellow Fever Mosquito, Its Life History, Bionomics and Structure; Cambridge University Press: London, UK, 1960. [Google Scholar]
- Iltis, C.; Louâpre, P.; Vogelweith, F.; Thiéry, D.; Moreau, J. How to stand the heat? Post-stress nutrition and developmental stage determine insect response to a heat wave. J. Insect Physiol. 2021, 131, e104214. [Google Scholar] [CrossRef]
- Salgado, J.F.M.; Premkrishnan, B.N.V.; Oliveira, E.L.; Vettath, V.K.; Goh, F.G.; Hou, X.; Drautz-Moses, D.I.; Cai, Y.; Schuster, S.C.; Junqueira, A.C.M. The dynamics of the midgut microbiome in Aedes aegypti during digestion reveal putative symbionts. PNAS Nexus 2024, 3, e317. [Google Scholar] [CrossRef]
- Al-Tahhan, R.A.; Sandrin, T.R.; Bodour, A.A.; Maier, R.M. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: Effect on cell surface properties and interaction with hydrophobic substrates. Appl. Environ. Microbiol. 2000, 66, 3262–3268. [Google Scholar] [CrossRef]
- Zouache, K.; Martin, E.; Rahola, N.; Gangue, M.F.; Minard, G.; Dubost, A.; Van, V.T.; Dickson, L.; Ayala, D.; Lambrechts, L.; et al. Larval habitat determines the bacterial and fungal microbiota of the mosquito vector Aedes aegypti. FEMS Microbiol. Ecol. 2022, 98, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, T.; Wu, Y.; Zhong, D.; Zhou, G.; Su, X.; Xu, J.; Sotero, C.F.; Sadruddin, A.A.; Wu, K.; et al. Bacterial microbiota assemblage in Aedes albopictus mosquitoes and its impacts on larval development. Mol. Ecol. 2018, 27, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.E.M.; Radl, J.; Dimopoulos, G.; Short, S.M. Bacterial communities of Aedes aegypti mosquitoes differ between crop and midgut tissues. PLoS Negl. Trop. Dis. 2023, 17, e0011218. [Google Scholar] [CrossRef]
- Zhang, P.; Edgar, B.A. Insect Gut Regeneration. Cold Spring Harb. Perspect. Biol. 2022, 14, a040915. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 950.46, Moisture in Meat; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. AOAC Official Method 923.03, Ash of Flour. Direct Method; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. AOAC Official Method 981.10, Crude Protein in Meat. Block Digestion Method; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sá, G.C.S.; Silva, M.A.T.; Silva, D.F.; Santi-Gadelha, T.; Fragoso, S.P.; Madruga, M.S.; Pacheco, M.T.B.; Lima, E.O.; Uchôa, A.F.; Gadelha, C.A.A. Nutritional composition and biological activities (antioxidant and antifungal) of Sesbania virgata (Cav.) Pers. seeds. RBTA 2021, 15, 1–20. [Google Scholar] [CrossRef]
- Camilios-Neto, D.; Bugay, C.; Santana-Filho, A.P.; Joslin, T.; Souza, L.M.; Sassaki, G.L.; Mitchell, D.A.; Krieger, N. Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl. Microbiol. Biotechnol. 2011, 89, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Springer, J. Determination of interfacial tension from the profile of a pendant drop using computer-aided image processing. J. Colloid. Interface Sci. 1996, 184, 64–76. [Google Scholar] [CrossRef]
- WHO Guidelines for Laboratory and Field Testing of Mosquito Larvicides: Communicable Disease Control, Prevention and Eradication, and WHO Pesticide Evaluation Scheme; WHO: Geneva, Switzerland, 2005.
- Quiroz-Martínez, H.; Garza-Rodríguez, M.I.; Trujillo-González, M.I.; Zepeda-Cavazos, I.G.; Siller-Aguillon, I.; Martínez-Perales, J.F.; Rodríguez-Castro, V.A. Selection of oviposition sites by female Aedes aegypti exposed to two larvicides. J. Am. Mosq. Control Assoc. 2012, 28, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Almeida, W.A.; Nova, I.C.V.; Nascimento, J.S.; Moura, M.C.; Agra-Neto, A.C.; Costa, H.N.; Cruz, G.S.; Teixeira, Á.A.C.; Wanderley-Teixeira, V.; Ferreira, M.R.A.; et al. Effects of Plectranthus barbatus leaf extract on survival, digestive proteases, midgut morphophysiology and gut microbiota homeostasis of Aedes aegypti larvae. S Afr. J. Bot. 2021, 141, 116–125. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard M07-A10; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Bartholomew, J.W.; Mittwer, T. The Gram Stain. Bacteriol. Rev. 1952, 16, 1–29. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Emulsification Index (%) | Surface Tension (mN/m) | Interfacial Tension (mN/m) |
|---|---|---|---|
| BSAW | 59.30 ± 1.00 | 28.52 ± 0.22 | 3.27 ± 0.10 |
| VSM | 0.00 ± 0.00 | 69.90 ± 0.74 | 17.00 ± 0.30 |
| SDS | 69.90 ± 1.10 | Nt | Nt |
| Parameter | Values (%) |
|---|---|
| Total lipids | 57.02 ± 0.58 |
| Carbohydrates | 25.06 ± 0.46 |
| Crude protein | 10.76 ± 0.23 |
| Ash | 4.42 ± 0.08 |
| Moisture | 2.75 ± 0.03 |
| Lethal Concentration (LC) | Slope (SE) | χ2 | R2 | |
|---|---|---|---|---|
| 24 h post-exposure | ||||
| LC50 | 4.656 (1.448–14.967) | 1.256 (0.259) | 0.143 | 0.908 |
| LC90 | 47.792 (14.865–153.648) | |||
| LC99 | 319.075 (99.247–1025.812) | |||
| 48 h post-exposure | ||||
| LC50 | 1.189 (0.480–2.944) | 1.253 (0.201) | 0.035 | 0.883 |
| LC90 | 14.534 (5.868–35.998) | |||
| LC99 | 119.893 (45.175–277.145) | |||
| Oviposition Profile (Days Post-Exposure) | BSAW | Control |
|---|---|---|
| 2 | 0 (0–0) ± 0.00 | 0 (0–1) ± 0.58 |
| 4 | 2 (0–7) ± 4.04 | 90 (0–169) ± 85.10 |
| 6 | 7 (3–16) ± 7.51 | 87 (62–117) ± 27.75 |
| 8 | 4 (0–10) ± 5.51 | 30 (3–48) ± 23.63 |
| Total eggs | 41 | 624 |
| ODI = −79% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, G.C.d.S.; Bezerra, P.V.V.; Ramos, E.O.; Orsato, A.; Leite, K.; Feio, A.M.; Pimentel, L.M.S.; Alves, J.d.A.; Gomes, G.S.; Rodrigues, P.D.; et al. Pseudomonas aeruginosa Rhamnolipids Produced by Andiroba (Carapa guianensis Aubl.) (Sapindales: Meliaceae) Biomass Waste from Amazon: A Potential Weapon Against Aedes aegypti L. (Diptera: Culicidae). Molecules 2025, 30, 618. https://doi.org/10.3390/molecules30030618
Sá GCdS, Bezerra PVV, Ramos EO, Orsato A, Leite K, Feio AM, Pimentel LMS, Alves JdA, Gomes GS, Rodrigues PD, et al. Pseudomonas aeruginosa Rhamnolipids Produced by Andiroba (Carapa guianensis Aubl.) (Sapindales: Meliaceae) Biomass Waste from Amazon: A Potential Weapon Against Aedes aegypti L. (Diptera: Culicidae). Molecules. 2025; 30(3):618. https://doi.org/10.3390/molecules30030618
Chicago/Turabian StyleSá, Giulian César da Silva, Pedro Vitor Vale Bezerra, Evelly Oliveira Ramos, Alexandre Orsato, Karoline Leite, Alan Moura Feio, Lucas Mariano Siqueira Pimentel, Joane de Almeida Alves, Glenda Soares Gomes, Pamela Dias Rodrigues, and et al. 2025. "Pseudomonas aeruginosa Rhamnolipids Produced by Andiroba (Carapa guianensis Aubl.) (Sapindales: Meliaceae) Biomass Waste from Amazon: A Potential Weapon Against Aedes aegypti L. (Diptera: Culicidae)" Molecules 30, no. 3: 618. https://doi.org/10.3390/molecules30030618
APA StyleSá, G. C. d. S., Bezerra, P. V. V., Ramos, E. O., Orsato, A., Leite, K., Feio, A. M., Pimentel, L. M. S., Alves, J. d. A., Gomes, G. S., Rodrigues, P. D., Quintella, C. M., Fragoso, S. P., da Silva, E. C., Uchôa, A. F., & dos Santos, S. C. (2025). Pseudomonas aeruginosa Rhamnolipids Produced by Andiroba (Carapa guianensis Aubl.) (Sapindales: Meliaceae) Biomass Waste from Amazon: A Potential Weapon Against Aedes aegypti L. (Diptera: Culicidae). Molecules, 30(3), 618. https://doi.org/10.3390/molecules30030618








