Effects of Biased Analogues of the Kappa Opioid Receptor Agonist, U50,488, in Preclinical Models of Pain and Side Effects
Abstract
1. Introduction
2. Results
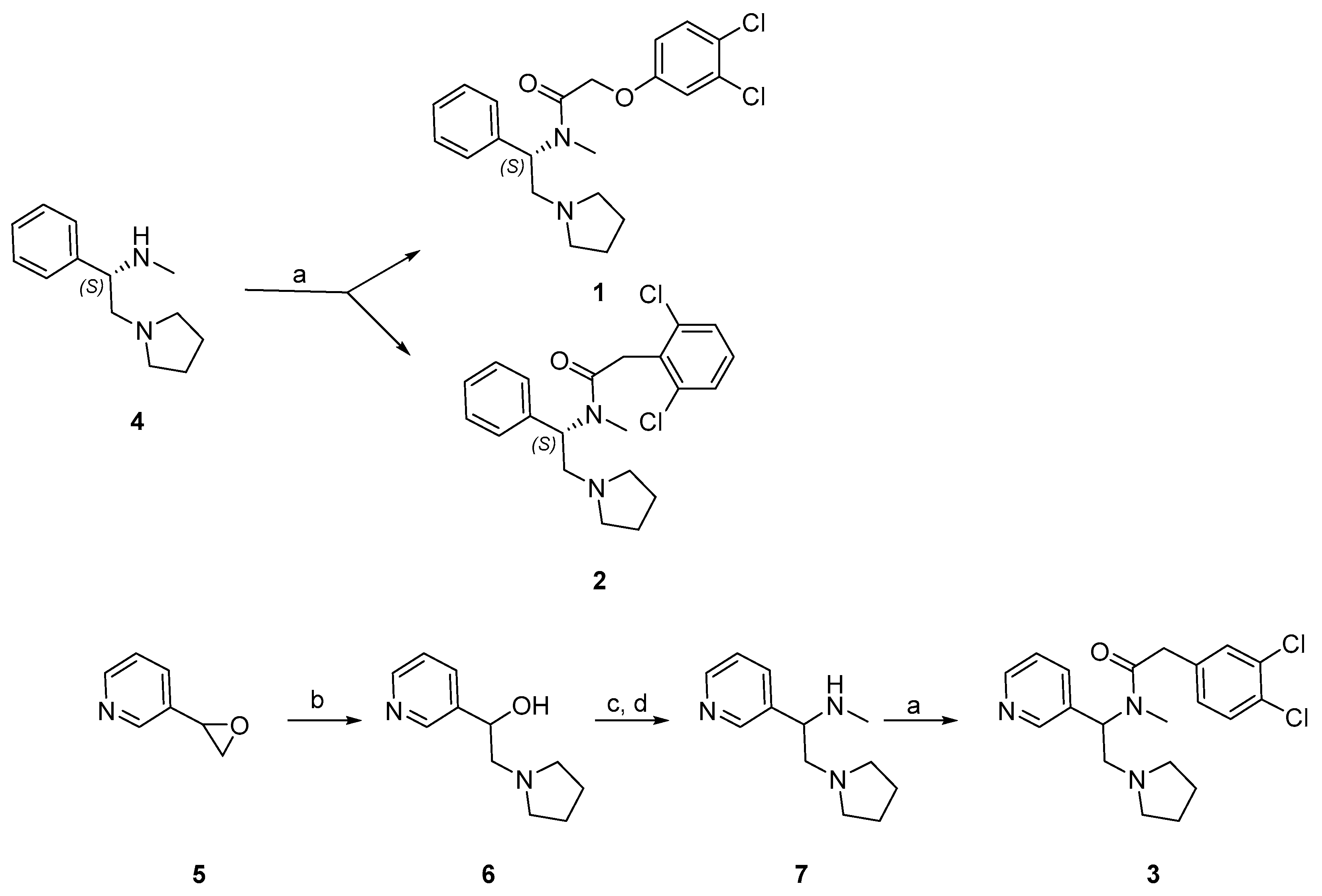
2.1. Synthesis
2.2. Functional Activity and Signaling Bias
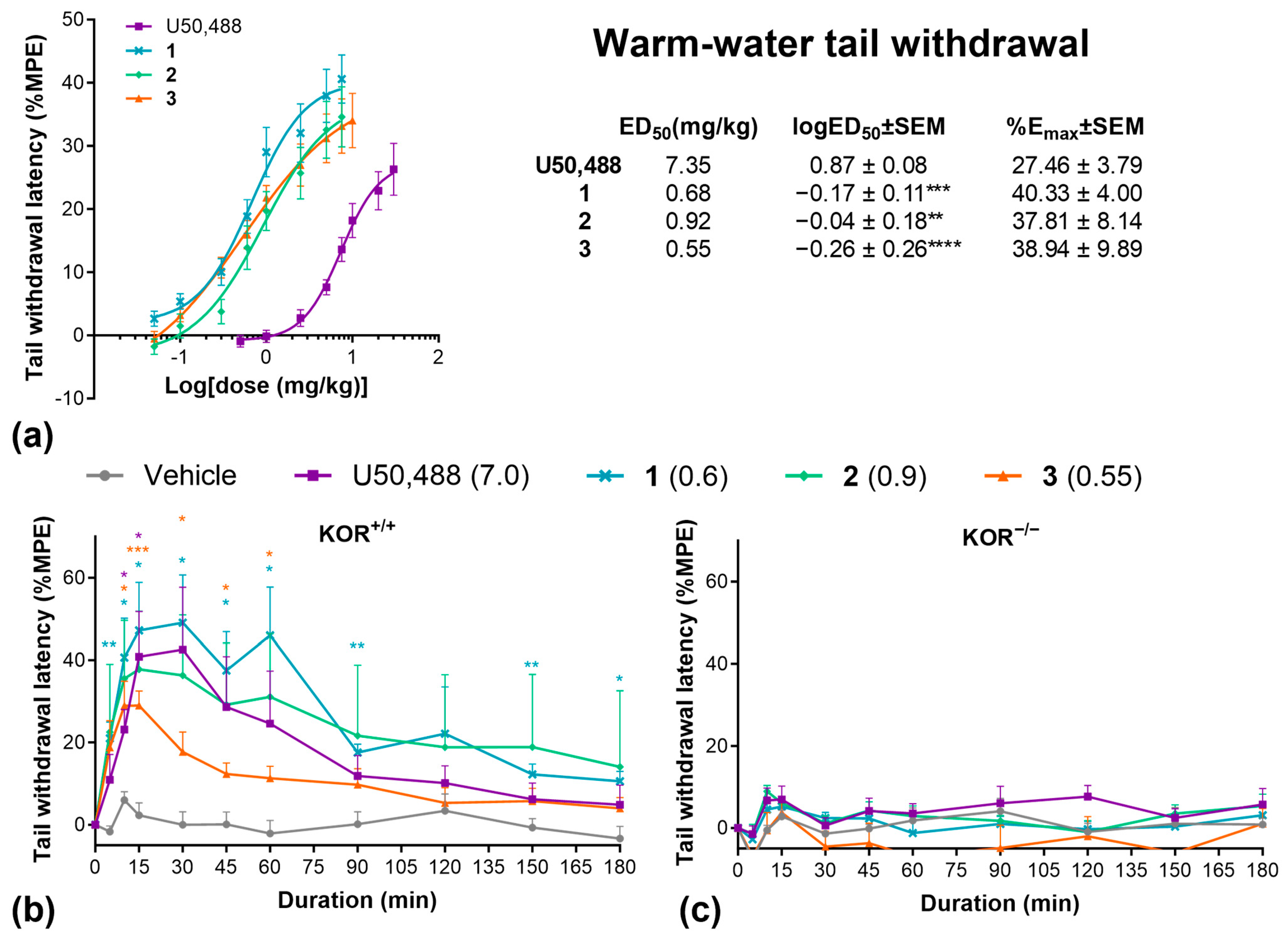
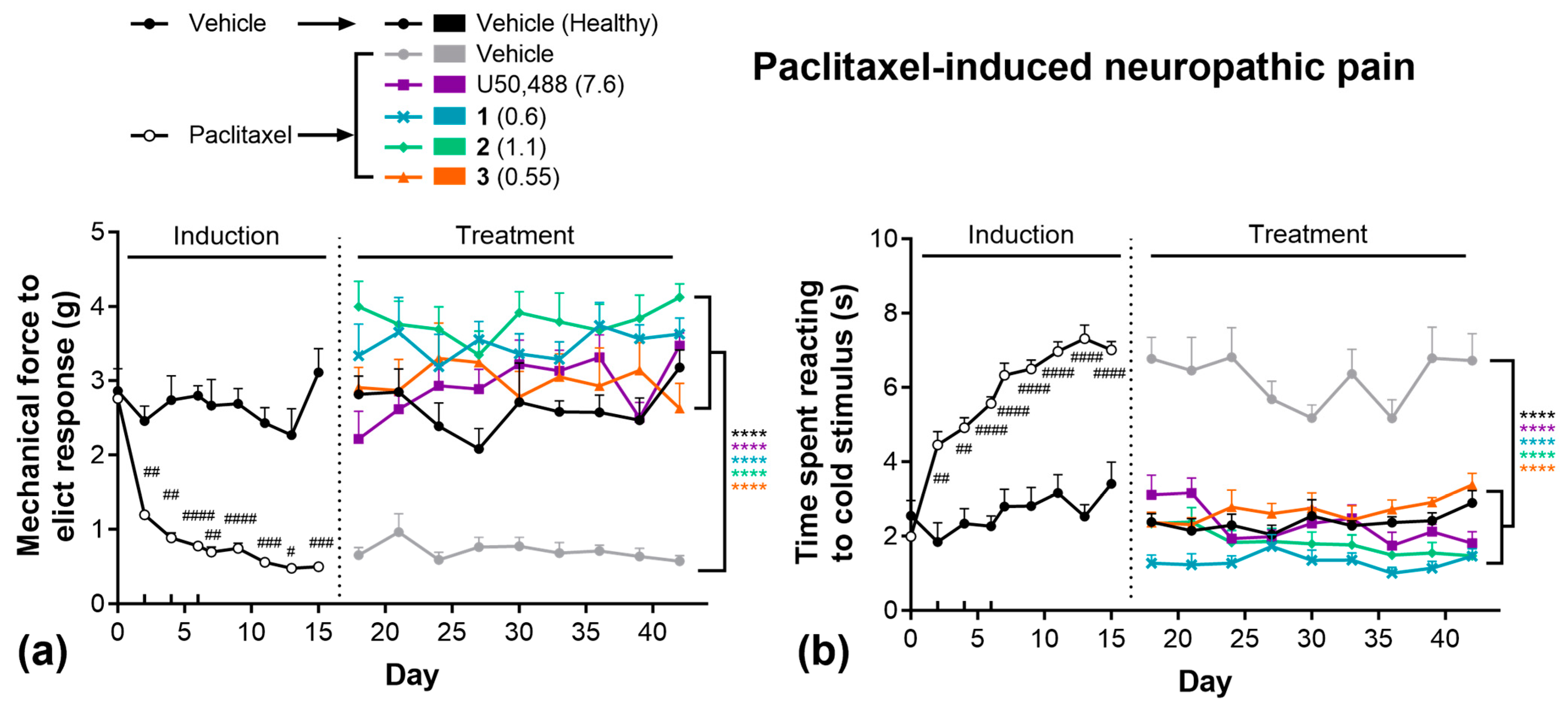
2.3. Pain Models
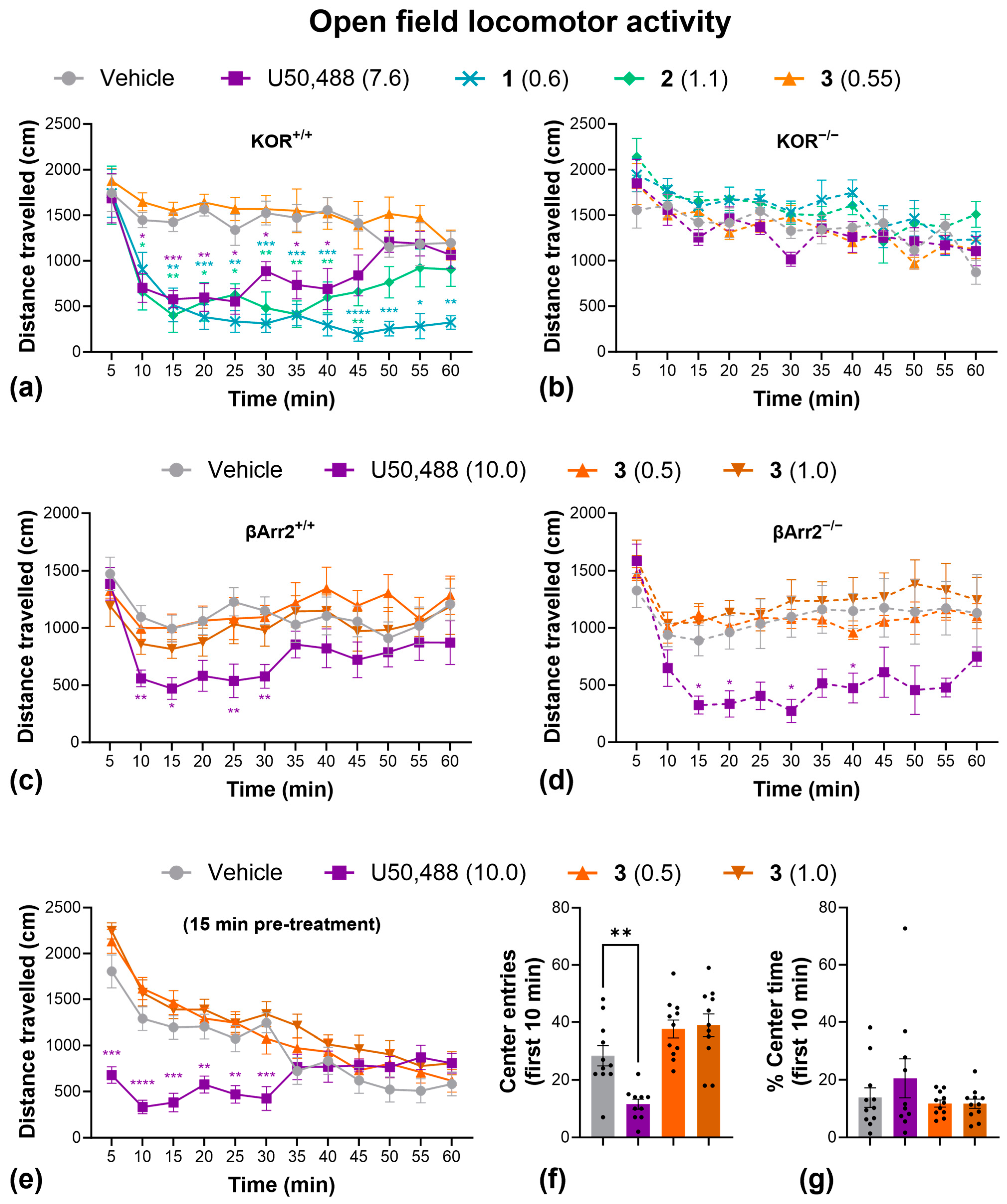
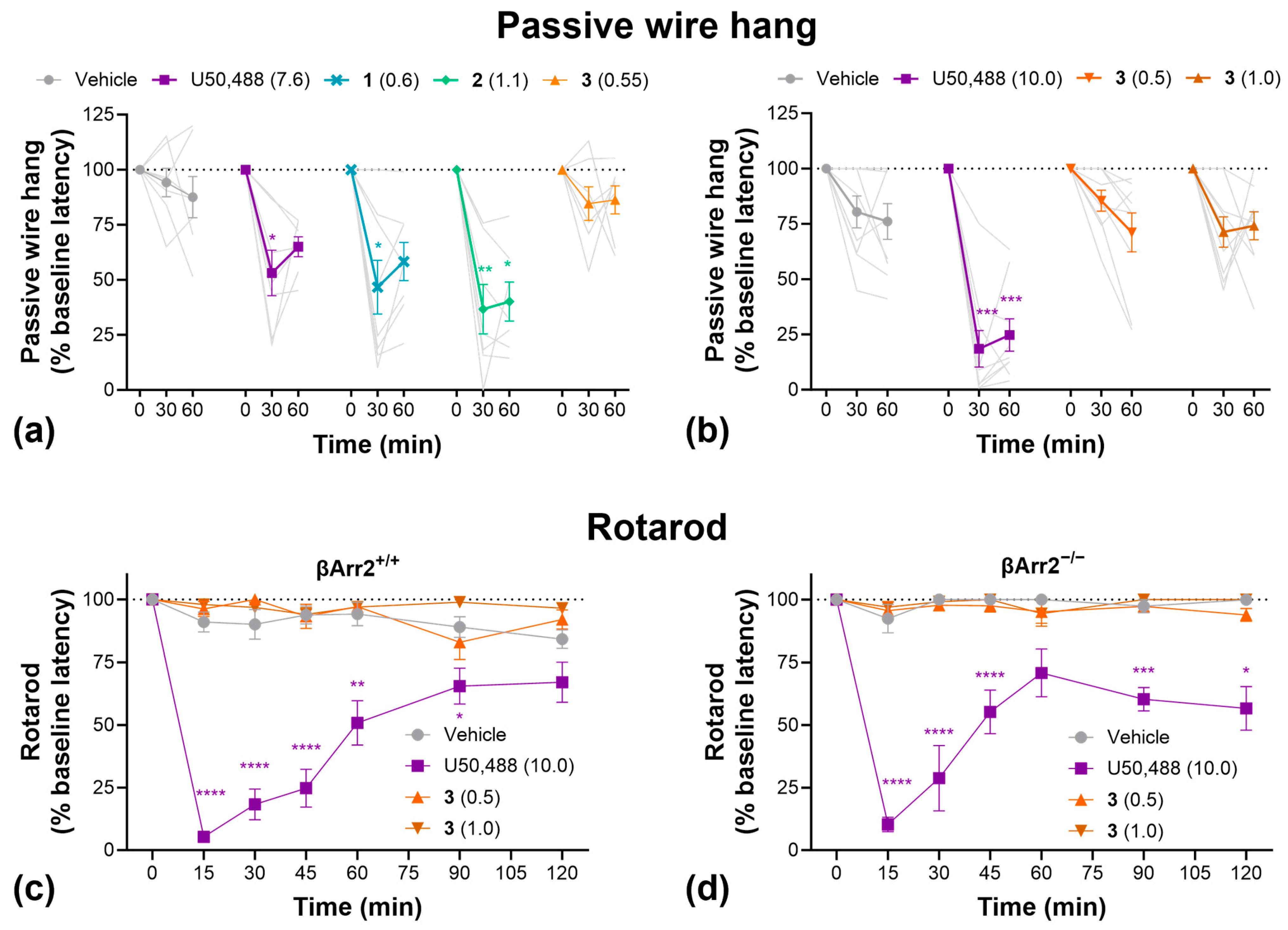
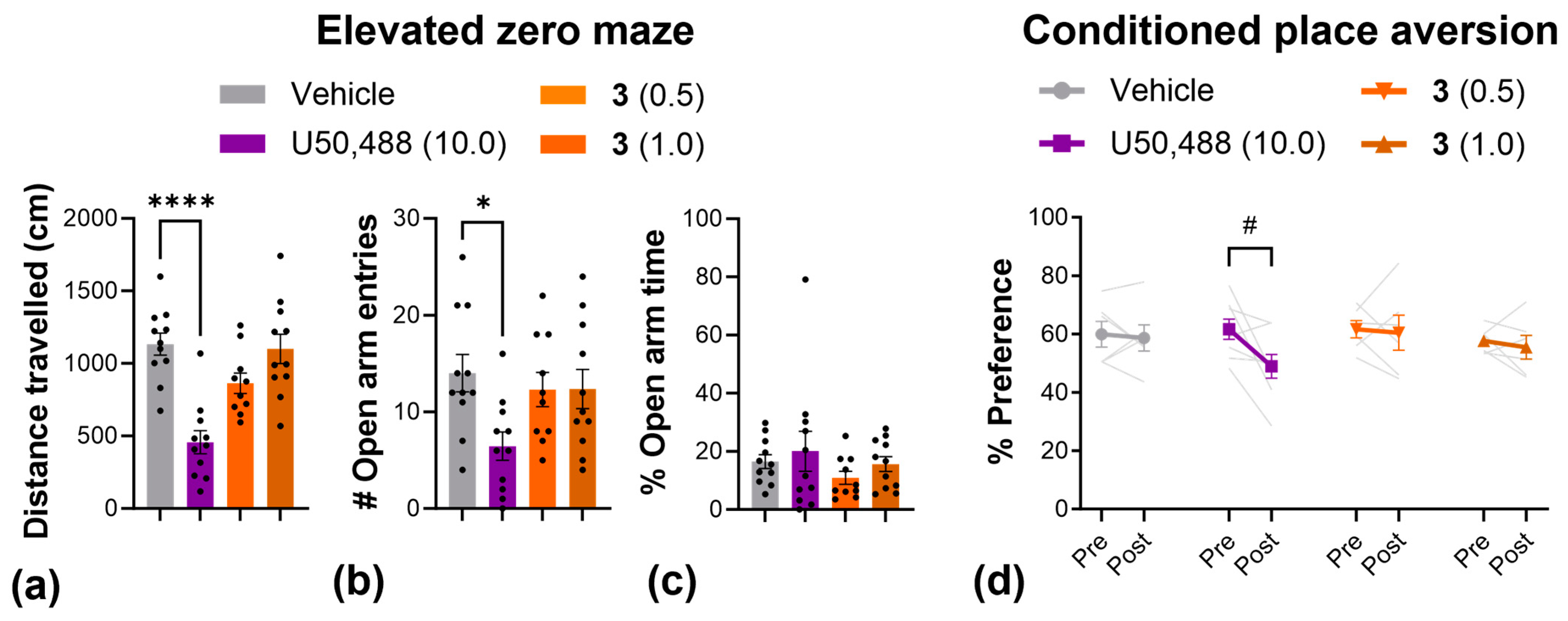
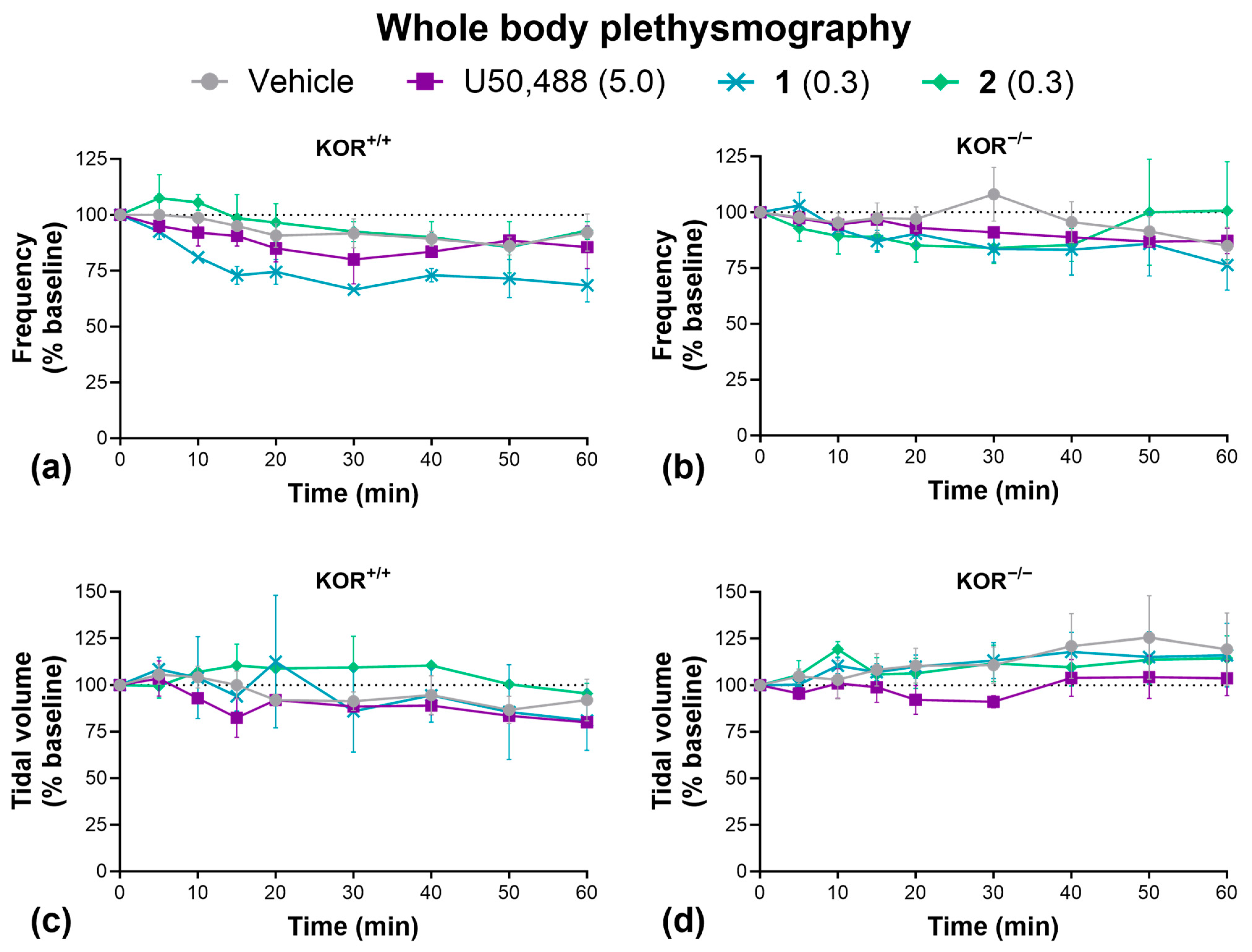
2.4. Side Effects
3. Discussion
3.1. Therapeutic Potential of 1, 2, and 3
3.2. G-Protein Bias and Role of β-Arrestin2 Signaling
4. Materials and Methods
4.1. Drugs
4.2. Cell Lines and Cell Culture
4.3. Forskolin-Induced cAMP Accumulation
4.4. β-Arrestin2 Recruitment Assay
4.5. Bias Calculation
4.6. Subjects
4.7. Warm Water Tail-Withdrawal
4.8. Paclitaxel-Induced Allodynia
4.9. Open-Field Locomotor Activity
4.10. Passive Wire Hang
4.11. Rotarod
4.12. Elevated Zero Maze
4.13. Conditioned Place Aversion
4.14. Whole Body Plethysmography
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peckys, D.; Landwehrmeyer, G.B. Expression of Mu, Kappa, and Delta Opioid Receptor Messenger RNA in the Human CNS: A 33P in Situ Hybridization Study. Neuroscience 1999, 88, 1093–1135. [Google Scholar] [CrossRef]
- Peng, J.; Sarkar, S.; Chang, S.L. Opioid Receptor Expression in Human Brain and Peripheral Tissues Using Absolute Quantitative Real-Time RT-PCR. Drug Alcohol. Depend. 2012, 124, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Simonin, F.; Gavériaux-Ruff, C.; Befort, K.; Matthes, H.; Lannes, B.; Micheletti, G.; Mattéi, M.G.; Charron, G.; Bloch, B.; Kieffer, B. Kappa-Opioid Receptor in Humans: CDNA and Genomic Cloning, Chromosomal Assignment, Functional Expression, Pharmacology, and Expression Pattern in the Central Nervous System. Proc. Natl. Acad. Sci. USA 1995, 92, 7006–7010. [Google Scholar] [CrossRef]
- Mei, F.; Mayoral, S.R.; Nobuta, H.; Wang, F.; Desponts, C.; Lorrain, D.S.; Xiao, L.; Green, A.J.; Rowitch, D.; Whistler, J.; et al. Identification of the Kappa-Opioid Receptor as a Therapeutic Target for Oligodendrocyte Remyelination. J. Neurosci. 2016, 36, 7925–7935. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Duan, Y.; Wei, W.; Cai, Y.; Chai, H.; Lv, J.; Du, X.; Zhu, J.; Xie, X. Kappa Opioid Receptor Activation Alleviates Experimental Autoimmune Encephalomyelitis and Promotes Oligodendrocyte-Mediated Remyelination. Nat. Commun. 2016, 7, 11120. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, R.; Bibi, R.; Biggerstaff, A.; Hong, S.; Pengelly, B.; Prisinzano, T.E.; La Flamme, A.C.; Kivell, B.M. Nalfurafine Promotes Myelination in Vitro and Facilitates Recovery from Cuprizone + Rapamycin-Induced Demyelination in Mice. Glia 2024, 72, 1801–1820. [Google Scholar] [CrossRef]
- Tangherlini, G.; Kalinin, D.V.; Schepmann, D.; Che, T.; Mykicki, N.; Ständer, S.; Loser, K.; Wünsch, B. Development of Novel Quinoxaline-Based κ-Opioid Receptor Agonists for the Treatment of Neuroinflammation. J. Med. Chem. 2019, 62, 893–907. [Google Scholar] [CrossRef]
- Schrader, T.O.; Lorrain, K.I.; Bagnol, D.; Edu, G.C.; Broadhead, A.; Baccei, C.; Poon, M.M.; Stebbins, K.J.; Xiong, Y.; Lorenzana, A.O.; et al. Identification and In Vivo Evaluation of Myelination Agent PIPE-3297, a Selective Kappa Opioid Receptor Agonist Devoid of β-Arrestin-2 Recruitment Efficacy. ACS Chem. Neurosci. 2023, 15, 685–698. [Google Scholar] [CrossRef]
- Beck, T.C.; Wilson, E.M.; Wilkes, E.; Lee, L.W.; Norris, R.; Valdebran, M. Kappa Opioid Agonists in the Treatment of Itch: Just Scratching the Surface? Itch 2023, 8, e0072. [Google Scholar] [CrossRef]
- Inui, S. Nalfurafine Hydrochloride for the Treatment of Pruritus. Clin. Cosmet. Investig. Dermatol. 2012, 13, 1507–1513. [Google Scholar] [CrossRef]
- Deeks, E.D. Difelikefalin: First Approval. Drugs 2021, 81, 1937–1944. [Google Scholar] [CrossRef]
- Paton, K.F.; Atigari, D.V.; Kaska, S.; Prisinzano, T.; Kivell, B.M. Strategies for Developing κ Opioid Receptor Agonists for the Treatment of Pain with Fewer Side Effects. J. Pharmacol. Exp. Ther. 2020, 375, 332–348. [Google Scholar] [CrossRef]
- Dalefield, M.L.; Scouller, B.; Bibi, R.; Kivell, B.M. The Kappa Opioid Receptor: A Promising Therapeutic Target for Multiple Pathologies. Front. Pharmacol. 2022, 13, 837671. [Google Scholar] [CrossRef]
- French, A.R.; van Rijn, R.M. An Updated Assessment of the Translational Promise of G-Protein-Biased Kappa Opioid Receptor Agonists to Treat Pain and Other Indications without Debilitating Adverse Effects. Pharmacol. Res. 2022, 177, 106091. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Sawyer, B.J.; Akins, N.S.; Le, H.V. A Systematic Review on the Kappa Opioid Receptor and Its Ligands: New Directions for the Treatment of Pain, Anxiety, Depression, and Drug Abuse. Eur. J. Med. Chem. 2022, 243, 114785. [Google Scholar] [CrossRef]
- Mores, K.L.; Cummins, B.R.; Cassell, R.J.; Van Rijn, R.M. A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists. Front. Pharmacol. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.F. Biased Ligands at the Kappa Opioid Receptor: Fine-Tuning Receptor Pharmacology. Handb. Exp. Pharmacol. 2022, 271, 115–135. [Google Scholar] [CrossRef]
- Paton, K.F.; Biggerstaff, A.; Kaska, S.; Crowley, R.S.; La Flamme, A.C.; Prisinzano, T.E.; Kivell, B.M. Evaluation of Biased and Balanced Salvinorin A Analogs in Preclinical Models of Pain. Front. Neurosci. 2020, 14, 765. [Google Scholar] [CrossRef]
- Paton, K.F.; Luo, D.; La Flamme, A.C.; Prisinzano, T.E.; Kivell, B.M. Sex Differences in Kappa Opioid Receptor Agonist Mediated Attenuation of Chemotherapy-Induced Neuropathic Pain in Mice. Front. Pharmacol. 2022, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.F.; Kumar, N.; Crowley, R.S.; Harper, J.L.; Prisinzano, T.E.; Kivell, B.M. The Analgesic and Anti-Inflammatory Effects of Salvinorin A Analogue β-Tetrahydropyran Salvinorin B in Mice. Eur. J. Pain 2017, 21, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.A.; Havelin, J.J.; Mcintosh, M.I.; Ciccone, H.A.; Pangilinan, K.; Imbert, I.; Largent-Milnes, T.M.; King, T.; Vanderah, T.W.; Streicher, J.M. A Kappa Opioid Receptor Agonist Blocks Bone Cancer Pain Without Altering Bone Loss, Tumor Size, or Cancer Cell Proliferation in a Mouse Model of Cancer-Induced Bone Pain. J. Pain 2018, 19, 612–625. [Google Scholar] [CrossRef]
- Sounvoravong, S.; Takahashi, M.; Nakashima, M.N.; Nakashima, K. Disability of Development of Tolerance to Morphine and U-50,488H, a Selective κ-Opioid Receptor Agonist, in Neuropathic Pain Model Mice. J. Pharmacol. Sci. 2004, 94, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Lara, A.; Cabañero, D.; Maldonado, R. Kappa Opioid Receptor Modulation of Endometriosis Pain in Mice. Neuropharmacology 2021, 195, 108677. [Google Scholar] [CrossRef]
- Beck, T.C.; Reichel, C.M.; Helke, K.L.; Bhadsavle, S.S.; Dix, T.A. Non-Addictive Orally-Active Kappa Opioid Agonists for the Treatment of Peripheral Pain in Rats. Eur. J. Pharmacol. 2019, 856, 172396. [Google Scholar] [CrossRef] [PubMed]
- Briggs, S.L.; Rech, R.H.; Sawyer, D.C. Kappa Antinociceptive Activity of Spiradoline in the Cold-Water Tail-Flick Assay in Rats. Pharmacol. Biochem. Behav. 1998, 60, 467–472. [Google Scholar] [CrossRef]
- Tang, A.H.; Collins, R.J. Behavioral Effects of a Novel Kappa Opioid Analgesic, U-50488, in Rats and Rhesus Monkeys. Psychopharmacology 1985, 85, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Mori, A.; Yanagita, T. One Year Long-Term Study on Abuse Liability of Nalfurafine in Hemodialysis Patients. Int. J. Clin. Pharmacol. Ther. 2013, 51, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Shook, J.E.; Watkins, W.D.; Camporesi, E.M. Differential Roles of Opioid Receptors in Respiration, Respiratory Disease, and Opiate-Induced Respiratory Depression. Am. Rev. Respir. Dis. 2012, 142, 909. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, E.R.; Torjman, M.C.; Munera, C.L.; Stauffer, J.W.; Setnik, B.S.; Bagal, S.N. Effect of Difelikefalin, a Selective Kappa Opioid Receptor Agonist, on Respiratory Depression: A Randomized, Double-blind, Placebo-controlled Trial. Clin. Transl. Sci. 2021, 14, 1886. [Google Scholar] [CrossRef]
- Porreca, F.; Mosberg, H.I.; Hurst, R.; Hruby, V.J.; Burks, T.F. Roles of Mu, Delta and Kappa Opioid Receptors in Spinal and Supraspinal Mediation of Gastrointestinal Transit Effects and Hot-Plate Analgesia in the Mouse. J. Pharmacol. Exp. Ther. 1984, 230, 341–348. [Google Scholar] [CrossRef]
- Bals-Kubik, R.; Ableitner, A.; Herz, A.; Shippenberg, T.S. Neuroanatomical Sites Mediating the Motivational Effects of Opioids as Mapped by the Conditioned Place Preference Paradigm in Rats. J. Pharmacol. Exp. Ther. 1993, 264, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ehrich, J.M.; Messinger, D.I.; Knakal, C.R.; Kuhar, J.R.; Schattauer, S.S.; Bruchas, M.R.; Zweifel, L.S.; Kieffer, B.L.; Phillips, P.E.M.; Chavkin, C. Kappa Opioid Receptor-Induced Aversion Requires P38 MAPK Activation in VTA Dopamine Neurons. J. Neurosci. 2015, 35, 12917–12931. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, K.; Kawai, K.; Suzuki, T.; Kawamura, K.; Tanaka, T.; Narita, M.; Nagase, H.; Suzuki, T. Possible Pharmacotherapy of the Opioid κ Receptor Agonist for Drug Dependence. Ann. N. Y. Acad. Sci. 2004, 1025, 404–413. [Google Scholar] [CrossRef]
- Mague, S.D.; Pliakas, A.M.; Todtenkopf, M.S.; Tomasiewicz, H.C.; Zhang, Y.; Stevens, W.C.; Jones, R.M.; Portoghese, P.S.; Carlezon, W.A. Antidepressant-Like Effects of κ-Opioid Receptor Antagonists in the Forced Swim Test in Rats. J. Pharmacol. Exp. Ther. 2003, 305, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mello, N.K.; Negus, S.S. Interactions between Kappa Opioid Agonists and Cocaine: Preclinical Studies. Ann. N. Y. Acad. Sci. 2000, 909, 104–132. [Google Scholar] [CrossRef]
- Mori, T.; Nomura, M.; Yoshizawa, K.; Nagase, H.; Narita, M.; Suzuki, T. Differential Properties between TRK-820 and U-50,488H on the Discriminative Stimulus Effects in Rats. Life Sci. 2004, 75, 2473–2482. [Google Scholar] [CrossRef]
- Nagase, H.; Hayakawa, J.; Kawamura, K.; Kawai, K.; Takezawa, Y.; Matsuura, H.; Tajima, C.; Endo, T. Discovery of a Structurally Novel Opioid K-Agonist Derived from 4, 5-Epoxymorphinan. Chem. Pharm. Bull. 1998, 46, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shiozaki, Y.; Masukawa, Y.; Misawa, M.; Nagase, H. The Role of Mu- and Kappa-Opioid Receptors in Cocaine-Induced Conditioned Place Preference. Jpn. J. Pharmacol. 1992, 58, 435–442. [Google Scholar] [CrossRef]
- Todtenkopf, M.S.; Marcus, J.F.; Portoghese, P.S.; Carlezon, W.A. Effects of κ-Opioid Receptor Ligands on Intracranial Self-Stimulation in Rats. Psychopharmacology 2004, 172, 463–470. [Google Scholar] [CrossRef]
- Walsh, S.L.; Strain, E.C.; Abreu, M.E.; Bigelow, G.E. Enadoline, a Selective Kappa Opioid Agonist: Comparison with Butorphanol and Hydromorphone in Humans. Psychopharmacology 2001, 157, 151–162. [Google Scholar] [CrossRef]
- van de Wetering, R.; Ewald, A.; Welsh, S.; Kornberger, L.; Williamson, S.E.; McElroy, B.D.; Butelman, E.R.; Prisinzano, T.E.; Kivell, B.M. The Kappa Opioid Receptor Agonist 16-Bromo Salvinorin A Has Anti-Cocaine Effects without Significant Effects on Locomotion, Food Reward, Learning and Memory, or Anxiety and Depressive-like Behaviors. Molecules 2023, 28, 4848. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Land, B.B.; Aita, M.; Xu, M.; Barot, S.K.; Li, S.; Chavkin, C. Stress-Induced P38 Mitogen-Activated Protein Kinase Activation Mediates κ-Opioid-Dependent Dysphoria. J. Neurosci. 2007, 27, 11614–11623. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Macey, T.A.; Lowe, J.D.; Chavkin, C. Kappa Opioid Receptor Activation of P38 MAPK Is GRK3- and Arrestin-Dependent in Neurons and Astrocytes. J. Biol. Chem. 2006, 281, 18081–18089. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, C.; Ormazábal, M.J.; Abalo, R.; Alfaro, M.J.; Martín, M.I. Calcitonin Reverts Pertussis Toxin Blockade of the Opioid Analgesia in Mice. Neurosci. Lett. 1999, 273, 175–178. [Google Scholar] [CrossRef]
- Gullapalli, S.; Ramarao, P. Role of L-Type Ca2+ Channels in Pertussis Toxin Induced Antagonism of U50,488H Analgesia and Hypothermia. Brain Res. 2002, 946, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Soto-Moyano, R.; Mestre, C.; Eschalier, A.; Pelissier, T.; Paeile, C.; Contreras, E. Intrathecal Pertussis Toxin but Not Cyclic AMP Blocks Kappa Opioid-Induced Antinociception in Rat. Int. J. Neurosci. 1995, 81, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Chavkin, C. Kinase Cascades and Ligand-Directed Signaling at the Kappa Opioid Receptor. Psychopharmacology 2010, 210, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Spetea, M.; Eans, S.O.; Ganno, M.L.; Lantero, A.; Mairegger, M.; Toll, L.; Schmidhammer, H.; McLaughlin, J.P. Selective κ Receptor Partial Agonist HS666 Produces Potent Antinociception without Inducing Aversion after i.c.v. Administration in Mice. Br. J. Pharmacol. 2017, 174, 2444–2456. [Google Scholar] [CrossRef]
- White, K.L.; Robinson, J.E.; Zhu, H.; DiBerto, J.F.; Polepally, P.R.; Zjawiony, J.K.; Nichols, D.E.; Malanga, C.J.; Roth, B.L. The G Protein–Biased κ-Opioid Receptor Agonist RB-64 Is Analgesic with a Unique Spectrum of Activities In Vivo. J. Pharmacol. Exp. Ther. 2015, 352, 98–109. [Google Scholar] [CrossRef]
- Weerawarna, S.A.; Davis, R.D.; Nelson, W.L. Isothiocyanate-Substituted κ-Selective Opioid Receptor Ligands Derived from N-Methyl-N-[(1S)-1-Phenyl-2-(1-Pyrrolidinyl)Ethyl]Phenylacetamide. J. Med. Chem. 1994, 37, 2856–2864. [Google Scholar] [CrossRef]
- Costello, G.F.; Main, B.G.; Barlow, J.J.; Carroll, J.A.; Shaw, J.S. A Novel Series of Potent and Selective Agonists at the Opioid κ-Receptor. Eur. J. Pharmacol. 1988, 151, 475–478. [Google Scholar] [CrossRef]
- Gottschlich, R.; Prucher, H.; Ackermann, K.-A.; Barber, A.; Greiner, H.; Haase, A. Nitrogen-Containing Cyclic Compounds. U.S. Patent 0374756, 27 June 1990. [Google Scholar]
- Leeson, P.D.; Springthorpe, B. The Influence of Drug-like Concepts on Decision-Making in Medicinal Chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, M.A.M.; Meanwell, N.A. Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem. 2021, 64, 14046–14128. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.F. Heterocyclic Replacements for Benzene: Maximising ADME Benefits by Considering Individual Ring Isomers. Eur. J. Med. Chem. 2016, 124, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Vecchietti, V.; Giardina, G. Heterocyclic Derivatives. U.S. Patent EP 0333427 A1, 25 July 1989. [Google Scholar]
- Schmid, C.L.; Kennedy, N.M.; Ross, N.C.; Lovell, K.M.; Yue, Z.; Morgenweck, J.; Cameron, M.D.; Bannister, T.D.; Bohn, L.M. Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell 2017, 171, 1165–1175.e13. [Google Scholar] [CrossRef]
- Millan, M.J. κ-Opioid Receptors and Analgesia. Trends Pharmacol. Sci. 1990, 11, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Juranek, J.K.; Zygulska, A.L. Chemotherapy-Induced Neuropathies—A Growing Problem for Patients and Health Care Providers. Brain Behav. 2017, 7, e00558. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of Paclitaxel-Induced Peripheral Neuropathy: A Current Review of in Vitro and in Vivo Findings Using Rodent and Human Model Systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef]
- Ferris, C.F.; Nodine, S.; Pottala, T.; Cai, X.; Knox, T.M.; Fofana, F.H.; Kim, S.; Kulkarni, P.; Crystal, J.D.; Hohmann, A.G. Alterations in Brain Neurocircuitry Following Treatment with the Chemotherapeutic Agent Paclitaxel in Rats. Neurobiol. Pain. 2019, 6, 100034. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Gallego, R.A.; Edwards, M.P. Lipophilic Efficiency as an Important Metric in Drug Design. J. Med. Chem. 2018, 61, 6401–6420. [Google Scholar] [CrossRef] [PubMed]
- El Daibani, A.; Paggi, J.M.; Kim, K.; Laloudakis, Y.D.; Popov, P.; Bernhard, S.M.; Krumm, B.E.; Olsen, R.H.J.; Diberto, J.; Carroll, F.I.; et al. Molecular Mechanism of Biased Signaling at the Kappa Opioid Receptor. Nat. Commun. 2023, 14, 1338. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Charlton, S.J. Biased Agonism in Drug Discovery—Is It Too Soon to Choose a Path? Mol. Pharmacol. 2018, 93, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Conibear, A.; Henderson, G. Biased Agonism: Lessons from Studies of Opioid Receptor Agonists. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.; Gondin, A.B.; Kliewer, A.; Sanchez, J.; Lim, H.D.; Alamein, C.; Manandhar, P.; Santiago, M.; Fritzwanker, S.; Schmiedel, F.; et al. Low Intrinsic Efficacy for G Protein Activation Can Explain the Improved Side Effect Profiles of New Opioid Agonists. Sci. Signal. 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.T.; Semache, M.; Gross, F.; Da Fonte, D.F.; Runtz, L.; Colley, C.; Mezni, A.; Le Gouill, C.; Lukasheva, V.; Hogue, M.; et al. Biased Signaling of the Mu Opioid Receptor Revealed in Native Neurons. iScience 2019, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Stahl, E.L.; Schmid, C.L.; Scarry, S.M.; Aubé, J.; Bohn, L.M. G Protein Signaling–Biased Agonism at the κ-Opioid Receptor Is Maintained in Striatal Neurons. Sci. Signal. 2018, 11, eaar4309. [Google Scholar] [CrossRef]
- Jamshidi, R.J.; Jacobs, B.A.; Sullivan, L.C.; Chavera, T.A.; Saylor, R.M.; Prisinzano, T.E.; Clarke, W.P.; Berg, K.A. Functional Selectivity of Kappa Opioid Receptor Agonists in Peripheral Sensory Neurons. J. Pharmacol. Exp. Ther. 2015, 355, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Winpenny, D.; Clark, M.; Cawkill, D. Biased Ligand Quantification in Drug Discovery: From Theory to High Throughput Screening to Identify New Biased μ Opioid Receptor Agonists. Br. J. Pharmacol. 2016, 173, 1393–1403. [Google Scholar] [CrossRef]
- Kenakin, T. The Effective Application of Biased Signaling to New Drug Discovery. Mol. Pharmacol. 2015, 88, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Bölcskei, K.; Kriszta, G.; Sághy, É.; Payrits, M.; Sipos, É.; Vranesics, A.; Berente, Z.; Ábrahám, H.; Ács, P.; Komoly, S.; et al. Behavioural Alterations and Morphological Changes Are Attenuated by the Lack of TRPA1 Receptors in the Cuprizone-Induced Demyelination Model in Mice. J. Neuroimmunol. 2018, 320, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A.; van Putten, M. Assessing Functional Performance in the Mdx Mouse Model. J. Vis. Exp. 2014, 85, 51303. [Google Scholar] [CrossRef]
- McLaughlin, J.; See, R.E. Selective Inactivation of the Dorsomedial Prefrontal Cortex and the Basolateral Amygdala Attenuates Conditioned-Cued Reinstatement of Extinguished Cocaine-Seeking Behavior in Rats. Psychopharmacology 2003, 168, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Zavou, M.J.; Milton, P.L.; Chan, S.T.; Tan, J.L.; Dickinson, H.; Murphy, S.V.; Jenkin, G.; Wallace, E.M. Measuring Respiratory Function in Mice Using Unrestrained Whole-Body Plethysmography. J. Vis. Exp. 2014, 51755. [Google Scholar] [CrossRef]
- Zysman-Colman, Z.; Lands, L.C. Whole Body Plethysmography: Practical Considerations. Paediatr. Respir. Rev. 2016, 19, 39–41. [Google Scholar] [CrossRef]
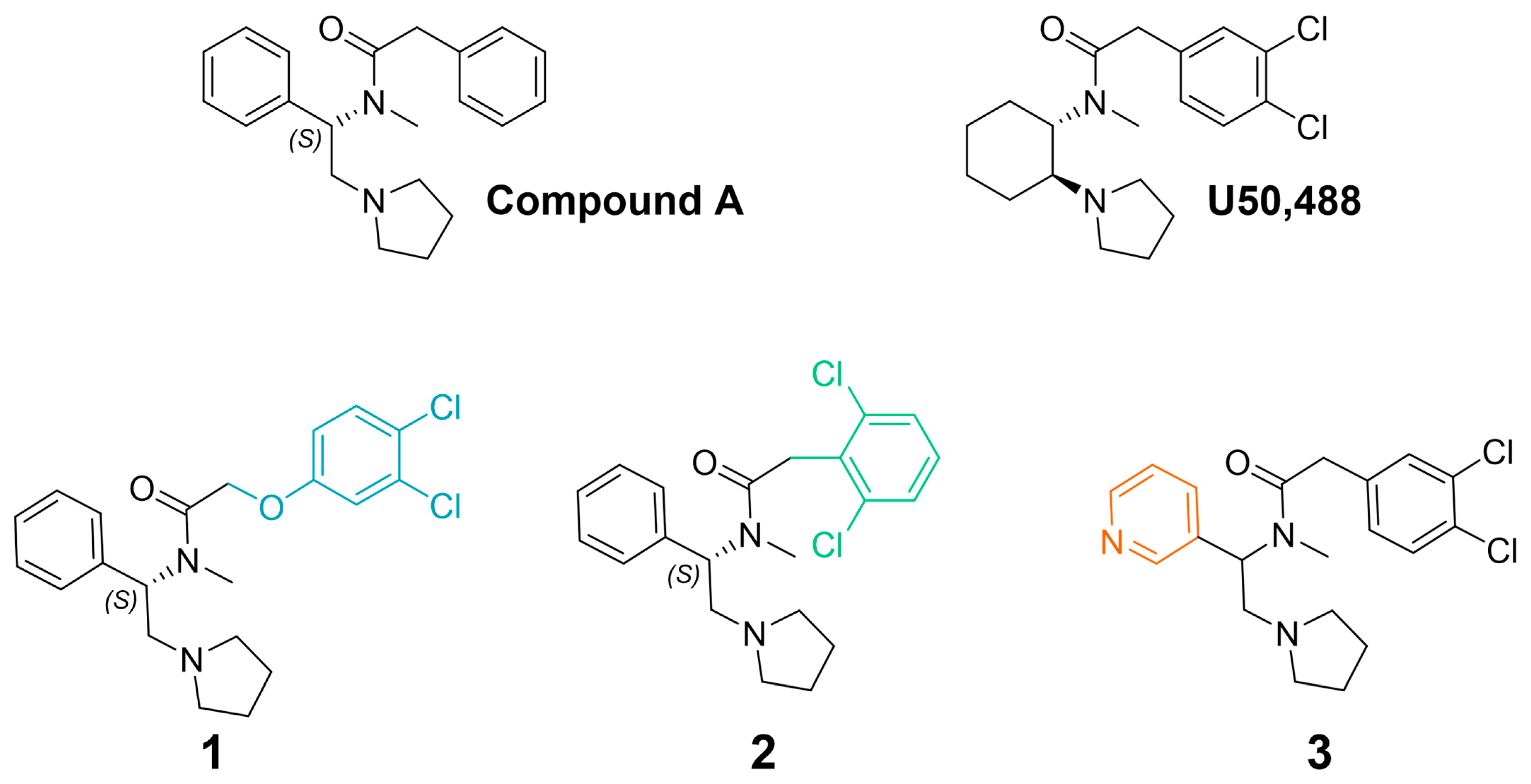
| Compound | KOR cAMP | KOR β-Arrestin2 | Bias Factor * | ||||
|---|---|---|---|---|---|---|---|
| EC50 (nM) | pEC50 ± SEM | %Emax ± SEM | EC50 (nM) | pEC50 ± SEM | %Emax ± SEM | ||
| U50,488 | 0.53 | 9.3 ± 0.2 | 100.5 ± 0.5 | 75 | 7.1 ± 0.2 | 100.4 ± 1.0 | 1.0 |
| 1 | 0.016 | 10.8 ± 0.2 | 100.7 ± 0.3 | 2.1 | 8.7 ± 0.1 | 107.0 ± 6.8 | 0.9 |
| 2 | 0.020 | 10.7 ± 0.1 | 101.2 ± 0.4 | 6.4 | 8.2 ± 0.1 | 106.0 ± 4.4 | 2.2 |
| 3 | 0.12 | 9.9 ± 0.2 | 98.6 ± 1.8 | 28 | 7.6 ± 0.1 | 90.4 ± 5.6 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Wetering, R.; Vu, L.Y.; Kornberger, L.D.; Luo, D.; Scouller, B.; Hong, S.; Paton, K.; Prisinzano, T.E.; Kivell, B.M. Effects of Biased Analogues of the Kappa Opioid Receptor Agonist, U50,488, in Preclinical Models of Pain and Side Effects. Molecules 2025, 30, 604. https://doi.org/10.3390/molecules30030604
van de Wetering R, Vu LY, Kornberger LD, Luo D, Scouller B, Hong S, Paton K, Prisinzano TE, Kivell BM. Effects of Biased Analogues of the Kappa Opioid Receptor Agonist, U50,488, in Preclinical Models of Pain and Side Effects. Molecules. 2025; 30(3):604. https://doi.org/10.3390/molecules30030604
Chicago/Turabian Stylevan de Wetering, Ross, Loan Y. Vu, Lindsay D. Kornberger, Dan Luo, Brittany Scouller, Sheein Hong, Kelly Paton, Thomas E. Prisinzano, and Bronwyn M. Kivell. 2025. "Effects of Biased Analogues of the Kappa Opioid Receptor Agonist, U50,488, in Preclinical Models of Pain and Side Effects" Molecules 30, no. 3: 604. https://doi.org/10.3390/molecules30030604
APA Stylevan de Wetering, R., Vu, L. Y., Kornberger, L. D., Luo, D., Scouller, B., Hong, S., Paton, K., Prisinzano, T. E., & Kivell, B. M. (2025). Effects of Biased Analogues of the Kappa Opioid Receptor Agonist, U50,488, in Preclinical Models of Pain and Side Effects. Molecules, 30(3), 604. https://doi.org/10.3390/molecules30030604







