4.1. Chemistry
Reagents were obtained from Sigma Aldrich and Enamine were used without further purification. All organic solvents used were of pure analytical grade. Dioxane was dried over molecular sieves (4 Å). THF was dried by refluxing under Argon with Na metal and benzophenone as an indicator for dryness. Column chromatography was carried out using silica-gel 40–60 μm mesh with CHCl3:MeOH:NH4OH, DCM:MeOH, EtOAc:Heptane, CHCl3:MeOH or EtOAc:MeOH:NH4OH. Mass spectrometry was performed on APCI-MS (Advion expressionS CMS; direct analysis probe), the flow rate used was 10 μL/min, the super soft method was used to avoid fragmentation and the m/z range was 10 to 1000 with an acquisition speed of 10,000 m/z units/sec; for ESI-MS (LCQ-Classic, Thermo Finnigan; direct injection), the flow rate was 20 μL/min, the scan range was 50–2000 m/z, the voltage was 5 KV and the capillary temperature was 220 °C. TLC was stained with KMnO4 for the detection of spots. 1H-NMR and 13C-NMR spectra were recorded at 400 MHz and 101 MHz, respectively, using a Varian VNMS-MR 400 spectrometer. 1H shifts are referenced to the residual protonated solvent signal (δ 7.26 for CDCl3, δ 3.31, 4.87 for CD3OD and δ 2.5 for DMSO-d6) and 13C shifts are referenced to the deuterated solvent signal (δ 77.0 for CDCl3, δ 49.0 for CD3OD and δ 39.5 for DMSO-d6). Chemical shifts are given in parts per million (ppm), and all coupling constants (J) are given in Hz.
The purities of compounds 9, 12, 18, 19 and 21 were determined by HPLC (Jasco.). Pump: PU-980 intelligent HPLC pump, Detector: UV-975 intelligent UV/VIS detector and Sampler: 851-AS intelligent sampler. HPLC-method: Flow rate 1 mL/min. The solvent system used was acetonitrile: water, 60:40 + 0.05% TFA. The pressure was 67 kg/cm2. Peaks were detected at λ = 220 nm.
The purities of compounds 10, 11, 13, 14, 15 and 20 were determined by HPLC Shimadzu (Kyoto, Japan). Pump: two LC-10AD pumps, Detector: SPD-M10A VP PDA detector and Sampler: SIL-HT autosampler. Analytical HPLC: a LiChrospher column RP-18, 5 μm particle size and 10 cm length were used, and the solvent system was MeOH:H2O, with 5 to 95% + 0.05% TFA over 30 min. The solvent system used for compound 20 was acetonitrile: water, 5 to 95%, with a flow rate of 1.2 mL/min over 6 min. Peaks were detected at λ = 254 nm.
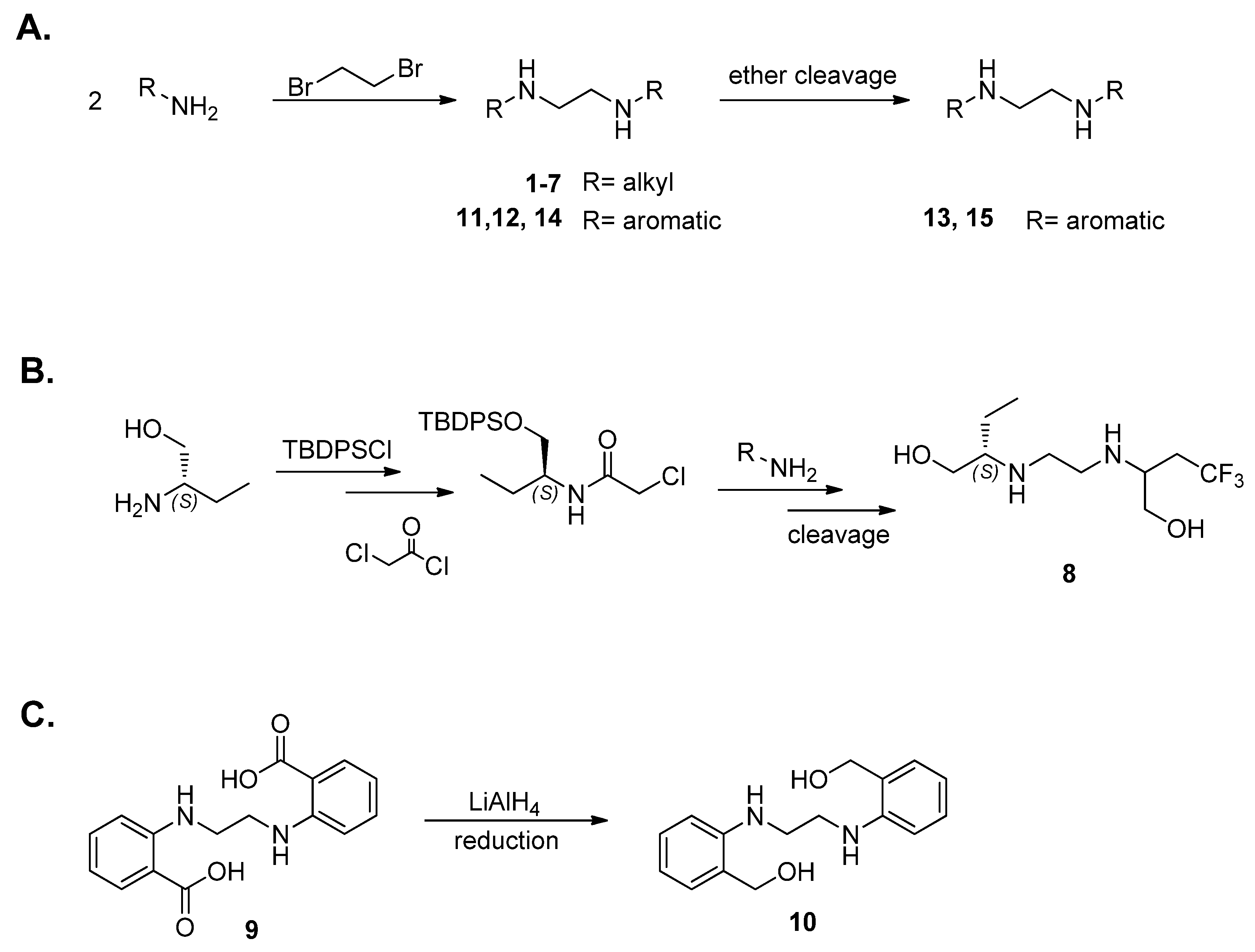
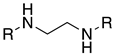
4.1.1. General Procedures for Synthesis of 2,2′-(Ethylene-1,2-diamino)dibutan-1-ol Analogs (Scheme 1A)
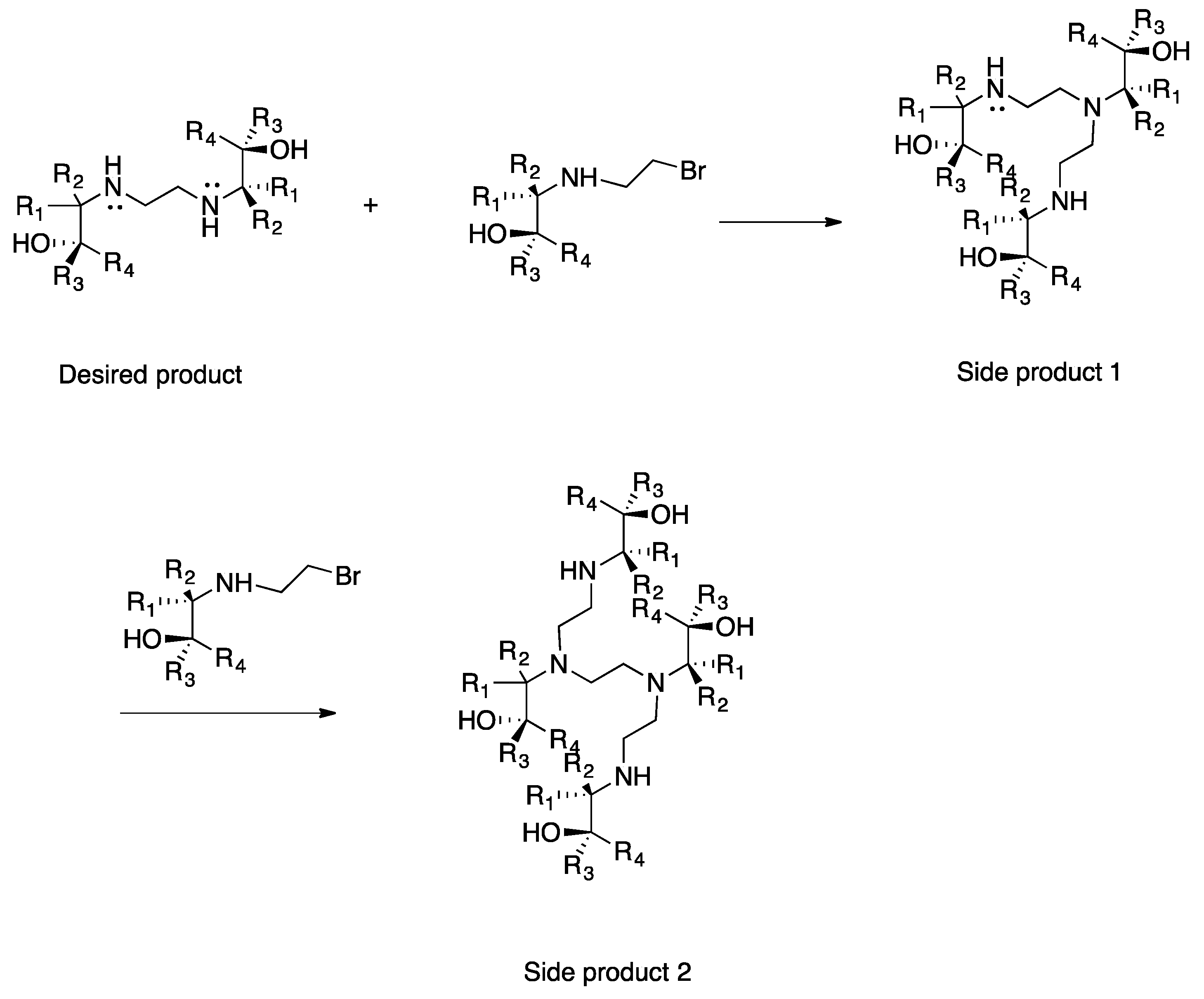
Dibromoethane (DBE) was added dropwise to the respective amino alcohol in a 25 mL two-necked round bottom flask (1:2 moles). After the addition of DBE was complete, the reaction was left to stir at 80 °C and stopped when side products were detected in the APCI-MS spectra (
Figure 4). After cooling down, one of two work-up procedures was carried out.
Method 1. The solid or sticky oil produced was dissolved in H
2O and washed with CHCl
3. The aqueous phase was basified with 2 equiv. NaOH solution and stirred for 10 min. The free base of the product was extracted with CHCl
3 (50 mL × 3) and combined organic phases were dried over anhydrous Na
2SO
4. The solvent was evaporated and the product was purified by column chromatography (CHCl
3:MeOH:NH
4OH, 88:10:2) [
18].
Method 2. In total, 1.75 M of KOH in ethanol was added and stirring was continued at room temperature for 1 h. The precipitated KBr was then filtered, and ethanol was evaporated under reduced pressure. The resulting oil was purified by recrystallization/precipitation. The oil was dissolved in the least amount of CHCl
3 and then heptane was added dropwise until the solution became turbid and then left overnight. The formed precipitate was filtered by vacuum filtration [
19].
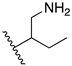
(2S,2′S)-2,2′-(ethylene-1,2-diamino)di(3-methylbutan-1-ol) (1): The synthesis followed the general procedure mentioned above, but the reaction was left for 10 h until all start (S-valinol) was consumed and method 1 was used for the work-up. C12H28N2O2, yellow oil; yield: 24%; 1H NMR (400 MHz, CDCl3) δ 3.62 (dd, J = 10.8, 3.9 Hz, 2H), 3.38 (dd, J = 10.8, 7.9 Hz, 2H), 2.89–2.83 (m, 2H), 2.73–2.63 (m, 6H), 2.39 (ddd, J = 7.9, 6.4, 3.9 Hz, 2H), 1.76 (dq, J = 13.5, 6.8 Hz, 2H), 0.92 (dd, J = 24.0, 6.8 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 64.5, 61.7, 47.1, 29.2, 19.3, 18.5; MS (APCI): m/z 233.3 (M+ + 1); Rf = 0.24 (CHCl3:MeOH: NH4OH, 88:10:2).
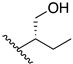
(2S,2′S)-2,2′-(ethylene-1,2-diamino)di(propan-1-ol) (2): It followed the general procedure using S-alaninol, and the second procedure for work-up was used. C8H20N2O2, white powder; yield: 10%; m.p. 92–95 °C; 1H NMR (400 MHz, CD3OD) δ 3.48 (dd, J = 10.9, 4.9 Hz, 2H), 3.39 (dd, J = 10.8, 6.8 Hz, 2H), 2.85–2.77 (m, 2H), 2.77–2.70 (m, 2H), 2.70–2.62 (m, 2H), 1.04 (d, J = 6.5 Hz, 6H); 13C NMR (101 MHz, CD3OD) δ 66.4, 55.7, 47.1, 16.7; MS (APCI): m/z 177.2 (M+ + 1); Rf = 0.35 (CHCl3:MeOH:NH4OH, 88:10:2).
1,1′-(Ethylene-1,2-diamino)di(2-methylpropan-2-ol) (3): It followed the general procedure using 1-amino-2-methyl-2-propanol, but the reaction was carried out after 1.5 h. The second work-up method was used. Note that this time, crystallization took longer than for other derivatives. C10H24N2O2, off-white semi-solid; yield: 16.5%; 1H NMR (400 MHz, CDCl3) δ 2.78 (s, 4H), 2.54 (s, 4H), 1.17 (s, 12H); 13C NMR (101 MHz, CDCl3) δ 69.5, 60.0, 49.7, 27.4. MS (APCI): 205.18 m/z (M+ + 1).
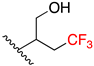
2,2′-(Ethylene-1,2-diamino)di(4,4,4-trifluorobutan-1-ol) (4): 2-amino-4,4,4-triflourobutan-1-ol.HCl (2.828 mmol) was stirred with DIPEA (2.828 mmol) at 60 °C and then DBE (1.414 mmol) was added dropwise. The mixture was left to stir for 4 h. After cooling down, the second work-up method mentioned in the general procedure was used; however, only CHCl3 precipitate started to form and was filtered after being left overnight. C10H18F6N2O2, white powder; yield: 27%; m.p. 117–122 °C; 1H NMR (400 MHz, CD3OD) δ 3.63 (dd, J = 11.2, 4.7 Hz, 2H), 3.50 (dd, J = 11.2, 5.8 Hz, 2H), 2.90 (qd, J = 5.9, 4.6 Hz, 2H), 2.74 (s, 4H), 2.45–2.19 (m, 4H); 13C NMR (101 MHz, CD3OD) δ 126.9 (d, 1JCF = 275.8 Hz), 62.4, 53.9 (d, 3JCF = 2.5 Hz), 45.8, 45.7, 34.9 (q, 2JCF = 27.4 Hz); MS (APCI): m/z 313.3 (M+ + 1); Rf = 0.23 (CHCl3:MeOH:NH4OH, 88:10:2).
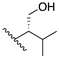
2,2′-(Ethylene-1,2-diamino)di(4-fluoro-2-methylbutan-1-ol) (5): The synthesis followed the general procedure using 2-amino-4-fluoro-2-methylbutan-1-ol and the second work-up method was carried out exactly as for compound 4. C12H26F2N2O2, white powder; yield: 4.4%; m.p. 115–123 °C; 1H NMR (400 MHz, CD3OD) δ 4.65 (td, J = 6.4, 1.8 Hz, 2H), 4.53 (td, J = 6.4, 1.8 Hz, 2H), 3.41 (s, 4H), 2.62 (s, 4H), 1.86 (t, J = 6.3 Hz, 2H), 1.80 (t, J = 6.3 Hz, 2H), 1.07 (s, 6H); 13C NMR (101 MHz, CD3OD) δ 80.6 (d, 1JCF = 162.1 Hz), 65.8, 55.0 (d, 4JCF = 3.9 Hz), 41.0, 36.1 (dd, 2JCF = 18.7, 3.0 Hz), 20.4; MS (APCI): m/z 269.3 (M+ + 1); Rf = 0.14 (CHCl3:MeOH:NH4OH, 88:10:2).
2,2′-(Ethylene-1,2-diamino)di(4,4-difluorobutan-1-ol) (6): The compound was synthesized with the same procedure as compound 4 using 2-amino-4,4,-difluorobutan-1-ol but precipitation failed and column chromatography was used instead to purify the product (CHCl3:MeOH:NH4OH, 78:20:2). C10H20F4N2O2, beige, semi-solid; yield: 6%; 1H NMR (400 MHz, CD3OD) δ 6.33–5.82 (m, 2H), 3.70–3.57 (m, 2H), 3.56–3.45 (m, 2H), 2.94–2.83 (m, 2H), 2.86–2.63 (m, 4H), 2.08–1.89 (m, 4H).; 13C NMR (101 MHz, CD3OD) δ 16.5 (t, J = 237.2 Hz), 113.8 (t, 1JCF = 237.4 Hz), 54.1 (t, 3JCF = 5.5 Hz), 54.0 (t, 3JCF = 5.5 Hz), 36.2 (t, 2JCF = 21.2 Hz), 35.7 (t, 2JCF = 21.0 Hz); MS(APCI): m/z 277.1 (M+ + 1); Rf = 0.35 (CHCl3:MeOH:NH4OH, 78:20:2).
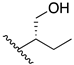
(2S,2′S)-2,2′-(ethane-1,2-diylbis(azanediyl))bis(but-3-en-1-ol) (7) The compound was synthesised by adding dibromoethane (DBE) dropwise to (S)-2-aminobut-3-en1-ol hydrochloride in a 25 mL two-necked round bottom flask (1:2 moles). After the addition of DBE was complete, the reaction was left to stir at 60 °C for 2 h. The sticky oil produced was dissolved in H2O and washed with CHCl3. The aqueous phase was basified with 2 equiv. NaOH solution and stirred for 30 min. The free base of the product was extracted with CHCl3/EtOH (2:1) and combined organic phases were dried over anhydrous Na2SO4. C10H20N2O2, white, semi-solid; yield: 5%; 1H NMR (400 MHz, CD3OD) δ 5.63 (ddd, J = 17.3, 10.3, 8.1 Hz, 2H), 5.24 (ddd, J = 17.3, 1.8, 0.9 Hz, 2H), 5.19 (ddd, J = 10.4, 1.8, 0.7 Hz, 2H), 3.53 (dd, J = 10.9, 4.9 Hz, 2H), 3.44 (dd, J = 10.9, 7.6 Hz, 2H), 3.19–3.09 (m, 2H), 2.84–2.71 (m, 2H), 2.67–2.54 (m, 2H), 13C NMR (101 MHz, CD3OD) 138.7, 118.6, 65.7, 64.7, 47.3; MS(APCI): m/z 201.158 (M+ + 1); Rf = 0.33 (DCM:MeOH:NH4OH, 79:20:1).
4.1.2. Synthesis of Asymmetric EMB Analog (8, Scheme 1B) [11]
Synthesis of [(2S)-2-aminobutoxy](tert-butyl)diphenylsilane (1i): TBDSiCl (1.1 equiv.) was added dropwise to a mixture of imidazole (1.1 equiv.) in DCM at 0 °C. After stirring for 15 min, (s)-(+), amino alcohol was added. The solution was left to stir for 16 h at room temperature before being poured into a saturated NaHCO3 solution. The pH was kept below 12 to avoid the cleavage of the tert-butyldiphenylsilane. The organic phase was extracted with DCM (3×) and the collected organic phases were dried over MgSO4. DCM was evaporated under vacuum and the crude oil was purified by column chromatography (DCM:1% MeOH + drops of NH4OH) to give 1i oil. C20H29NOSi, 77% yield, APCI-MS: m/z 328.3 [M + H]+, 1H NMR (400 MHz, CDCl3) δ 7.72–7.63 (m, 4H), 7.48–7.33 (m, 6H), 3.63 (dd, J = 9.9, 4.1 Hz, 1H), 3.43 (dd, J = 9.8, 7.1 Hz, 1H), 2.85–2.74 (m, 1H), 1.52–1.36 (m, 2H), 1.07 (s, 9H), 0.89 (t, J = 7.5 Hz, 3H).
Synthesis of N-[(2S)-1-[(tert-butyldiphenylsilyl)oxy]butan-2-yl]-2-chloroacetamide (1j): Chloroacetyl chloride (1.1 equiv.) was added dropwise to a cooled solution (0 °C) of 1i and DIPEA (2 equiv.) in DCM. The solution was left to stir overnight at room temperature. Water was added to quench the reaction. The organic phase was extracted with EtOAc (3×) and dried over MgSO4. After the evaporation of the solvent, flash chromatography (tert-butylmethylether:heptane, 1:2) was used to obtain the pure 1j. C22H30ClNO2Si, 50% yield, APCI-MS: m/z 404.2 [M]+, 1H NMR (400 MHz, CDCl3) δ 7.70–7.60 (m, 4H), 7.50–7.33 (m, 6H), 6.96–6.89 (m, 1H), 4.12–3.96 (m, 2H), 3.98–3.85 (m, 1H), 3.70 (d, J = 3.3 Hz, 2H), 1.79–1.56 (m, 2H), 1.12–1.05 (m, 9H), 0.95–0.81 (m, 3H).
Synthesis of N-[(2S)-1-[(tert-butyldiphenylsilyl)oxy]butan-2-yl]-2-[(4,4,4-trifluoro-1-hydroxybutan-2-yl)-amino]acetamide (1k): 2-amino-4,4,4-triflourobutan-1-ol.HCl (1 equiv.) was added to a cooled solution (0 °C) of 1j and DIPEA (3 equiv.) in DMF. The reaction was stirred at 70 °C for 14 h before quenching with water. The organic phase was extracted with CHCl3 and brine (3×) to get rid of DIPEA and DMF. The collected organic phases were dried over MgSO4. Flash chromatography (CHCl3:MeOH + 1% NH4OH, 0 → 5%) yielded 1k. C26H37F3N2O3Si, orange oil, 40% yield, APCI-MS: m/z 510.3 [M]+, 1H NMR (400 MHz, CDCl3) δ 7.64 (dq, J = 8.0, 1.8 Hz, 4H), 7.49–7.33 (m, 6H), 7.15 (d, J = 9.3 Hz, 1H), 7.03 (d, J = 9.1 Hz, 1H), 3.97–3.85 (m, 1H), 3.76–3.59 (m, 3H), 3.48 (dd, J = 11.1, 4.8 Hz, 1H), 3.26 (d, J = 1.7 Hz, 2H), 3.00–2.88 (m, 1H), 2.45 (s, 1H), 2.41–2.12 (m, 2H), 1.76–1.46 (m, 2H), 1.07 (s, 9H), 0.86 (td, J = 7.5, 2.7 Hz, 3H).
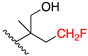
Synthesis of 4,4,4-trifluoro-2-[(2-{[(2S)-1-hydroxybutan-2-yl]amino}ethyl)amino]butan-1-ol (8) A two-necked round-bottom flask was dried and flushed with Argon. LiAlH4 (6 equiv.) was suspended in dry dioxane and cooled to 0 °C. 1k (1 equiv.) was diluted in a small amount of dry dioxane before being added dropwise to the suspension. The mixture was then left to stir under Reflux (100 °C) overnight. For the workup, the reaction flask was cooled to 0 °C and H2O (H2O:LiAlH4, 1:1), NaOH 15% aq. Solution (NaOH:LiAlH4, 1:1) and H2O (H2O:LiAlH4, 3:1) were cautiously added and left to stir overnight at room temperature. The solids were removed by vacuum filtration and the filtrate was concentrated under reduced pressure. The crude oil was dissolved in CHCl3 and then drops of heptane were added until 8 crushed out as a white precipitate. It was filtered and left overnight in the desiccator to dry. C10H21F3N2O2, white solid, 9% yield, m.p. 118–120 °C, calculated monoisotopic mass m/z 258.15551, ESI-HRMS: m/z 281.1448 [M + Na]+, 1H NMR (400 MHz, CD3OD) δ 3.71–3.60 (m, 2H), 3.53 (dd, J = 11.2, 5.7 Hz, 1H), 3.45 (dd, J = 11.0, 6.6 Hz, 1H), 2.94 (m, 1H), 2.82–2.68 (m, 4H), 2.52 (tt, J = 6.8, 5.0 Hz, 1H), 2.46–2.25 (m, 2H), 1.63–1.39 (m, 2H), 0.97 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 126.9 (q, CF3, 1JCF = 275.9 Hz), 62.6, 62.4 (d, 4JCF = 1.2 Hz), 60.6–60.5 (m), 53.9 (q, 3JCF = 2.4 Hz), 46.0, 45.8, 34.9 (q, 2JCF = 27.2 Hz), 23.4, 9.3. The HPLC purity was 89% and was not further purified since it did not show any in vitro activity.
4.1.3. Synthesis of Symmetric EMB Analogs Having N-Atoms as Substituents on Aromatic Systems (Scheme 1A,C)
N,N′-Ethylenedianthranilic acid (9) was commercially available. C
16H
16N
2O
4, white powder; m.p.228 °C [
20];
1H NMR (400 MHz, DMSO-
d6) δ 7.78 (dd,
J = 8.0, 1.7 Hz, 2H), 7.35 (ddd,
J = 8.7, 7.1, 1.8 Hz, 2H), 6.82 (dd,
J = 8.6, 1.0 Hz, 2H), 6.56 (ddd,
J = 8.0, 7.1, 1.0 Hz, 2H), 3.45 (s, 4H);
13C NMR (101 MHz, DMSO-
d6) δ 170.3, 151.2, 134.9, 132.2, 114.8, 111.7, 110.6, 41.7; MS (APCI):
m/
z 301.1 (M
+ + 1).
2,2′-(ethylene-1,2-diamino)-di(phenylmethanol) (10): In a two-necked 25 mL round bottom flask under Argon, LiAlH4 (1.99 mmol, 6 equiv.) was suspended in 10 mL dry THF. N,N′-ethylenedianthranilic acid (0.33 mmol) was added and the mixture was heated under reflux for 1 h. The completion of the reaction was concluded using TLC (EtOAc:Heptane, 1:1). The reaction was cooled to 0 °C with an ice/water bath. Then, it was treated cautiously in sequence with H2O (0.1 mL), NaOH 15% aq. solution (0.1 mL) and H2O (0.3 mL) and left to stir overnight. THF was decanted and the residue was filtered under vacuum and washed with EtOAc and CHCl3. Organic layers were combined and dried over anhydrous Na2SO4. When evaporating some of the solvent under reduced pressure, crystals started to appear, and the flask was removed and left to crystallize for 3 days. Crystals of pure 10 were then filtered. C16H20N2O2, yellow crystals; yield: 67%; m.p. 123–124 °C; 1H NMR (400 MHz, CD3OD) δ 7.19–7.10 (m, 2H), 7.07 (dd, J = 7.4, 1.6 Hz, 2H), 6.74 (dd, J = 8.1, 1.1 Hz, 2H), 6.63 (td, J = 7.4, 1.1 Hz, 2H), 4.56 (s, 4H), 3.44 (s, 4H), 3.34 (s, 4H); 13C NMR (101 MHz, CD3OD) δ 148.5, 130.0, 129.86, 126.8, 117.9, 111.8, 64.1, 43.8; MS (APCI): m/z 273.2 (M+ + 1); Rf = 0.22 (EtOAc:Heptane, 1:1).
N1,N2-di(2-methoxyphenethyl)ethane-1,2-diamine dihydrochloride (11): The compound was synthesized following the general procedure mentioned above, but the reaction was left for 16 h at 100 °C until all 2-methoxyphenethylamine was consumed and method one was used for the work-up, except the solid obtained after drying was dissolved in EtOH, neutralized with dil. HCl and then recrystallized using diethylether; C20H30N2O2Cl2, white solid; yield: 13%; m.p. 236–241 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.55 (s, 4H), 7.26 (t, J = 7.8 Hz, 2H), 6.98–6.75 (m, 6H), 3.76 (s, 6H), 3.33 (s, 4H), 3.27–3.16 (m, 4H), 2.98 (dd, J = 9.8, 6.5 Hz, 4H); 13C NMR (126 MHz, DMSO-d6) δ 159.9, 138.9, 130.2, 121.3, 114.8, 112.8, 55.5, 48.1, 43.2, 32.0; MS (ESI): m/z 329.4 (M+ + 1); Rf = 0.59 (MeOH:CHCl3 3% + 1% TFA).
N1,N2-bis(3,4-dimethoxyphenethyl)ethane-1,2-diamine dihydrochloride (12): The synthesis was the same as for compound 11, using 3,4-Dimethoxyphenylethylamine as the start. C22H34N2O4Cl2, white solid; yield 9%; m.p. 226–230 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 4H), 6.91–6.85 (m, 4H), 6.77 (dd, J = 8.2, 2.0 Hz, 2H), 3.76 (s, 6H), 3.73 (s, 6H), 3.34 (s, 4H), 3.18 (s, 4H), 2.90 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 148.8, 147.8, 129.2, 120.6, 112.6, 112.1, 55.6, 47.9, 42.8, 31.1. MS (ESI): m/z 389.3 (M+ + 1); Rf = 0.66 (CHCl3:3%MeOH:TEA 0.5%).
N1,N2-di((2-hydroxyphenyl)ethyl)ethane-1,2-diamine dihydrobromide (13): An aqueous solution HBr (48%, excess) was added carefully to 11. The mixture was left to stir for 3 h at 130 °C and then left overnight at room temperature. The reaction was monitored with thin-layer chromatography (TLC, CHCl3:MeOH 2%:NH4OH 0.05%). Water was evaporated under vacuum and the solid was purified by preparative HPLC. C18H26N2O22+, grey solid, 23% yield, melts with decomposition at 250 °C, calculated monoisotopic mass m/z 302.19943, ESI-HRMS: m/z 301.1912 (C18H25N2O2) [M + H]+, 1H NMR (400 MHz, Deuterium Oxide) δ 7.33 (t, J = 7.8 Hz, 2H), 6.95–6.84 (m, 6H), 3.46 (s, 4H), 3.42 (t, J = 7.3 Hz, 4H), 3.03 (t, J = 7.3 Hz, 4H); 13C NMR (101 MHz, Deuterium Oxide) δ 155.9, 137.9, 130.5, 120.9, 115.7, 114.3, 49.0, 43.1, 31.6.
N1,N2-bis(5-chloro-2-methoxyphenyl)ethane-1,2-diamine (14): Under an inert atmosphere, dibromoethane (DBE, 6.34 mmole) was added dropwise to a solution of 5-chloro-2-methoxyaniline (4 equiv.) in dry DMF. CaCO
3 (1.66 equiv.) was added and the mixture was stirred vigorously at 100 °C for 2 h [
16]. After the removal of DMF under vacuum, the mixture was extracted three times with DCM and dried over Na
2SO
4. The crude oil was purified by column chromatography (
tert-butylmethylether:heptane, 1:2 +1% TFA). C
16H
18Cl
2N
2O
2, White solid, 15% yield, m.p. 135–136 °C, calculated monoisotopic mass
m/
z 340.07453, ESI-HRMS:
m/
z 341.0814 [M + H]
+,
1H NMR (500 MHz, CDCl
3) δ 6.64 (d,
J = 8.3 Hz, 2H), 6.63 (dd,
J = 8.3, 2.2 Hz, 2H), 6.58 (d,
J = 2.2 Hz, 2H), 4.77–4.72 (m, 2H), 3.81 (s, 6H), 3.40 (s, 4H).
13C NMR (126 MHz, CDCl
3) δ 145.5, 138.9, 126.4, 115.9, 110.2, 109.8, 55.6, 42.7.
N1,N2-bis(5-chloro-2-hydroxyphenyl)ethane-1,2-diamine dihydrobromide (15): To cleave the ether, 5 equiv. of BBr
3 (1M in DCM) were slowly added to
14 and left to stir at 0 °C—room temperature for 5 h [
21]. The reaction progress was monitored by the disappearance of the start signal in the APCI-MS. MeOH was added to quench the reaction, and after the evaporation of the solvent, the pure
15 was obtained without further purification. C
14H
16Cl
2N
2O
22+, brown solid, 78% yield, m.p. 135–136 °C, calculated monoisotopic mass
m/
z 312.04323, ESI-HRMS:
m/
z 311.0366 [M − H]
−,392.9605 [M + Br]
−, 313.0506 [M + H]
+, 392.9588 [M + Br]
+.
1H NMR (400 MHz, CD
3OD) δ 7.18 (d,
J = 2.5 Hz, 2H), 7.08 (dd,
J = 8.7, 2.5 Hz, 2H), 6.90 (d,
J = 8.6 Hz, 2H), 3.65 (s, 4H);
13C NMR (101 MHz, CD
3OD) δ 148.9, 129.1, 127.4, 125.9, 120.4, 117.8, 45.8.
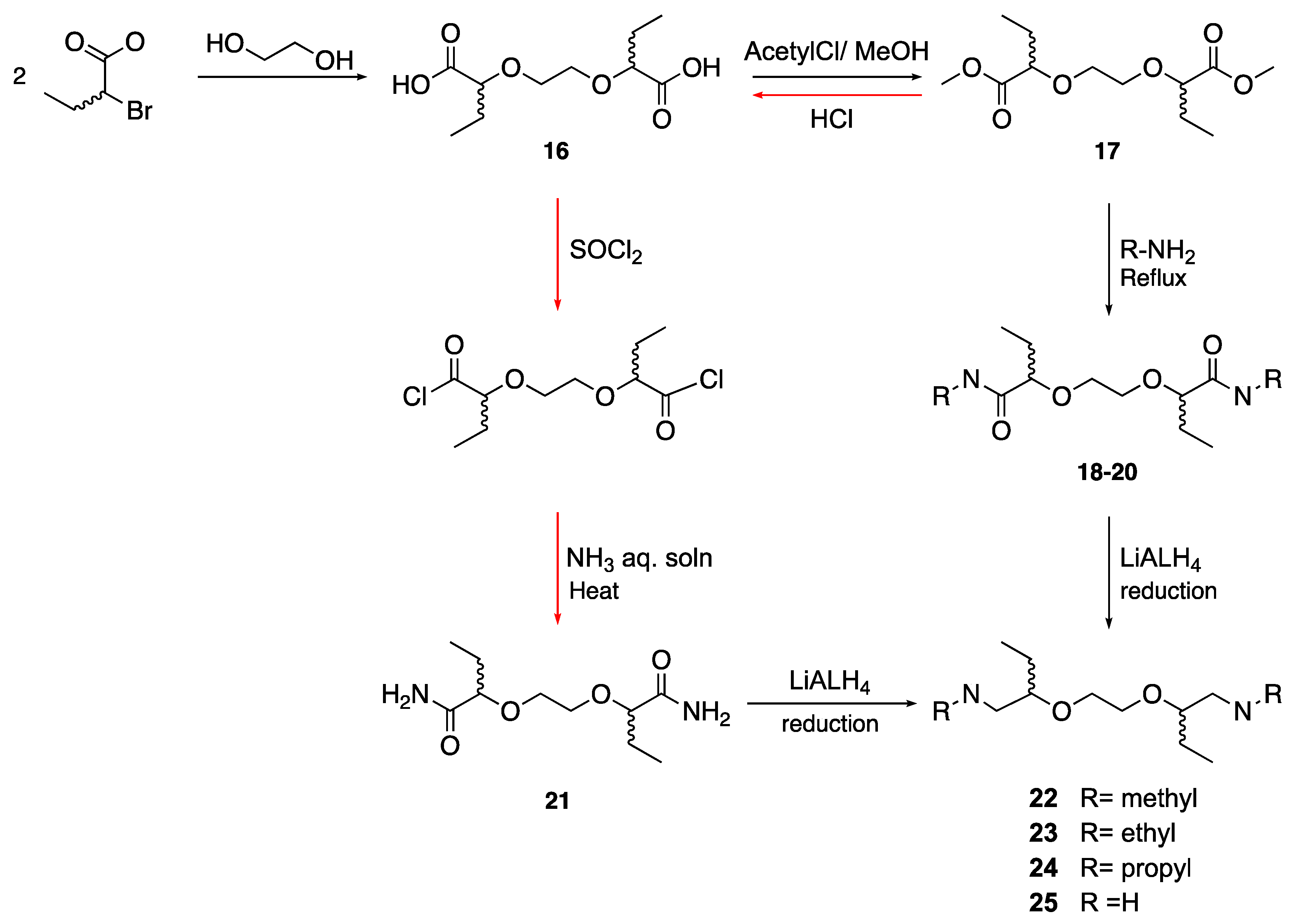
4.1.4. General Procedures for Synthesis of 2,2′-(Ethylene-1,2-dioxo)dibutan-1-amine Analogs (Scheme 2)
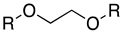
Synthesis of 2,2′-(ethylene-1,2-dioxo)dibutanoic acid. Method A: In a three-necked 250 mL round bottom flask and under Argon, NaH (4 equiv.) 60% in paraffin was washed with heptane (2 × 20 mL) to remove paraffin. After decanting the heptane, dry THF was added then ethylene glycol (1 equiv.) was added gradually, and the mixture was left to stir at room temperature for 30 min. The reaction flask was put in an ice bath (0 °C) and 2-bromobutyric acid (2 equiv.) was added dropwise after being diluted in dry THF. After the addition was complete, the reaction was left to reflux overnight. The reaction was quenched by the addition of distilled water. The acidification to pH~2 was accomplished by the dropwise addition of conc. HCl. The aqueous phase was extracted with ethyl acetate (3×), and then the combined organic fractions were dried over anhydrous Na
2SO
4. Ethyl acetate was removed under reduced pressure, leaving the yellow oil. The crude oil was taken to the next step without further purification [
22].
Method B. In a three-necked 250 mL round bottom flask under Argon, NaH (80 mmol) 60% in paraffin was suspended in 10 mL dry DMF. Ethylene glycol (10 mmol) was added, and the mixture was left at room temperature to stir for 30 min. The reaction flask was put in an ice bath (0 °C) and 2-bromobutyric acid (20 mmol) was added dropwise. After the addition was complete, the reaction was left at 60 °C. The reaction was quenched by the addition of distilled water. The acidification went to pH~2 by the dropwise addition of conc. HCl. The aqueous phase was extracted with ethyl acetate (×3), and then the combined organic fractions were passed over anhydrous Na2SO4. Ethyl acetate was evaporated. The oil obtained was purified by column chromatography (wash with 400 mL hexane to remove paraffin, DCM:MeOH 5%).
2,2′-(Ethylene-1,2-dioxo)-dibutanoic acid (16): C10H18O6, brown crystals; yield: 21%; melts with decomposition between 180–230 °C; 1H NMR (400 MHz, CD3OD) δ 3.86 (s, 4H), 3.77–3.65 (m, 2H), 1.84 (tt, J = 14.3, 7.1 Hz, 4H), 1.01–0.90 (m, 6H); 13C NMR (101 MHz, CD3OD) δ 178.2, 83.1, 68.5, 24.7, 8.4; MS(ESI): m/z 233.032 (M+ − 1); Rf = 0.31 (DCM:MeOH, 1:1).
Synthesis of dimethyl 2,2′-(ethylene-1,2-dioxo)dibutanoate: A solution of MeOH (20 mL) and acetyl chloride (1 mL) at 0 °C was left to stir for 15 min. before adding the diaicid (16) (2 mmol) portionwise. The mixture was heated to reflux overnight. MeOH was evaporated under vacuum, followed by the addition of water and saturated NaHCO3 to neutralize the aq. phase. This was followed by extraction with ethyl acetate (50 mL × 3); the combined organic layers were dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure to give an oily product. The resulting dimethylester was purified by column chromatography (EtOAc:Heptane, 1:1).
Dimethyl 2,2′-(ethylene-1,2-dioxo)dibutanoate (17): C10H22O6, oil; yield: 24%; 1H NMR (400 MHz, CDCl3) δ 3.90 (dt, J = 7.3, 5.4 Hz, 2H), 3.81–3.73 (m, 2H), 3.72 (s, 6H), 3.61–3.53 (m, 2H), 1.83–1.66 (m, 4H), 1.00–0.90 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 173.2, 173.2, 80.6, 80.4, 77.3, 77.0, 76.7, 69.9, 69.7, 51.7, 51.7, 26.2, 26.1, 9.6, 9.5; MS (ESI): m/z 285.79 (M+ + 23); MS (APCI): m/z 263.1 (M+ + 1); Rf = 0.54 (EtOAc:Heptane, 1:1).
Synthesis of 2,2′-(ethylene-1,2-dioxo)dibutanamide derivatives: In a 10 mL round bottom flask, the purified ester was added gradually to aqueous alkyl amine solution (4 equiv.). The mixture was left to stir at 70 °C (reflux) until the reaction was complete. For methyl amine (40% solution), the reaction took 3 h, while for ethylamine (70% solution), it took 3 days, and for propyl amine, (98% solution) it took 7 days. After the reaction was complete, the product was concentrated under reduced pressure and left in the desiccator overnight to remove water. The diamide was purified by column chromatography (EtOAc:MeOH:NH4OH, 94.5:5:0.5 18, CHCl3:MeOH:NH4OH, 89:10:1 19, CHCl3:MeOH, 95:5 20).
2,2′-(Ethylene-1,2-dioxo)di(N-methylbutanamide) (18): C12H24N2O4, oil; yield: 57%; 1H NMR (400 MHz, CDCl3) δ 6.81 (d, J = 65.5 Hz, 2H), 3.77 (ddd, J = 6.9, 5.5, 4.2 Hz, 2H), 3.73–3.68 (m, 2H), 3.68–3.59 (m, 2H), 2.84 (d, J = 4.9 Hz, 6H), 1.97–1.82 (m, 2H), 1.77–1.64 (m, 2H), 0.96 (td, J = 7.4, 3.9 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 172.9, 172.8, 82.1, 69.8, 69.6, 26.3, 25.9, 25.6, 9.3, 9.2; MS (APCI): m/z 261.1 (M+ + 1); Rf = 0.12 (EtOAc:MeOH:NH4OH, 94.5:5:0.5).
2,2′-(Ethylene-1,2-dioxo)di(N-ethylbutanamide) (19): C14H28N2O4, oil; yield: 18%; 1H NMR (400 MHz, CDCl3) δ 6.76 (d, J = 55.2 Hz, 2H), 3.75–3.63 (m, 4H), 3.64–3.59 (m, 2H), 3.36–3.17 (m, 4H), 1.94–1.78 (m, 2H), 1.75–1.55 (m, 2H), 1.12 (t, J = 7.3 Hz, 6H), 0.93 (td, J = 7.4, 4.0 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 172.0, 172.0, 82.0, 82.0, 69.7, 69.5, 33.8, 33.7, 26.3, 25.9, 15.0, 14.9, 9.4, 9.2; MS (APCI): m/z 289.3 (M+ + 1); Rf = 0.41 (CHCl3:MeOH:NH4OH, 97:2:1).
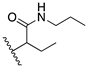
2,2′-(Ethylene-1,2-dioxo)di(N-propylbutanamide) (20): C16H32N2O4, oil; yield: 59%; 1H NMR (400 MHz, CDCl3) δ 6.79 (d, J = 60.5 Hz, 2H), 3.76–3.73 (m, 2H), 3.71–3.57 (m, 4H), 3.29–3.13 (m, 4H), 1.93–1.81 (m, 2H), 1.69 (tt, J = 14.6, 7.3 Hz, 2H), 1.58–1.47 (m, 4H), 1.02–0.87 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 172.2, 172.17, 82.1, 69.8, 69.6, 40.7, 40.6, 26.4, 26.0, 22.94, 22.90, 11.4, 11.3, 9.5, 9.3; MS (APCI): m/z 317.4 (M+ + 1); Rf = 0.4 (CHCl3:MeOH, 95:5).
Synthesis of 2,2′-(ethylene-1,2-dioxo)dibutanamide: The diester was hydrolyzed to the dicarboxylic acid again by adding 1N HCl (2 equiv.) and refluxed overnight. The mixture was concentrated under reduced pressure and then extracted with diethylether (20 mL × 5) and dried over anhydrous Na2SO4. The solvent was evaporated and the obtained oil was left in the desiccator overnight. Afterwards, SOCl2 (2 equiv.) was added together with a few small crystals of NaCl and left to reflux overnight. Excess SOCl2 was removed by adding toluene, followed by evaporation under reduced pressure. The final step was adding NH3 (25% aq. solution) and heating to 70 °C. The reaction was monitored with APCI until completion. The resulted diamide was purified by column chromatography (CHCl3:MeOH:NH4OH, 89:10:1).
2,2′-(Ethylene-1,2-dioxo)dibutanamide (21): C10H20N2O4, white powder; yield: 59.45%; m.p. 119–120 °C; 1H NMR (400 MHz, CDCl3) δ 6.73 (d, J = 38.6 Hz, 2H), 5.96 (s, 2H), 3.77–3.71 (m, 4H), 3.71–3.65 (m, 2H), 1.85 (ddt, J = 12.1, 4.6, 2.8 Hz, 2H), 1.73 (dq, J = 14.3, 7.1 Hz, 2H), 0.97 (td, J = 7.4, 3.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 175.41, 175.40, 82.01, 82.0, 69.8, 69.7, 26.0, 25.7, 9.3, 9.2; MS (APCI): m/z 233. 2 (M+ + 1); Rf = 0.35 (CHCl3:MeOH:NH4OH, 89:10:1).
Synthesis of 2,2′-(ethylene-1,2-dioxo)di(butan-1-amine) derivatives: In a two-necked 50 mL round bottom flask under Argon, LiAlH4 (6 equiv.) was suspended in 5 mL dioxane. The flask was cooled to 0 °C in an ice/water bath, and the amide derivative was first dissolved in 5 mL dioxane and then added dropwise while stirring. After the complete addition, the reaction was left to reflux at 100 °C overnight. The reaction was cooled to 0 °C and treated cautiously in sequence with H2O (H2O:LiAlH4, 1:1), NaOH 15% aq. solution (NaOH:LiAlH4, 1:1), H2O (H2O:LiAlH4, 3:1) and left to stir overnight. The solids were removed by vacuum filtration over a glass filter and washed with dioxane and EtOAc. The filtrate was concentrated under reduced pressure and left overnight in the desiccator. No further purification of the diamine produced was required.
2,2′-(Ethylene-1,2-dioxo)di(N-methylbutan-1-amine) (22): C12H28N2O2, yellow oil; yield: 85.48%; 1H NMR (400 MHz, CDCl3) δ 3.70–3.61 (m, 2H), 3.56–3.48 (m, 2H), 3.36 (p, J = 6.1 Hz, 2H), 2.56 (d, J = 5.6 Hz, 4H), 2.40 (s, 6H), 1.96 (s, 2H), 1.59–1.41 (m, 4H), 0.87 (t, J = 7.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 80.6, 68.96, 68.9, 55.1, 55.06, 36.5, 25.1, 25.05, 9.6; MS (APCI): m/z 233.3 (M+ + 1).
2,2′-(Ethylene-1,2-dioxo)di(N-ethylbutan-1-amine) (23): C14H32N2O2, yellow oil; yield: 69.7%; 1H NMR (400 MHz, CDCl3) δ 3.72–3.65 (m, 2H), 3.62–3.52 (m, 2H), 3.46–3.35 (m, 2H), 2.64 (dddd, J = 8.9, 7.4, 5.6, 1.9 Hz, 8H), 1.62–1.39 (m, 4H), 1.18–1.05 (m, 6H), 0.94–0.80 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 80.5, 80.45, 68.8, 68.7, 52.64, 52.62, 44.03, 44.00, 25.0, 24.94, 14.9, 9.48, 9.46.; MS (APCI): m/z 261.3 (M+ + 1).
2,2′-(Ethylene-1,2-dioxo)di(N-propylbutan-1-amine) (24): C16H36N2O2, yellow oil; yield: 69.7%; 1H NMR (400 MHz, CDCl3) δ 3.71–3.64 (m, 2H), 3.62–3.54 (m, 2H), 3.42 (dddd, J = 13.5, 12.0, 6.9, 3.9 Hz, 2H), 2.68–2.59 (m, 4H), 2.58–2.48 (m, 4H), 1.65–1.37 (m, 8H), 0.92–0.87 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 80.8, 69.1, 69.0, 53.11, 53.1, 52.18, 52.16, 25.3, 25.25, 23.2, 11.9, 9.8, 9.78.; MS (APCI): m/z 289.4 (M+ + 1).
2,2′-(Ethylene-1,2-dioxo)di(butan-1-amine) (25): C10H24N2O2, yellow oil; yield: 98.3%; 1H NMR (500 MHz, CDCl3) δ 3.69–3.60 (m, 2H), 3.61–3.52 (m, 2H), 3.19 (tt, J = 8.6, 4.5 Hz, 2H), 2.75 (dd, J = 13.2, 3.7 Hz, 2H), 2.64 (ddd, J = 13.2, 6.9, 1.4 Hz, 2H), 1.61–1.49 (m, 2H), 1.51–1.37 (m, 2H), 0.97–0.76 (m, 6H); 13C NMR (126 MHz, CDCl3) δ 82.9, 82.8, 68.9, 44.6, 24.5, 9.7; MS(APCI): m/z 205.2 (M+ + 1).