Complete Assignments of 1H and 13C NMR Chemical Shift Changes Observed upon Protection of Hydroxy Group in Borneol and Isoborneol and Their DFT Verification
Abstract
1. Introduction
2. Results and Discussion
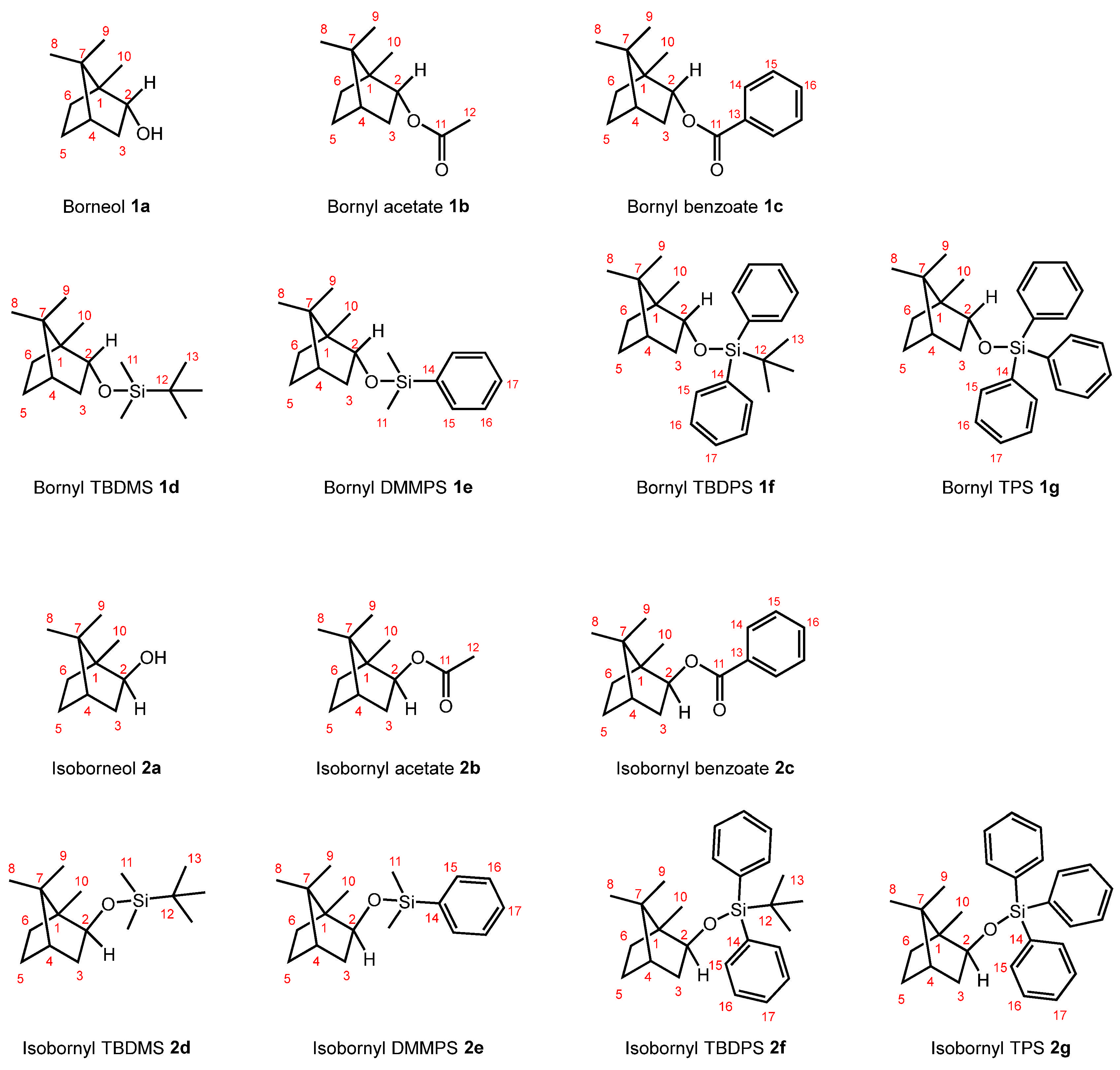
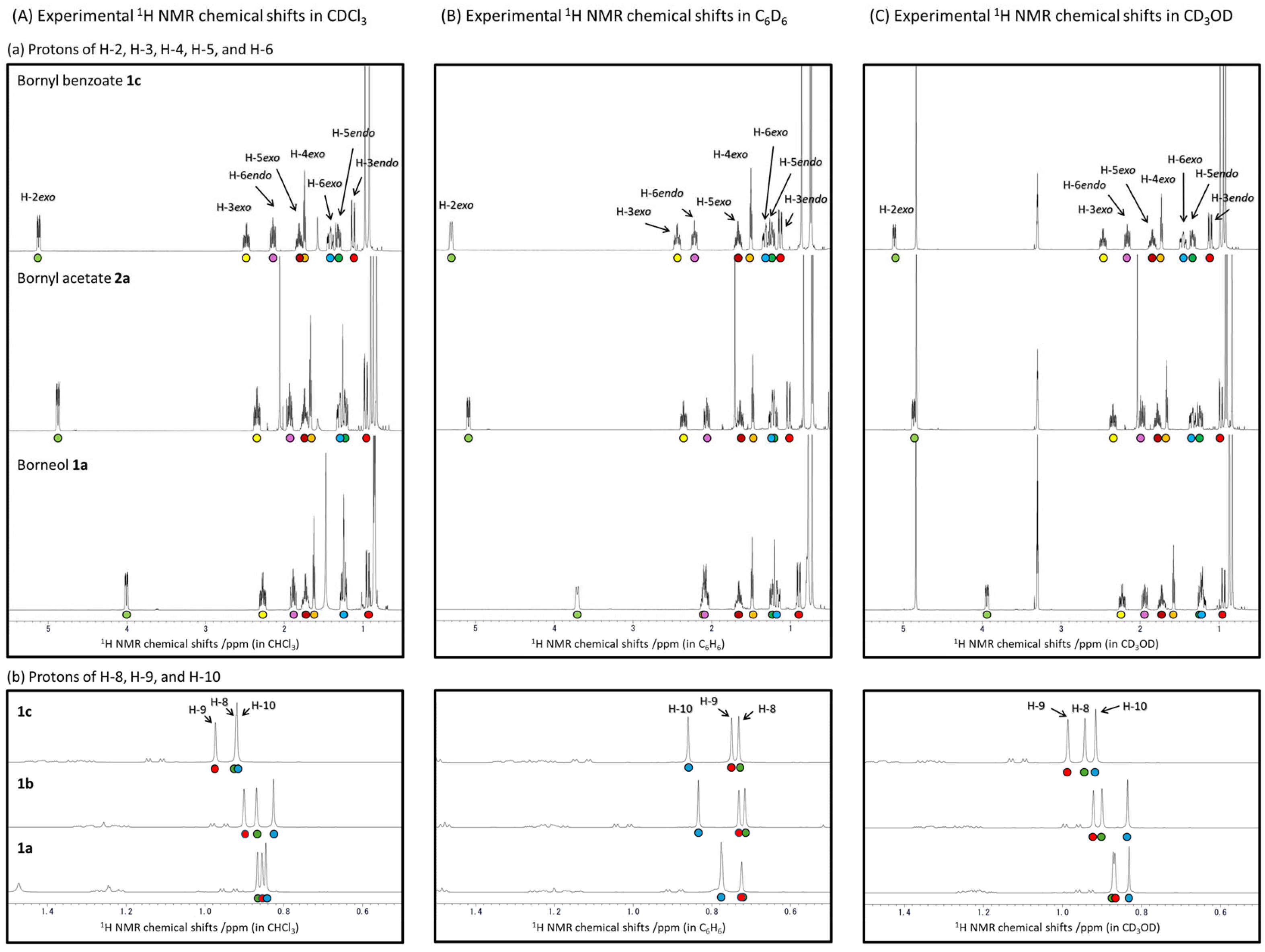
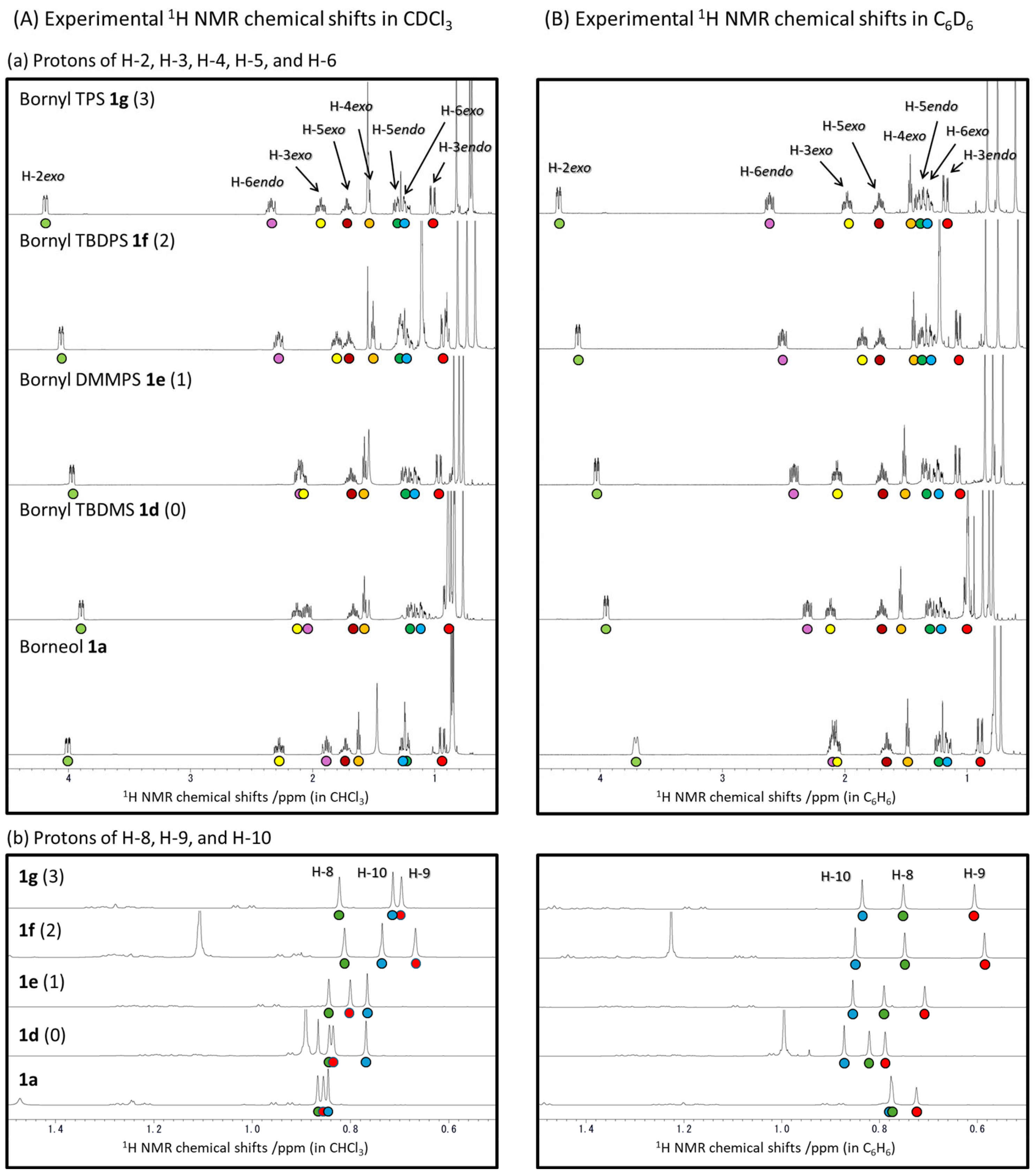
2.1. The Correlation Between the Experimentally Obtained and DFT-Calculated 1H and 13C NMR Chemical Shifts of Borneol 1a, Isoborneol 2a, and Their Derivatives 1b–1g and 2b–2g
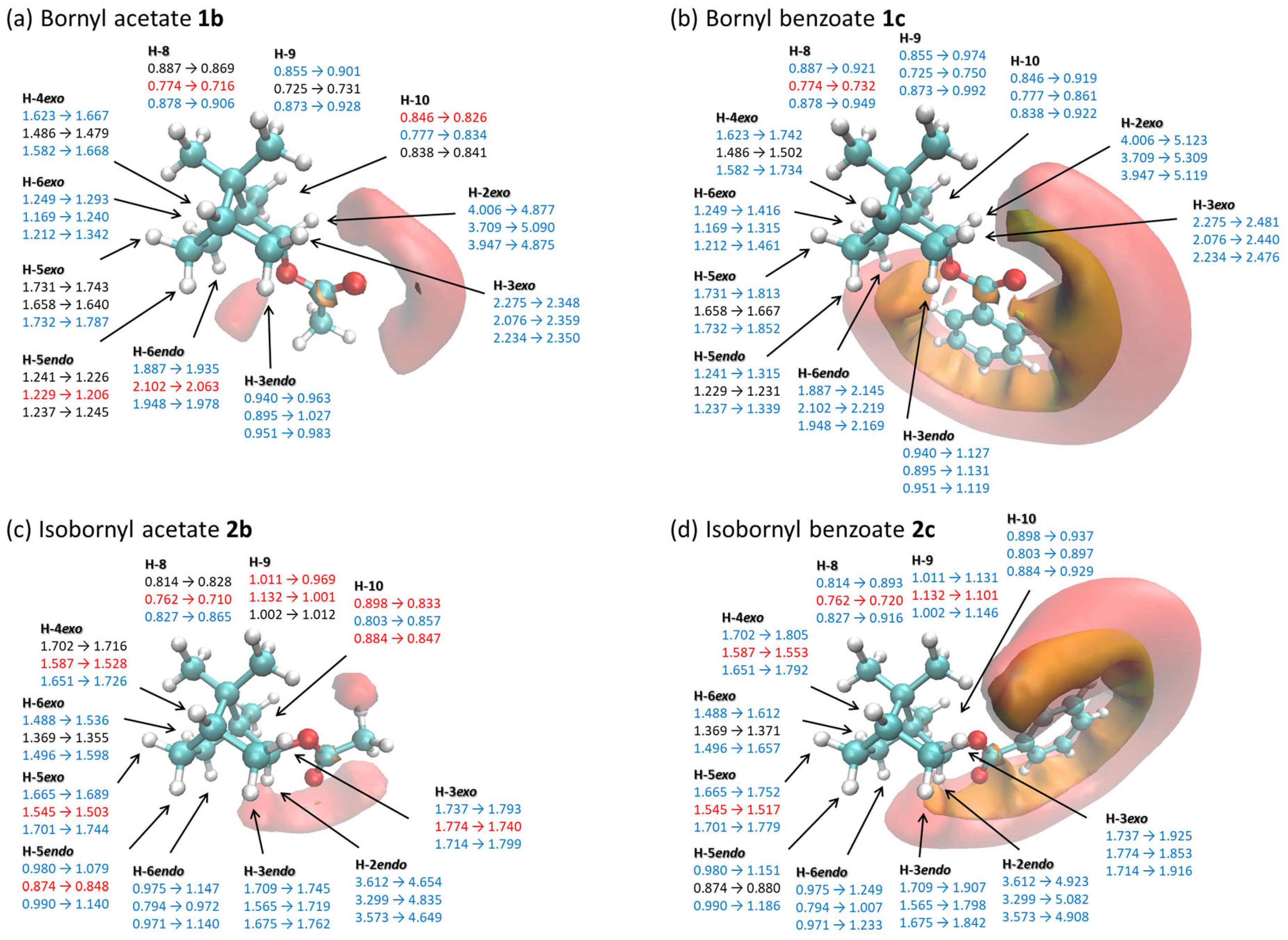
2.2. Relationship Between the Changes in 1H NMR Chemical Shifts of Borneol 1a, Isoborneol 2a, and Their Derivatives Containing an Acetyl Group or a Benzoyl Group 1b–1c or 2b–2c and the Iso-Chemical Shielding Surface (ICSS)
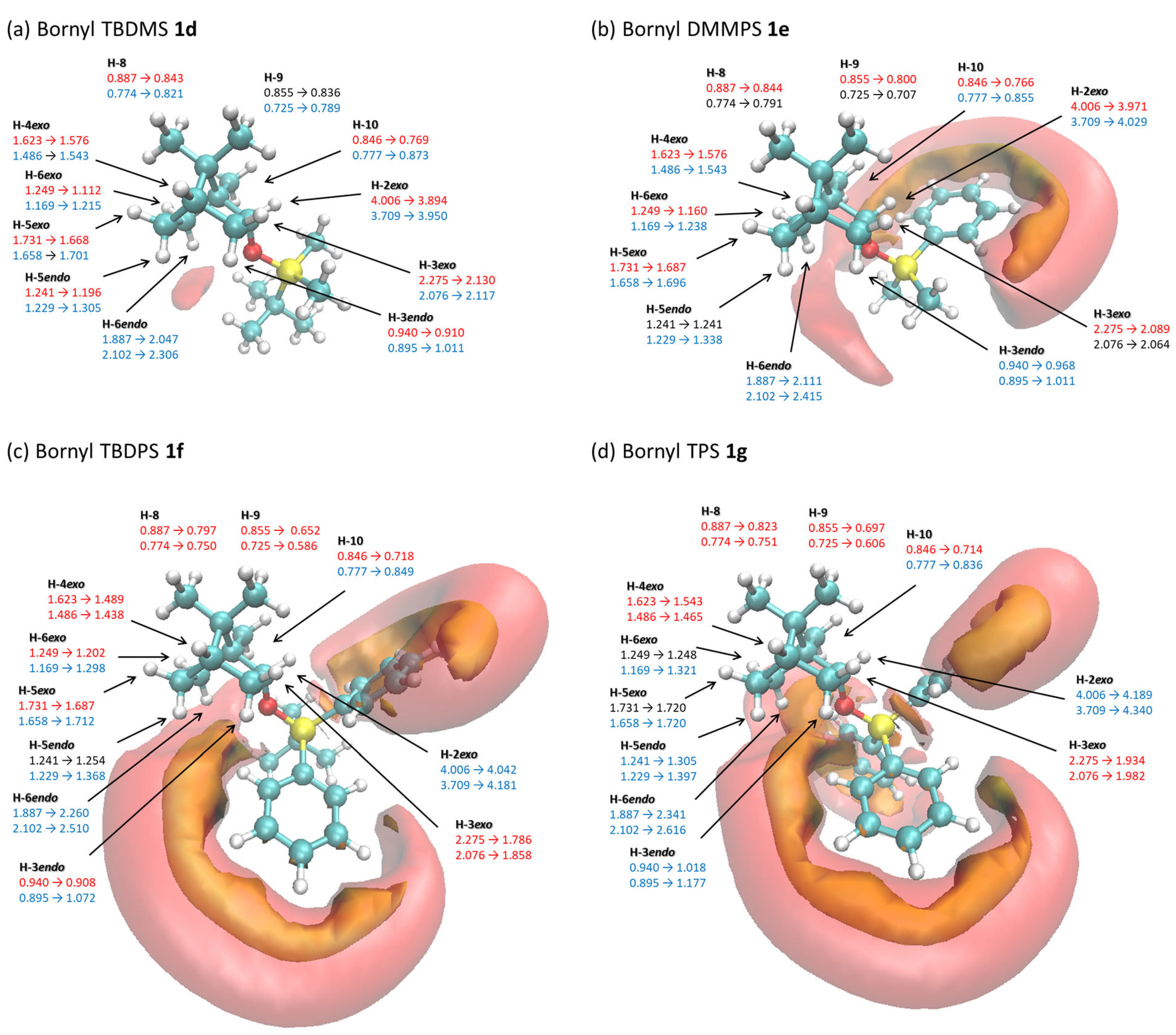
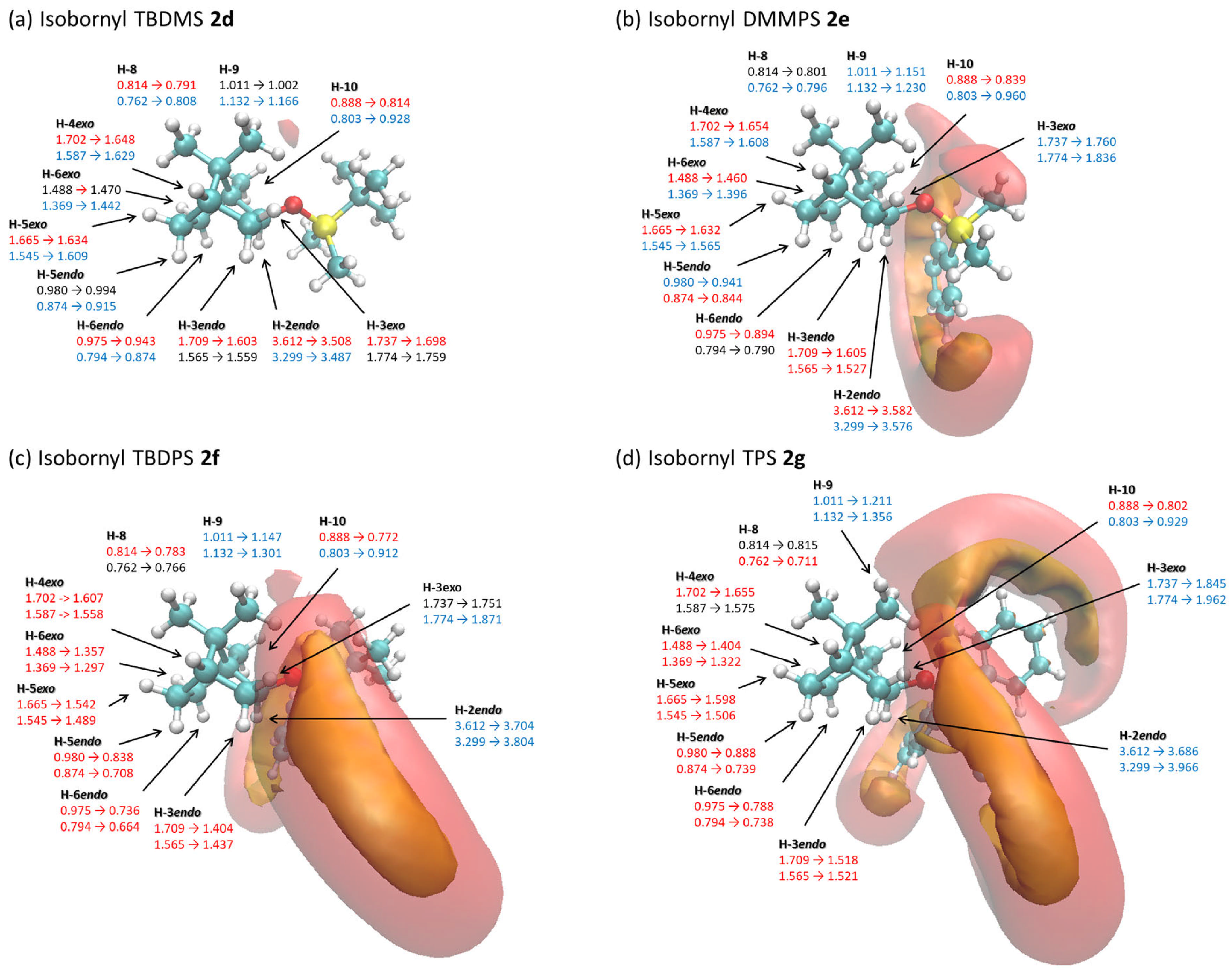
2.3. Relationship Between the Changes in 1H NMR Chemical Shifts of Borneol 1a, Isoborneol 2a, and Their Derivatives Containing Various Silyl Protective Groups 1d–1g and 2d–2g and the Iso-Chemical Shielding Surface (ICSS)
3. Materials and Methods
3.1. Chemicals
3.2. NMR Measurements
3.3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on borneol. Food Chem. Toxicol. 2008, 46, S77–S80. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-J.; Hung, C.-C.; Shih, T.-L.; Yiin, L.-M.; Chen, H.-P. Investigation of borneols sold in Taiwan by chiral gas chromatography. J. Food Drug Anal. 2016, 26, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives-Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Hanrahan, J. Chapter 7—Biologically active compounds from Lamiaceae family: Central nervous system effects. Stud. Nat. Prod. Chem. 2021, 68, 255–315. [Google Scholar]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS PharmSciTech 2021, 22, 145. [Google Scholar] [CrossRef]
- Li, Y.; Ren, M.; Wang, J.; Ma, R.; Chen, H.; Xie, Q.; Li, H.; Li, J.; Wang, J. Progress in Borneol Intervention for Ischemic Stroke: A Systematic Review. Front. Pharmacol. 2021, 12, 606682. [Google Scholar] [CrossRef]
- Rajput, A.; Kasar, A.; Thorat, S.; Kulkarni, M. Borneol: A Plant-Sourced Terpene with a Variety of Promising Pharmacological Effects. Nat. Prod. J. 2023, 13, 13–28. [Google Scholar]
- Hu, X.; Yan, Y.; Liu, W.; Liu, J.; Fan, T.; Deng, H.; Cai, Y. Advances and perspectives on pharmacological activities and mechanisms of the monoterpene borneol. Phytomedicine 2024, 132, 155848. [Google Scholar] [CrossRef]
- Martínez, A.G.; Vilar, E.T.; Fraile, A.G.; de la, M.; Cerero, S.; Maroto, B.L. Synthesis and catalytic activity of 10-(aminomethyl) isoborneol-based catalysts: The role of the C(2)-group on the asymmetric induction. Tetrahedron Asymmetry 2003, 14, 1959–1963. [Google Scholar] [CrossRef]
- Arrayás, R.G.; Adrio, J.; Carretero, J.C. Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Fu, B.M.; Zhang, Z.-J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Khine, A.A.; Liu, J.-W.; Cheng, H.-C.; Hu, A.; Chen, H.-P.; Shih, T.-L. Resolution of isoborneol and its isomers by GC/MS to identify “synthetic” and “semi-synthetic” borneol products. Chirality 2018, 30, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Gansow, O.A.; Willcott, M.R.; Lenkinski, R.E. Carbon magnetic resonance. Signal assignment by alternately pulsed nuclear magnetic resonance and lanthanide-induced chemical shifts. J. Am. Chem. Soc. 1971, 93, 4295–4297. [Google Scholar] [CrossRef]
- Briggs, J.; Hart, F.A.; Moss, G.P.; Randall, E.W. A ready method of assignment for 13C nuclear magnetic resonance spectra: The complete assignment of the 13C spectrum of borneol. J. Chem. Soc. D Chem. Commun. 1971, 364–365. [Google Scholar] [CrossRef]
- Hawkes, G.E.; Leibfritz, D.; Roberts, D.W.; Roberts, J.D. Nuclear magnetic resonance shift reagents. Question of the orientation of the magnetic axis in lanthanide-substrate complexes. J. Am. Chem. Soc. 1973, 95, 1659–1661. [Google Scholar] [CrossRef]
- Levy, G.C.; Komoroski, R.A. Paramagnetic relaxation reagents. Alternatives or complements to lanthanide shift reagents in nuclear magnetic resonance spectral analysis. J. Am. Chem. Soc. 1974, 96, 678–681. [Google Scholar] [CrossRef]
- Simova, S. Application of to the measurement of homonuclear HSQC coupling constants, J(H,H). Magn. Reson. Chem. 1998, 36, 505–510. [Google Scholar] [CrossRef]
- Baldovini, N.; Tomi, F.; Casanova, J. Enantiomeric Differentiation of Bornyl Acetate by 13C-NMR Using a Chiral Lanthanide Shift Reagent. Phytochem. Anal. 2003, 14, 241–244. [Google Scholar] [CrossRef]
- Khandelwal, D.; Hooda, S.; Brar, A.S.; Shankar, R. Stereochemical assignments of the nuclear magnetic resonance spectra of isobornyl acrylate/methacrylonitrile copolymers. J. Appl. Polym. Sci. 2012, 126, 916–928. [Google Scholar] [CrossRef]
- Lyu, B.; Sako, H.; Sugiura, M.; Hiraga, Y.; Takagi, R.; Niwayama, S. 1H NMR Chemical Shift Changes as a Result of Introduction of Carbonyl-Containing Protecting Groups Observed in Bornol and Isoborneol. Molbank 2024, 2024, M1899. [Google Scholar] [CrossRef]
- Lyu, B.; Sugiura, M.; Tayama, K.; Hiraga, Y.; Takagi, R.; Niwayama, S. The Shielding Effect of Phenyl Groups in the Silyl-Protecting Groups Introduced into Borneol and Isoborneol. Molbank 2024, 2024, M1908. [Google Scholar] [CrossRef]
- Gaussian 09, Revision D.01. Available online: https://gaussian.com/glossary/g09/ (accessed on 24 December 2024).
- Klod, S.; Kleinpeter, E. Ab initio calculation of the anisotropy effect of multiple bonds and the ring current effect of arenes-application in conformational and configurational analysis. J. Chem. Soc. Perkin Trans. 2001, 2, 1893–1898. [Google Scholar]
- Schleyer, P.v.R.; Maerker, C.; Dansfeld, A.; Jiao, H.; Hommes, N.J.R.v.E. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6518. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Schleyer, P.v.R.; Mo, Y.; McAllister, M.A.; Tidwell, T.T. Magnetic Evidence for the Aromaticity and Antiaromaticity of Charged Fluorenyl, Indenyl, and Cyclopentadienyl Systems. J. Am. Chem. Soc. 1997, 119, 7075–7083. [Google Scholar] [CrossRef]
- Zywietz, T.K.; Jiao, H.; Schleyer, P.v.R.; de Meijere, A. Aromaticity and Antiaromaticity in Oligocyclic Annelated Five-Membered Ring Systems. J. Org. Chem. 1998, 63, 3417–3422. [Google Scholar] [CrossRef]
- Nendel, M.; Houk, K.N.; Tolbert, L.M.; Vogel, E.; Jiao, H.; Schleyer, P.v.R. Bond Alternation and Aromatic Character in Cyclic Polyenes: Assessment of Theoretical Methods for Computing the Structures and Energies of Bismethano [14]annulenes. J. Phys. Chem. A 1998, 102, 7191–7198. [Google Scholar] [CrossRef]
- Schulmann, J.M.; Disch, R.L.; Jiao, H.; Schleyer, P.v.R. Chemical Shifts of the [N]Phenylenes and Related Compounds. J. Phys. Chem. A 1998, 102, 8051–8055. [Google Scholar] [CrossRef]
- Stanger, A. Aromaticity, NICS—Past and Present. Eur. J. Org. Chem. 2020, 2020, 3120–3127. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Holzberger, A. Theoretical study of conformation and dynamic behaviour of [3.3]orthocyclophane and heterocyclic analogues. Tetrahedron 2001, 57, 6941–6946. [Google Scholar] [CrossRef]
- Germer, A.; Klod, S.; Peter, M.G.; Kleinpeter, E. NMR spectroscopic and theoretical study of the complexation of the inhibitor allosamidin in the binding pocket of the plant chitinase hevamine. J. Mol. Model. 2002, 8, 231–236. [Google Scholar] [CrossRef]
- Liu, S.-F.; Mao, J.-D.; Schmidt-Rohr, K. A Robust Technique for Two-Dimensional Separation of Undistorted Chemical-Shift Anisotropy Powder Patterns in Magic-Angle-Spinning NMR. J. Mag. Res. 2002, 155, 15–28. [Google Scholar] [CrossRef]
- Corminboeuf, C.; Heine, T.; Seifert, G.; Schleyer, P.v.C.; Weber, J. Induced magnetic fields in aromatic [n]-annulenes-interpretation of NICS tensor components. Phys. Chem. Chem. Phys. 2004, 6, 273–276. [Google Scholar] [CrossRef]
- Geuenich, D.; Hess, K.; Köhler, F.; Herges, R. Anisotropy of the Induced Current Density (ACID), a General Method To Quantify and Visualize Electronic Delocalization. Chem. Rev. 2005, 105, 3758–3772. [Google Scholar] [CrossRef] [PubMed]
- Kleinpeter, E. Chapter Three—Quantification and Visualization of the Anisotropy Effect in NMR Spectroscopy by Through-Space NMR Shieldings. Annu. Rep. NMR Spectrosc. 2014, 82, 115–166. [Google Scholar]
- Baranac-Stojanović, M. New insight into the anisotropic effects in solution-state NMR spectroscopy. RSC Adv. 2014, 4, 308–321. [Google Scholar] [CrossRef]
- Jorner, K.; Jahn, B.O.; Bultinck, P.; Ottosson, H. Triplet state homoaromaticity: Concept, computational validation and experimental relevance. Chem. Sci. 2018, 9, 3165–3176. [Google Scholar] [CrossRef]
- Jung, S.; Podlech, J. Stereoelectronic Effects: The γ-Gauche Effect in Sulfoxides. J. Phys. Chem. A 2018, 122, 5764–5772. [Google Scholar] [CrossRef]
- Francesca Nardelli, F.; Borsacchi, S.; Calucci, L.; Carignani, E.; Martini, F.; Geppi, M. Anisotropy and NMR spectroscopy. Rend. Lincei. Sci. Fis. E Nat. 2020, 31, 999–1010. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo [18]carbon: Bondingcharacter, electron delocalization, and aromaticity. Carbon 2020, 165, 468–475. [Google Scholar] [CrossRef]
- Hansen, P.E. The Synergy between Nuclear Magnetic Resonance and Density Functional Theory Calculations. Molecules 2024, 29, 336. [Google Scholar] [CrossRef]
- Chavelas-Hernández, L.; Valdéz-Camacho, J.R.; Hernández-Vázquez, L.G.; Dominguez-Mendoza, B.E.G.; Vasquez-Ríos, M.; Escalante, J. A New Approach Using Aromatic-Solvent-Induced Shifts in NMR Spectroscopy to Analyze β-Lactams with Various Substitution Patterns. Synlett 2020, 31, 158–164. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar]
- Singh, S.; Singh, H.; Srivastava, A.; Tandon, P.; Sinha, K.; Bharti, P.; Kumar, S.; Kumar, P.; Maurya, R. Study of conformational stability, structural, electronic and charge transfer properties of cladrin using vibrational spectroscopy and DFT calculations. Spectrochim. Acta A 2014, 132, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Bursch, M.; Mewes, J.-M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry, Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for N.M.R. chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Touw, S.I.E.; de Groot, H.J.M.; Buda, F. DFT calculations of the 1H NMR chemical shifts and 13C chemical shifts tensors of retinal isomers. J. Mol. Struct. 2004, 711, 141–147. [Google Scholar] [CrossRef]
- Sugimura, N.; Furuya, A.; Yatsu, T.; Shibue, T. Application of Density Functional Theory (DFT) and Empirical Scaling to Practical Prediction of 13C-NMR of (−)-Napyradiomycin A1. Bunseki Kagaku 2015, 64, 147–150. [Google Scholar] [CrossRef][Green Version]
- Le, P.Q.; Nguyen, N.Q.; Nguyen, T.T. DFT approach towards accurate prediction of 1H/13C NMR chemical shifts for dipterocarpol oxime. RSC Adv. 2023, 13, 31811–31819. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of Solvent Effects in Isotropic and Anisotropic Dielectrics and in Ionic Solutions with a Unified Integral Equation Method: Theoretical Bases, Computational Implementation, and Numerical Applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- PCM Mennucci, B.; Tomasi, J.; Cammi, R.; Cheeseman, J.R.; Frisch, M.J.; Devlin, F.J.; Gabriel, S.; Stephens, P.J. Polarizable Continuum Model (PCM) Calculations of Solvent Effects on Optical Rotations of Chiral Molecules. J. Phys. Chem. A 2002, 106, 6102–6113. [Google Scholar] [CrossRef]
- Tomasi, J. Selected features of the polarizable continuum model for the representation of solvation. WIREs Comput. Mol. Sci. 2011, 1, 855–867. [Google Scholar] [CrossRef]
- Mennucci, B. Polarizable continuum model. WIREs Comput. Mol. Sci. 2012, 2, 386–404. [Google Scholar] [CrossRef]
- Eilme, A. Solvatochromic Probe in Molecular Solvents: Implicit Versus Explicit Solvent Model. Theor. Chem. Acc. 2014, 133, 1538. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef]
- Herbert, J.M. Dielectric continuum methods for quantum chemistry. WIREs Comput. Mol. Sci. 2021, 11, e1519. [Google Scholar] [CrossRef]
- Saitô, H.; Nukada, K. NMR measurements of the anisotropy effect of the phenylimino group. Tetrahedron 1966, 22, 3313–3320. [Google Scholar] [CrossRef]
- Haseltine, R.; Huang, K.; Ranganayakulu, K.; Sorensen, T.S. The Observable Thirtyfold Degenerate Camphenehydro Cation. A Stereospecific exo-3,2-Methyl Shift and endo-6,2(exo 6,l)-Hydride Shift. Can. J. Chem. 1975, 53, 1056–1066. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Szatmári, I.; Lázár, L.; Koch, A.; Heydenreich, M.; Fülöp, F. Visualization and quantification of anisotropic effects on the 1H NMR spectra of 1,3-oxazino [4,3-a]isoquinolines- indirect estimates of steric compression. Tetrahedron 2009, 65, 8021–8027. [Google Scholar]
- Bols, M.; Pedersen, C.M. Silyl-protective groups influencing the reactivity and selectivity in glycosylations. Beilstein J. Org. Chem. 2017, 13, 93–105. [Google Scholar] [CrossRef]
- Lynch, C.C.; Sripada, A.; Wolf, C. Asymmetric Synthesis with Ynamides: Unique Reaction Control, Chemical Diversity and Applications. Chem. Soc. Rev. 2020, 49, 8543–8583. [Google Scholar] [CrossRef] [PubMed]
- Harriswangler, C.; Lucio-Martínez, F.; Godec, L.; Soro, L.K.; Fernández-Fariña, S.; Valencia, L.; Rodríguez-Rodríguez, A.; Esteban-Gómez, D.; Charbonnière, L.J.; Platas-Iglesias, C. Effect of Magnetic Anisotropy on the 1H NMR Paramagnetic Shifts and Relaxation Rates of Small Dysprosium(III) Complexes. Inorg. Chem. 2023, 62, 14326–14338. [Google Scholar] [CrossRef] [PubMed]
- Klinck, R.E.; Stothers, J.B. Nuclear Magnetic Resonance Studies. Part 1. The Chemical Shift of the Formyl Proton in Aromatic Aldehydes. Can. J. Chem. 1962, 40, 1071–1081. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Proton NMR Spectroscopy. In Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 140–141. [Google Scholar]
- Narzarski, R.B. On the Use of Deuterated Organic Solvents without TMS to Report 1H/13C NMR Spectral Data of Organic Compounds: Current State of the Method, Its Pitfalls and Benefits, and Related Issues. Molecules 2023, 28, 4369. [Google Scholar] [CrossRef]
- Spartan’06; Wavefunction, Inc.: Irvine, CA, USA, 2006; Available online: https://www.yumpu.com/en/document/view/28593290/spartan06-wavefunction-inc (accessed on 24 December 2024).
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, B.; Hiraga, Y.; Takagi, R.; Niwayama, S. Complete Assignments of 1H and 13C NMR Chemical Shift Changes Observed upon Protection of Hydroxy Group in Borneol and Isoborneol and Their DFT Verification. Molecules 2025, 30, 597. https://doi.org/10.3390/molecules30030597
Lyu B, Hiraga Y, Takagi R, Niwayama S. Complete Assignments of 1H and 13C NMR Chemical Shift Changes Observed upon Protection of Hydroxy Group in Borneol and Isoborneol and Their DFT Verification. Molecules. 2025; 30(3):597. https://doi.org/10.3390/molecules30030597
Chicago/Turabian StyleLyu, Baohe, Yoshikazu Hiraga, Ryukichi Takagi, and Satomi Niwayama. 2025. "Complete Assignments of 1H and 13C NMR Chemical Shift Changes Observed upon Protection of Hydroxy Group in Borneol and Isoborneol and Their DFT Verification" Molecules 30, no. 3: 597. https://doi.org/10.3390/molecules30030597
APA StyleLyu, B., Hiraga, Y., Takagi, R., & Niwayama, S. (2025). Complete Assignments of 1H and 13C NMR Chemical Shift Changes Observed upon Protection of Hydroxy Group in Borneol and Isoborneol and Their DFT Verification. Molecules, 30(3), 597. https://doi.org/10.3390/molecules30030597







