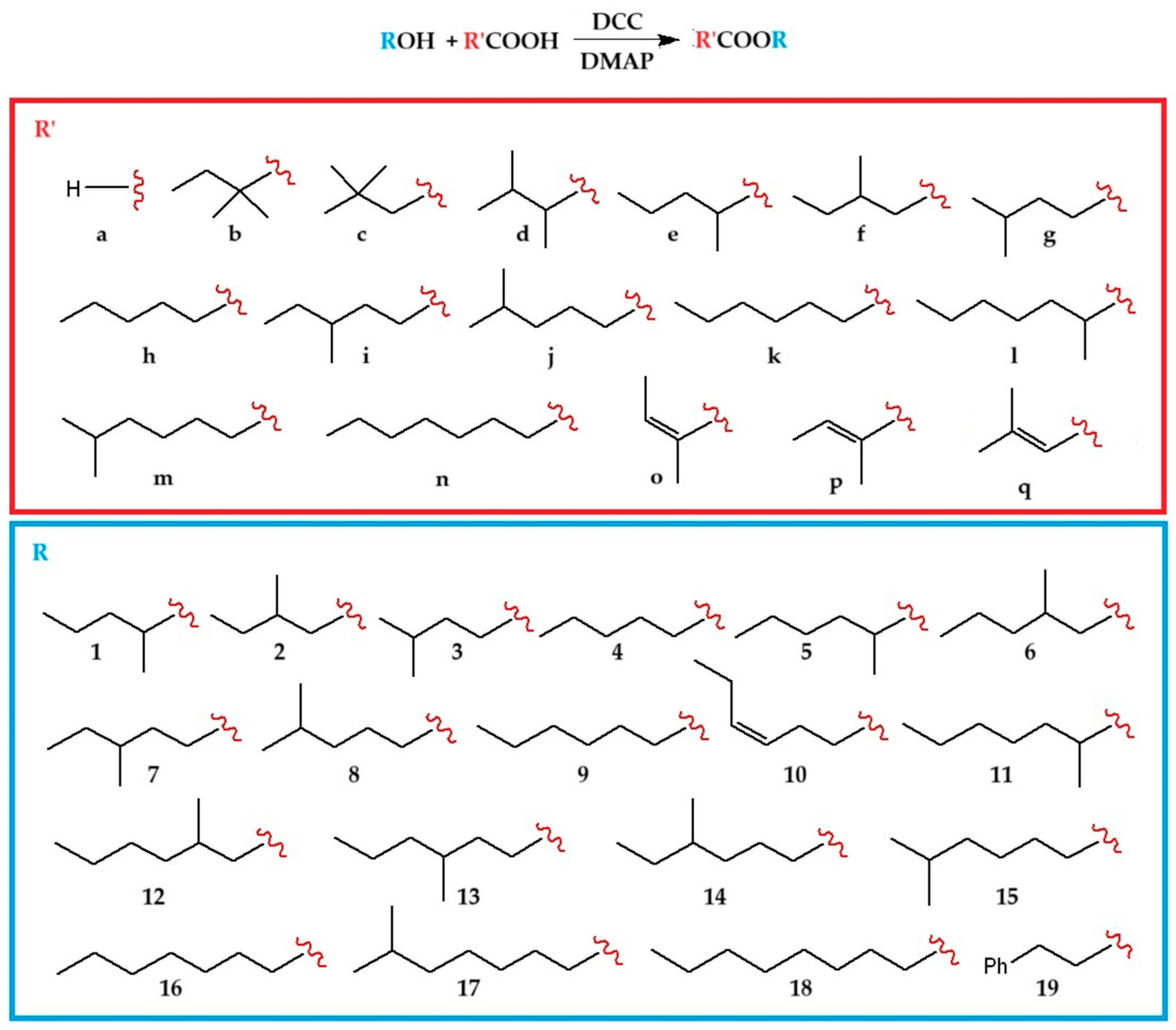
3.7. Synthesis of Esters
A solution of the appropriate alcohol (pentan-2-ol (
1), 2-methylbutan-1-ol (
2), 3-methylbutan-1-ol (
3), pentan-1-ol (
4), hexan-2-ol (
5), 2-methylpentan-1-ol (
6), 3-methylpentan-1-ol (
7), 4-methylpentan-1-ol (
8), hexan-1-ol (
9), (
Z)-hex-3-en-1-ol (
10), heptan-2-ol (
11), 2-methylhexan-1-ol (
12), 3-methylhexan-1-ol (
13), 4-methylhexan-1-ol (
14), 5-methylhexan-1-ol (
15), heptan-1-ol (
16), 6-methylheptan-1-ol (
17), octan-1-ol (
18), 2-phenylethan-1-ol (
19)), carboxylic acid (1.1 eq; formic (
a), 2,2-dimethylbutanoic (
b), 3,3-dimethylbutanoic (
c), 2,3-dimethylbutanoic (
d), 2-methylpentanoic (
e), 3-methylpentanoic (
f), 4-methylpentanoic (
g), hexanoic (
h), 4-methylhexanoic (
i), 5-methylhexanoic (
j), heptanoic (
k), 2-methylheptanoic (
l), 6-methylheptanoic (
m), octanoic (
n), angelic (
o), tiglic (
p), and senecioic (
q) acid), DMAP (0.3 eq) and DCC (1.1 eq) in 1.5 mL of dry DCM was left overnight at room temperature, and after filtration of the precipitated
N,
N′-dicyclohexylurea, the remaining solution was immediately analyzed by GC–MS [
5]. In the case of the esters detected in
P. graveolens essential oil fraction and several other esters from the library, the mentioned synthetic procedures were repeated on a somewhat larger scale to provide appropriate amounts of the compounds for full spectral characterization. A solution of the appropriate alcohol, carboxylic acid (1.1 eq), DMAP (0.3 eq) and DCC (1.1 eq) in 20 mL of dry DCM was stirred overnight, at room temperature, in a round bottom flask equipped with a CaCl
2 guard tube. The resulting residue was purified by gradient dry-flash column chromatography; mixtures of hexane and diethyl ether of increasing polarity were used for elution. Esters were washed from the column with 5% (
v/
v) diethyl ether in hexane [
5]. The purity of the ester fractions was checked by GC-MS. Spectral data (NMR, IR, and/or MS), and assignments of
1H and
13C signals for the synthesized esters are given below and in the
Supplementary Materials (Figures S1–S159 and Tables S3–S9).
1-Methylhexyl formate (11a): RI = 955 (DB-5MS column); MS (EI), m/z (%) 99 (6), 98 (23), 88 (8), 86 (51), 84 (79), 83 (20), 73 (15), 71 (5), 70 (39), 69 (33), 57 (28), 56 (65), 55 (47), 51 (29), 49 (89), 47 (19), 45 (100), 43 (35), 42 (16), 41 (50), 39 (17).
2-Methylhexyl formate (12a): RI = 980 (DB-5MS column); MS (EI), m/z (%) 98 (5), 83 (13), 74 (11), 71 (13), 70 (84), 69 (71), 57 (14), 56 (63), 55 (100), 54 (6), 44 (5), 43 (78), 42 (17), 41 (57), 40 (6), 39 (20).
3-Methylhexyl formate (13a): RI = 986 (DB-5MS column); MS (EI), m/z (%) 87 (5), 83 (12), 74 (9), 71 (12), 70 (100), 69 (84), 68 (7), 59 (6), 57 (26), 56 (59), 55 (81), 53 (7), 44 (14), 43 (61), 42 (29), 41 (82), 40 (23), 39 (22).
4-Methylhexyl formate (14a): RI = 1007 (DB-5MS column); MS (EI), m/z (%) 98 (6), 85 (5), 83 (11), 74 (17), 73 (7), 71 (14), 70 (94), 69 (76), 67 (7), 59 (11), 57 (23), 56 (68), 55 (100), 53 (8), 45 (13), 44 (30), 43 (82), 42 (25), 41 (79), 40 (50), 39 (24).
5-Methylhexyl formate (15a): RI = 994 (DB-5MS column); MS (EI), m/z (%) 101 (5), 83 (24), 70 (36), 69 (35), 57 (24), 56 (100), 55 (66), 43 (43), 42 (15), 41 (50), 39 (14).
Heptyl formate (16a): RI = 1026 (DB-5MS column); MS (EI), m/z (%) 98 (6), 86 (9), 84 (16), 83 (11), 71 (5), 70 (100), 69 (57), 68 (16), 67 (5), 57 (23), 56 (90), 55 (63), 54 (7), 51 (6), 49 (16), 47 (6), 43 (35), 42 (36), 41 (69), 39 (20).
1-Methylbutyl 2,2-dimethylbutanoate (1b): MS (EI), m/z (%) 117 (16), 116 (19), 101 (24), 100 (8), 99 (95), 84 (11), 71 (33), 70 (36), 61 (7), 60 (15), 59 (22), 58 (22), 57 (100), 56 (15), 55 (24), 49 (11), 45 (5), 43 (73), 42 (13), 41 (41), 39 (16).
2-Methylbutyl 2,2-dimethylbutanoate (2b): MS (EI), m/z (%) 117 (8), 99 (18), 88 (9), 72 (6), 71 (100), 70 (53), 55 (15), 43 (54), 42 (8), 41 (19).
3-Methylbutyl 2,2-dimethylbutanoate (3b): MS (EI), m/z (%) 158 (7), 117 (11), 99 (9), 88 (8), 72 (6), 71 (100), 70 (54), 55 (19), 43 (59), 42 (7), 41 (23), 39 (9).
Pentyl 2,2-dimethylbutanoate (4b): MS (EI), m/z (%) 158 (10), 117 (40), 101 (5), 99 (14), 88 (11), 72 (5), 71 (100), 70 (27), 55 (16), 43 (59), 42 (9), 41 (25), 39 (10).
1-Methylbutyl 3,3-dimethylbutanoate (1c): MS (EI), m/z (%) 143 (5), 117 (16), 116 (7), 101 (22), 100 (5), 99 (78), 87 (5), 71 (27), 70 (37), 61 (6), 60 (17), 59 (18), 57 (100), 56 (12), 55 (26), 45 (5), 44 (18), 43 (52), 42 (13), 41 (35), 40 (35), 39 (11).
2-Methylbutyl 3,3-dimethylbutanoate (2c): MS (EI), m/z (%) 117 (6), 101 (16), 100 (7), 99 (100), 71 (57), 70 (78), 59 (14), 57 (73), 56 (18), 55 (25), 43 (44), 42 (11), 41 (43), 39 (16).
3-Methylbutyl 3,3-dimethylbutanoate (3c): MS (EI), m/z (%) 117 (9), 101 (7), 100 (5), 99 (50), 72 (5), 71 (58), 70 (100), 69 (5), 59 (10), 57 (54), 56 (12), 55 (31), 43 (51), 42 (11), 41 (45), 39 (16).
Pentyl 3,3-dimethylbutanoate (4c): MS (EI), m/z (%) 130 (11), 117 (51), 115 (8), 102 (5), 101 (33), 100 (9), 99 (95), 83 (5), 72 (5), 71 (34), 70 (100), 69 (8), 61 (23), 60 (20), 59 (29), 57 (96), 56 (16), 55 (35), 53 (6), 43 (70), 42 (24), 41 (71), 40 (5), 39 (25).
1-Methylbutyl 2,3-dimethylbutanoate (1d): MS (EI), m/z (%) 101 (22), 99 (6), 98 (19), 87 (5), 81 (8), 74 (11), 70 (16), 69 (17), 67 (6), 59 (7), 58 (6), 57 (100), 56 (35), 55 (33), 54 (7), 45 (15), 44 (48), 43 (35), 42 (13), 41 (32), 40 (91), 39 (14).
2-Methylbutyl 2,3-dimethylbutanoate (2d): MS (EI), m/z (%) 144 (5), 117 (11), 101 (8), 99 (55), 74 (38), 72 (5), 71 (100), 70 (73), 57 (8), 56 (14), 55 (36), 53 (5), 43 (76), 42 (13), 41 (31), 40 (24), 39 (14).
3-Methylbutyl 2,3-dimethylbutanoate (3d): MS (EI), m/z (%) 117 (19), 101 (8), 99 (27), 74 (23), 71 (97), 70 (100), 69 (6), 59 (7), 56 (11), 55 (31), 43 (77), 42 (9), 41 (30), 39 (10).
Pentyl 2,3-dimethylbutanoate (4d): MS (EI), m/z (%) 144 (6), 118 (5), 117 (66), 115 (5), 101 (12), 99 (37), 75 (12), 74 (69), 73 (5), 72 (5), 71 (70), 70 (45), 69 (6), 57 (7), 56 (14), 55 (34), 53 (5), 44 (15), 43 (100), 42 (20), 41 (40), 40 (24), 39 (16).
1-Methylbutyl 2-methylpentanoate (1e epimer I): MS (EI), m/z (%) 144 (5), 117 (19), 99 (44), 87 (6), 74 (31), 71 (74), 70 (23), 56 (6), 55 (13), 45 (8), 44 (46), 43 (67), 42 (9), 41 (21), 40 (100).
1-Methylbutyl 2-methylpentanoate (1e epimer II): MS (EI), m/z (%) 117 (28), 116 (6), 100 (5), 99 (50), 87 (7), 74 (35), 71 (100), 70 (29), 69 (6), 56 (6), 55 (19), 45 (11), 44 (47), 43 (78), 42 (12), 41 (28), 40 (99), 39 (9).
2-Methylbutyl 2-methylpentanoate (2e): MS (EI), m/z (%) 144 (6), 117 (17), 99 (65), 87 (9), 74 (30), 71 (100), 70 (71), 69 (5), 56 (8), 55 (27), 43 (73), 41 (35), 42 (13), 39 (13).
3-Methylbutyl 2-methylpentanoate (3e): MS (EI), m/z (%) 117 (25), 99 (31), 87 (7), 74 (14), 71 (86), 70 (100), 69 (8), 56 (8), 55 (35), 43 (83), 42 (13), 41 (40), 39 (15).
Pentyl 2-methylpentanoate (4e): MS (EI), m/z (%) 144 (8), 118 (7), 117 (100), 115 (11), 99 (42), 87 (15), 75 (7), 74 (50), 71 (65), 70 (39), 69 (9), 56 (9), 55 (27), 43 (86), 42 (19), 41 (43), 39 (16).
1-Methylbutyl 3-methylpentanoate (1f): MS (EI), m/z (%) 117 (22), 116 (7), 99 (60), 87 (13), 71 (37), 70 (30), 69 (6), 61 (10), 60 (23), 56 (12), 55 (19), 45 (8), 44 (45), 43 (47), 42 (13), 41 (29), 40 (100), 39 (9).
2-Methylbutyl 3-methylpentanoate (2f): MS (EI), m/z (%) 117 (11), 100 (7), 99 (100), 87 (11), 72 (5), 71 (72), 70 (91), 69 (7), 61 (5), 60 (14), 57 (12), 56 (6), 55 (25), 43 (55), 42 (16), 41 (34), 39 (12).
3-Methylbutyl 3-methylpentanoate (3f): Yield 75%; IR (cm−1) 2961, 2877, 1734, 1463, 1382, 1368, 1286, 1245, 1181, 1125, 1097, 1052, 987, 774, 738, 706; 1H NMR (400 MHz, CDCl3) 4.10 (triplet, J = 6.9 Hz, 2H, H-1′), 2.31 (doublet of doublets, J = 14.6, 8.1 Hz, 1H, H-2a), 2.09 (doublet of doublets, J = 14.6, 8.1 Hz, 1H, H-2b), 1.94–1.82 (multiplet, 1H, H-3), 1.68 (nonet, J = 6.9 Hz, 1H, H-3′), 1.52 (pseudo quartet, J = 6.9 Hz, 2H, H-2′), 1.47–1.31 (multiplet, 1H, H-4a), 1.29–1.13 (multiplet, 1H, H-4b), 0.93 (doublet, J = 6.7 Hz, 3H, H-6), 0.92 (doublet, J = 6.9 Hz, 6H, H-4′ and H-5′), 0.88 (triplet, J = 7.4 Hz, 3H, H-5); 13C NMR (101 MHz, CDCl3) 173.53 (C-1), 62.82 (C-1′), 41.61 (C-2), 37.39 (C-2′), 31.97 (C-3), 29.34 (C-4), 25.08 (C-3′), 22.46 (C-4′ and C-5′), 19.28 (C-6), 11.28 (C-5); MS (EI), m/z (%) 117 (15), 99 (46), 87 (7), 71 (57), 70 (100), 69 (8), 57 (7), 56 (5), 55 (29), 43 (54), 42 (14), 41 (33), 39 (13).
Pentyl 3-methylpentanoate (4f): MS (EI), m/z (%) 130 (6), 118 (7), 117 (95), 115 (7), 100 (7), 99 (92), 87 (26), 73 (9), 71 (64), 70 (100), 69 (15), 61 (22), 60 (24), 57 (14), 56 (8), 55 (36), 43 (81), 42 (31), 41 (57), 40 (5), 39 (20).
1-Methylbutyl 4-methylpentanoate (1g): MS (EI), m/z (%) 143 (7), 117 (31), 116 (10), 101 (11), 100 (7), 99 (100), 87 (12), 81 (37), 74 (6), 73 (16), 71 (39), 70 (54), 69 (9), 60 (7), 57 (23), 56 (12), 55 (45), 53 (6), 45 (7), 44 (17), 43 (95), 42 (19), 41 (42), 40 (25), 39 (13).
2-Methylbutyl 4-methylpentanoate (2g): MS (EI), m/z (%) 117 (13), 101 (11), 100 (9), 99 (100), 83 (6), 81 (45), 73 (12), 71 (46), 70 (95), 69 (8), 60 (5), 57 (14), 56 (13), 55 (37), 53 (5), 43 (72), 42 (13), 41 (44), 39 (16).
3-Methylbutyl 4-methylpentanoate (3g): MS (EI), m/z (%) 143 (5), 117 (15), 101 (9), 99 (49), 83 (5), 81 (30), 73 (7), 71 (42), 70 (100), 69 (9), 56 (9), 55 (40), 43 (68), 42 (12), 41 (39), 39 (14).
Pentyl 4-methylpentanoate (4g): MS (EI), m/z (%) 143 (5), 118 (6), 117 (100), 115 (6), 101 (18), 100 (6), 99 (71), 87 (5), 83 (12), 81 (45), 74 (7), 73 (25), 71 (27), 70 (74), 69 (14), 56 (12), 55 (43), 53 (5), 43 (83), 42 (23), 41 (55), 39 (20).
1-Methylbutyl hexanoate (1h): MS (EI), m/z (%) 117 (18), 116 (7), 100 (5), 99 (48), 87 (5), 71 (23), 70 (25), 60 (10), 56 (7), 55 (15), 45 (5), 44 (41), 43 (44), 42 (10), 41 (18), 40 (100), 39 (8).
2-Methylbutyl hexanoate (2h): MS (EI), m/z (%) 117 (12), 100 (7), 99 (100), 87 (7), 73 (7), 71 (49), 70 (81), 69 (5), 60 (9), 56 (5), 55 (27), 43 (55), 42 (16), 41 (31), 39 (12).
3-Methylbutyl hexanoate (3h): MS (EI), m/z (%) 117 (17), 99 (51), 73 (5), 71 (44), 70 (100), 69 (8), 60 (5), 55 (35), 43 (59), 42 (16), 41 (32), 39 (13).
Pentyl hexanoate (4h): MS (EI), m/z (%) 118 (6), 117 (100), 115 (5), 100 (6), 99 (80), 87 (13), 73 (15), 71 (38), 70 (77), 69 (13), 60 (14), 56 (5), 55 (35), 43 (72), 42 (28), 41 (46), 39 (17).
1-Methylpentyl 2,2-dimethylbutanoate (5b): MS (EI), m/z (%) 117 (15), 101 (24), 100 (8), 99 (95), 85 (15), 84 (40), 83 (7), 71 (13), 69 (19), 67 (6), 61 (5), 60 (13), 59 (17), 57 (100), 56 (32), 55 (49), 53 (6), 44 (10), 43 (56), 42 (22), 41 (49), 40 (18), 39 (17).
2-Methylpentyl 2,2-dimethylbutanoate (6b): MS (EI), m/z (%) 117 (11), 99 (20), 88 (10), 85 (12), 84 (33), 72 (6), 71 (100), 70 (17), 69 (9), 57 (6), 56 (19), 55 (15), 43 (66), 42 (9), 41 (26), 39 (9).
3-Methylpentyl 2,2-dimethylbutanoate (7b): MS (EI), m/z (%) 172 (7), 117 (16), 99 (8), 88 (8), 85 (15), 84 (47), 72 (6), 71 (100), 70 (20), 69 (23), 57 (11), 56 (10), 55 (22), 43 (65), 42 (8), 41 (31), 39 (10).
4-Methylpentyl 2,2-dimethylbutanoate (8b): MS (EI), m/z (%) 172 (7), 117 (42), 99 (11), 88 (9), 85 (11), 84 (21), 72 (6), 71 (100), 70 (20), 69 (11), 57 (5), 56 (12), 55 (16), 43 (73), 42 (10), 41 (30), 39 (10).
Hexyl 2,2-dimethylbutanoate (9b): MS (EI), m/z (%) 172 (7), 117 (34), 99 (23), 88 (9), 72 (6), 71 (100), 70 (15), 69 (5), 56 (7), 55 (15), 43 (53), 42 (8), 41 (24), 39 (9).
1-Methylpentyl 3,3-dimethylbutanoate (5c): MS (EI), m/z (%) 143 (5), 117 (13), 101 (23), 100 (7), 99 (86), 85 (13), 84 (41), 83 (6), 71 (9), 69 (19), 60 (13), 59 (22), 57 (100), 56 (32), 55 (52), 53 (7), 45 (6), 44 (18), 43 (68), 42 (28), 41 (58), 40 (44), 39 (25).
2-Methylpentyl 3,3-dimethylbutanoate (6c): MS (EI), m/z (%) 129 (5), 117 (9), 101 (16), 100 (7), 99 (100), 85 (41), 84 (62), 71 (14), 69 (13), 59 (13), 57 (73), 56 (40), 55 (21), 53 (5), 43 (58), 42 (12), 41 (42), 39 (16).
3-Methylpentyl 3,3-dimethylbutanoate (7c): MS (EI), m/z (%) 117 (16), 101 (6), 100 (5), 99 (49), 85 (53), 84 (100), 83 (6), 71 (10), 69 (44), 59 (10), 57 (76), 56 (32), 55 (33), 53 (6), 43 (45), 42 (9), 41 (55), 39 (16).
4-Methylpentyl 3,3-dimethylbutanoate (8c): MS (EI), m/z (%) 157 (5), 117 (49), 116 (8), 101 (13), 100 (7), 99 (67), 85 (63), 84 (81), 83 (9), 71 (13), 69 (32), 61 (7), 59 (14), 57 (100), 56 (71), 55 (30), 53 (6), 43 (83), 42 (17), 41 (80), 40 (5), 39 (22).
Hexyl 3,3-dimethylbutanoate (9c): MS (EI), m/z (%) 117 (58), 101 (34), 100 (8), 99 (83), 85 (17), 84 (62), 83 (8), 71 (13), 69 (22), 61 (40), 60 (12), 59 (23), 57 (100), 56 (52), 55 (35), 53 (6), 43 (73), 42 (19), 41 (72), 40 (5), 39 (22).
1-Methylpentyl 2,3-dimethylbutanoate (5d): MS (EI), m/z (%) 117 (19), 112 (11), 101 (26), 99 (48), 85 (25), 84 (27), 83 (12), 75 (10), 74 (43), 72 (5), 71 (57), 70 (23), 69 (19), 68 (5), 67 (6), 59 (5), 58 (6), 57 (100), 56 (40), 55 (53), 53 (6), 45 (10), 44 (31), 43 (85), 42 (21), 41 (57), 40 (69), 39 (17).
2-Methylpentyl 2,3-dimethylbutanoate (6d): MS (EI), m/z (%) 158 (5), 141 (5), 129 (5), 117 (21), 101 (11), 100 (5), 99 (79), 85 (39), 84 (62), 83 (8), 75 (7), 74 (49), 72 (6), 71 (92), 70 (8), 69 (19), 59 (8), 57 (23), 56 (63), 55 (31), 43 (100), 42 (11), 41 (46), 39 (15).
3-Methylpentyl 2,3-dimethylbutanoate (7d): MS (EI), m/z (%) 117 (19), 101 (5), 99 (22), 85 (39), 84 (93), 74 (14), 71 (51), 70 (10), 69 (55), 67 (9), 57 (25), 56 (32), 55 (36), 53 (7), 49 (6), 44 (22), 43 (100), 42 (13), 41 (57), 40 (57), 39 (17).
4-Methylpentyl 2,3-dimethylbutanoate (8d): MS (EI), m/z (%) 141 (5), 117 (27), 101 (7), 99 (34), 86 (5), 85 (50), 84 (100), 83 (8), 75 (8), 74 (25), 71 (63), 70 (10), 69 (50), 59 (10), 57 (35), 56 (36), 55 (39), 45 (7), 43 (86), 42 (8), 41 (44), 39 (11).
Hexyl 2,3-dimethylbutanoate (9d): MS (EI), m/z (%) 158 (5), 118 (6), 117 (62), 101 (9), 99 (29), 85 (10), 84 (35), 83 (8), 82 (6), 75 (12), 74 (57), 71 (54), 70 (9), 69 (22), 67 (10), 57 (16), 56 (40), 55 (48), 54 (7), 53 (9), 51 (7), 49 (9), 45 (5), 44 (47), 43 (100), 42 (26), 41 (62), 40 (97), 39 (23).
1-Methylpentyl 2-methylpentanoate (5e epimer I): MS (EI), m/z (%) 117 (28), 100 (5), 99 (72), 97 (6), 87 (7), 85 (41), 84 (29), 83 (16), 81 (6), 74 (38), 71 (68), 69 (23), 67 (8), 57 (24), 56 (24), 55 (43), 53 (5), 44 (9), 43 (100), 42 (17), 41 (46), 40 (10), 39 (15).
1-Methylpentyl 2-methylpentanoate (5e epimer II): MS (EI), m/z (%) 143 (5), 117 (28), 100 (5), 99 (74), 97 (5), 87 (7), 85 (38), 84 (28), 83 (9), 74 (37), 71 (63), 70 (7), 69 (20), 57 (17), 56 (23), 55 (34), 44 (8), 43 (100), 42 (15), 41 (38), 40 (10), 39 (11).
2-Methylpentyl 2-methylpentanoate (6e): MS (EI), m/z (%) 129 (6), 117 (25), 100 (5), 99 (77), 87 (9), 85 (32), 84 (58), 74 (33), 71 (84), 69 (21), 57 (11), 56 (48), 55 (24), 43 (100), 42 (16), 41 (49), 39 (15).
3-Methylpentyl 2-methylpentanoate (7e): MS (EI), m/z (%) 117 (35), 99 (28), 87 (6), 85 (37), 84 (100), 74 (12), 71 (55), 70 (6), 69 (56), 57 (20), 56 (27), 55 (34), 53 (5), 43 (80), 41 (50), 39 (15).
4-Methylpentyl 2-methylpentanoate (8e): MS (EI), m/z (%) 118 (6), 117 (90), 99 (32), 87 (7), 85 (29), 84 (53), 83 (5), 75 (6), 74 (16), 71 (54), 69 (28), 57 (13), 56 (39), 55 (22), 43 (100), 42 (15), 41 (50), 39 (14).
Hexyl 2-methylpentanoate (9e): MS (EI), m/z (%) 158 (5), 129 (6), 118 (7), 117 (100), 99 (35), 87 (13), 85 (7), 84 (30), 75 (12), 74 (45), 71 (52), 69 (17), 57 (10), 56 (29), 55 (27), 43 (86), 42 (16), 41 (44), 39 (14).
1-Methylpentyl 3-methylpentanoate (5f): MS (EI), m/z (%) 143 (6), 117 (24), 116 (13), 100 (6), 99 (100), 87 (10), 85 (15), 84 (32), 71 (33), 69 (12), 61 (8), 60 (22), 57 (13), 56 (26), 55 (21), 45 (5), 44 (10), 43 (65), 42 (15), 41 (32), 40 (21), 39 (9).
2-Methylpentyl 3-methylpentanoate (6f): MS (EI), m/z (%) 117 (14), 100 (7), 99 (100), 87 (10), 85 (27), 84 (60), 71 (42), 69 (18), 57 (15), 56 (36), 55 (19), 43 (67), 42 (17), 41 (42), 39 (13).
3-Methylpentyl 3-methylpentanoate (7f): MS (EI), m/z (%) 117 (26), 99 (51), 87 (7), 85 (32), 84 (100), 83 (5), 71 (33), 70 (6), 69 (57), 60 (5), 57 (25), 56 (28), 55 (33), 53 (5), 43 (58), 42 (14), 41 (49), 39 (15).
4-Methylpentyl 3-methylpentanoate (8f): MS (EI), m/z (%) 157 (8), 118 (6), 117 (89), 116 (9), 100 (5), 99 (69), 87 (12), 85 (36), 84 (72), 83 (9), 73 (6), 71 (41), 70 (5), 69 (38), 61 (8), 60 (8), 57 (25), 56 (71), 55 (27), 53 (5), 43 (100), 42 (23), 41 (68), 40 (5), 39 (20).
Hexyl 3-methylpentanoate (9f): MS (EI), m/z (%) 118 (7), 117 (100), 116 (6), 101 (6), 99 (80), 87 (23), 85 (14), 84 (59), 83 (6), 73 (8), 71 (43), 70 (6), 69 (31), 61 (33), 60 (16), 57 (22), 56 (52), 55 (36), 53 (5), 43 (88), 42 (25), 41 (63), 39 (18).
1-Methylpentyl 4-methylpentanoate (5g): MS (EI), m/z (%) 143 (8), 117 (25), 116 (12), 101 (16), 100 (9), 99 (100), 85 (17), 84 (37), 81 (32), 73 (12), 71 (13), 69 (16), 57 (25), 56 (25), 55 (28), 44 (5), 43 (75), 42 (13), 41 (32), 39 (9).
2-Methylpentyl 4-methylpentanoate (6g): MS (EI), m/z (%) 117 (18), 101 (12), 100 (7), 99 (100), 85 (26), 84 (65), 81 (45), 73 (10), 71 (17), 70 (5), 69 (22), 57 (14), 56 (48), 55 (29), 43 (86), 42 (14), 41 (49), 39 (16).
3-Methylpentyl 4-methylpentanoate (7g): Yield 78%; IR (cm−1) 2959, 2931, 2874, 1737, 1464, 1368, 1329, 1265, 1176, 1104, 1060, 974, 775; 1H NMR (400 MHz, CDCl3) 4.16–4.03 (multiplet, 2H, H-1′), 2.32–2.27 (multiplet, 2H, H-2), 1.72–1.61 (multiplet, 1H, H-2′a), 1.61–1.48 (overlapping peaks, 3H, H-3 and H-4), 1.48–1.41 (overlapping peaks, 2H, H-2′b and H-3′), 1.41–1.31 (multiplet, 1H, H-4′a), 1.19 (pseudo doublet of quintets, J = 13.5, 7.3 Hz, 1H, H-4′b), 0.90 (doublet, J = 6.4 Hz, 9H, H-5, H-6 and H-6′), 0.88 (triplet, J = 7.4 Hz, 3H, H-5′); 13C NMR (101 MHz, CDCl3) 174.19 (C-1), 62.87 (C-1′), 35.13 (C-2′), 33.82 (C-3), 32.48 (C-2), 31.41 (C-3′), 29.38 (C-4′), 27.68 (C-4), 22.23 (C-5 and C-6), 19.02 (C-6′), 11.22 (C-5′); MS (EI), m/z (%) 143 (6), 117 (30), 101 (11), 99 (44), 85 (34), 84 (100), 83 (10), 81 (34), 73 (6), 71 (13), 70 (7), 69 (65), 57 (22), 56 (33), 55 (44), 53 (6), 43 (72), 42 (10), 41 (54), 39 (16).
4-Methylpentyl 4-methylpentanoate (8g): MS (EI), m/z (%) 157 (10), 118 (5), 117 (73), 101 (14), 99 (51), 85 (33), 84 (71), 83 (15), 81 (39), 71 (14), 70 (6), 69 (45), 57 (19), 56 (59), 55 (37), 53 (6), 43 (100), 42 (15), 41 (64), 39 (18).
Hexyl 4-methylpentanoate (9g): MS (EI), m/z (%) 118 (6), 117 (100), 101 (19), 100 (5), 99 (61), 85 (10), 84 (48), 83 (14), 81 (41), 74 (6), 73 (20), 71 (14), 70 (5), 69 (29), 61 (12), 60 (5), 57 (15), 56 (50), 55 (42), 53 (5), 43 (89), 42 (18), 41 (59), 39 (17).
1-Methylpentyl hexanoate (5h): MS (EI), m/z (%) 143 (8), 117 (21), 116 (13), 100 (7), 99 (100), 85 (14), 84 (32), 73 (7), 71 (23), 69 (13), 60 (16), 57 (8), 56 (23), 55 (23), 44 (9), 43 (68), 42 (17), 41 (26), 40 (17), 39 (8).
2-Methylpentyl hexanoate (6h): MS (EI), m/z (%) 117 (17), 100 (7), 99 (100), 87 (6), 85 (19), 84 (52), 73 (6), 71 (29), 69 (18), 60 (7), 57 (7), 56 (36), 55 (20), 43 (66), 42 (16), 41 (36), 39 (12).
3-Methylpentyl hexanoate (7h): MS (EI), m/z (%) 143 (6), 117 (32), 99 (55), 85 (25), 84 (100), 83 (5), 73 (5), 71 (25), 70 (6), 69 (66), 60 (5), 57 (17), 56 (28), 55 (38), 43 (61), 42 (15), 41 (47), 39 (14).
4-Methylpentyl hexanoate (8h): MS (EI), m/z (%) 157 (11), 118 (6), 117 (98), 116 (6), 100 (5), 99 (70), 87 (7), 85 (27), 84 (76), 83 (9), 73 (8), 71 (29), 70 (6), 69 (48), 60 (6), 57 (15), 56 (62), 55 (32), 53 (5), 43 (100), 42 (24), 41 (64), 39 (17).
Hexyl hexanoate (9h): MS (EI), m/z (%) 118 (6), 117 (100), 116 (5), 100 (5), 99 (71), 87 (11), 85 (9), 84 (50), 73 (14), 71 (27), 70 (5), 69 (28), 61 (14), 60 (10), 57 (9), 56 (45), 55 (35), 43 (79), 42 (22), 41 (50), 39 (15).
(Z)-Hex-3-en-1-yl 2,2-dimethylbutanoate (10b): MS (EI), m/z (%) 99 (29), 72 (6), 71 (100), 70 (10), 55 (23), 53 (7), 43 (46), 42 (12), 41 (32), 40 (5), 39 (25).
(Z)-Hex-3-en-1-yl 3,3-dimethylbutanoate (10c): MS (EI), m/z (%) 101 (5), 99 (27), 83 (26), 82 (69), 81 (7), 71 (10), 67 (81), 59 (9), 57 (76), 56 (19), 55 (69), 54 (12), 53 (18), 51 (6), 43 (22), 42 (17), 41 (100), 40 (11), 39 (55).
(Z)-Hex-3-en-1-yl 2,3-dimethylbutanoate (10d): MS (EI), m/z (%) 107 (6), 106, (6), 105 (7), 99 (16), 94 (6), 83 (16), 82 (100), 81 (7), 80 (5), 79 (8), 78 (14), 74 (6), 71 (56), 68 (7), 67 (80), 56 (10), 55 (31), 54 (7), 53 (10), 52 (7), 51 (18), 50 (6), 44 (18), 43 (46), 42 (15), 41 (36), 40 (25), 39 (18).
(Z)-Hex-3-en-1-yl 2-methylpentanoate (10e): MS (EI), m/z (%) 99 (15), 83 (8), 82 (86), 81 (7), 79 (8), 74 (5), 71 (56), 69 (9), 68 (8), 67 (81), 65 (8), 56 (9), 55 (51), 54 (16), 53 (24), 51 (9), 44 (7), 43 (87), 42 (25), 41 (100), 40 (20), 39 (67), 38 (7).
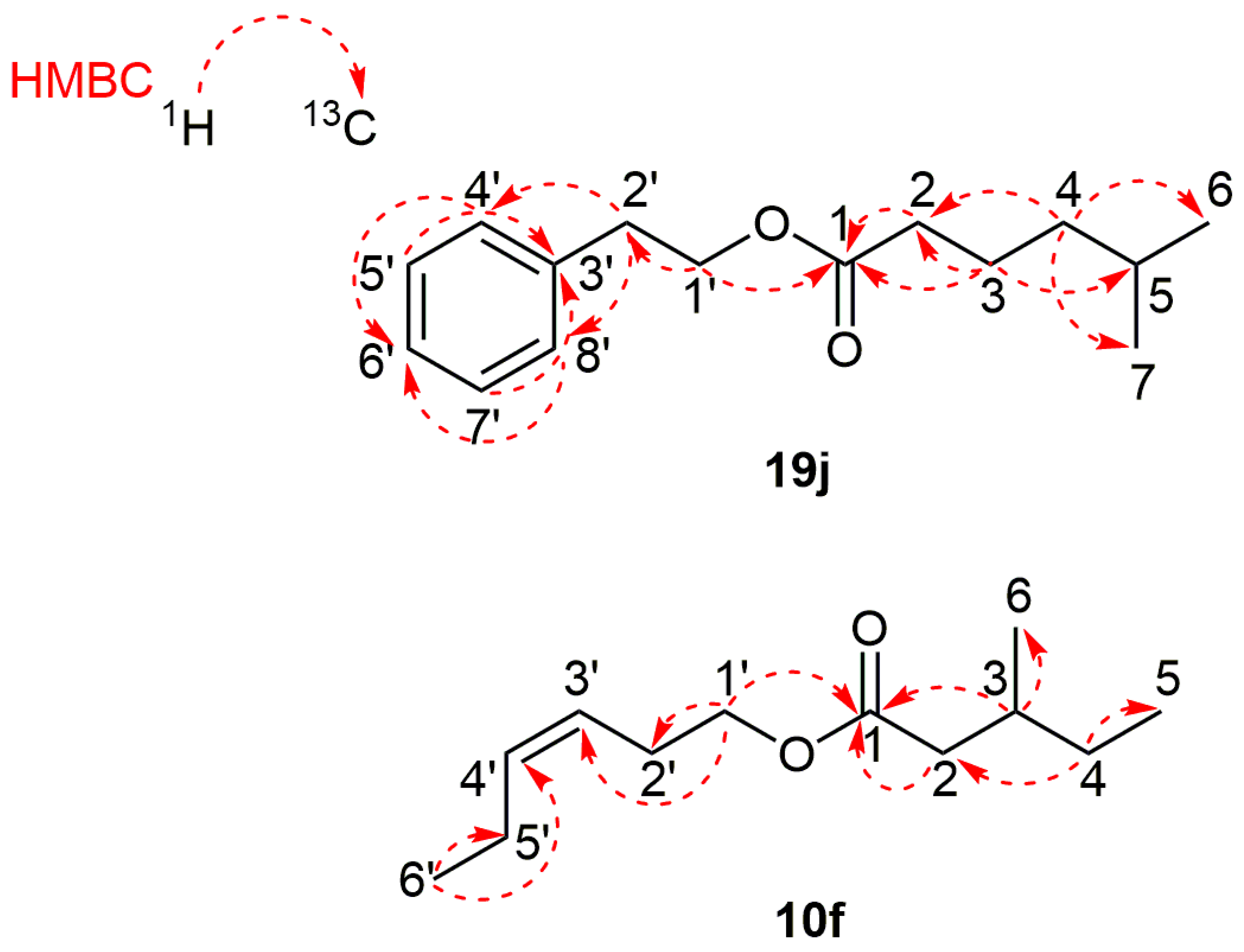
(Z)-Hex-3-en-1-yl 3-methylpentanoate (10f): Yield: 68%; IR (cm−1) 2963, 2934, 2877, 1736, 1461, 1382, 1286, 1243, 1178, 1125, 1097, 1045, 1007, 720; 1H NMR (400 MHz, CDCl3) 5.54–5.46 (multiplet, 1H, H-4′), 5.36–5.28 (multiplet, 1H, H-3′), 4.07 (triplet, J = 6.9 Hz, 2H, H-1′), 2.38 (pseudo quartet of doublets of triplets, J = 7.0, 1.4, 0.7 Hz, 2H, H-2′), 2.30 (doublet of doublets, J = 14.7, 6.1 Hz, 1H, H-2a), 2.10 (doublet of doublets, J = 14.7, 8.2 Hz, 1H, H-2b), 2.10–2.02 (multiplet, 1H, H-5′), 1.89 (doublet of doublets of quartets of doublets of doublets, J = 8.2, 7.4, 6.7, 6.1, 5.7 Hz, 1H, H-3), 1.36 (doublet of quartets of doublets, J = 13.4, 7.4, 5.7 Hz, 1H, H-4a), 1.22 (pseudo doublet of quintets, J = 13.4, 7.4 Hz, 1H, H-4b), 0.97 (pseudo triplet, J = 7.5 Hz, 3H, H-6′), 0.93 (doublet, J = 6.7 Hz, 3H, H-6), 0.89 (pseudo triplet, J = 7.4 Hz, 3H, H-5); 13C NMR (101 MHz, CDCl3) 173.42 (C-1), 134.47 (C-4′), 123.81 (C-3′), 63.70 (C-1′), 41.52 (C-2), 31.92 (C-3), 29.33 (C-4), 26.79 (C-2′), 20.61 (C-5′), 19.27 (C-6), 14.23 (C-6′), 11.28 (C-5); MS (EI), m/z (%) 99 (41), 83 (16), 82 (100), 81 (8), 71 (32), 68 (6), 67 (80), 57 (6), 55 (27), 54 (6), 42 (8), 41 (29), 39 (13).
(Z)-Hex-3-en-1-yl 4-methylpentanoate (10g): MS (EI), m/z (%) 101 (5), 99 (24), 83 (15), 82 (87), 81 (36), 71 (12), 67 (100), 56 (11), 55 (58), 54 (12), 53 (16), 51 (5), 43 (78), 42 (15), 41 (92), 40 (9), 39 (45).
(Z)-Hex-3-en-1-yl hexanoate (10h): MS (EI), m/z (%) 99 (38), 83 (12), 82 (100), 81 (7), 71 (24), 68 (7), 67 (93), 55 (30), 54 (7), 43 (35), 42 (10), 41 (36), 39 (15).
1-Methylhexyl angelate (11o): MS (EI), m/z (%) 100 (8), 84 (7), 83 (100), 55 (59), 53 (7), 39 (10).
2-Methylhexyl angelate (12o): MS (EI), m/z (%) 101 (7), 100 (35), 99 (5), 98 (15), 91 (6), 84 (5), 83 (27), 81 (9), 79 (9), 70 (13), 69 (25), 67 (10), 57 (29), 56 (65), 55 (54), 53 (11), 44 (31), 43 (19), 42 (11), 41 (51), 40 (100), 39 (27).
3-Methylhexyl angelate (13o): MS (EI), m/z (%) 101 (27), 100 (64), 99 (7), 98 (19), 85 (5), 83 (60), 82 (11), 71 (5), 70 (26), 69 (34), 58 (5), 57 (100), 56 (22), 55 (79), 53 (11), 44 (10), 43 (39), 41 (32), 40 (19), 39 (17).
4-Methylhexyl angelate (14o): MS (EI), m/z (%) 101 (6), 100 (14), 83 (13), 70 (8), 69 (15), 57 (23), 56 (7), 55 (24), 54 (5), 44 (30), 43 (10), 41 (30), 40 (100), 39 (10).
5-Methylhexyl angelate (15o): MS (EI), m/z (%) 101 (11), 100 (39), 84 (10), 83 (100), 82 (9), 69 (6), 57 (27), 56 (13), 55 (73), 54 (9), 53 (9), 51 (5), 49 (7), 43 (13), 41 (13), 39 (15).
Heptyl angelate (16o): MS (EI), m/z (%) 101 (20), 100 (100), 85 (7), 83 (53), 82 (14), 69 (5), 57 (28), 56 (10), 55 (54), 43 (15), 42 (5), 41 (18), 39 (12).
1-Methylhexyl tiglate (11p): MS (EI), m/z (%) 101 (16), 98 (6), 86 (5), 84 (13), 83 (100), 82 (6), 69 (6), 56 (9), 55 (58), 53 (8), 49 (9), 43 (6), 39 (12).
2-Methylhexyl tiglate (12p): MS (EI), m/z (%) 101 (35), 100 (9), 99 (7), 98 (44), 84 (6), 83 (99), 81 (8), 70 (24), 69 (42), 67 (6), 58 (5), 57 (29), 56 (100), 55 (83), 54 (10), 53 (14), 51 (5), 44 (11), 43 (28), 42 (13), 41 (67), 40 (30). 39 (33).
3-Methylhexyl tiglate (13p): MS (EI), m/z (%) 101 (65), 100 (8). 99 (6), 98 (65), 84 (7), 83 (100), 82 (5), 71 (6), 70 (58), 69 (66), 57 (23), 56 (35), 55 (93), 54 (8), 53 (11), 43 (25), 42 (6), 41 (30), 40 (8), 39 (16).
4-Methylhexyl tiglate (14p): MS (EI), m/z (%) 102 (6), 101 (89), 100 (14), 99 (5), 98 (40), 84 (7), 83 (81), 82 (7), 81 (8), 71 (7), 70 (77), 69 (53), 67 (9), 57 (53), 56 (37), 55 (100), 54 (9), 53 (19), 44 (16), 43 (28), 42 (15), 41 (77), 40 (39), 39 (34).
5-Methylhexyl tiglate (15p): Yield: 75%; IR (cm−1) 2955, 2870, 1712, 1654, 1467, 1385, 1367, 1255, 1136, 1079, 734, 656; UV (MeCN, 0.05 M) λmax nm (log ε): 199 (2.87), 216 (1.65), 274 (0.21); 1H NMR (400 MHz, CDCl3) 6.85 (quartet of quartets, J = 7.0, 1.4 Hz, 1H, H-3), 4.12 (triplet, J = 6.7 Hz, 2H, H-1′), 1.83 (pseudo quintet, J = 1.4 Hz, 3H, H-5), 1.79 (doublet of quartets, J = 7.0, 1.4 Hz, 3H, H-4), 1.65 (pseudo quintet, J = 6.7 Hz, 2H, H-2′), 1.54 (nonet, J = 6.6 Hz, 1H, H-5′), 1.41–1.33 (multiplet, 2H, H-3′), 1.27–1.17 (multiplet, 2H, H-4′), 0.88 (doublet, J = 6.6 Hz, 6H, H-6′ and H-7′); 13C NMR (101 MHz, CDCl3) 168.25 (C-1), 136.81 (C-3), 128.79 (C-2), 64.57 (C-1′), 38.54 (C-4′), 28.92 (C-2′), 27.86 (C-5′), 23.80 (C-3′), 22.55 (C-6′ and C-7′), 14.32 (C-4), 12.03 (C-5); MS (EI), m/z (%) 102 (6), 101 (100), 100 (11), 84 (6), 83 (76), 82 (5), 70 (19), 69 (18), 57 (22), 56 (35), 55 (59), 53 (7), 43 (18), 41 (19), 39 (10).
Heptyl tiglate (16p): MS (EI), m/z (%) 102 (5), 101 (87), 100 (17), 84 (9), 83 (100), 82 (8), 70 (12), 69 (9), 57 (12), 56 (19), 55 (76), 54 (8), 53 (10), 43 (11), 42 (6), 41 (19), 39 (14).
1-Methylhexyl senecioate (11q): MS (EI), m/z (%) 101 (28), 100 (44), 98 (8), 84 (6), 83 (100), 82 (7), 70 (5), 69 (8), 57 (17), 56 (17), 55 (27), 53 (5), 43 (13), 42 (5), 41 (18), 39 (11).
2-Methylhexyl senecioate (12q): MS (EI), m/z (%) 101 (22), 100 (36), 98 (12), 84 (6), 83 (100), 82 (6), 69 (5), 57 (19), 56 (17), 55 (21), 43 (8), 41 (11), 39 (7).
3-Methylhexyl senecioate (13q): MS (EI), m/z (%) 101 (36), 100 (38). 99 (5), 98 (20), 84 (6), 83 (100), 82 (8), 70 (16), 69 (19), 57 (42), 56 (11), 55 (34), 53 (7), 43 (18), 41 (18), 39 (11).
4-Methylhexyl senecioate (14q): MS (EI), m/z (%) 101 (52), 100 (37), 98 (9), 84 (6), 83 (100), 82 (9), 70 (24), 69 (17), 57 (64), 56 (12), 55 (40), 53 (9), 43 (17), 42 (7), 41 (34), 40 (10), 39 (17).
5-Methylhexyl senecioate (15q): MS (EI), m/z (%) 101 (54), 100 (61), 85 (5), 84 (6), 83 (100), 82 (11), 70 (6), 69 (7), 57 (37), 56 (13), 55 (33), 53 (6), 43 (19), 41 (18), 39 (11).
Heptyl senecioate (16q): MS (EI), m/z (%) 198 (5), 101 (59), 100 (92), 85 (8), 84 (6), 83 (100), 82 (15), 70 (5), 57 (23), 56 (9), 55 (30), 53 (6), 43 (13), 42 (5), 41 (17), 39 (11).
1-Methylbutyl 2-methylheptanoate (1l epimer I): RI = 1337 (DB-5MS column); MS (EI), m/z (%) 146 (5), 145 (43), 144 (17), 127 (56), 99 (13), 87 (15), 74 (74), 71 (58), 70 (44), 69 (9), 57 (84), 56 (13), 55 (49), 53 (6), 45 (9), 44 (35), 43 (100), 42 (25), 41 (48), 40 (65), 39 (17).
1-Methylbutyl 2-methylheptanoate (1l epimer II): RI = 1341 (DB-5MS column); MS (EI), m/z (%) 146 (6), 145 (46), 144 (14), 128 (5), 127 (57), 99 (13), 87 (21), 74 (85), 71 (62), 70 (46), 69 (10), 57 (88), 56 (11), 55 (43), 45 (8), 44 (32), 43 (100), 42 (21), 41 (48), 40 (46), 39 (19).
2-Methylbutyl 2-methylheptanoate (2l): RI = 1379 (DB-5MS column); MS (EI), m/z (%) 145 (25), 144 (18), 128 (5), 127 (53), 101 (7), 99 (17), 87 (16), 74 (50), 71 (52), 70 (100), 69 (9), 58 (5), 57 (92), 56 (14), 55 (37), 43 (59), 42 (16), 41 (52), 39 (14).
3-Methylbutyl 2-methylheptanoate (3l): RI = 1372 (DB-5MS column); MS (EI), m/z (%) 145 (17), 144 (6), 127 (14), 99 (6), 87 (8), 74 (15), 71 (39), 70 (100), 69 (7), 57 (44), 56 (8), 55 (27), 43 (46), 42 (9), 41 (30).
Pentyl 2-methylheptanoate (4l): RI = 1412 (DB-5MS column); MS (EI), m/z (%) 146 (9), 145 (100), 144 (19), 127 (30), 115 (15), 103 (5), 101 (9), 99 (10), 87 (24), 75 (12), 74 (80), 73 (5), 71 (15), 70 (55), 69 (13), 57 (76), 56 (13), 55 (34), 43 (64), 42 (20), 41 (48), 39 (11).
1-Methylbutyl 6-methylheptanoate (1m): RI = 1390 (DB-5MS column); MS (EI), m/z (%) 145 (45), 128 (7), 127 (86), 126 (12), 117 (33), 111 (14), 109 (99), 104 (76), 101 (13), 99 (24), 98 (6), 89 (5), 87 (13), 86 (37), 84 (24), 83 (58), 82 (62), 74 (9), 69 (31), 67 (20), 61 (44), 60 (21), 59 (8), 57 (68), 56 (31), 55 (98), 53 (6), 45 (47), 44 (44), 43 (100), 42 (25), 41 (78), 40 (7), 39 (21).
2-Methylbutyl 6-methylheptanoate (2m): RI = 1413 (DB-5MS column); MS (EI), m/z (%) 145 (6), 127 (30), 126 (5), 109 (40), 101 (6), 85 (9), 82 (16), 73 (7), 71 (37), 70 (100), 69 (6), 60 (9), 57 (22), 56 (9), 55 (34), 44 (10), 43 (49), 42 (11), 41 (31), 40 (19), 39 (8).
3-Methylbutyl 6-methylheptanoate (3m): RI = 1410 (DB-5MS column); MS (EI), m/z (%) 145 (5), 127 (9), 109 (18), 82 (10), 71 (31), 70 (100), 67 (6), 57 (14), 56 (6), 55 (39), 44 (24), 43 (51), 42 (11), 41 (30), 40 (42), 39 (11).
Pentyl 6-methylheptanoate (4m): RI = 1449 (DB-5MS column); MS (EI), m/z (%) 146 (5), 145 (54), 127 (24), 111 (6), 109 (35), 101 (9), 85 (8), 83 (17), 82 (26), 73 (17), 71 (19), 70 (100), 69 (11), 67 (8), 61 (13), 60 (16), 57 (24), 56 (8), 55 (44), 44 (8), 43 (71), 42 (21), 41 (46), 40 (11), 39 (10).
1-Methylbutyl octanoate (1n): RI = 1414 (DB-5MS column); MS (EI), m/z (%) 145 (49), 144 (28), 128 (8), 127 (100), 115 (6), 101 (17), 87 (31), 85 (10), 84 (19), 73 (12), 71 (33), 70 (76), 69 (11), 61 (7), 60 (26), 57 (62), 56 (10), 55 (73), 45 (9), 44 (29), 43 (84), 42 (36), 41 (56), 40 (44), 39 (20).
2-Methylbutyl octanoate (2n): RI = 1451 (DB-5MS column); MS (EI), m/z (%) 145 (15), 144 (9), 128 (9), 127 (100), 115 (5), 109 (5), 101 (9), 87 (7), 73 (8), 71 (32), 70 (97), 69 (7), 60 (9), 57 (57), 56 (7), 55 (39), 43 (44), 42 (16), 41 (46), 39 (14).
3-Methylbutyl octanoate (3n): RI = 1448 (DB-5MS column); MS (EI), m/z (%) 145 (11), 127 (29), 73 (5), 71 (28), 70 (100), 69 (6), 57 (26), 55 (32), 43 (40), 42 (10), 41 (27), 39 (7).
Pentyl octanoate (4n): RI = 1486 (DB-5MS column); MS (EI), m/z (%) 146 (9), 145 (100), 144 (9), 128 (6), 127 (65), 115 (10), 103 (5), 101 (13), 97 (5), 89 (9), 87 (9), 73 (19), 71 (16), 70 (90), 69 (13), 67 (5), 61 (11), 60 (17), 57 (47), 56 (7), 55 (48), 43 (57), 42 (27), 41 (53), 39 (14).
1-Methylhexyl 2,2-dimethylbutanoate (11b): MS (EI), m/z (%) 117 (16), 99 (26), 98 (12), 88 (6), 84 (5), 72 (5), 71 (100), 70 (14), 69 (5), 57 (57), 56 (7), 55 (20), 49 (9), 44 (26), 43 (61), 42 (11), 41 (30), 40 (47), 39 (11).
2-Methylhexyl 2,2-dimethylbutanoate (12b): MS (EI), m/z (%) 117 (15), 99 (24), 98 (33), 88 (11), 81 (9), 72 (7), 71 (100), 70 (47), 69 (39), 67 (9), 58 (5), 57 (48), 56 (83), 55 (64), 53 (16), 44 (33), 43 (70), 42 (23), 41 (78), 40 (62), 39 (31).
3-Methylhexyl 2,2-dimethylbutanoate (13b): MS (EI), m/z (%) 117 (16), 99 (14), 98 (40), 88 (6), 81 (10), 72 (6), 71 (100), 70 (64), 69 (50), 67 (10), 57 (39), 56 (34), 55 (68), 54 (5), 53 (12), 44 (29), 43 (81), 42 (21), 41 (64), 40 (49), 39 (27).
4-Methylhexyl 2,2-dimethylbutanoate (14b): MS (EI), m/z (%) 117 (7), 98 (8), 81 (6), 71 (25), 70 (26), 69 (16), 67 (7), 57 (15), 56 (13), 55 (30), 44 (47), 43 (22), 42 (13). 41 (37), 40 (100), 39 (17).
5-Methylhexyl 2,2-dimethylbutanoate (15b): MS (EI), m/z (%) 117 (41), 99 (10), 98 (8), 88 (9), 83 (6), 72 (6), 71 (100), 70 (41), 69 (11), 57 (34), 56 (30), 55 (29), 44 (12), 43 (74), 42 (10), 41 (36), 40 (22), 39 (11).
Heptyl 2,2-dimethylbutanoate (16b): MS (EI), m/z (%) 186 (7), 122 (34), 121 (42), 117 (52), 101 (6), 99 (17), 98 (10), 97 (10), 96 (7), 88 (14), 83 (5), 82 (7), 81 (12), 78 (7), 72 (6), 71 (100), 70 (32), 69 (13), 68 (7), 66 (13), 57 (28), 56 (17), 55 (29), 54 (9), 51 (9), 44 (5), 43 (61), 42 (16), 41 (40), 39 (14).
1-Methylhexyl 3,3-dimethylbutanoate (11c): MS (EI), m/z (%) 143 (6), 117 (15), 116 (10), 101 (21), 100 (7), 99 (87), 98 (26), 71 (10), 70 (10), 69 (10), 60 (8), 59 (13), 58 (5), 57 (100), 56 (22), 55 (20), 43 (25), 42 (9), 41 (32), 39 (9).
2-Methylhexyl 3,3-dimethylbutanoate (12c): MS (EI), m/z (%) 117 (7), 101 (9), 99 (65), 98 (26), 71 (8), 70 (16), 69 (10), 61 (9), 59 (8), 57 (100), 56 (41), 55 (17), 43 (28), 42 (8), 41 (34), 40 (12), 39 (9).
3-Methylhexyl 3,3-dimethylbutanoate (13c): MS (EI), m/z (%) 117 (10), 99 (36), 98 (43), 83 (8), 71 (12), 70 (46), 69 (41), 61 (8), 57 (100), 56 (37), 55 (39), 53 (6), 44 (15), 43 (37), 42 (11), 41 (54), 40 (27), 39 (15).
4-Methylhexyl 3,3-dimethylbutanoate (14c): MS (EI), m/z (%) 117 (15), 99 (29), 98 (32), 96 (6), 83 (11), 81 (9), 79 (8), 71 (8), 70 (60), 69 (53), 67 (9), 59 (6), 57 (100), 56 (36), 55 (52), 53 (9), 44 (31), 43 (25), 42 (12), 41 (77), 40 (69), 39 (27).
5-Methylhexyl 3,3-dimethylbutanoate (15c): MS (EI), m/z (%) 117 (21), 101 (7), 99 (38), 98 (28), 83 (15), 71 (8), 70 (30), 69 (16), 61 (7), 57 (100), 56 (41), 55 (21), 43 (28), 42 (5), 41 (30), 39 (7).
Heptyl 3,3-dimethylbutanoate (16c): MS (EI), m/z (%) 117 (40), 101 (22), 100 (5), 99 (51), 98 (35), 83 (9), 71 (11), 70 (30), 69 (16), 61 (27), 60 (6), 59 (14), 57 (100), 56 (30), 55 (29), 53 (5), 43 (35), 42 (12), 41 (59), 39 (15).
1-Methylhexyl 2,3-dimethylbutanoate (11d): MS (EI), m/z (%) 117 (9), 99 (22), 98 (16), 85 (16), 84 (10), 83 (6), 81 (6), 74 (16), 73 (9), 71 (46), 70 (21), 69 (32), 67 (5), 57 (64), 56 (45), 55 (48), 54 (9), 53 (7), 49 (5), 44 (37), 43 (66), 42 (15), 41 (62), 40 (100), 39 (19).
2-Methylhexyl 2,3-dimethylbutanoate (12d): MS (EI), m/z (%) 117 (5), 99 (19), 98 (11), 84 (6), 81 (5), 74 (7), 71 (23), 70 (9), 69 (8), 57 (24), 56 (23), 55 (17), 49 (8), 44 (42), 43 (27), 42 (6), 41 (27), 40 (100), 39 (9).
3-Methylhexyl 2,3-dimethylbutanoate (13d): MS (EI), m/z (%) 117 (8), 99 (11), 98 (28), 86 (5), 84 (8), 81 (8), 79 (5), 74 (5), 71 (24), 70 (23), 69 (25), 68 (6), 57 (26), 56 (24), 55 (27), 53 (6), 51 (5), 49 (7), 44 (50), 43 (33), 42 (9), 41 (32), 40 (100), 39 (11).
4-Methylhexyl 2,3-dimethylbutanoate (14d): MS (EI), m/z (%) 84 (5), 57 (6), 49 (9), 44 (50), 43 (7), 41 (6), 40 (100).
5-Methylhexyl 2,3-dimethylbutanoate (15d): MS (EI), m/z (%) 118 (5), 117 (72), 101 (8), 99 (35), 98 (28), 83 (17), 81 (6), 75 (11), 74 (32), 71 (66), 70 (46), 69 (28), 58 (5), 57 (100), 56 (78), 55 (49), 53 (6), 44 (11), 43 (96), 42 (10), 41 (59), 40 (21), 39 (17).
Heptyl 2,3-dimethylbutanoate (16d): MS (EI), m/z (%) 118 (6), 117 (100), 116 (5), 101 (14), 99 (42), 98 (35), 83 (7), 75 (20), 74 (78), 71 (74), 70 (37), 69 (25), 68 (7), 57 (76), 56 (60), 55 (22), 54 (7), 44 (16), 43 (96), 42 (22), 41 (75), 40 (31), 39 (21).
1-Methylhexyl 2-methylpentanoate (11e epimer I): MS (EI), m/z (%) 143 (7), 117 (33), 116 (5), 100 (6), 99 (90), 98 (28), 87 (7), 74 (48), 71 (72), 70 (16), 69 (16), 58 (5), 57 (100), 56 (32), 55 (29), 45 (7), 43 (69), 42 (13), 41 (43), 39 (11).
1-Methylhexyl 2-methylpentanoate (11e epimer II): MS (EI), m/z (%) 143 (10), 117 (41), 116 (6), 100 (7), 99 (100), 98 (30), 87 (8), 74 (44), 72 (5), 71 (74), 70 (15), 69 (14), 58 (5), 57 (93), 56 (27), 55 (26), 45 (6), 43 (64), 42 (13), 41 (41), 39 (12).
2-Methylhexyl 2-methylpentanoate (12e): MS (EI), m/z (%) 117 (31), 100 (6), 99 (91), 98 (44), 87 (8), 83 (6), 74 (41), 71 (92), 70 (38), 69 (31), 58 (5), 57 (86), 56 (100), 55 (35), 53 (5), 44 (5), 43 (89), 42 (17), 41 (62), 40 (5), 39 (17).
3-Methylhexyl 2-methylpentanoate (13e): MS (EI), m/z (%) 143 (8), 117 (48), 99 (45), 98 (93), 87 (5), 83 (16), 74 (16), 72 (5), 71 (82). 70 (87), 69 (85), 68 (5), 57 (97), 56 (57), 55 (53), 53 (5), 44 (5), 43 (100), 42 (16), 41 (58), 39 (14).
4-Methylhexyl 2-methylpentanoate (14e): MS (EI), m/z (%) 118 (6), 117 (80), 99 (33), 98 (48), 87 (5), 83 (8), 74 (14), 71 (62), 70 (97), 69 (54), 67 (6), 58 (5), 57 (100), 56 (45), 55 (44), 53 (7), 44 (8), 43 (76), 42 (20), 41 (76), 40 (11), 39 (19).
5-Methylhexyl 2-methylpentanoate (15e): MS (EI), m/z (%) 118 (7), 117 (96), 99 (43), 98 (36), 87 (8), 83 (20), 75 (9), 74 (29), 71 (71), 70 (51), 69 (33), 58 (5), 57 (100), 56 (84), 55 (44), 44 (5), 43 (98), 42 (13), 41 (54), 39 (13).
Heptyl 2-methylpentanoate (16e): MS (EI), m/z (%) 143 (7), 118 (6), 117 (100), 99 (31), 98 (28), 87 (11), 75 (12), 74 (38), 71 (48), 70 (24), 69 (16), 57 (38), 56 (26), 55 (29), 43 (65), 42 (15), 41 (52), 39 (14).
1-Methylhexyl 3-methylpentanoate (11f): MS (EI), m/z (%) 143 (7), 117 (23), 116 (14), 100 (7), 99 (100), 98 (28), 87 (10), 71 (30), 70 (13), 69 (15), 61 (7), 60 (18), 57 (40), 56 (29), 55 (21), 43 (34), 42 (11), 41 (33), 39 (10).
2-Methylhexyl 3-methylpentanoate (12f): MS (EI), m/z (%) 117 (18), 100 (7), 99 (100), 98 (38), 87 (7), 83 (5), 71 (37), 70 (30), 69 (21), 61 (12), 60 (12), 57 (73), 56 (72), 55 (26), 44 (6), 43 (61), 42 (16), 41 (51), 40 (11), 39 (13).
3-Methylhexyl 3-methylpentanoate (13f): MS (EI), m/z (%) 143 (5), 117 (35), 100 (5), 99 (63), 98 (80), 97 (5), 87 (6), 83 (17), 81 (7), 71 (47), 70 (93), 69 (100), 68 (7), 61 (12), 60 (7), 57 (91), 56 (65), 55 (71), 53 (9), 44 (16), 43 (89), 42 (25), 41 (90), 40 (27), 39 (27).
4-Methylhexyl 3-methylpentanoate (14f): MS (EI), m/z (%) 117 (35), 99 (32), 98 (51), 96 (6), 87 (5), 83 (13), 81 (8), 79 (7), 71 (26), 70 (87), 69 (79), 68 (7), 67 (12), 61 (6), 60 (5), 58 (5), 57 (74), 56 (42), 55 (62), 54 (6), 53 (12), 44 (26), 43 (41), 42 (24), 41 (100), 40 (50), 39 (29).
5-Methylhexyl 3-methylpentanoate (15f): MS (EI), m/z (%) 118 (5), 117 (69), 100 (5), 99 (69), 98 (46), 87 (11), 83 (28), 71 (39), 70 (63), 69 (41), 61 (11), 57 (100), 56 (87), 55 (43), 43 (69), 42 (13), 41 (54), 39 (12).
Heptyl 3-methylpentanoate (16f): MS (EI), m/z (%) 118 (7), 117 (100), 116 (8), 101 (5), 99 (71), 98 (46), 87 (21), 83 (11), 73 (8), 71 (42), 70 (46), 69 (30), 68 (8), 61 (27), 60 (12), 57 (62), 56 (41), 55 (40), 53 (6), 43 (61), 42 (24), 41 (75), 39 (20).
1-Methylhexyl 4-methylpentanoate (11g): MS (EI), m/z (%) 143 (8), 117 (22), 116 (10), 101 (10), 100 (7), 99 (100), 98 (33), 83 (6), 81 (30), 73 (11), 71 (13), 70 (17), 69 (21), 57 (59), 56 (39), 55 (32), 43 (52), 42 (10), 41 (39), 39 (10).
2-Methylhexyl 4-methylpentanoate (12g): MS (EI), m/z (%) 117 (23), 101 (10), 100 (7), 99 (100), 98 (43), 83 (9), 81 (39), 73 (10), 71 (18), 70 (39), 69 (24), 61 (6), 57 (76), 56 (89), 55 (32), 43 (76), 42 (12), 41 (49), 40 (5), 39 (12).
3-Methylhexyl 4-methylpentanoate (13g): MS (EI), m/z (%) 143 (7), 117 (32), 101 (11), 99 (46), 98 (73), 97 (7), 83 (22), 81 (33), 73 (7), 71 (23), 70 (95), 69 (96), 67 (6), 61 (5), 57 (82), 56 (65), 55 (76), 54 (5), 53 (7), 43 (100), 42 (20), 41 (77), 40 (38), 39 (21).
4-Methylhexyl 4-methylpentanoate (14g): MS (EI), m/z (%) 157 (6), 117 (55), 115 (5), 101 (8), 99 (36). 98 (40), 97 (6), 83 (12), 81 (26), 73 (6), 71 (16), 70 (100), 69 (53), 67 (5), 57 (76), 56 (41), 55 (43), 53 (6), 44 (5), 43 (53), 42 (15), 41 (61), 40 (7), 39 (15).
5-Methylhexyl 4-methylpentanoate (15g): MS (EI), m/z (%) 118 (5), 117 (80), 101 (18), 100 (5), 99 (66), 98 (42), 97 (7), 83 (39), 81 (42), 73 (12), 71 (19), 70 (70), 69 (47), 58 (5), 57 (97), 56 (100), 55 (60), 53 (5), 44 (5), 43 (90), 42 (12), 41 (62), 39 (14).
Heptyl 4-methylpentanoate (16g): MS (EI), m/z (%) 118 (7), 117 (100), 116 (8), 101 (19), 100 (5), 99 (57), 98 (43), 97 (7), 83 (17), 81 (40), 74 (5), 73 (17), 71 (15), 70 (43), 69 (28), 68 (7), 61 (10), 57 (45), 56 (40), 55 (41), 53 (5), 43 (67), 42 (15), 41 (64), 39 (16).
1-Methylhexyl hexanoate (11h): MS (EI), m/z (%) 143 (7), 117 (20), 116 (11), 100 (6), 99 (100), 98 (26), 73 (7), 71 (21), 70 (15), 69 (15), 60 (15), 57 (33), 56 (29), 55 (22), 43 (39), 42 (11), 41 (27), 39 (8).
2-Methylhexyl hexanoate (12h): MS (EI), m/z (%) 117 (22), 100 (7), 99 (100), 98 (37), 87 (6), 83 (5), 73 (6), 71 (27), 70 (33), 69 (20), 61 (6), 60 (9), 57 (55), 56 (76), 55 (26), 43 (68), 42 (15), 41 (44), 40 (5), 39 (10).
3-Methylhexyl hexanoate (13h): MS (EI), m/z (%) 143 (7), 117 (36), 99 (57), 98 (73), 97 (6), 83 (17), 81 (6), 73 (5), 71 (34), 70 (95), 69 (100), 68 (7), 61 (7), 57 (67), 56 (65), 55 (72), 53 (8), 44 (11), 43 (95), 42 (25), 41 (79), 40 (16), 39 (22).
4-Methylhexyl hexanoate (14h): MS (EI), m/z (%) 157 (5), 117 (51), 99 (38), 98 (37), 97 (5), 83 (11), 71 (22), 70 (100), 69 (68), 67 (8), 57 (63), 56 (38), 55 (47), 53 (7), 44 (11), 43 (44), 42 (21), 41 (68), 40 (20), 39 (21).
5-Methylhexyl hexanoate (15h): Yield 77%; IR (cm−1) 2956, 2932, 2870, 1737, 1467, 1385, 1367, 1245, 1170, 1099, 1009, 734; H NMR (400 MHz, CDCl3) 4.06 (triplet, J = 6.7 Hz, 2H, H-1′), 2.29 (triplet, J = 7.5 Hz, 2H, H-2), 1.69–1.57 (overlapping peaks, 4H, H-2′ and H-3), 1.55 (nonet, J = 6.6 Hz, 1H, H-5′), 1.39–1.24 (overlapping peaks, 6H, H-3′, H-4 and H-5), 1.23–1.15 (multiplet, 2H, H-4′), 0.90 (triplet, J = 6.9 Hz, 3H, H-6), 0.87 (doublet, J = 6.6 Hz, 6H, H-6′ and H-7′); 13C NMR (101 MHz, CDCl3) 174.03 (C-1), 64.40 (C-1′), 38.53 (C-4′), 34.39 (C-2), 31.34 (C-4), 28.89 (C-2′), 27.89 (C-5′), 24.73 (C-3), 23.73 (C-3′), 22.55 (C-6′ and C-7′), 22.34 (C-5), 13.92 (C-6); MS (EI), m/z (%) 117 (63), 99 (57), 98 (36), 87 (5), 83 (26), 73 (8), 71 (25), 70 (62), 69 (42), 68 (5), 60 (9), 57 (78), 56 (100), 55 (53), 44 (14), 43 (86), 42 (20), 41 (59), 40 (13), 39 (15).
Heptyl hexanoate (16h): MS (EI), m/z (%) 118 (6), 117 (100), 99 (65), 98 (41), 97 (5), 89 (6), 87 (11), 83 (8), 73 (12), 71 (29), 70 (44), 69 (29), 68 (7), 61 (13), 60 (8), 57 (39), 56 (39), 55 (40), 53 (5), 43 (63), 42 (22), 41 (63), 39 (17).
6-Methylheptyl angelate (17o): MS (EI), m/z (%) 101 (16), 100 (42), 95 (9), 91 (7), 84 (7), 83 (23), 82 (9), 81 (8), 79 (6), 71 (16), 70 (11), 69 (32), 68 (6), 67 (12), 57 (34), 56 (39), 55 (52), 54 (10), 53 (13), 51 (6), 49 (7), 44 (40), 43 (35), 42 (14), 41 (52), 40 (100), 39 (24).
Octyl angelate (18o): MS (EI), m/z (%) 101 (29), 100 (100), 85 (5), 83 (31), 82 (10), 71 (12), 70 (5), 57 (19), 56 (8), 55 (37), 43 (19), 42 (5), 41 (15), 39 (7).
6-Methylheptyl tiglate (17p): MS (EI), m/z (%) 102 (9), 101 (100), 100 (10), 97 (8), 84 (12), 83 (59), 82 (5), 71 (5), 70 (10), 69 (23), 57 (18), 56 (27), 55 (48), 53 (5), 43 (20), 42 (5), 41 (18), 39 (8).
Octyl tiglate (18p): MS (EI), m/z (%) 102 (6), 101 (100), 100 (12), 84 (9), 83 (57), 82 (6), 70 (11), 69 (9), 57 (9), 56 (12), 55 (45), 53 (6), 43 (12), 42 (5), 41 (16), 39 (8).
6-Methylheptyl senecioate (17q): MS (EI), m/z (%) 101 (60), 100 (69), 85 (6), 84 (9), 83 (100), 82 (12), 71 (20), 70 (6), 69 (17), 57 (33), 56 (20), 55 (40), 53 (8), 43 (35), 42 (8), 41 (29), 40 (5), 39 (14).
Octyl senecioate (18q): MS (EI), m/z (%) 212 (5), 101 (67), 100 (98), 84 (8), 83 (100), 82 (15), 71 (10), 70 (7), 69 (7), 57 (15), 56 (8), 55 (31), 53 (7), 43 (18), 42 (6), 41 (19), 39 (10).
2-Phenylethyl 4-methylhexanoate (19i): Yield 80%; RI = 1720 (DB-5MS column); IR (cm−1) 3030, 2960, 2875, 1735, 1605, 1498, 1455, 1381, 1364, 1237, 1169, 1104, 1031, 748, 698; UV (MeCN, 0.05 M) λmax nm (log ε): 204 (4.50), 224 (4.20), 275 (3.67), 281 (3.69); 1H NMR (400 MHz, CDCl3) 7.34–7.27 (multiplet, 2H, H-5′ and H-7′), 7.25–7.20 (overlapping peaks, 3H, H-4′, H-6′, and H-8′), 4.28 (triplet, J = 7.1 Hz, 2H, H-1′), 2.94 (triplet, J = 7.1 Hz, 2H, H-2′), 2.37–2.20 (multiplet, 2H, H-2), 1.69–1.58 (multiplet, 1H, H-3a), 1.45–1.36 (multiplet, 1H, H-3b), 1.36–1.25 (overlapping peaks, 2H, H-4 and H-5a), 1.20–1.08 (multiplet, 1H, H-5b), 0.86 (triplet, J = 7.1 Hz, 3H, H-6), 0.85 (doublet, J = 6.4 Hz, 3H, H-7); 13C NMR (101 MHz, CDCl3) 174.09 (C-1), 137.90 (C-3′), 128.91 (C-4′ and C-8′), 128.48 (C-5′ and C-7′), 126.53 (C-6′), 64,74 (C-1′), 35.16 (C-2′), 33.97 (C-4), 32.14 (C-2), 31.49 (C-3), 29.13 (C-5), 18.79 (C-7), 11.28 (C-6); MS (EI), m/z (%) 105 (33), 104 (100), 103 (5), 95 (9), 83 (5), 77 (6), 55 (10), 43 (10).
2-Phenylethyl 5-methylhexanoate (19j): Yield 82%; RI = 1711 (DB-5MS column); IR (cm−1) 3030, 2955, 2871, 1735, 1605, 1498, 1455, 1386, 1367, 1311, 1246, 1168, 1107, 1056, 1016, 747, 698; MS (EI), m/z (%) 105 (31), 104 (100), 103 (5), 95 (11), 77 (6), 43 (11); UV (MeCN, 0.05 M) λmax nm (log ε): 205 (4.55), 223 (4.22), 275 (3.71), 280 (3.71); 1H NMR (400 MHz, CDCl3) 7.33–7.27 (multiplet, 2H, H-5′ and H-7′), 7.25–7.19 (overlapping peaks, 3H, H-4′, H-6′, and H-8′), 4.29 (triplet, J = 7.1 Hz, 2H, H-1′), 2.93 (triplet, J = 7.1 Hz, 2H, H-2′), 2.26 (triplet, J = 7.6 Hz, 2H, H-2), 1.63–1.55 (multiplet, 2H, H-3), 1.53 (nonet, J = 6.6 Hz, 1H, H-5), 1.19–1.12 (multiplet, 2H, H-4), 0.87 (doublet, 6H, J = 6.6 Hz, H-6 and H-7); 13C NMR (101 MHz, CDCl3) 173.82 (C-1), 137.90 (C-3′), 128.92 (C-4′ and C-8′), 128.49 (C-5′ and C-7′), 126.54 (C-6′), 64.72 (C-1′), 38.35 (C-4), 35.17 (C-2′), 34.57 (C-2), 27.78 (C-5), 22.86 (C-3), 22.50 (C-6 and C-7); MS (EI), m/z (%) 105 (31), 104 (100), 103 (5), 95 (11), 77 (6), 43 (11).
2-Phenylethyl heptanoate (19k): RI = 1749 (DB-5MS column); MS (EI), m/z (%) 113 (5), 105 (27), 104 (100), 103 (7), 91 (9), 77 (7), 43 (10).
2-Phenylethyl 6-methylheptanoate (19m): RI = 1812 (DB-5MS column); MS (EI), m/z (%) 109 (6), 105 (32), 104 (100), 103 (5), 91 (6), 77 (5), 57 (6), 43 (7).
2-Phenylethyl octanoate (19n): RI = 1853 (DB-5MS column); MS (EI), m/z (%) 105 (27), 104 (100), 103 (6), 91 (7), 77 (6), 57 (11), 41 (7).