Impact of Sm3+ Ions on Oxygen Vacancy Formation in Ceria Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Sm-Doped CeO2(111) Surface
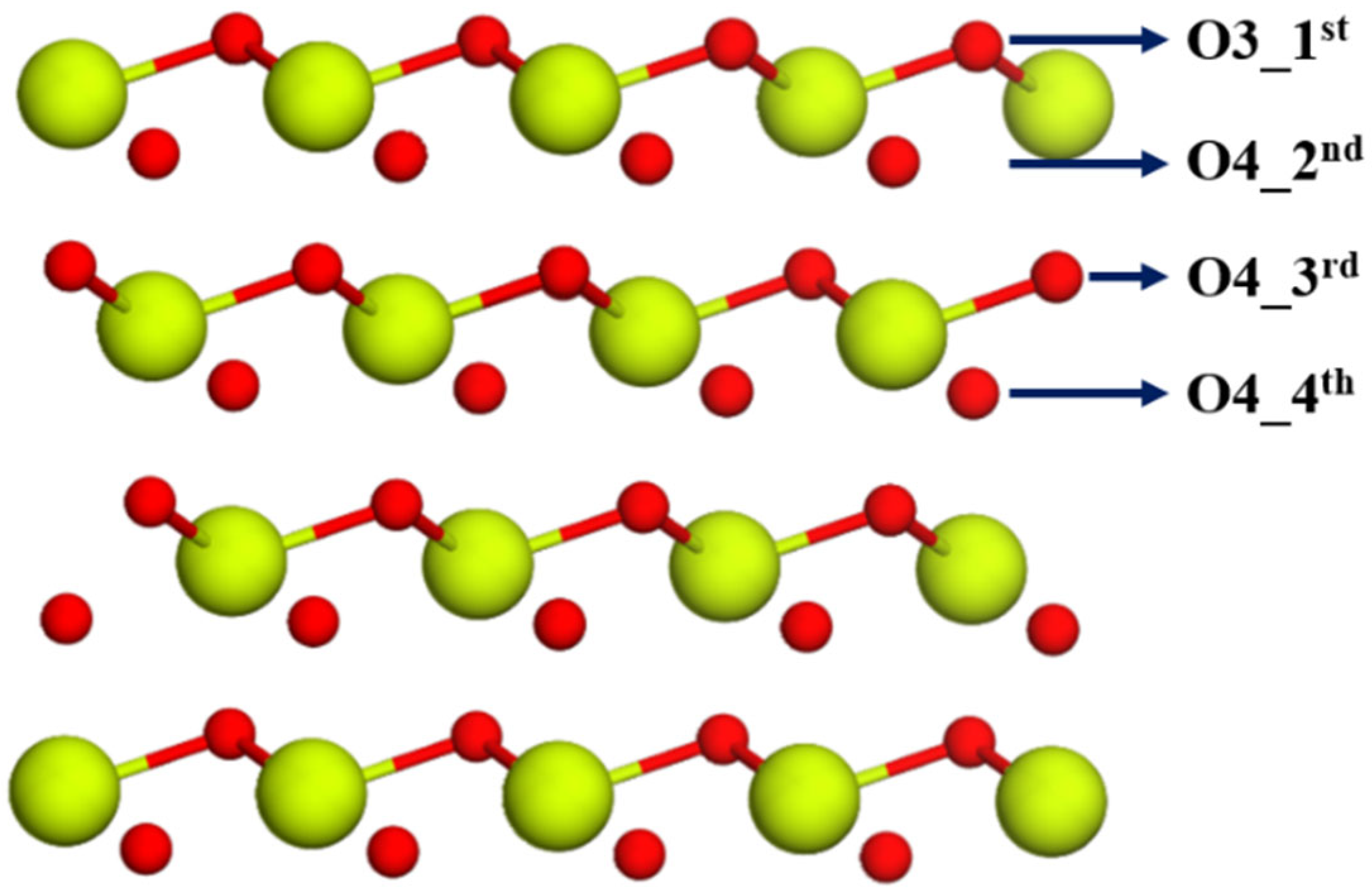
2.1.1. Sm Mono- and Bi-Doped Surfaces
2.1.2. Reducibility of Surfaces with Sm Mono- and Bi-Doping
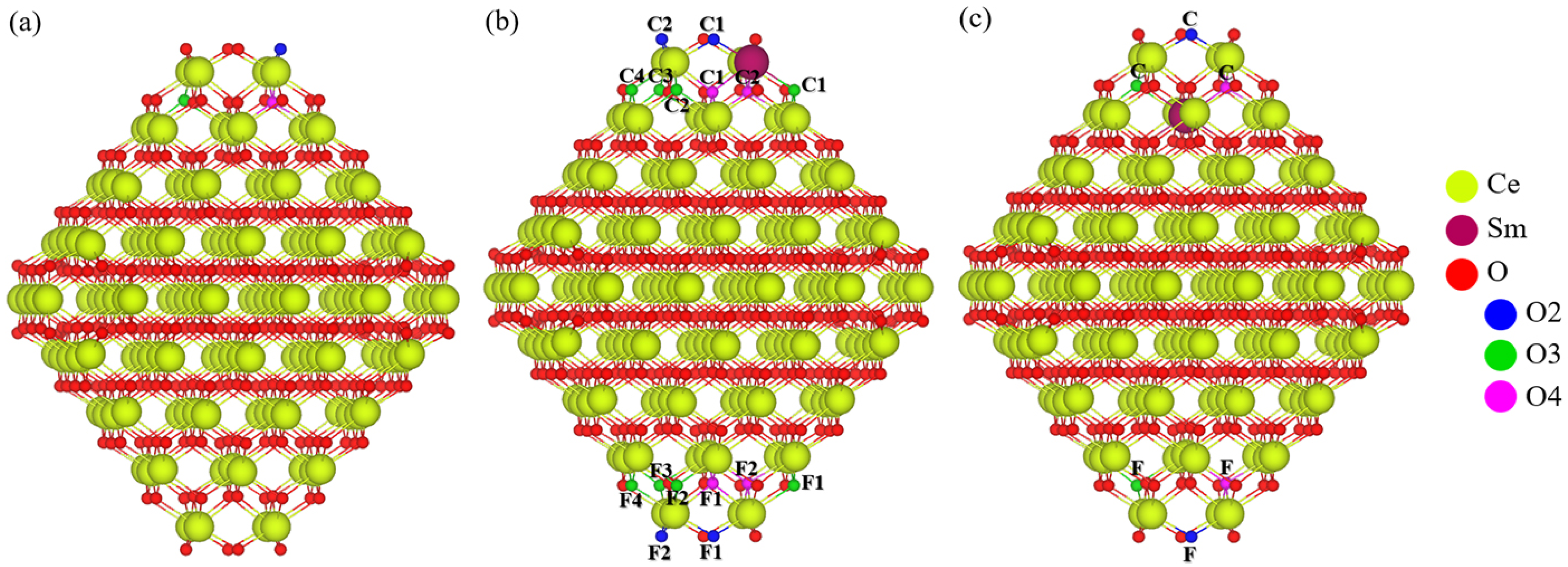
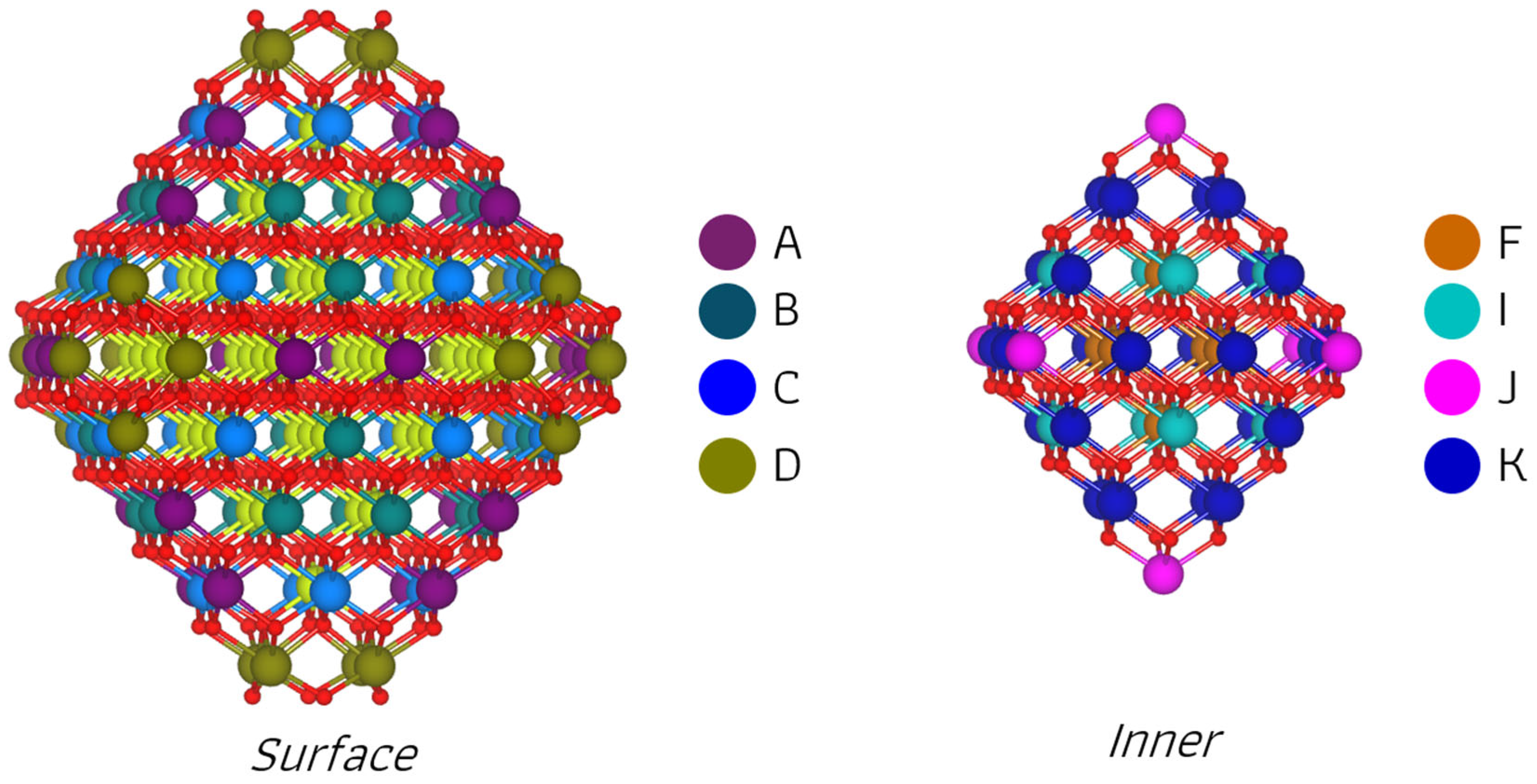
2.2. Sm-Doped Ce140O280 Nanoparticle
2.2.1. Sm Mono- and Bi-Doped Nanoparticle
2.2.2. Reducibility of Nanoparticles with Sm Mono- and Bi-Doping
3. Method and Models
3.1. Computational Details
3.2. CeO2(111) Slab Model
3.3. Ce140O280 Nanoparticle Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, D.; Babucci, M.; Casey, W.H.; Gates, B.C. The Surface Chemistry of Metal Oxide Clusters: From Metal–Organic Frameworks to Minerals. ACS Cent. Sci. 2020, 6, 1523–1533. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmad, T.; Naaz, F.; Islam, S. ul Review on Metals and Metal Oxides in Sustainable Energy Production: Progress and Perspectives. Energy Fuels 2023, 37, 1577–1632. [Google Scholar] [CrossRef]
- Vinchhi, P.; Ray, A.; Mallik, K.; Pati, R. Gd-Doped Ceria with Extraordinary Oxygen-Ion Conductivity for Low Temperature Solid Oxide Fuel Cells. Sci. Rep. 2024, 14, 19010. [Google Scholar] [CrossRef]
- Putla, S.B.; Kamali, M.; Swapna, B.; Reddy, B.M.; Sudarsanam, P. Review of Shape-Controlled CeO2 Nanocatalysts for Purification of Auto-Exhaust Pollutants. ACS Appl. Nano Mater. 2024, 7, 6749–6771. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Y.; Zhang, Z.; An, J.; Wang, F. Transformations of Biomass, Its Derivatives, and Downstream Chemicals over Ceria Catalysts. ACS Catal. 2020, 10, 8788–8814. [Google Scholar] [CrossRef]
- Weng, Q.; Sun, H.; Fang, C.; Xia, F.; Liao, H.; Lee, J.; Wang, J.; Xie, A.; Ren, J.; Guo, X. Catalytic Activity Tunable Ceria Nanoparticles Prevent Chemotherapy-Induced Acute Kidney Injury without Interference with Chemotherapeutics. Nat. Commun. 2021, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, Y.; Lee, N.; Soh, M.; Kim, D.; Hyeon, T. Ceria-based Therapeutic Antioxidants for Biomedical Applications. Adv. Mater. 2024, 36, 2210819. [Google Scholar] [CrossRef]
- Khivantsev, K.; Pham, H.; Engelhard, M.H.; Aleksandrov, H.A.; Kovarik, L.; Bowden, M.; Li, X.S.; Tian, J.; Koleva, I.Z.; Song, I.; et al. Transforming Ceria into 2D Clusters Enhances Catalytic Activity. Nature 2025, 640, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.K.; Liu, Z.; Lee, H.; Hong, S.; Song, H.; Abbas, H.G.; Kwon, Y.; Ringe, S.; Oh, J. Boosting Electrochemical CO2 Reduction to Methane via Tuning Oxygen Vacancy Concentration and Surface Termination on a Copper/Ceria Catalyst. ACS Catal. 2022, 12, 10973–10983. [Google Scholar] [CrossRef]
- Ziemba, M.; Schilling, C.; Ganduglia-Pirovano, M.V.; Hess, C. Toward an Atomic-Level Understanding of Ceria-Based Catalysts: When Experiment and Theory Go Hand in Hand. Acc. Chem. Res. 2021, 54, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Schaube, M.; Merkle, R.; Maier, J. Interdependence of Point Defects and Reaction Kinetics: CO and CH4 Oxidation on Ceria and Zirconia. J. Phys. Chem. C 2020, 124, 18544–18556. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C. Support Nanostructure Boosts Oxygen Transfer to Catalytically Active Platinum Nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Wu, Z. In Situ Spectroscopic Insights into the Redox and Acid-Base Properties of Ceria Catalysts. Chin. J. Catal. 2021, 42, 2122–2140. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Nolan, M.; Fearon, J.E.; Watson, G.W. Oxygen Vacancy Formation and Migration in Ceria. Solid State Ion 2006, 177, 3069–3074. [Google Scholar] [CrossRef]
- Han, Z.-K.; Liu, W.; Gao, Y. Advancing the Understanding of Oxygen Vacancies in Ceria: Insights into Their Formation, Behavior, and Catalytic Roles. JACS Au 2025, 5, 1549–1569. [Google Scholar] [CrossRef]
- Lee, J.G.; Park, J.H.; Shul, Y.G. Tailoring Gadolinium-Doped Ceria-Based Solid Oxide Fuel Cells to Achieve 2 W Cm−2 at 550 C. Nat. Commun. 2014, 5, 4045. [Google Scholar] [CrossRef]
- Burbano, M.; Norberg, S.T.; Hull, S.; Eriksson, S.G.; Marrocchelli, D.; Madden, P.A.; Watson, G.W. Oxygen Vacancy Ordering and the Conductivity Maximum in Y2O3-Doped CeO2. Chem. Mater. 2012, 24, 222–229. [Google Scholar] [CrossRef]
- Abdulwahab, K.O.; Khan, M.M.; Jennings, J.R. Doped Ceria Nanomaterials: Preparation, Properties, and Uses. ACS Omega 2023, 8, 30802–30823. [Google Scholar] [CrossRef]
- Fauzi, A.A.; Jalil, A.A.; Hassan, N.S.; Aziz, F.F.A.; Azami, M.S.; Hussain, I.; Saravanan, R.; Vo, D.-V. A Critical Review on Relationship of CeO2-Based Photocatalyst towards Mechanistic Degradation of Organic Pollutant. Chemosphere 2022, 286, 131651. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent Activity of Ceria in Soot Combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen Vacancies and Catalysis on Ceria Surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and Crystal-plane Effects of Nanoscale Ceria on the Activity of Au-CeO2 Catalysts for the Water–Gas Shift Reaction. Angew. Chem. Int. Ed. 2008, 47, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.Z.; Aleksandrov, H.A.; Vayssilov, G.N. Comparison of the Reactivity of Platinum Cations and Clusters Supported on Ceria or Alumina in Carbon Monoxide Oxidation. ACS Catal. 2023, 13, 5358–5374. [Google Scholar] [CrossRef]
- Hu, Z.; Metiu, H. Effect of Dopants on the Energy of Oxygen-Vacancy Formation at the Surface of Ceria: Local or Global? J. Phys. Chem. C 2011, 115, 17898–17909. [Google Scholar] [CrossRef]
- Dholabhai, P.P.; Adams, J.B.; Crozier, P.; Sharma, R. Oxygen Vacancy Migration in Ceria and Pr-Doped Ceria: A DFT+ U Study. J. Chem. Phys. 2010, 132, 094104. [Google Scholar] [CrossRef]
- Sardesai, N.P.; Ganesana, M.; Karimi, A.; Leiter, J.C.; Andreescu, S. Platinum-Doped Ceria Based Biosensor for in Vitro and in Vivo Monitoring of Lactate during Hypoxia. Anal. Chem. 2015, 87, 2996–3003. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y.; Zhao, J. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef]
- Figueroba, A.; Bruix, A.; Kovács, G.; Neyman, K.M. Metal-Doped Ceria Nanoparticles: Stability and Redox Processes. Phys. Chem. Chem. Phys. 2017, 19, 21729–21738. [Google Scholar] [CrossRef]
- Baek, J.W.; Han, S.; Lee, S.E.; Ahn, J.; Park, C.; Nam, J.S.; Kim, Y.H.; Shin, E.; Kim, M.; Jang, J.-S. Cobalt-Doped Ceria Sensitizer Effects on Metal Oxide Nanofibers: Heightened Surface Reactivity for High-Performing Chemiresistive Sensors. ACS Nano 2024, 18, 19568–19580. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Khan, J.A.; Muhammad, N.; Khan, Z.U.H.; Naseem, M.; Howari, F.M.; Nazzal, Y.; Niazi, N.K. Synthesis of Nitrogen-Doped Ceria Nanoparticles in Deep Eutectic Solvent for the Degradation of Sulfamethaxazole under Solar Irradiation and Additional Antibacterial Activities. Chem. Eng. J. 2020, 394, 124869. [Google Scholar] [CrossRef]
- Yang, H.; Jia, L.; Haraguchi, J.; Wang, Y.; Xu, B.; Zhang, Q.; Nan, Z.; Zhang, M.; Ohno, T. Nitrogen and Sulfur Co-Doped CeO2 Nanorods for Efficient Photocatalytic VOCs Degradation. Catal. Sci. Technol. 2022, 12, 5203–5209. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 Semiconductor as a Photocatalytic and Photoelectrocatalytic Material for the Remediation of Pollutants in Industrial Wastewater: A Review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Reddy, B.M.; Bharali, P.; Saikia, P.; Khan, A.; Loridant, S.; Muhler, M.; Grünert, W. Hafnium Doped Ceria Nanocomposite Oxide as a Novel Redox Additive for Three-Way Catalysts. J. Phys. Chem. C 2007, 111, 1878–1881. [Google Scholar] [CrossRef]
- Koleva, I.Z.; Aleksandrov, H.A.; Neyman, K.M.; Vayssilov, G.N. Preferential Location of Zirconium Dopants in Cerium Dioxide Nanoparticles and the Effects of Doping on Their Reducibility: A DFT Study. Phys. Chem. Chem. Phys. 2020, 22, 26568–26582. [Google Scholar] [CrossRef] [PubMed]
- Karapenchev, B.S.; Koleva, I.Z.; Neyman, K.M.; Aleksandrov, H.A. DFT Study of the Stability and Reducibility of Hf-Doped Ceria Nanoparticles. J. Phys. Chem. C 2025, 129, 7704–7716. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Bultinck, P.; Cottenier, S.; Van Speybroeck, V.; Van Driessche, I. Aliovalent Doping of CeO2: DFT Study of Oxidation State and Vacancy Effects. J. Mater. Chem. A 2014, 2, 13723–13737. [Google Scholar] [CrossRef]
- Xie, J.; Alomar, M.; Shah, M.A.K.Y.; Arshad, M.S.; Mushtaq, N. Optimizing Oxygen Vacancies and Electrochemical Performance of CeO2−δ Nanosheets through the Combination of Di-and Tri-Valent Doping. RSC adv. 2023, 13, 27233–27243. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; AlKhoori, A.A.; Efstathiou, A.M.; Jaoude, M.A.; Damaskinos, C.M.; Baker, M.A.; Almutawa, A.; Anjum, D.H.; Vasiliades, M.A.; Belabbes, A. Design Aspects of Doped CeO2 for Low-Temperature Catalytic CO Oxidation: Transient Kinetics and DFT Approach. ACS Appl. Mater. Interfaces 2021, 13, 22391–22415. [Google Scholar] [CrossRef]
- Aleksandrov, H.A.; Koleva, I.Z.; Neyman, K.M.; Tabakova, T.T.; Vayssilov, G.N. Structure and Reducibility of Yttrium-Doped Cerium Dioxide Nanoparticles and (111) Surface. RSC Adv. 2018, 8, 33728–33741. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, C.; Xiao, Y.; Xie, A.; Pang, Y.U.E.; Yang, Y. One-Step Synthesis of Samarium-Doped Ceria and Its CO Catalysis. Bull. Mater. Sci. 2015, 38, 1149–1154. [Google Scholar] [CrossRef]
- Chanapattharapol, K.C.; Krachuamram, S.; Kidkhunthod, P.; Poo-arporn, Y. The Effect of Sm Addition on Structure, Redox Properties and Catalytic Activities for Water Gas Shift Reaction of Ceria-Based Support. Solid State Sci. 2020, 99, 106066. [Google Scholar] [CrossRef]
- Anjaneya, K.C.; Nayaka, G.P.; Manjanna, J.; Govindaraj, G.; Ganesha, K.N. Studies on Structural, Morphological and Electrical Properties of Ce0.8Ln0.2O2−δ (Ln= Y3+, Gd3+, Sm3+, Nd3+ and La3+) Solid Solutions Prepared by Citrate Complexation Method. J. Alloys Compd. 2014, 585, 594–601. [Google Scholar] [CrossRef]
- Kim, D. Lattice Parameters, Ionic Conductivities, and Solubility Limits in Fluorite-structure MO2 Oxide [M= Hf4+, Zr4+, Ce4+, Th4+, U4+] Solid Solutions. J. Am. Ceram. Soc. 1989, 72, 1415–1421. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, R.-J.; Lee, W.; Dong, C.-L.; Gloter, A. Spectromicroscopic Evidence of Interstitial and Substitutional Dopants in Association with Oxygen Vacancies in Sm-Doped Ceria Nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 3274–3281. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Y.; Ding, L.; Xu, L.; Wu, Z.; Yuan, Q.; Huang, W. Reactivity of Hydroxyls and Water on a CeO2 (111) Thin Film Surface: The Role of Oxygen Vacancy. J. Phys. Chem. C 2013, 117, 5800–5810. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Mihaylov, M.; Petkov, P.S.; Hadjiivanov, K.I.; Neyman, K.M. Reassignment of the Vibrational Spectra of Carbonates, Formates, and Related Surface Species on Ceria: A Combined Density Functional and Infrared Spectroscopy Investigation. J. Phys. Chem. C 2011, 115, 23435–23454. [Google Scholar] [CrossRef]
- Hahn, K.R.; Iannuzzi, M.; Seitsonen, A.P.; Hutter, J. Coverage Effect of the CO2 Adsorption Mechanisms on CeO2 (111) by First Principles Analysis. J. Phys. Chem. C 2013, 117, 1701–1711. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Erratum: Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1993, 48, 4978. [Google Scholar] [CrossRef]
- Bao, J.L.; Gagliardi, L.; Truhlar, D.G. Self-Interaction Error in Density Functional Theory: An Appraisal. J. Phys. Chem. Lett. 2018, 9, 2353–2358. [Google Scholar] [CrossRef]
- Sharkas, K.; Wagle, K.; Santra, B.; Akter, S.; Zope, R.R.; Baruah, T.; Jackson, K.A.; Perdew, J.P.; Peralta, J.E. Self-Interaction Error Overbinds Water Clusters but Cancels in Structural Energy Differences. Proc. Natl. Acad. Sci. USA 2020, 117, 11283–11288. [Google Scholar] [CrossRef]
- Lundberg, M.; Siegbahn, P.E.M. Quantifying the Effects of the Self-Interaction Error in DFT: When Do the Delocalized States Appear? J. Chem. Phys. 2005, 122, 224103. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Atienzar, P.; García, H.; Chane-Ching, J.-Y. Hierarchically Mesostructured Doped CeO2 with Potential for Solar-Cell Use. Nat. Mater. 2004, 3, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.A.; Kozlov, S.M.; Lim, K.H.; Migani, A.; Neyman, K.M. Oxygen Vacancies in Self-Assemblies of Ceria Nanoparticles. J. Mater. Chem. A 2014, 2, 18329–18338. [Google Scholar] [CrossRef]
- Bruix, A.; Neyman, K.M. Modeling Ceria-Based Nanomaterials for Catalysis and Related Applications. Catal. Lett. 2016, 146, 2053–2080. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An LSDA+ U Study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Aryasetiawan, F.; Lichtenstein, A.I. First-Principles Calculations of the Electronic Structure and Spectra of Strongly Correlated Systems: The LDA+ U Method. J. Phys. Condens. Matter 1997, 9, 767. [Google Scholar] [CrossRef]
- Wang, Z.L.; Feng, X. Polyhedral Shapes of CeO2 Nanoparticles. J. Phys. Chem. B 2003, 107, 13563–13566. [Google Scholar] [CrossRef]
- Migani, A.; Neyman, K.M.; Bromley, S.T. Octahedrality versus Tetrahedrality in Stoichiometric Ceria Nanoparticles. Chem. Commun. 2012, 48, 4199–4201. [Google Scholar] [CrossRef] [PubMed]


| System | Position a | ΔE/eV | d(Sm-Sm)/pm | CN | µ(Sm)/µB |
|---|---|---|---|---|---|
| Ce63SmO128 | Surf | 0.00 | - | 7 | 4.88 |
| Sub | 0.09 | - | 8 | 4.82 | |
| Ce62Sm2O128 | 11_C | 0.00 | 384 | 7, 7 | 4.89, 4.89 |
| 11_F | 0.07 | 774 | 7, 7 | 4.88, 4.88 | |
| 12_C | 0.08 | 382 | 7, 8 | 4.88, 4.84 | |
| 12_F | 0.15 | 670 | 7, 8 | 4.88, 4.82 | |
| 13_C | 0.16 | 671 | 7, 8 | 4.88, 4.82 | |
| 13_F | 0.16 | 866 | 7, 8 | 4.88, 4.82 | |
| 14_C | 0.07 | 948 | 7, 7 | 4.88, 4.88 | |
| 14_F | 0.07 | 1224 | 7, 7 | 4.88, 4.88 | |
| 22_C | 0.13 | 384 | 8, 8 | 4.83, 4.83 | |
| 22_F | 0.25 | 774 | 8, 8 | 4.83, 4.82 | |
| 23_C | 0.14 | 386 | 8, 8 | 4.83, 4.83 | |
| 23_F | 0.25 | 866 | 8, 8 | 4.83, 4.81 |
| System | Model | Evac Range/eV | ΔEvac Range/eV | #Ce3+ | #Sm3+ |
|---|---|---|---|---|---|
| Ce64O127 | 2.43–3.25 (4) | 0.00–0.82 | 2 | - | |
| Ce63SmO127 | Surf | 1.41–1.90 (6) | 0.18–0.66 | 1 | 1 |
| Sub | 1.24–2.04 (8) | 0.00–0.81 | 1 | 1 | |
| Ce62Sm2O127 | 11_C | 0.50–0.84 (6) | 0.22–0.55 | 0 | 2 |
| 11_F | 0.45–0.80 (6) | 0.17–0.52 | 0 | 2 | |
| 22_C | 0.29–0.98 (6) | 0.00–0.69 | 0 | 2 |
| System | Position | ΔE/eV | d(Sm-Sm)/pm | CN | µ(Sm)/µB |
|---|---|---|---|---|---|
| Ce139SmO280 | S_D | 0.00 | - | 6 | 4.92 |
| S_A | 0.07 | - | 6 | 4.94 | |
| S_B | 0.12 | - | 7 | 4.91 | |
| S_C | 0.12 | - | 7 | 4.91 | |
| I_J | 0.04 | - | 8 | 4.78 | |
| I_K | 0.14 | - | 8 | 4.83 | |
| I_F | 0.17 | - | 8 | 4.80 | |
| I_I | 0.17 | - | 8 | 4.85 | |
| Ce138Sm2O280 | DD_C | 0.00 | 374 | 6, 6 | 4.92, 4.93 |
| DD_F | 0.19 | 2213 | 6, 6 | 4.92, 4.92 | |
| DJ_C | 0.07 | 377 | 6, 8 | 4.88, 4.83 | |
| DJ_F | 0.17 | 1890 | 6, 8 | 4.89, 4.81 |
| System | Model | Evac Range/eV | ΔEvac Range/eV | #Ce3+ | #Sm3+ |
|---|---|---|---|---|---|
| Ce140O279 | 1.04–2.22 (3) | 0.00–1.18 | 2 | - | |
| Ce139SmO279 | S_D | 0.34–1.99 (16) | 0.10–1.74 | 1 | 1 |
| I_J | 0.25–1.71 (6) | 0.00–1.46 | 1 | 1 | |
| Ce138Sm2O279 | DD_C | −0.10–1.81 (8) | 0.11–2.01 | 0 or 1 | 2 |
| DD_F | 0.09–1.65 (5) | 0.30–1.86 | 0 or 1 | 2 | |
| DJ_C | −0.21–1.71 (10) | 0.00–1.92 | 0 or 1 | 2 | |
| DJ_F | −0.06–1.21 (10) | 0.14–1.42 | 0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keyhanian, M.; Koleva, I.Z.; Aleksandrov, H.A. Impact of Sm3+ Ions on Oxygen Vacancy Formation in Ceria Systems. Molecules 2025, 30, 4615. https://doi.org/10.3390/molecules30234615
Keyhanian M, Koleva IZ, Aleksandrov HA. Impact of Sm3+ Ions on Oxygen Vacancy Formation in Ceria Systems. Molecules. 2025; 30(23):4615. https://doi.org/10.3390/molecules30234615
Chicago/Turabian StyleKeyhanian, Masoomeh, Iskra Z. Koleva, and Hristiyan A. Aleksandrov. 2025. "Impact of Sm3+ Ions on Oxygen Vacancy Formation in Ceria Systems" Molecules 30, no. 23: 4615. https://doi.org/10.3390/molecules30234615
APA StyleKeyhanian, M., Koleva, I. Z., & Aleksandrov, H. A. (2025). Impact of Sm3+ Ions on Oxygen Vacancy Formation in Ceria Systems. Molecules, 30(23), 4615. https://doi.org/10.3390/molecules30234615









