PET-Driven Fluorescence Modulation in Halochromic Styryl Hemicyanine Dyes Targeting DNA Minor Groove
Abstract
1. Introduction
2. Results and Discussion
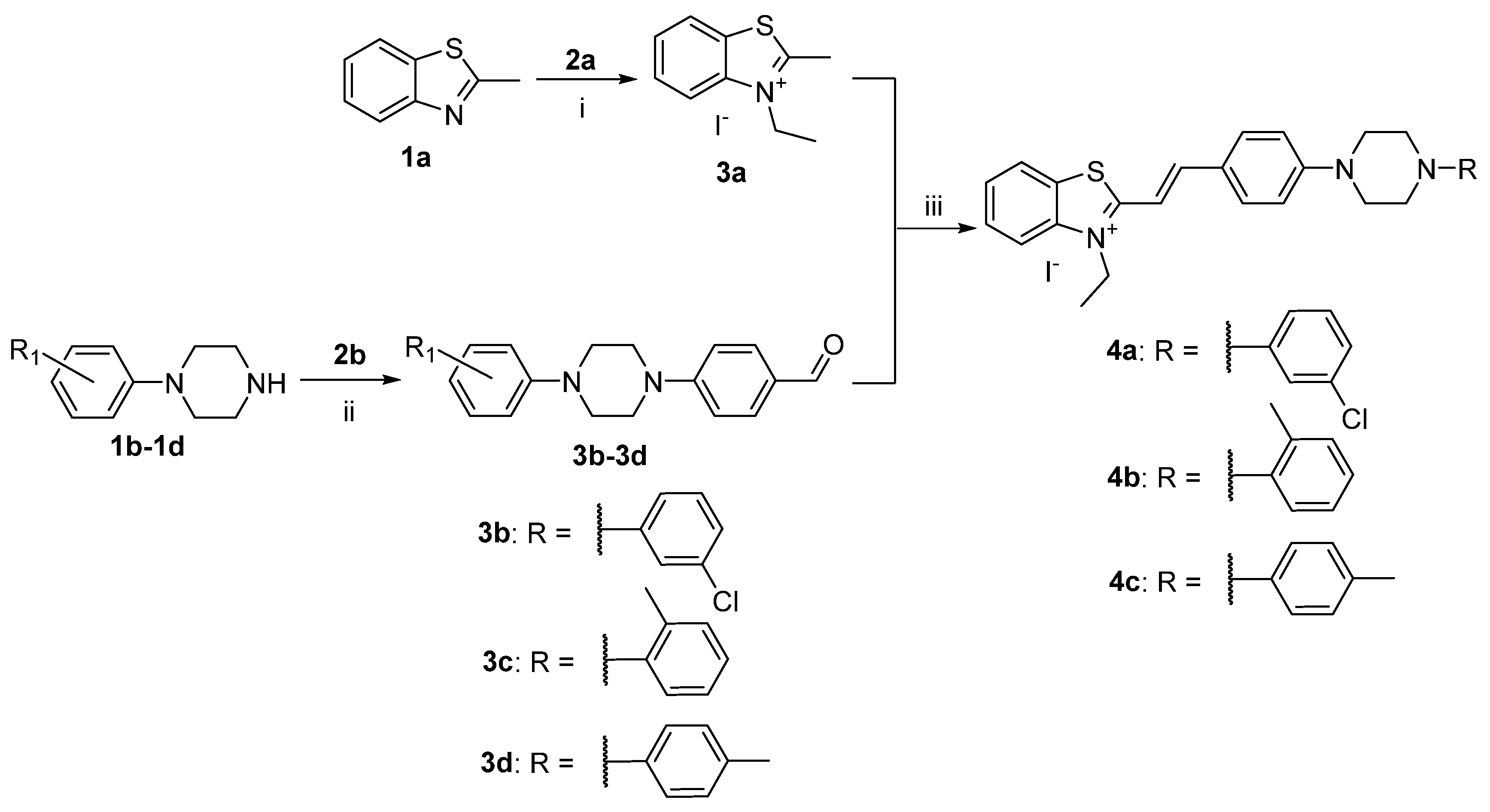
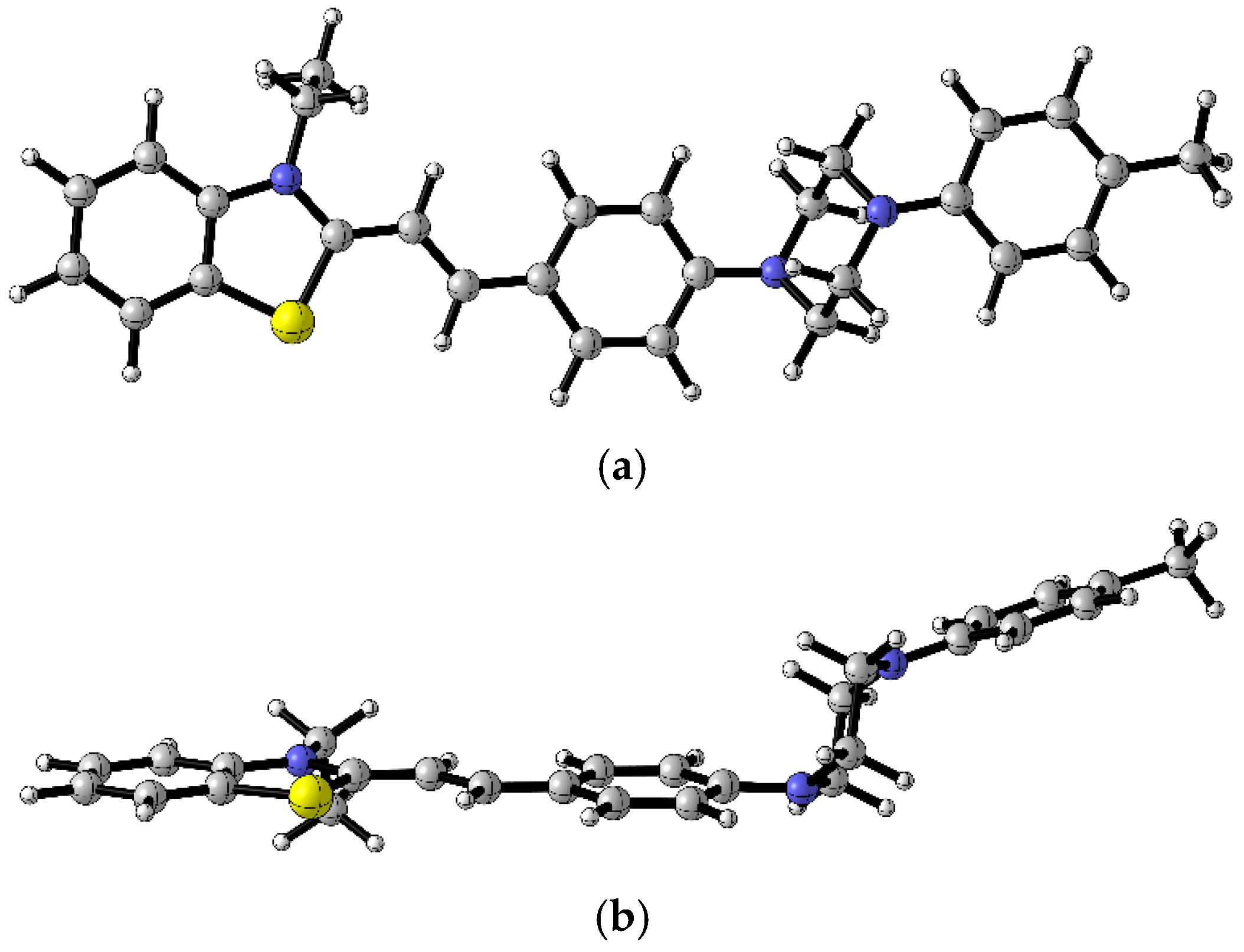
2.1. Synthesis of Target Molecules
2.2. Photophysical Properties of Dyes 4a–4c in Solution and in the Presence of HCl and DNA
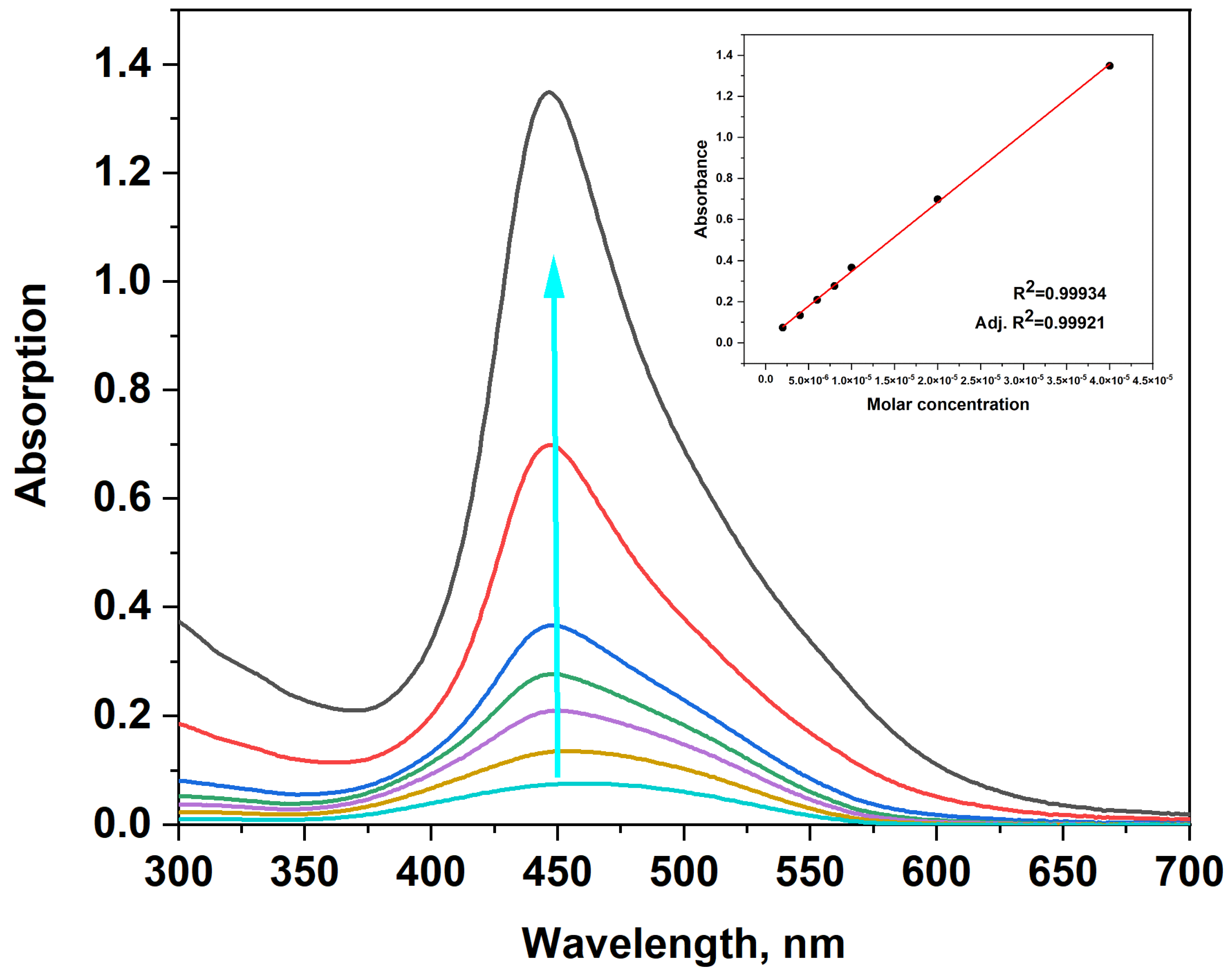
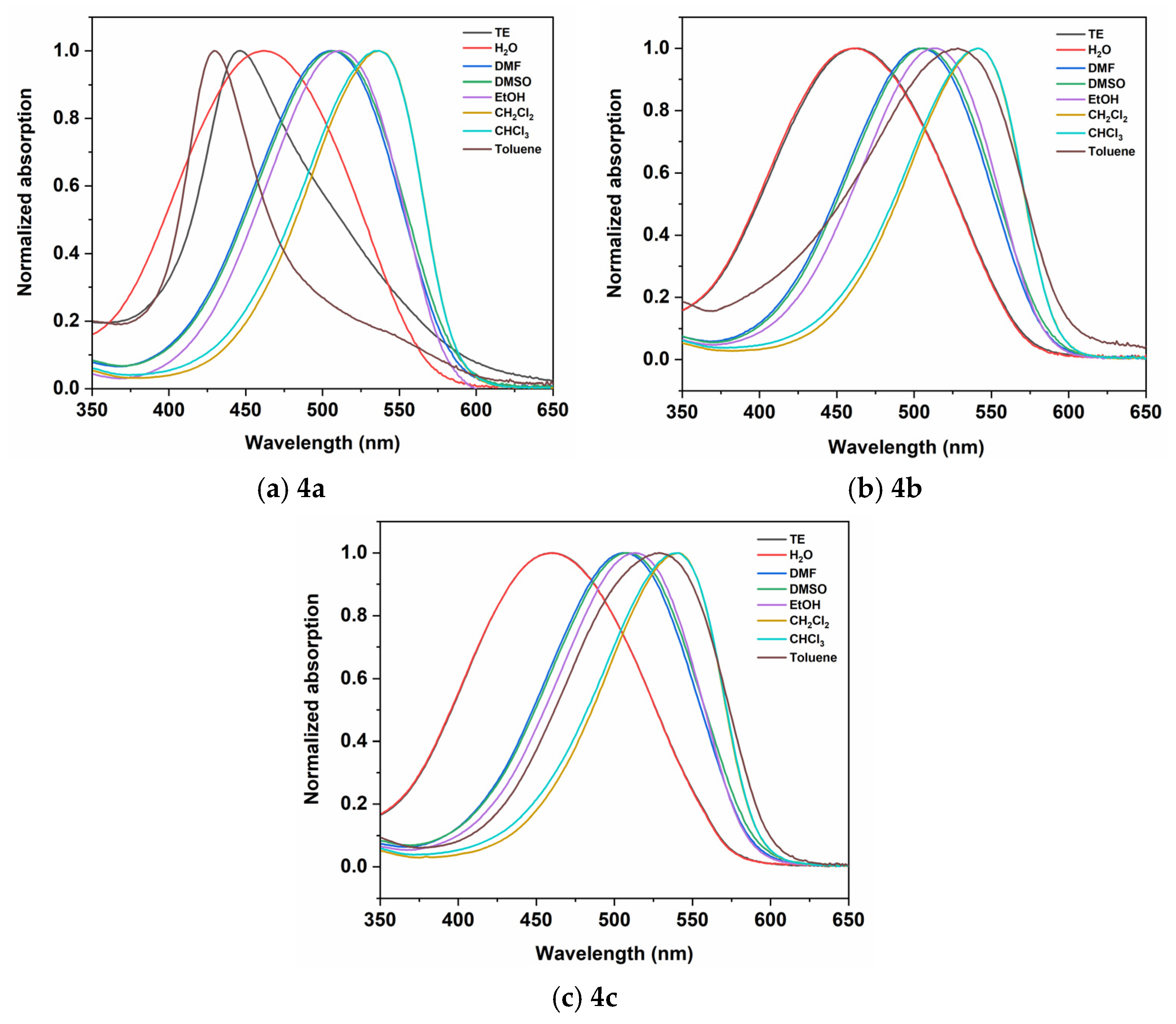
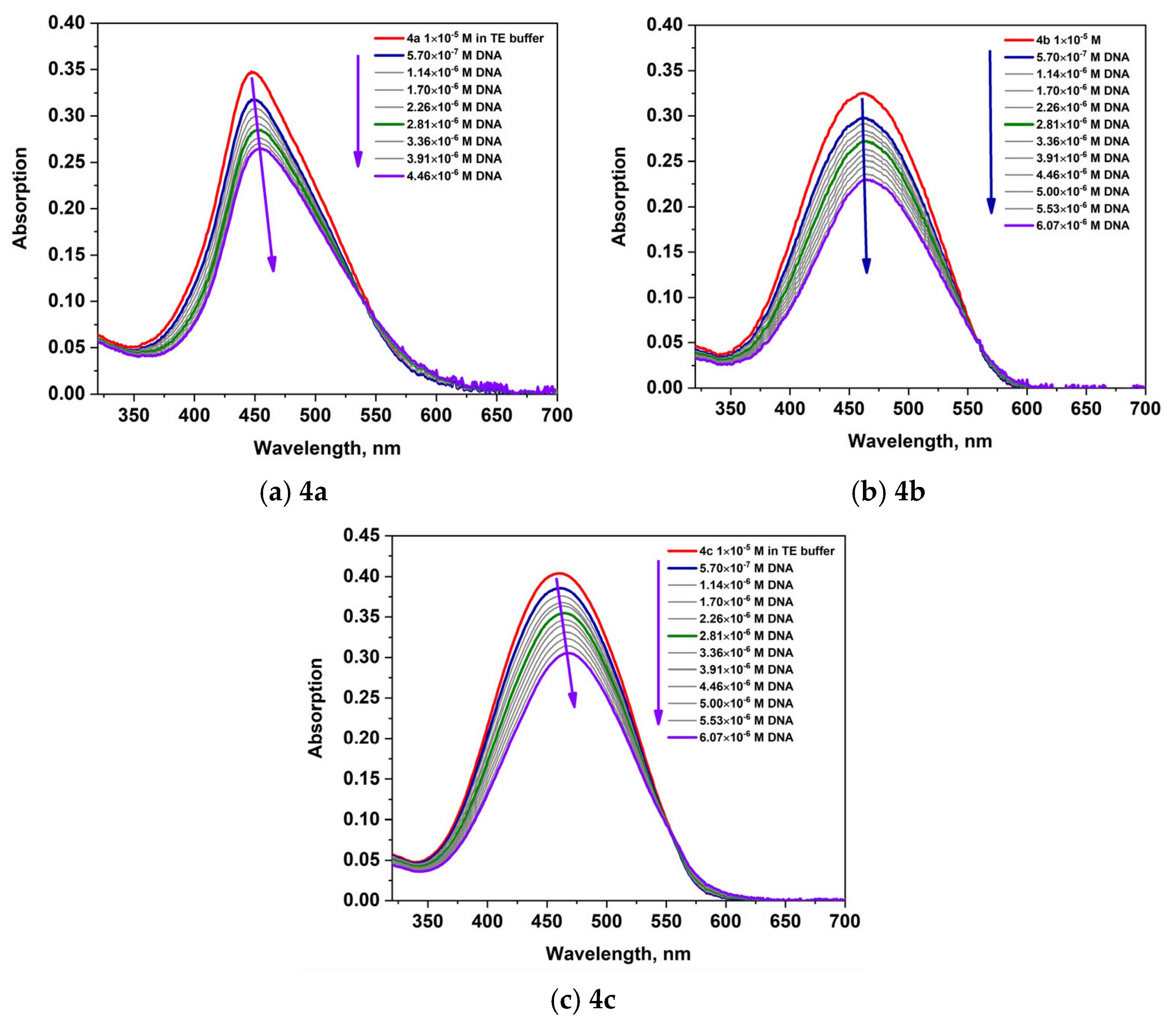
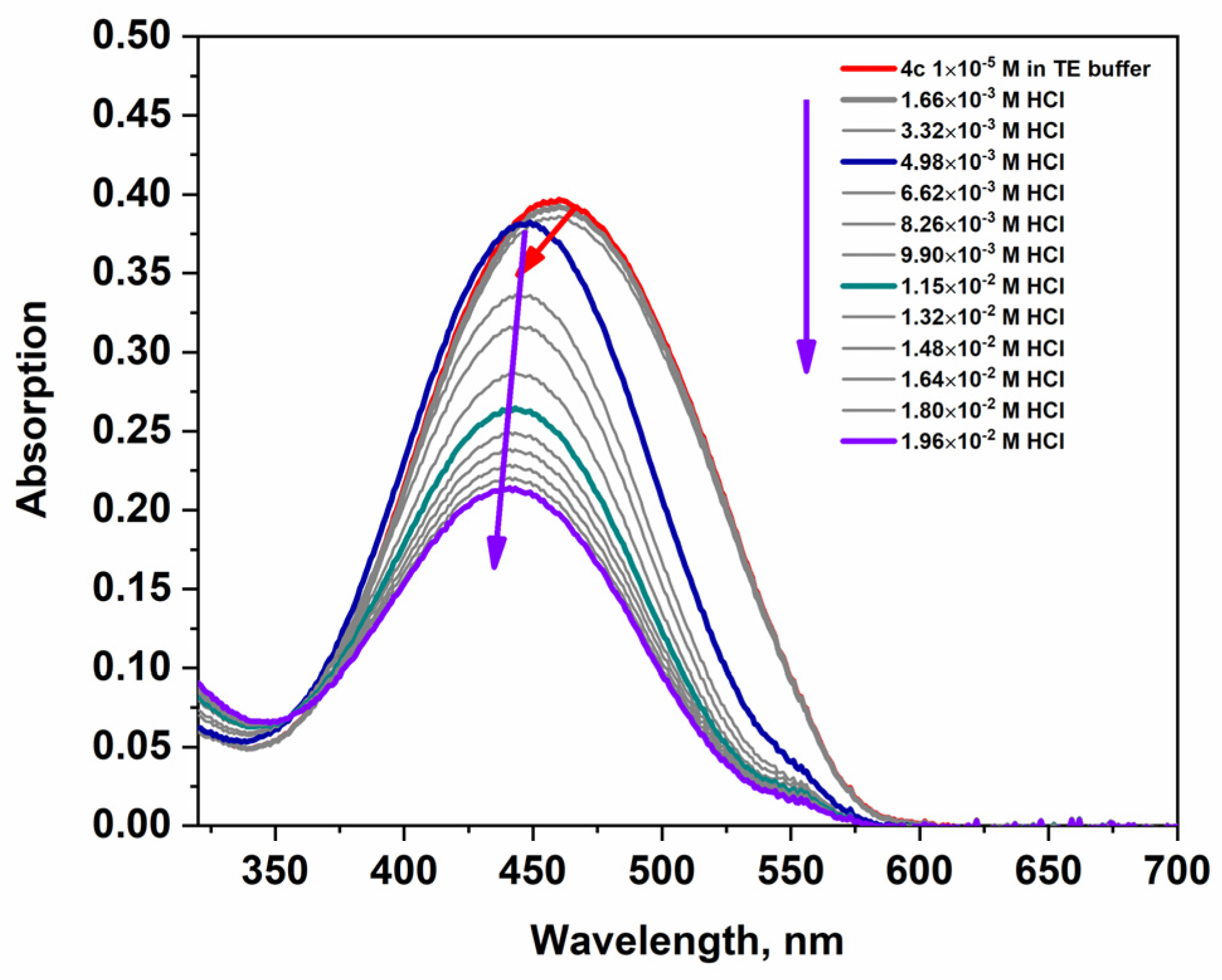
2.2.1. Absorption
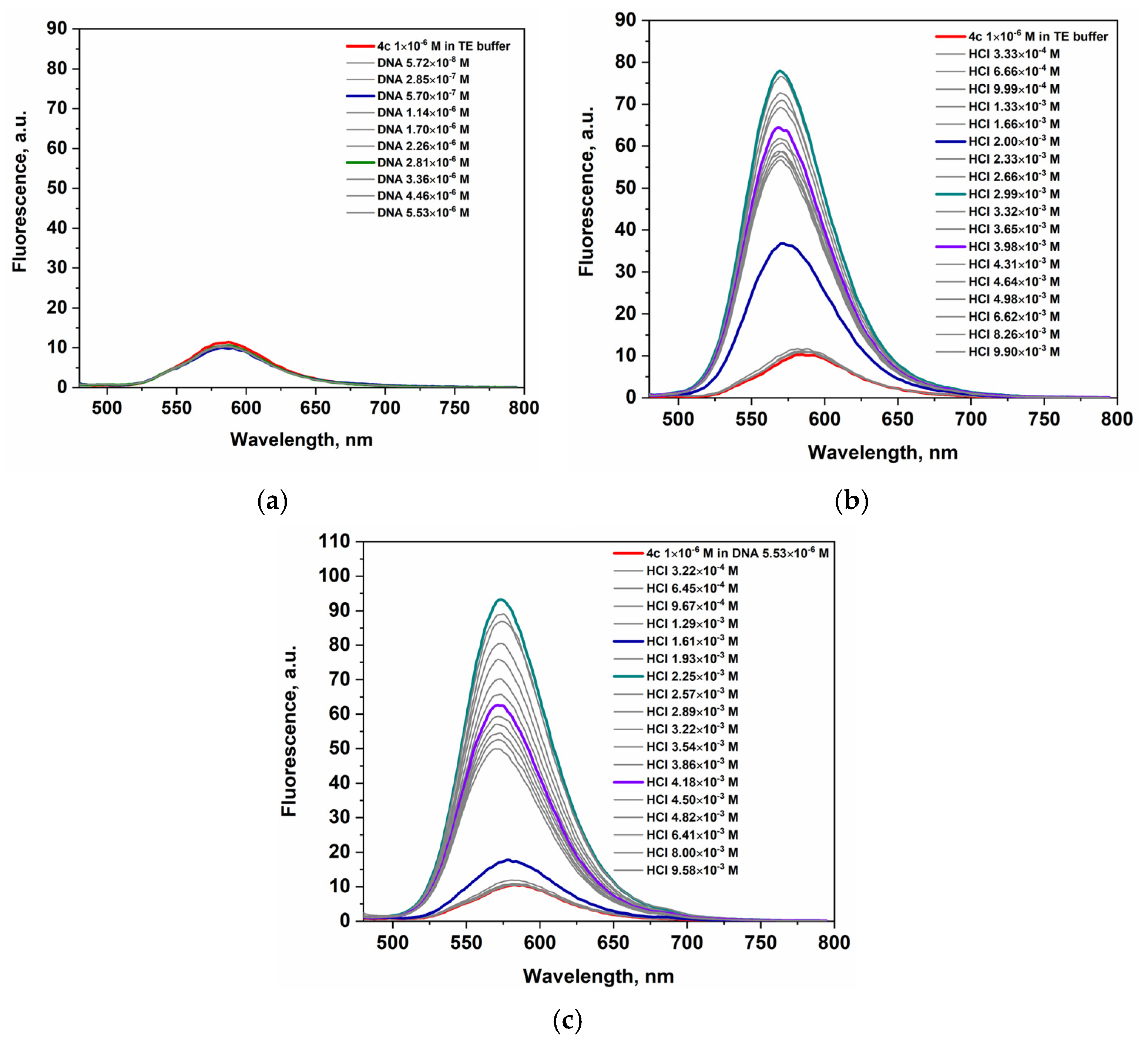
2.2.2. Fluorescence
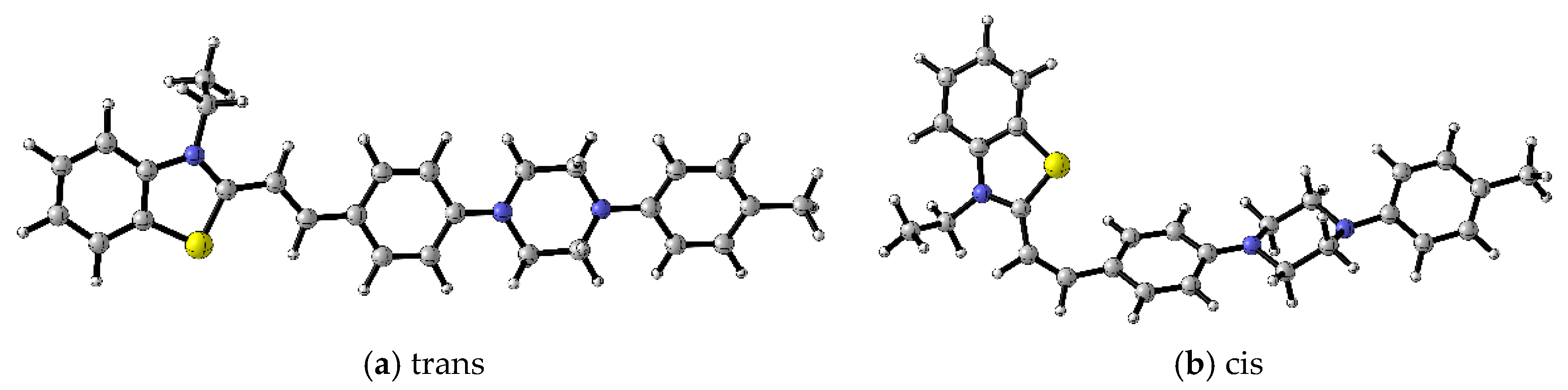
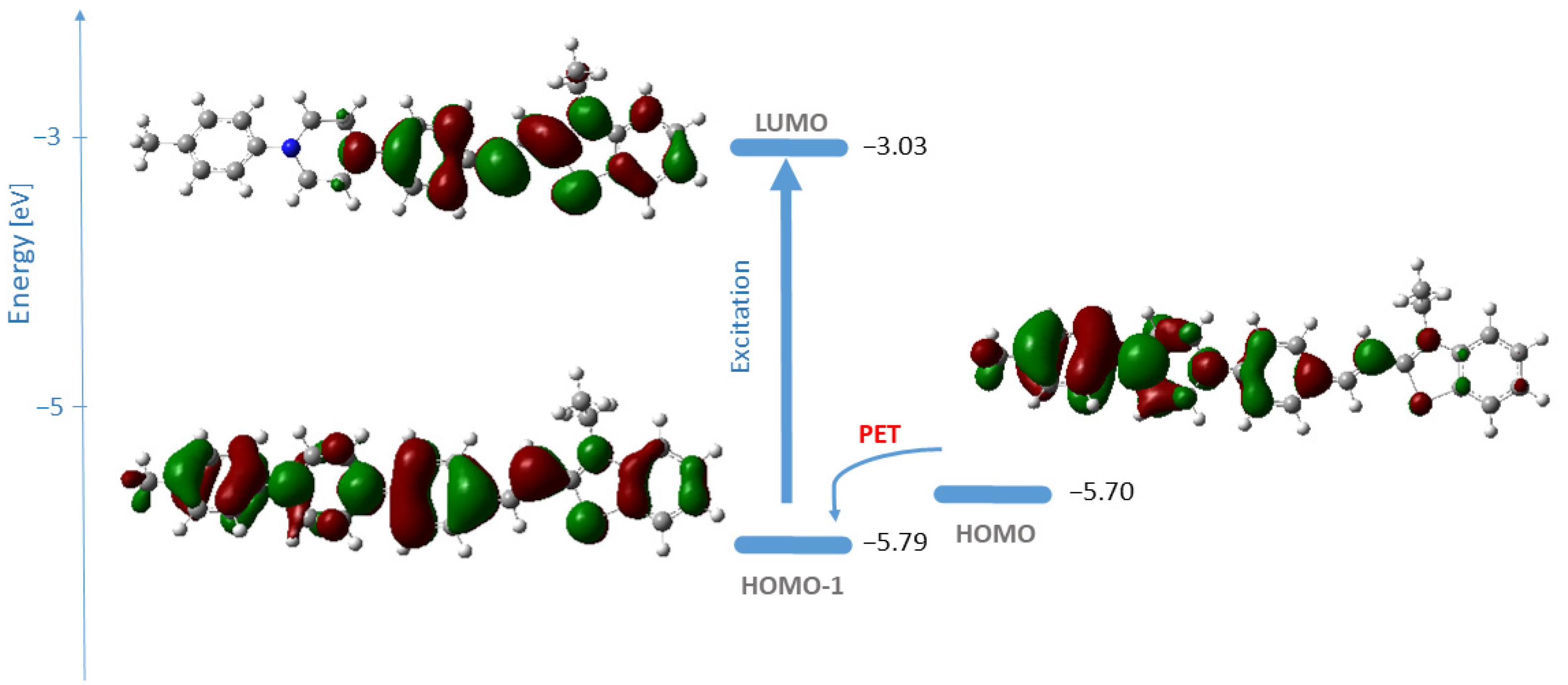
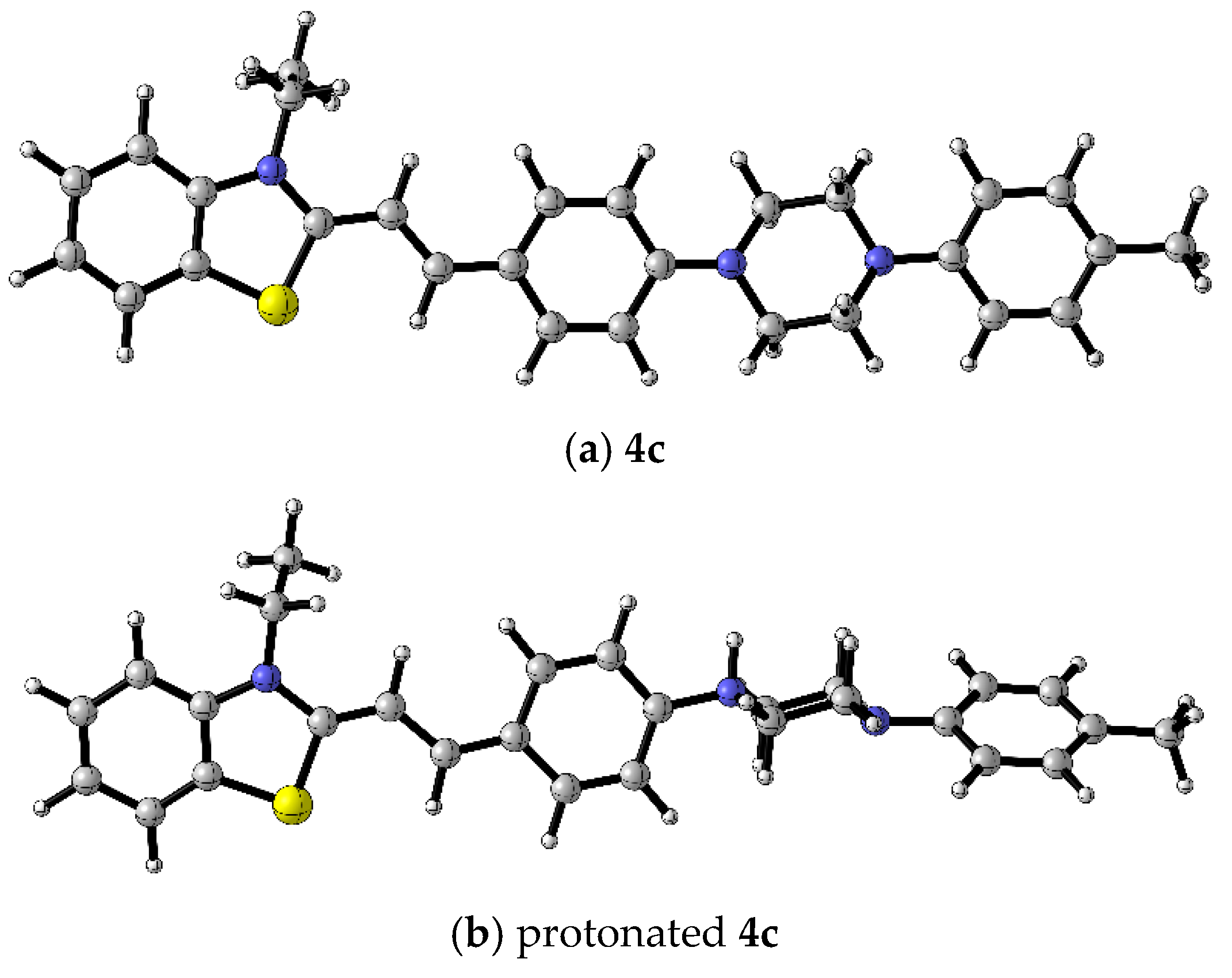
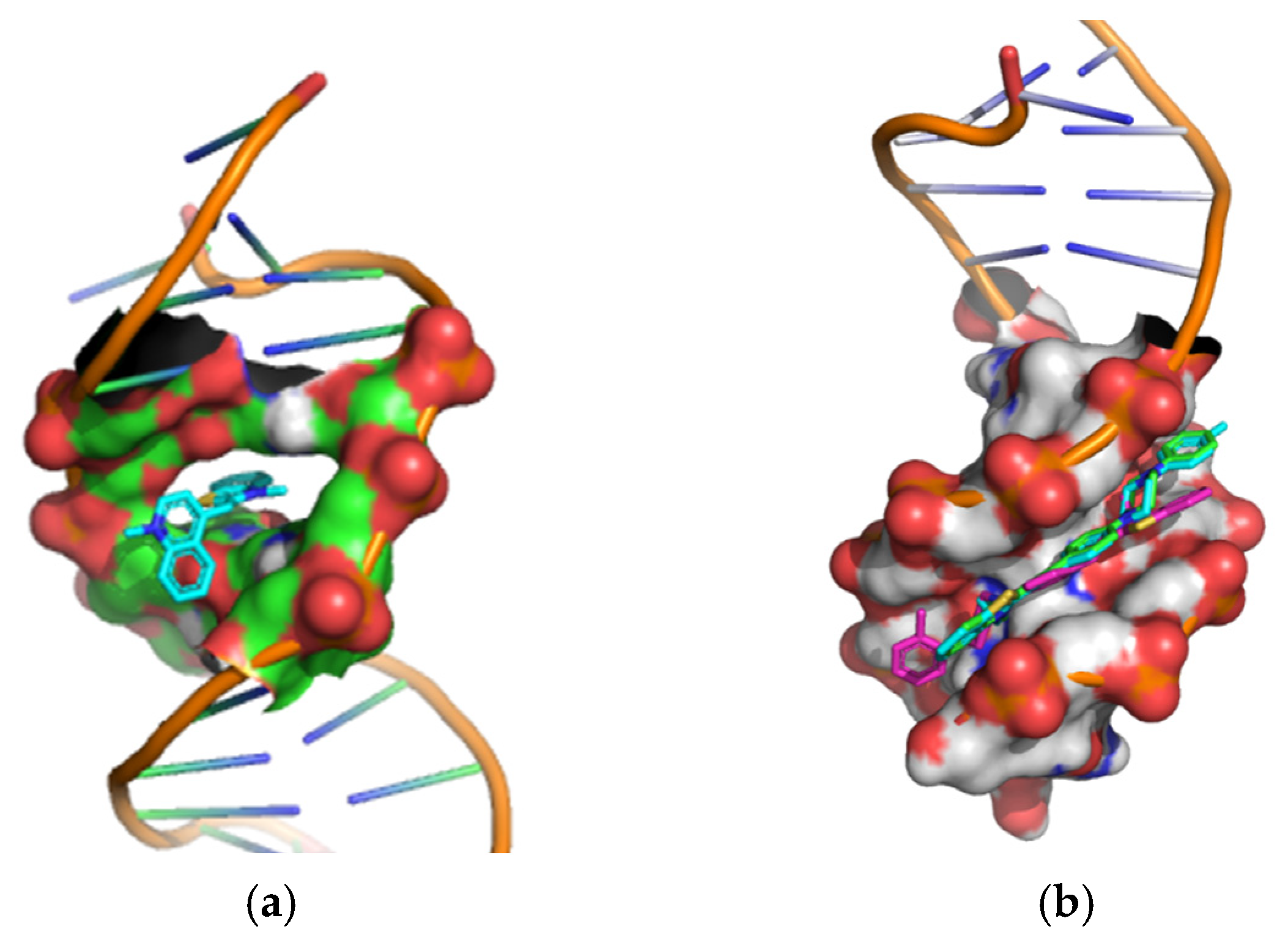
2.3. Molecular Docking Analysis
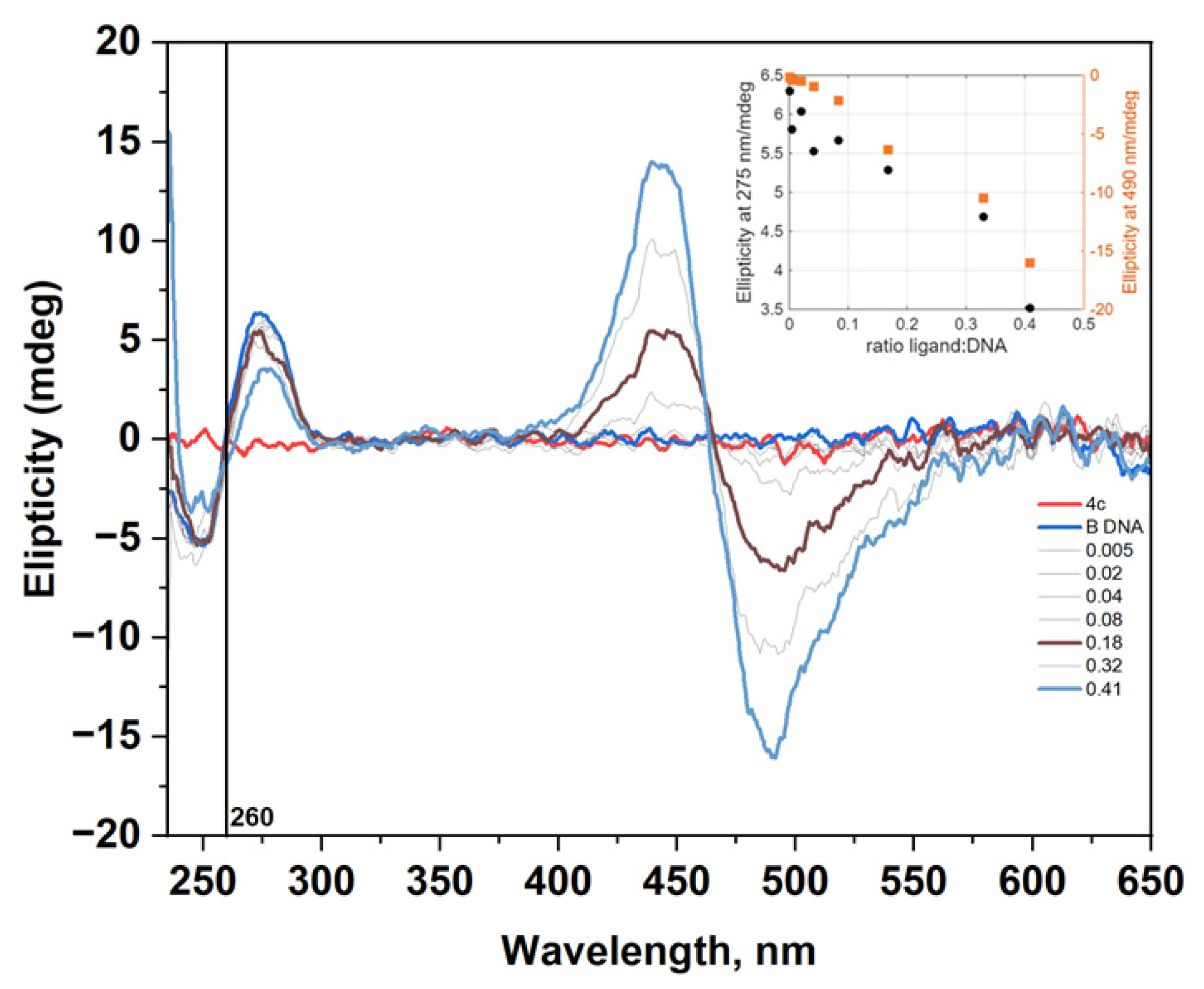
2.4. Circular Dichromism Measurements
3. Materials and Methods
3.1. Synthesis of Dyes 4a, 4b, 4c
3.2. Photophysical Properties of Dyes 4a, 4b, 4c
3.3. Molecular Docking of Dyes 4a, 4b, 4c
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | Minimum Inhibitory Concentration |
| AChE | Acetylcholinesterase |
| BuChE | Butyrylcholinesterase |
References
- Sirim, M.M.; Krishna, V.S.; Sriram, D.; Unsal Tan, O. Novel benzimidazole-acrylonitrile hybrids and their derivatives: Design, synthesis and antimycobacterial activity. Eur. J. Med. Chem. 2020, 188, 112010. [Google Scholar] [CrossRef]
- Mishra, C.B.; Manral, A.; Kumari, S.; Saini, V.; Tiwari, M. Design, synthesis and evaluation of novel indandione derivatives as multifunctional agents with cholinesterase inhibition, anti-β-amyloid aggregation, antioxidant and neuroprotection properties against Alzheimer’s disease. Bioorg. Med. Chem. 2016, 24, 3829–3841. [Google Scholar] [CrossRef]
- Kurutos, A.; Shindo, Y.; Hiruta, Y.; Oka, K.; Citterio, D. Near-infrared pH responsive heptamethine cyanine platforms: Modulating the proton acceptor. Dye. Pigm. 2020, 181, 108611. [Google Scholar] [CrossRef]
- Barbier, H. Sensitizing dyes derived from the quinolines, quinaldines and lepidines containing the dimethylamino and diethylamino radicals. J. Bull. Soc. Chim. Fr. 1920, 27, 427–439. [Google Scholar]
- König, W.; Treichel, O. Zur Frage der Konstitution der Cyanine. J. Prakt. Chem. 1921, 102, 63–84. [Google Scholar] [CrossRef]
- Mills, W.H.; Pope, W.J. CVIII.—2-p-Dimethylaminostyrylpyridine methiodide, a new photographic sensitiser. J. Chem. Soc. Trans. 1922, 121, 946–947. [Google Scholar] [CrossRef]
- Ephardt, H.; Fromherz, P. Fluorescence and photoisomerization of an amphiphilic aminostilbazolium dye as controlled by the sensitivity of radiationless deactivation to polarity and viscosity. J. Phys. Chem. 1989, 93, 7717–7725. [Google Scholar] [CrossRef]
- Kovalska, V.; Kryvorotenko, D.; Balanda, A.; Losytskyy, M.Y.; Tokar, V.; Yarmoluk, S. Fluorescent homodimer styrylcyanines: Synthesis and spectral-luminescent studies in nucleic acids and protein complexes. Dye. Pigm. 2005, 67, 47–54. [Google Scholar] [CrossRef]
- Kovalska, V.; Losytskyy, M.Y.; Kryvorotenko, D.; Balanda, A.; Tokar, V.; Yarmoluk, S. Synthesis of novel fluorescent styryl dyes based on the imidazo [1, 2-a] pyridinium chromophore and their spectral-fluorescent properties in the presence of nucleic acids and proteins. Dye. Pigm. 2006, 68, 39–45. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Tatarets, A.L.; Patsenker, L.D.; Kryvorotenko, D.V.; Yarmoluk, S.M. Spectroscopic study of squaraines as protein-sensitive fluorescent dyes. Dye. Pigm. 2007, 72, 285–292. [Google Scholar] [CrossRef]
- Balanda, A.; Volkova, K.; Kovalska, V.; Losytskyy, M.Y.; Tokar, V.; Prokopets, V.; Yarmoluk, S. Synthesis and spectral–luminescent studies of novel 4-oxo-4, 6, 7, 8-tetrahydropyrrolo [1, 2-a] thieno [2, 3-d] pyrimidinium styryls as fluorescent dyes for biomolecules detection. Dye. Pigm. 2007, 75, 25–31. [Google Scholar] [CrossRef]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigm. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Wang, B.-L.; Jiang, C. DNA G-quadruplexes as a template to direct cyanine dyes to form H-aggregates and application of the self-assembly entity as a new G-quadruplexes ligands screening platform. J. Anal. Chem. 2019, 91, 1541–1547. [Google Scholar] [CrossRef]
- Zonjić, I.; Radić Stojković, M.; Crnolatac, I.; Tomašić Paić, A.; Pšeničnik, S.; Vasilev, A.; Kandinska, M.; Mondeshki, M.; Baluschev, S.; Landfester, K.; et al. Styryl dyes with N-Methylpiperazine and N-Phenylpiperazine Functionality: AT-DNA and G-quadruplex binding ligands and theranostic agents. Bioorg. Chem. 2022, 127, 105999. [Google Scholar] [CrossRef]
- Said, A.I.; Kandinska, M.; Vasilev, A.; Grabchev, I. Styryl hemicyanine-DNA assembly for selective Hg2+ sensing and molecular computing. J. Photochem. Photobiol. A Chem. 2024, 452, 115590. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Legault, C.Y. CYLview, 1.0b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009; Available online: http://www.cylview.org.
- de Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef]
- Niu, H.; Liu, J.; O’Connor, H.M.; Gunnlaugsson, T.; James, T.D.; Zhang, H. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Abboud, J.L.; Kamlet, M.J.; Taft, R.W. Regarding a generalized scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 8325–8327. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, .pi.*, .alpha., and .beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. Linear solvation energy relationships. Part 3. Some reinterpretations of solvent effects based on correlations with solvent π* and α values. J. Chem. Soc. Perkin Trans. 2 1979, 3, 349–356. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The .beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Jaumot, J.; Gargallo, R.; de Juan, A.; Tauler, R. A graphical user-friendly interface for MCR-ALS: A new tool for multivariate curve resolution in MATLAB. Chemometr. Intell. Lab. Syst. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, M.; Niu, X.; Sun, W.; Jiao, K. Spectrophotometric Studies on the Interaction of Yeast RNA with Crystal Violet and Its Analytical Application. J. Chil. Chem. Soc. 2008, 53, 1594–1598. [Google Scholar] [CrossRef][Green Version]
| Entry | Dye 1 | in HCl Presence 2 | in DNA Presence 3 | |||
|---|---|---|---|---|---|---|
| ε (M−1 cm−1) | λabs | ε (M−1 cm−1) | λabs | ε (M−1 cm−1) | λabs | |
| 4a | 33,652 | 449 | 27,200 | 449 | 26,500 | 455 |
| 4b | 32,746 | 460 | 17,800 | 442 | 22,990 | 465 |
| 4c | 39,029 | 460 | 21,400 | 441 | 30,550 | 468 |
| Entry | λcomp (nm) | f | λexp (nm) | λcomp (nm) | f |
|---|---|---|---|---|---|
| Water | Toluene | ||||
| 4a | 475 | 1.6844 | 447 | — | — |
| 4b | 476 | 1.7013 | 460 | 487 | 1.6265 |
| 4c | 486 | 0.5439 | 460 | — | — |
| 469 | 1.2540 | — | 490 | 1.8043 | |
| Entry | λmax (nm) | |||||||
|---|---|---|---|---|---|---|---|---|
| TE | H2O | DMF | DMSO | EtOH | CH2Cl2 | CHCl3 | Toluene | |
| 4a | 447 | 462 | 505 | 507 | 511 | 537 | 534 | 430 |
| 4b | 460 | 461 | 505 | 507 | 512 | 541 | 542 | 524 |
| 4c | 460 | 461 | 506 | 509 | 513 | 541 | 541 | 528 |
| Entry | λcomp (nm) | f | λcomp (eV) | λexp (nm) |
|---|---|---|---|---|
| Water | TE Buffer | |||
| 4c | 486 | 0.5439 | 2.55 | — |
| 469 | 1.2540 | 2.64 | 460 | |
| 4cH+ | 524 | 0.0922 | 2.37 | 553 |
| 455 | 0.9244 | 2.72 | 442 | |
| Entry | Dye 1 | in HCl Presence 2 | in DNA Presence 3 | Dye–DNA Complex in the Presence of HCl 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λex | λem | λex | λem | ΔI | λex | λem | ΔI | λex | λem | ΔI | |
| 4a | 450 | 590 | 450 | 573 | 9.3 | 445 | 585 | 0.6 | 445 | 569 | 6.9 |
| 4b | 460 | 589 | 460 | 572 | 4.9 | 460 | 589 | 0.5 | 460 | 575 | 5.01 |
| 4c | 460 | 587 | 460 | 569 | 9.5 | 460 | 583 | 0.9 | 460 | 573 | 9.5 |
| Minor Groove-Binding | Intercalator | ||
|---|---|---|---|
| 4a | 4b | 4c | TO |
| −9.4 | −8.7 | −9.2 | −8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, T.; Pashev, A.; Ilieva, S.; Gargallo, R.; Cheshmedzhieva, D.; Vasilev, A. PET-Driven Fluorescence Modulation in Halochromic Styryl Hemicyanine Dyes Targeting DNA Minor Groove. Molecules 2025, 30, 4607. https://doi.org/10.3390/molecules30234607
Aleksandrova T, Pashev A, Ilieva S, Gargallo R, Cheshmedzhieva D, Vasilev A. PET-Driven Fluorescence Modulation in Halochromic Styryl Hemicyanine Dyes Targeting DNA Minor Groove. Molecules. 2025; 30(23):4607. https://doi.org/10.3390/molecules30234607
Chicago/Turabian StyleAleksandrova, Teodora, Aleksandar Pashev, Sonia Ilieva, Raimundo Gargallo, Diana Cheshmedzhieva, and Aleksey Vasilev. 2025. "PET-Driven Fluorescence Modulation in Halochromic Styryl Hemicyanine Dyes Targeting DNA Minor Groove" Molecules 30, no. 23: 4607. https://doi.org/10.3390/molecules30234607
APA StyleAleksandrova, T., Pashev, A., Ilieva, S., Gargallo, R., Cheshmedzhieva, D., & Vasilev, A. (2025). PET-Driven Fluorescence Modulation in Halochromic Styryl Hemicyanine Dyes Targeting DNA Minor Groove. Molecules, 30(23), 4607. https://doi.org/10.3390/molecules30234607






