Novel Ni/Zn MOFs for Sorbitol Production via Catalytic Transfer Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectroscopic Data
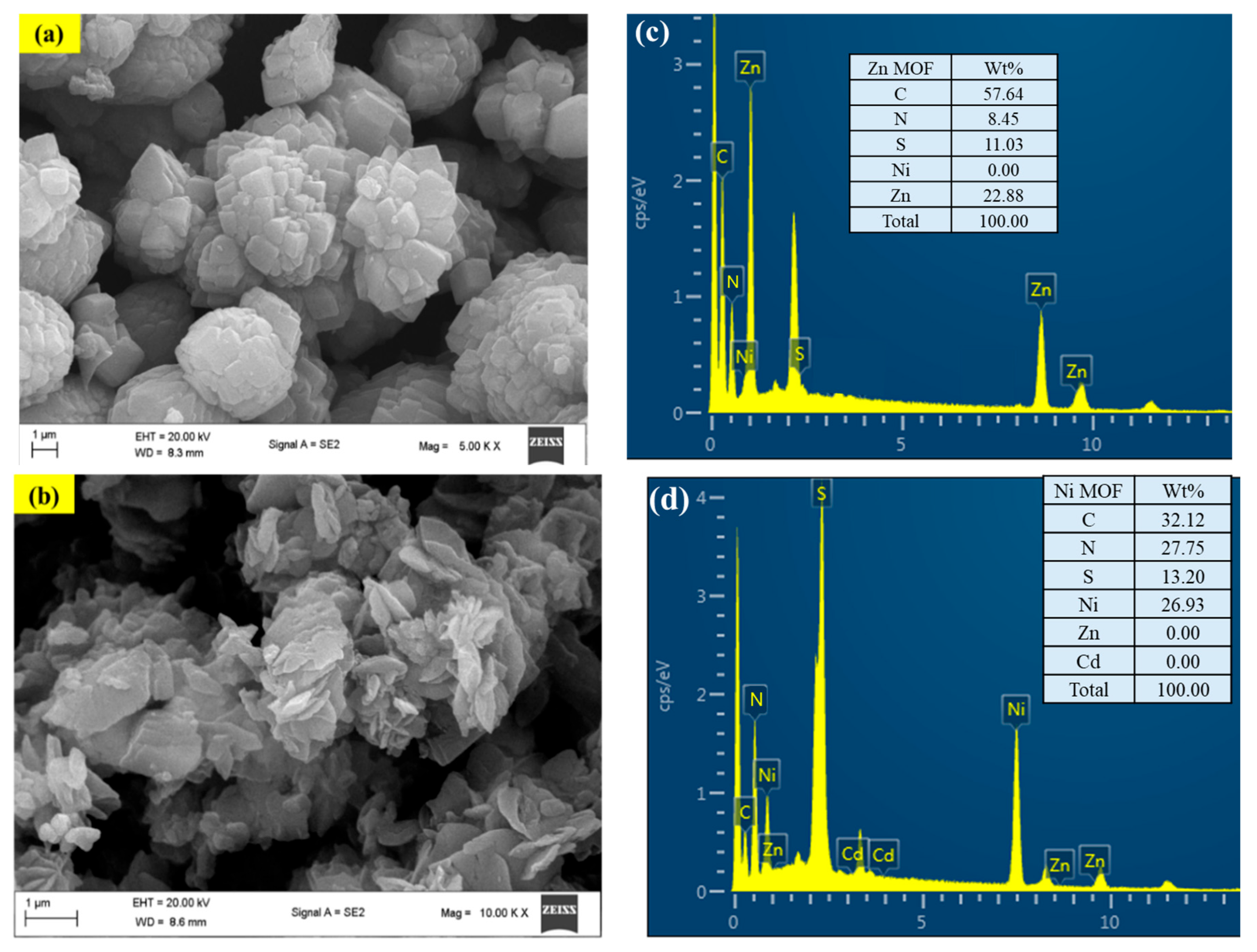
2.2. SEM Images and EDX Elemental Mapping
2.3. Particle Size Distribution and BET Analysis
2.4. Bronsted-Lewis Acid Sites Estimation
2.5. Thermal Stability
2.6. PXRD Patterns
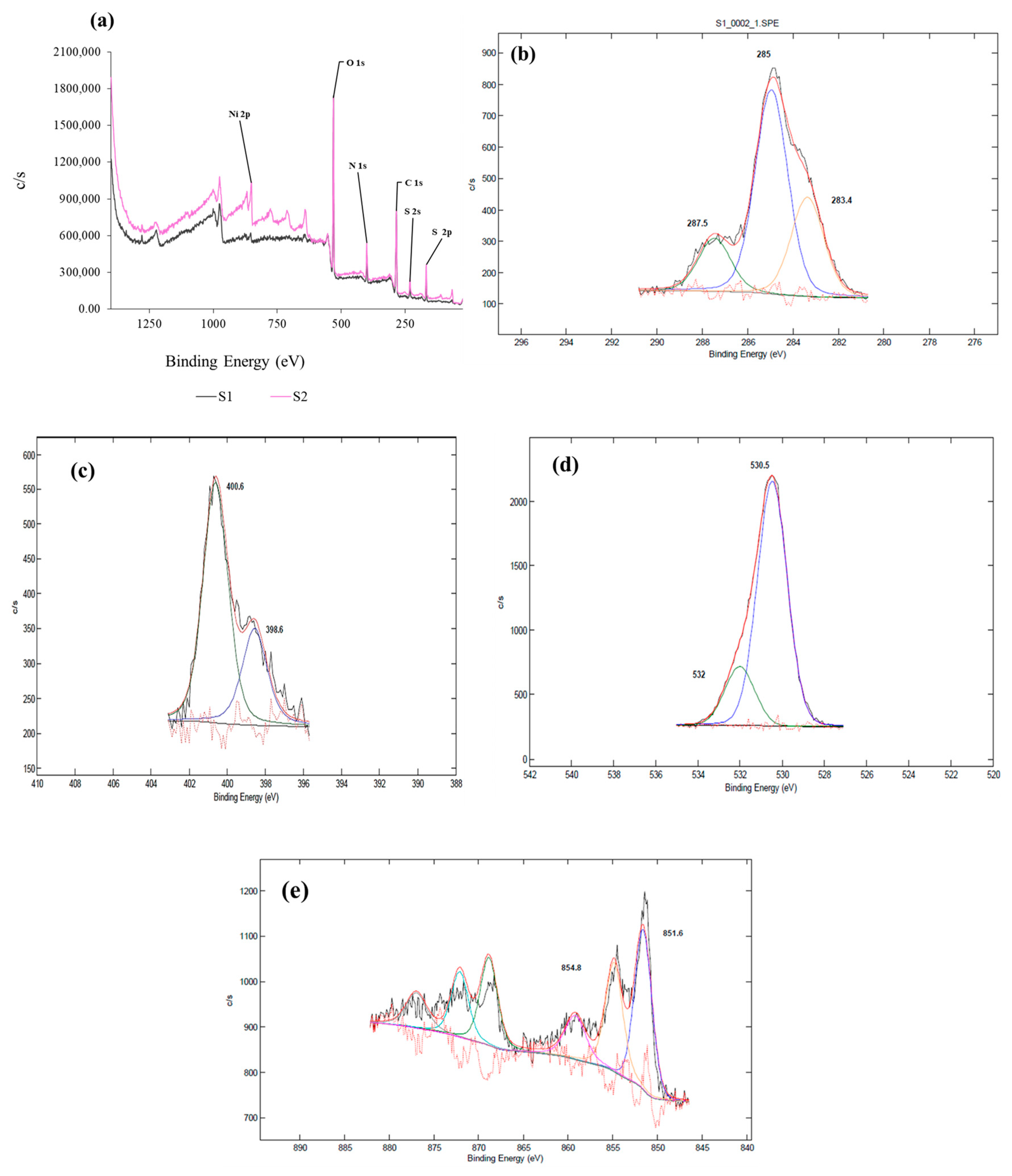
2.7. XPS
2.8. Catalytic Results
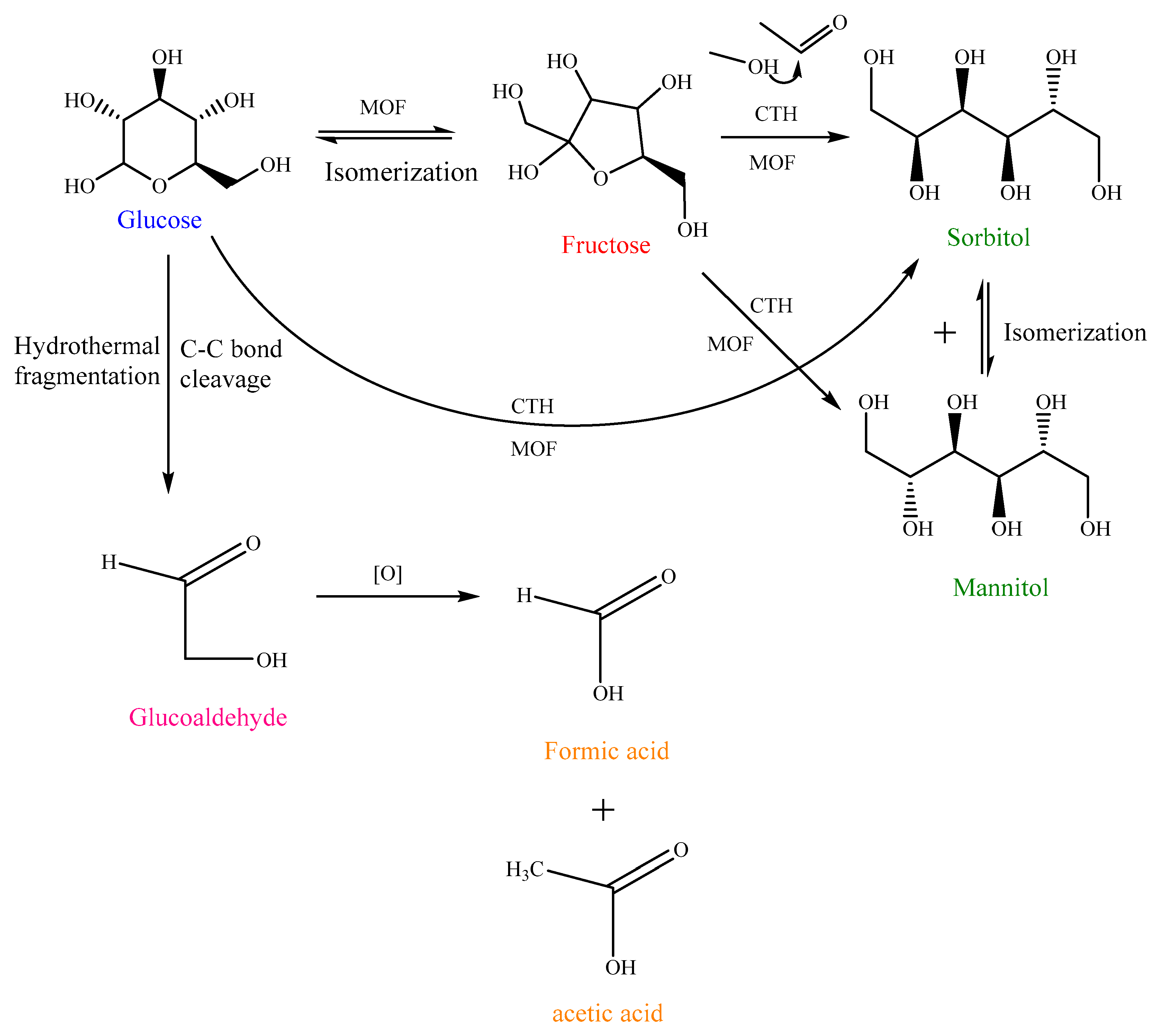
2.8.1. Produced Biochemicals
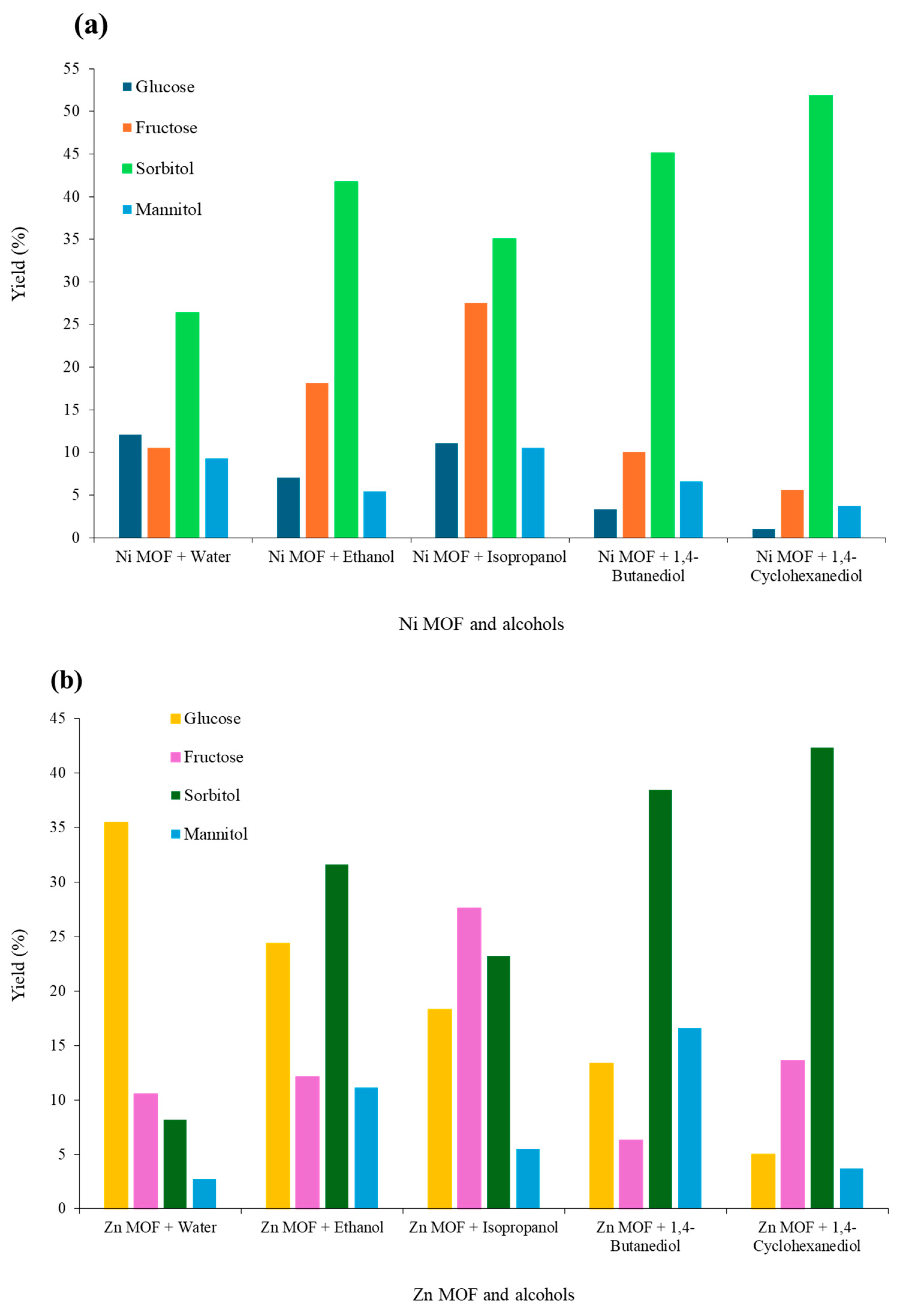
2.8.2. Effect of Hydrogen Donors
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalysts
3.3. Characterization Instrumentation
3.4. Determination of Acid Sites Using the Poisoning Test (Brønsted-Lewis)
3.5. Catalytic Tests
3.6. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexzman, Z.A.; Annuar, N.H.R. Hydrogenation of glucose into sorbitol using Ru-based catalyst: A Short Review. Malays. J. Catal. 2021, 5, 1–9. [Google Scholar]
- Gilkey, M.J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- García, B.; Moreno, J.; Morales, G.; Melero, J.A.; Iglesias, J. Production of sorbitol via catalytic transfer hydrogenation of glucose. Appl. Sci. 2020, 10, 1843. [Google Scholar] [CrossRef]
- García, B.; Orozco-Saumell, A.; López Granados, M.; Moreno, J.; Iglesias, J. Catalytic transfer hydrogenation of glucose to sorbitol with Raney nickel catalysts using biomass-derived diols as hydrogen donors. ACS Sustain. Chem. Eng. 2021, 9, 14857–14867. [Google Scholar] [CrossRef]
- Orak, C.; Sapmaz, A.; Yüksel, A. Selective catalytic hydrogenation of cellulose into sorbitol with Ru-based catalysts. Turk. J. Chem. 2022, 46, 434–445. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Huang, J.; Chen, L.; Ma, L.; Huang, X. Conversion of cellulose and cellobiose into sorbitol catalyzed by ruthenium supported on a polyoxometalate/metal-organic framework hybrid. ChemSusChem 2013, 6, 1545–1555. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, L.; Singleton, M.L.; Idrissi, H.; Hermans, S. Synergistic effects altering reaction pathways: The case of glucose hydrogenation over Fe-Ni catalysts. Appl. Catal. B Environ. 2021, 288, 119997. [Google Scholar] [CrossRef]
- Pandya, H.; Vinod, C.P.; Rathod, V.; Kantam, M.L. Kinetic model of hydrogenation of glucose to sorbitol on a Ni/Bentonite catalyst. Ind. Eng. Chem. Res. 2024, 63, 4771–4781. [Google Scholar] [CrossRef]
- Devred, F.; Gieske, A.H.; Adkins, N.; Dahlborg, U.; Bao, C.M.; Calvo-Dahlborg, M.; Bakker, J.W.; Nieuwenhuys, B.E. Influence of phase composition and particle size of atomised Ni–Al alloy samples on the catalytic performance of Raney-type nickel catalysts. Appl. Catal. A General. 2009, 356, 154–161. [Google Scholar] [CrossRef]
- Schimpf, S.; Louis, C.; Claus, P. Ni/SiO2 catalysts prepared with ethylenediamine nickel precursors: Influence of the pretreatment on the catalytic properties in glucose hydrogenation. Appl. Catal. A General. 2007, 318, 45–53. [Google Scholar] [CrossRef]
- Hijratur, R.; Novesar, J. Raney nickel synthesis for glucose hydrogenation without hydrogen gas. World J. Adv. Res. Rev. 2022, 15, 455–462. [Google Scholar] [CrossRef]
- Zhong, J.; Pérez-Ramírez, J.; Yan, N. Biomass valorisation over polyoxometalate-based catalysts. Green Chem. 2021, 23, 18–36. [Google Scholar] [CrossRef]
- Gundekari, S.; Desai, H.; Ravi, K.; Mitra, J.; Srinivasan, K. In situ generated Ru(0)-HRO@Na-beta from hydrous ruthenium oxide (HRO)/Na-beta: An energy-efficient catalyst for selective hydrogenation of sugars. Front. Chem. 2020, 8, 525277. [Google Scholar] [CrossRef]
- Kusserow, B.; Schimpf, S.; Claus, P. Hydrogenation of glucose to sorbitol over nickel and ruthenium catalysts. Adv. Synth. Catal. 2002, 345, 289–299. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Zhao, J.; Liang, J.; Zhu, J. Production of sorbitol via hydrogenation of glucose over ruthenium coordinated with amino styrene-co-maleic anhydride polymer encapsulated on activated carbon (Ru/ASMA@AC) Catalyst. Molecules 2023, 28, 4830. [Google Scholar] [CrossRef]
- Romero, A.; Díaz, J.A.; Nieto-Márquez, A.; Essayem, N.; Alonso, E.; Pinel, C. One-pot catalytic hydrolysis/hydrogenation of cellobiose into hexitols over Ru/Al-MCM-48. Microporous Mesoporous Mater. 2018, 271, 186–195. [Google Scholar] [CrossRef]
- Gong, J.; Katz, M.J.; Kerton, F.M. Catalytic conversion of glucose to 5-hydroxymethylfurfural using zirconium-containing metal-organic frameworks using microwave heating. RSC Adv. 2018, 8, 31618–31627. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Abdelkader-Fernández, V.K.; Cunha-Silva, L.; Balula, S.S. Isomerization of glucose to fructose catalyzed by metal–organic frameworks. Sustain. Energy Fuels 2021, 5, 3847–3857. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef]
- Athikaphan, P.; Jaitham, K.; Saneaha, J.; Nijpanich, S.; Neramittagapong, A.; Grisdanurak, N.; Neramittagapong, S. Optimization of Ru/ZSM-5 catalyst for selective D-glucose hydrogenation to sorbitol: A Box-behnken design approach. Results Eng. 2024, 24, 103541. [Google Scholar] [CrossRef]
- Alias, M.F.; Mahal, D.H.; Sadiq, A.S. Complexes of some transition metal with 2-benzoyl thiobenzimidazole and 1,10-phenanthroline and studying their antibacterial activity. Baghdad Sci. J. 2016, 13, 84–94. [Google Scholar] [CrossRef]
- Singh, K.; Ritu; Kumar, V. Synthesis, spectral and thermal studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes derived from 1,2,4-triazole Schiff bases. Int. J. Inorg. Bioinorg. Chem. 2014, 4, 32–39. [Google Scholar]
- Bashir, M.; Dar, A.A.; Yousuf, I. Syntheses, structural characterization, and cytotoxicity assessment of novel mn(ii) and zn(ii) complexes of aroyl-hydrazone schiff base ligand. ACS Omega 2023, 8, 3026–3042. [Google Scholar] [CrossRef]
- Konstantinovi, S.S.; Radovanovi, B.C.; Krklješ, A. Thermal behaviour of Co(II), Ni(II), Cu(II), Zn(II), Hg(II) and Pd(II) complexes with isatin-β-thiosemicarbazone. J. Therm. Anal. Calorim. 2007, 90, 525–531. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yi, C.; Xing, K.; Xu, K.; Yao, L.; Jia, P.; Wu, H.; Wen, L.; Cheng, Y.; Xu, Z. The Brønsted-Lewis acid sites in metal–organic framework biomimetic nanozyme for cooperatively enhancing the hydrolysis of lactose. Catal. Lett. 2024, 154, 5124–5133. [Google Scholar] [CrossRef]
- Liu, L.; Cao, L.; Niu, H. and Wang, J. Zinc metal–organic framework growing on the surface of fruit peels and its photocatalytic activity. ACS Omega 2021, 6, 10187–10195. [Google Scholar] [CrossRef]
- Deacon, A.; Briquet, L.; Malankowska, M.; Massingberd-Mundy, F.; Rudic, S.; Hyde, T.L.; Cavaye, H.; Coronas, J.; Poulston, S.; Johnson, T. Understanding the ZIF-L to ZIF-8 transformation from fundamentals to fully costed kilogram-scale production. Commun. Chem. 2022, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yao, J.; Liu, Q.; Wang, K.; Chen, F.; Wang, H. Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Microporous Mesoporous Mater. 2014, 184, 55–60. [Google Scholar] [CrossRef]
- Hall, J.N.; Bollini, P. Metal–organic framework MIL-100 catalyzed acetalization of benzaldehyde with methanol: Lewis or Brønsted acid catalysis? ACS Catal. 2020, 10, 3750–3763. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Fang, Z.-P.; Dai, Z.-J.; Cai, J.-M.; Shen, L.-J.; Zhang, Y.-F.; Au, C.-T.; Jiang, L.-L. Iron-based metal–organic frameworks as platform for H2S selective conversion: Structure-dependent desulfurization activity. Inorg. Chem. 2020, 59, 4483–4492. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Mondelli, C.; Pérez-Ramírez, J. Continuous transfer hydrogenation of sugars to alditols with bioderived donors over Cu–Ni–Al catalysts. ChemCatChem 2015, 7, 1551–1558. [Google Scholar] [CrossRef]
- Mosca, L.P.L.; Gapan, A.B.; Angeles, R.A.; Lopez, E.C.R. Stability of metal–organic frameworks: Recent advances and future trends. Eng. Proc. 2023, 56, 146. [Google Scholar]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Xiao, L.; Yao, S.; Liu, J.; Zou, H.; Xu, Y.; Cao, Y.; Chen, C. Efficient ultrasonic synthesis of Ni-based metal–organic framework for high performance battery-type supercapacitor electrodes. Energy Technol. 2022, 10, 2100350. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetic, M. A review of strategies for the synthesis of n-doped graphene-like materials. Nanomaterials 2020, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Boas, C.; Focassio, B.; Marinho, E., Jr.; Larrude, D.G.; Salvadori, M.C.; Leao, C.R.; Dos Santos, D.J. Characterization of nitrogen doped grapheme bilayers synthesized by fast, low temperature microwave plasma-enhanced chemical vapour deposition. Sci. Rep. 2019, 9, 13715. [Google Scholar]
- Song, X.Z.; Zhao, Y.H.; Zhang, F.; Ni, J.C.; Zhang, Z.; Tan, Z.; Wang, X.F.; Li, Y. Coupling plant polyphenol coordination assembly with Co(OH)2 to enhance electrocatalytic performance towards oxygen evolution reaction. Nanomaterials 2022, 12, 3972. [Google Scholar] [CrossRef]
- Finšgar, M.; Merl, D.K. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Yamamoto, S.; Bluhm, H.; Andersson, K.; Ketteler, G.; Ogasawara, H.; Salmeron, M.; Nilsson, A. In situ x-ray photoelectron spectroscopy studies of water on metals and oxides at ambient conditions. J. Phys. Condens. Matter 2008, 20, 184025. [Google Scholar] [CrossRef]
- Sivkov, D.V.; Petrova, O.V.; Nekipelov, S.V.; Vinogradov, A.S.; Skandakov, R.N.; Bakina, K.A.; Isaenko, S.I.; Ob’edkov, A.M.; Kaverin, B.S.; Vilkov, I.V.; et al. Quantitative characterization of oxygen-containing groups on the surface of carbon materials: XPS and NEXAFS study. Appl. Sci. 2022, 12, 7744. [Google Scholar] [CrossRef]
- Schiros, T.; Andersson, K.J.; Pettersson, L.G.M.; Nilsson, A.; Ogasawara, H. Chemical bonding of water to metal surfaces studied with core-level spectroscopies. J. Electron Spectrosc. Relat. Phenom. 2010, 177, 85–98. [Google Scholar] [CrossRef]
- Zu, S.; Zhang, H.; Zhang, T.; Zhang, M.; Song, L. Ni-Rh-based bimetallic conductive MOF as a high-performance electrocatalyst for the oxygen evolution reaction. Front. Chem. 2023, 11, 1242672. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bian, C.; Hu, L. Exploration of the corrosion behavior of electroless plated ni-p amorphous alloys via x-ray photoelectron spectroscopy. Molecules 2023, 28, 377. [Google Scholar] [CrossRef] [PubMed]
- Xi, R.; Tang, Y.; Smith, R.L.; Liu, X.; Liu, L.; Qi, X. Selective hydrogenation of glucose to sorbitol with tannic acid-based porous carbon sphere supported Ni–Ru bimetallic catalysts. Green Energy Environ. 2023, 8, 1719–1727. [Google Scholar] [CrossRef]
- Inan, A.; Sünbül, A.B.; Caylar, M.; Uruş, S.; Orhan, Z.; Köse, M.; Ispir, E. Highly effective aldose reductase mimetics: Catalytic transfer hydrogenation of D-glucose to D-sorbitol with novel azo-azomethine based Ru(II) complexes. J. Mol. Struct. 2023, 1277, 134844. [Google Scholar] [CrossRef]
- Oozeerally, R.; Ramkhelawan, S.D.K.; Burnett, D.L.; Tempelman, C.H.L.; Degirmenci, V. ZIF-8 metal organic framework for the conversion of glucose to fructose and 5-hydroxymethyl furfural. Catalysts 2019, 9, 812. [Google Scholar] [CrossRef]
- Zhou, Y.; Smith, R.L.; Qi, X. Chemocatalytic production of sorbitol from cellulose via sustainable chemistry—A tutorial review. Green Chem. 2024, 26, 202–243. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Guan, J.; Chen, X.; Qin, Z.; Mu, X.; Xian, M. Selective hydrogenation of D-glucose to D-sorbitol over Ru/ZSM-5 catalysts. Chin. J. Catal. 2014, 35, 733–740. [Google Scholar] [CrossRef]
- Bermejo-Deval, R.; Orazov, M.; Gounder, R.; Hwang, S.-J.; Davis, M.E. Active sites in Sn-Beta for glucose isomerization to fructose and epimerization to mannose. ACS Catal. 2014, 4, 2288–2297. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Z.; Hu, X.; Yang, S.; Rezayan, A.; Lu, T.; Chen, Z.; Zhang, Y. Highly efficient isomerization of glucose to fructose over Sn-doped silica nanotube. Resour. Chem. Mater. 2024, 3, 159–165. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Wang, W.; Liang, J.; Orooji, Y.; Dai, C.; Fu, X.; Yang, Y.; Xu, W.; Zhu, J. Efficient sorbitol producing process through glucose hydrogenation catalyzed by Ru supported amino poly (Styrene-co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3. Catalysts 2020, 10, 1068. [Google Scholar] [CrossRef]
- Mendes, P.C.D.; Costa-Amaral, R.; Gomes, J.F.; Da Silva, J.L.F. The influence of hydroxy groups on the adsorption of three-carbon alcohols on Ni(111), Pd(111) and Pt(111) surfaces: A density functional theory study within the D3 dispersion correction. Phys. Chem. Chem. Phys. 2019, 21, 8434–8444. [Google Scholar] [CrossRef]
- Mphuthi, L.E.; Erasmus, E.; Langner, E.H.G. Metal exchange of ZIF-8 and ZIF-67 nanoparticles with Fe(II) for enhanced photocatalytic performance. ACS Omega 2021, 6, 31632–31645. [Google Scholar] [CrossRef]


| Catalyst Type | Specific Catalyst | Hydrogen Donor/Solvent | Temp. (°C)/Time (h) | Key Performance (Sorbitol Yield) | Reference |
|---|---|---|---|---|---|
| Non-Noble | Mo-Promoted Raney Ni | Ethanol, 1,4-Butanediol or 1,5-Pentanediol (Diols) | 130/6 | Sorbitol yield: 87.3% (with 1,4-butanediol); high selectivity and stability with diols over 550 h. The most common CTH system in alcohol media. | [4] |
| Non-Noble | Raney Ni | Methanol, ethanol, i-Propanol | 130/6 | ∼58.2% sorbitol yield; feasibility demonstrated, but lower performance than with diols. | [3] |
| Non-Noble | Cu-Ni-Al2O3 | 1,4-Butanediol (Diol) | 150/Continuous Flow | ∼67% sorbitol yield; highlighted the efficiency of diol donors. | [33] |
| Bimetallic/Hybrid | Ni-Ru/Porous Carbon Sphere (Ni-Ru@PCS) | Water (Implied H2 or CTH with high performance) | 140/2.5 | 100% selectivity at 99% conversion. (Though synthesis is complex, the result was outstanding). | [47] |
| Noble Metal | Ru Complexes (Azo-azomethine-based Ru(II)) | i-Propanol | Not specified (Low-Pressure CTH) | Highly effective aldose reductase mimetics. | [48] |
| MOF-Derived | Ni(II)- or Zn(II)-MOFs | Water, ethanol, isopropanol, 1,4-butanediol, and 1,4-cyclohexanediol | 150/6 | ∼51.8% sorbitol yield (with 1,4-cyclohexanediol); feasibility demonstrated, but lower performance compared to Raney Ni with diols. | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokoyi, V.; Deenadayalu, N. Novel Ni/Zn MOFs for Sorbitol Production via Catalytic Transfer Hydrogenation. Molecules 2025, 30, 4565. https://doi.org/10.3390/molecules30234565
Tokoyi V, Deenadayalu N. Novel Ni/Zn MOFs for Sorbitol Production via Catalytic Transfer Hydrogenation. Molecules. 2025; 30(23):4565. https://doi.org/10.3390/molecules30234565
Chicago/Turabian StyleTokoyi, Vuyolwethu, and Nirmala Deenadayalu. 2025. "Novel Ni/Zn MOFs for Sorbitol Production via Catalytic Transfer Hydrogenation" Molecules 30, no. 23: 4565. https://doi.org/10.3390/molecules30234565
APA StyleTokoyi, V., & Deenadayalu, N. (2025). Novel Ni/Zn MOFs for Sorbitol Production via Catalytic Transfer Hydrogenation. Molecules, 30(23), 4565. https://doi.org/10.3390/molecules30234565







