Development of Prediction Capabilities for High-Throughput Screening of Physiochemical Properties by Biomimetic Chromatography
Abstract
1. Introduction
- Section 2 will explore the quantitative relationship between retention behaviour in these BC systems and their corresponding “gold standard” biological assays.
- Section 3 will introduce modern ML algorithms as tools to decode these complex, non-linear relationships and their use in QSSR.
- Section 4 will present selected applications from the recent literature, followed by our conclusions on the current state of the art and upcoming challenges.
2. Physiochemistry in Pharmacokinetics: From Gold Standards to Biomimetic Alternatives
2.1. Plasma Protein Binding (PPB) and Volume of Distribution (VD)
- Technical Details: AGP and HSA are both protein-based columns within Affinity chromatography, initially designed for chiral separation by exploiting the stereospecific binding pockets of the immobilised proteins. However, these columns have found additional applications in ADMET profiling and can be employed as tools for drug distribution and drug–drug interactions. AGP contains α1-acid glycoprotein, a major plasma protein that binds basic and neutral drugs. HSA contains immobilised human serum albumin, a key plasma protein that binds numerous drugs in the bloodstream [33]. Daicel Corporation offers a wide range of protein-based chiral selectors. HSA and AGP are available under the trade names CHIRALPAK HSA and CHIRALPAK AGP. They also supply columns with immobilised Cellobiohydrolase (CBH) and serum albumins from various animal species.
- Technical Details: The retention and separation process in MLC relies on a double equilibrium. This determines how the analyte distributes itself among three different microenvironments: (i) between the bulk aqueous mobile phase and the surfactant-coated stationary phase; and (ii) between the bulk aqueous mobile phase and the micellar aggregates in the mobile phase. Analytes that bind strongly to the micelles are slowed down compared to those in the aqueous phase [39]. Due to this mechanism, the retention factor log kw(MLC) is proportional to the compound’s partitioning into lipids on the surface of a stationary phase and micelles, providing results that directly measure membrane affinity.
2.2. Oral Bioavailability (F), Human Oral Absorption (%HOA), Membrane Permeability
- Technical Details: IAM columns are phospholipid-based columns. The first commercially available column was IAM.PC (phosphatidylcholine). IAM.PC.DD2 is the latest version [57]. A switch from “Type A” silica to “Type B” silica after 2018 caused significant differences in retention for acidic and basic compounds. Valko et al. [58] emphasises using new CHI(IAM) values for calibration of new columns, for better in vivo correlations. IAM columns can be further specialised by using other phospholipids as head groups; IAM.PE (phosphatidylethanolamine) shows differences in abundance in vivo [59] and IAM.SPH (sphingomyelin) can give unique insights into drug–neuron activity due to its rich presence in animal nerve tissue compared to phosphatidylcholine [60].
- Technical Details: The core of the CMC stationary phase consists of adsorbed (on activated silica gel) cell membranes, which were historically sourced from tissue cells (e.g., rabbit red and white cells, rabbit cardiomyocytes and rat vascular endothelial cells [72,73,74,75]), and now from high-expression recombinant cell lines with specific receptors (e.g., Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2), Fibroblast Growth Factor Receptor-1 (FGRF1) [76,77]). This approach preserves the biological structure and activity of receptors, allowing for accurate simulation of in vivo interactions [78,79]. The adsorption of high-expression cell lines significantly enhances the sensitivity and accuracy of the method. The mechanism of retention is based on the specific recognition between the analyte and the membrane receptor. Ligands, such as drugs, selectively interact with membrane receptors adsorbed on silica gel, achieving chromatographic separation. A key parameter that can be measured is the equilibrium dissociation constant (KD), which reflects the affinity strength between a drug and its receptor. Methods such as frontal analysis and zonal elution are used within the CMC framework to calculate these KD values. Although CMC is widely used, due to a lack of commercial availability, its usage in HTS is heavily limited. Column life is relatively short due to membrane receptors falling off the silica gel, thereby losing stability and reproducibility. Moreover, the amount of attached membrane receptors in CMC should be controlled for the accuracy improvement [80].
2.3. Toxicity (DIPL and hERG)
2.4. General Technical Considerations
3. Machine Learning (ML): Translating Chromatographic Data into Predictions
- Data acquisition—represents curation of comprehensive, representative datasets. The quality and integrity of training data directly influence model performance and can significantly compromise the model’s predictive capabilities and generalisation ability.
- Data preprocessing—transforms data, including feature scaling, normalisation and handling missing values. Many ML algorithms exhibit sensitivity to feature scale disparities, where features differing by orders of magnitude can disproportionately influence model training. Standard techniques include standardisation, min-max scaling, and log transforming for heavily skewed distributions.
- Data partitioning—involves division of the dataset into training (typically 60–80%), validation (10–20%) and test (10–20%) sets. Data partitioning can be performed randomly, which is most suitable for large, diverse, and evenly distributed datasets, or through rational splitting, such as scaffold splitting that divides by groups of molecules (with similar chemical scaffolds), thereby ensuring better model generalisation and reducing overfitting [102]. The exact proportion may vary based on dataset size and specific application requirements [103].
- Model training—an optimisation process where a loss function (e.g., Mean Squared Error (MSE), Cross-Entropy) quantifies the error between the model’s prediction and the actual values. This function acts as a performance indicator that models seek to minimise iteratively throughout the training process. The choice of loss function influences model behaviour, particularly in handling outliers.
- Model evaluation—assessment of the final model’s performance on the unseen test set using evaluation metrics (e.g., R2, Q2). Evaluation ensures an unbiased assessment of the model’s ability to generalise to new, unseen data points. If the data partitioning step is omitted, this step should include validation methods (e.g., Leave-one-out Cross-Validation, LOOCV). In specific frameworks, such as Quantitative Structure–Activity Relationship (QSAR)/QSRR, and in general scientific papers, statistical tests should also be evaluated (e.g., Fisher test, t-test) to ensure model significance.
3.1. Molecular Representations
- Cleaning—handling missing values and descriptors with no variance.
- Normalising/standardisation—some ML algorithms like Support Vector Machine (SVM) and Artificial Neural Network (ANN) require features to be on the same scale.
- Feature selection/dimensionality reduction—removing low-variance descriptors, eliminating highly correlated descriptors, usually by unsupervised learning.
- Handling categorical variables—presence/absence of functional group requires encoding to binary (0, 1).
- Handling outliers—deciding if outliers should be included in the model.
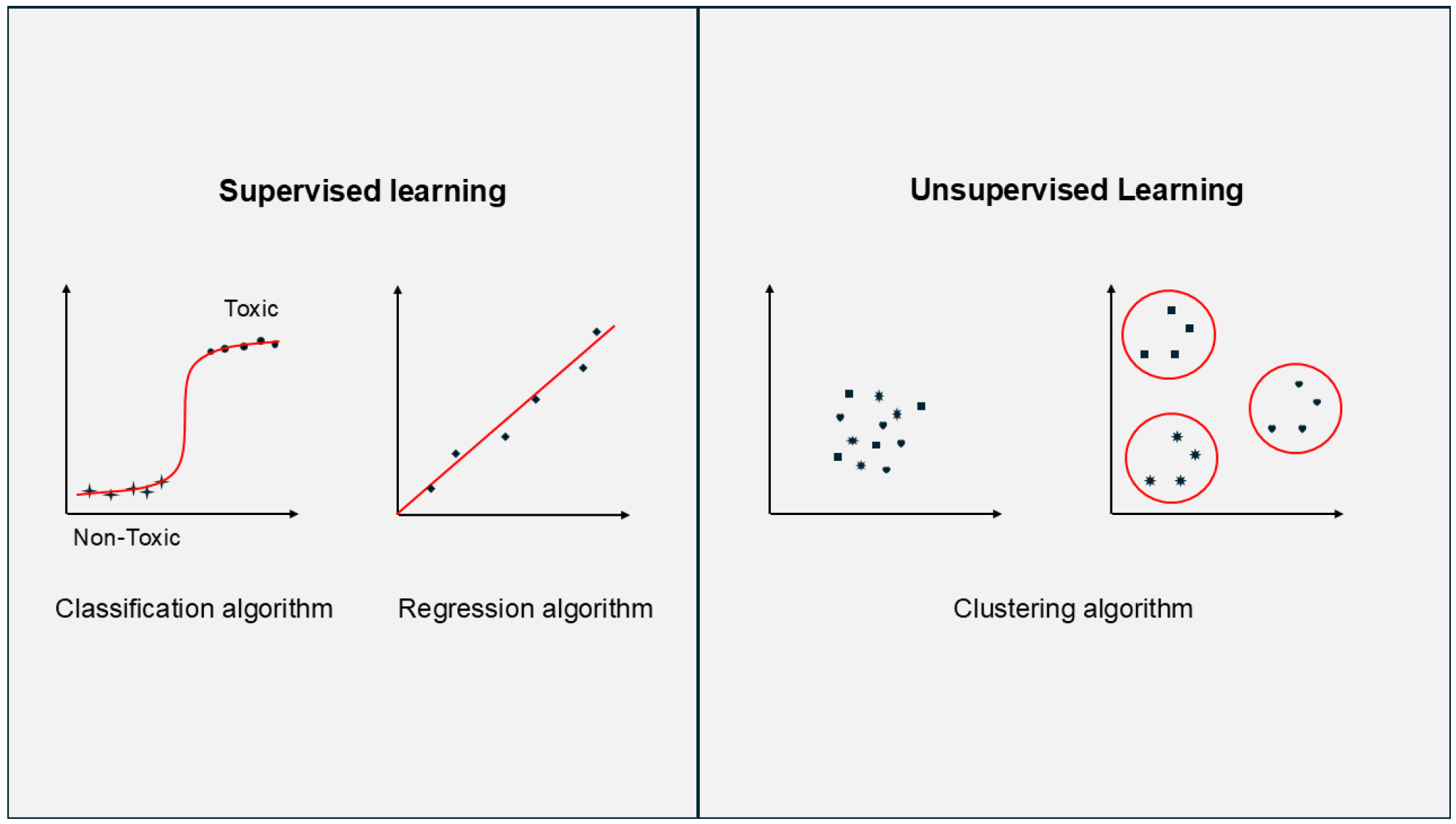
3.2. Unsupervised Learning
3.3. Supervised Learning
3.3.1. Regression Models
| Aspect | Linear Regression | Non-Linear Regression |
|---|---|---|
| Interpretability | Directly interpretable coefficients | Parameters are often context-dependent |
| Flexibility | Limited to linear trends | Captures saturating, sigmoidal or exponential relationships |
| Models | Ordinary Least Squares (OLS), PLS, MLR, SVR [126] | Polynomial Regression, SVR [127], RFR [128], Extreme Gradient Boosting (XGBoost) [129] |
| Loss Functions | Validation Method | Evaluation Metrics | Statistical Test |
|---|---|---|---|
| MSE—calculates the average of the squared differences between () and (y). Heavily penalises significant errors and is sensitive to outliers. Assuming errors follow a Gaussian distribution. | LOOCV trains on n-1 samples, tests on 1, and repeats n times. It is ideal for small datasets. Offers an unbiased estimate but comes with a high computational cost. | Sum of Squared Errors (SSE)—measures the total squared error between predictions and actual values. | F-test—overall model significance. Checks if the prediction is not due to chance alone. Standard threshold: p < 0.05. |
| Mean Absolute Error (MAE)—calculates the average absolute differences between () and (y). It is robust for outliers. Suitable for data containing outliers or errors that follow the Laplace distribution. | k-fold Cross-Validation (k-Fold CV). Splits data into k equal parts. Each fold is used only once as a test set. | R2 or R (e.g., “ML Model”)—measures the proportion of variance in the dependent variable that is explained by the independent variable. It ranges from 0 to 1, where R2 = 1 means that the model explains all the variance, and R2 = 0 implies that the model explains none of the variance. | t-test—individual variable significance. Provides p-value for each variable. Standard threshold: p < 0.05. |
| Huber Loss—combines MSE for minor errors (smooth and differentiable) and MAE for significant errors (robust to outliers) [130]. | EV (train–test split). Splits data into training set and independent set. The test set should never be used to train models. Gold standard for a prediction model. Provides R2_ext. | Q2 or R (e.g., “ML Model” with “Validation method”)—used to evaluate the predictive performance of a model, particularly in cross-validation or EV scenarios. It measures how well the model predicts new, unseen data. It ranges from −∞ to 1, where Q2 = 1 means strong predictive power, and Q2 < 0 implies that the model performs poorly on unseen data [131,132]. | Y-randomisation—permutation test. Checks robustness by verifying that R2 and Q2 remain similar after a random change to the output value. If R2 and Q2 drop in values, it is good because a relationship exists between the variables and the output [133]. |
3.3.2. Classification Models
4. Discussion and Future Perspectives
4.1. Throughput vs. Mechanistic Understanding
- Bunally et al. [12] marked a significant shift by introducing a 96/384-well plate format, integrating multiple parameters (ChromlogD, HSA binding, membrane interaction) into a single automated workflow. This addressed the speed bottleneck but potentially simplified the biological interpretation.
- Russo et al. [50] demonstrated the viability of 2D-LC systems combining HSA and IAM columns. Their work on a visual clustering approach for permeability characterisation offered an alternative to traditional statistical modelling.
- Vallianatou et al. [18] proposed complex HTS approach for early-stage CNS drug candidates.
- Conversely, Iwakuma et al. [84] dived into the detailed mechanism of drug–membrane interactions in chromatographic separation on IAM stationary phase. Investigating acetonitrile concentrations and salt effects.
- Alternative approaches like those by Ciura et al. [144] using micellar electrokinetic chromatography (MEKC) raises fundamental questions about whether complex biomimetic surfaces are even necessary, as high correlations (R2 = 0.904) were achieved with simplified surfactant systems.
4.2. Analytical Bottleneck
4.3. From Regression to Black Boxes
4.4. Will in Silico Replace Experimental?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (U)HPLC | (Ultra) High-Performance Liquid Chromatography |
| ADMET | Absorption, Distribution, Metabolism, Excretion, Toxicity |
| AGP | α-1-acid Glycoprotein |
| ANN | Artificial Neural Network |
| AUC | Area Under the Curve |
| BBB | Blood–Brain Barrier |
| BC | Biomimetic Chromatography |
| CA | Cluster Analysis |
| CHI | Chromatographic Hydrophobicity Index |
| Cl | Clearance |
| CMC | Cell Membrane Chromatography |
| CMK | Critical Micellar Concentration |
| CNS | Central Nervous System |
| CTAB | Cationic Cetyltrimethylammonium Bromide |
| CV | Cross-Validation |
| DIPL | Drug-Induced PhosphoLipidosis |
| ED | Equilibrium Dialysis |
| EV | External Validation |
| FGFR1 | Fibroblast Growth Factor Receptor-1 |
| GI | Gastrointestinal |
| HAc | Hydrogen bond Acceptors |
| HDo | Hydrogen bond Donors |
| hERG | human ether-a-go-go-related gene |
| HOA | Human Oral Absorption |
| HSA | Human-Serum Albumin |
| HTS | High-Throughput Screening |
| IAM | Immobilised Artificial Membrane |
| LLM | Large Language Model |
| LOC | Local Outlier Factor |
| LOOCV | Leave-One-Out Cross-Validation |
| MAE | Mean Absolute Error |
| MDCK | Madin-Darby canine kidney |
| MEKC | Micellar Electrokinetic Chromatography |
| ML | Machine Learning |
| MLC | Micellar Liquid Chromatography |
| MLP | Multilayer Perceptron |
| MLR | Multiple Linear Regression |
| MSE | Mean Squared Error |
| OLS | Ordinary Least Squares |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| PCA | Principal Component Analysis |
| PLS | Partial Least Square |
| PPB | Plasma Protein Binding |
| PSA | Polar Surface Area |
| QSAR | Quantitative Structure–Activity Relationship |
| QSRR | Quantitative Structure–Retention Relationship |
| RBF | Radial Basis Function |
| RFR | Random Forest Regression |
| SDS | Sodium Dodecyl Sulphate |
| SRD | Sum of Ranking Differences |
| SVM | Support Vector Machine |
| SVR | Support Vector Regression |
| t1/2 | Half-life |
| UV | Ultraviolet |
| Vd | Volume of distribution |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| XGBoost | Extreme Gradient Boosting |
References
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Corwin, H.; Toshio, F. p-σ-π Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Testa, B.; van de Waterbeemd, H.; Folkers, G.; Guy, R. Pharmacokinetic Optimization in Drug Research; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Avdeef, A. Physicochemical Profiling (Solubility, Permeability and Charge State). Curr. Top. Med. Chem. 2001, 1, 277–351. [Google Scholar] [CrossRef]
- Sangster, J.M. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry. Eur. J. Med. Chem. 1997, 11, 842. [Google Scholar] [CrossRef]
- Valkó, K.; Bevan, C.; Reynolds, D. Chromatographic Hydrophobicity Index by Fast-Gradient RP-HPLC: A High-Throughput Alternative to log P/log D. Anal. Chem. 1997, 69, 2022–2029. [Google Scholar] [CrossRef]
- Jeličić, M.-L.; Klarić, D.A.; Kovačić, J.; Verbanac, D.; Mornar, A. Accessing Lipophilicity and Biomimetic Chromatography Profile of Biologically Active Ingredients of Botanicals Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2022, 15, 965. [Google Scholar] [CrossRef]
- Pastewska, M.; Żołnowska, B.; Kovačević, S.; Kapica, H.; Gromelski, M.; Stoliński, F.; Sławiński, J.; Sawicki, W.; Ciura, K. Modeling of Anticancer Sulfonamide Derivatives Lipophilicity by Chemometric and Quantitative Structure-Retention Relationships Approaches. Molecules 2022, 27, 3965. [Google Scholar] [CrossRef]
- Pastewska, M.; Bednarczyk-Cwynar, B.; Kovačević, S.; Buławska, N.; Ulenberg, S.; Georgiev, P.; Kapica, H.; Kawczak, P.; Bączek, T.; Sawicki, W.; et al. Multivariate assessment of anticancer oleanane triterpenoids lipophilicity. J. Chromatogr. A 2021, 1656, 462552. [Google Scholar] [CrossRef]
- Valko, K.; Du, C.M.; Bevan, C.D.; Reynolds, D.P.; Abraham, M.H. Rapid-Gradient HPLC Method for Measuring Drug Interactions with Immobilized Artificial Membrane: Comparison with Other Lipophilicity Measures. J. Pharm. Sci. 2000, 89, 1085–1096. [Google Scholar] [CrossRef]
- Bunally, S.; Young, R.J. The role and impact of high throughput biomimetic measurements in drug discovery. ADMET DMPK 2018, 6, 74–84. [Google Scholar] [CrossRef]
- Chrysanthakopoulos, M.; Vallianatou, T.; Giaginis, C.; Tsantili-Kakoulidou, A. Investigation of the retention behavior of structurally diverse drugs on alpha1 acid glycoprotein column: Insight on the molecular factors involved and correlation with protein binding data. Eur. J. Pharm. Sci. 2014, 60, 24–31. [Google Scholar] [CrossRef]
- Chrysanthakopoulos, M.; Giaginis, C.; Tsantili-Kakoulidou, A. Retention of structurally diverse drugs in human serum albumin chromatography and its potential to simulate plasma protein binding. J. Chromatogr. A 2010, 1217, 5761–5768. [Google Scholar] [CrossRef]
- De Vrieze, M.; Lynen, F.; Chen, K.; Szucs, R.; Sandra, P. Predicting drug penetration across the blood–brain barrier: Comparison of micellar liquid chromatography and immobilized artificial membrane liquid chromatography. Anal. Bioanal. Chem. 2013, 405, 6029–6041. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, M.; Verzele, D.; Szucs, R.; Sandra, P.; Lynen, F. Evaluation of sphingomyelin, cholester, and phosphatidylcholine-based immobilized artificial membrane liquid chromatography to predict drug penetration across the blood-brain barrier. Anal. Bioanal. Chem. 2014, 406, 6179–6188. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Sztanke, M.; Sztanke, K. Modeling the Blood-Brain Barrier Permeability of Potential Heterocyclic Drugs via Biomimetic IAM Chromatography Technique Combined with QSAR Methodology. Molecules 2024, 29, 287. [Google Scholar] [CrossRef]
- Vallianatou, T.; Tsopelas, F.; Tsantili-Kakoulidou, A. Prediction Models for Brain Distribution of Drugs Based on Biomimetic Chromatographic Data. Molecules 2022, 27, 3668. [Google Scholar] [CrossRef]
- Kaliszan, R. QSRR: Quantitative Structure-(Chromatographic) Retention Relationships. Chem. Rev. 2007, 107, 3212–3246. [Google Scholar] [CrossRef]
- Tsopelas, F.; Stergiopoulos, C.; Danias, P.; Tsantili-Kakoulidou, A. Biomimetic separations in chemistry and life sciences. Microchim. Acta 2025, 192, 133. [Google Scholar] [CrossRef]
- Smith, D.A.; Di, L.; Kerns, E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef]
- Rolan, P. Plasma protein binding displacement interactions—Why are they still regarded as clinically important? Br. J. Clin. Pharmacol. 1994, 37, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.; Kulagowski, J.J.; Watt, A.P.; Rathbone, D.; Stevensons, G.I.; Carling, R.W.; Baker, R.; Marshall, G.R.; Kemp, J.A.; Foster, A.C.; et al. Effect of Plasma Protein Binding on in Vivo Activity and Brain Penetration of Glycine/NMDA Receptor Antagonists. J. Med. Chem. 1997, 40, 4053–4068. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Iwatsubo, T.; Kanamitsu, S.; Nakajima, Y.; Sugiyama, Y. Quantitative prediction of in vivo drug clearance and drug interactions from in vitro data on metabolism, together with binding and transport. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 461–499. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Eriksson, M.A.L.; Gabrielsson, J.; Nilsson, L.B. Studies of drug binding to plasma proteins using a variant of equilibrium dialysis. J. Pharm. Biomed. Anal. 2005, 38, 381–389. [Google Scholar] [CrossRef]
- Valko, K.; Nunhuck, S.; Bevan, C.; Abraham, M.H.; Reynolds, D.P. Fast Gradient HPLC Method to Determine Compounds Binding to Human Serum Albumin. Relationships with Octanol/Water and Immobilized Artificial Membrane Lipophilicity. J. Pharm. Sci. 2003, 92, 2236–2248. [Google Scholar] [CrossRef]
- Grumetto, L.; Barbato, F.; Russo, G. Scrutinizing the interactions between bisphenol analogues and plasma proteins: Insights from biomimetic liquid chromatography, molecular docking simulations and in silico predictions. Envion. Toxicol. Pharmacol. 2019, 68, 148–154. [Google Scholar] [CrossRef]
- Katopodi, A.; Tsotsou, E.; Iliou, T.; Deligiannidou, G.; Pontiki, E.; Kontogiorgis, C.; Tsopelas, F.; Detsi, A. Synthesis, Bioactivity, Pharmacokinetic and Biomimetic Properties of Multi-Substituted Coumarin Derivatives. Molecules 2021, 26, 5999. [Google Scholar] [CrossRef]
- Studziński, M.; Kozyra, P.; Pitucha, M.; Senczyna, B.; Matysiak, J. Retention Behavior of Anticancer Thiosemicarbazides in Biomimetic Chromatographic Systems and In Silico Calculations. Molecules 2023, 28, 7107. [Google Scholar] [CrossRef]
- Nisterenko, W.; Kułaga, D.; Woziński, M.; Singh, Y.R.; Judzińska, B.; Jagiello, K.; Greber, K.E.; Sawicki, W.; Ciura, K. Evaluation of Physicochemical Properties of Ipsapirone Derivatives Based on Chromatographic and Chemometric Approaches. Molecules 2024, 29, 1862. [Google Scholar] [CrossRef]
- Brusač, E.; Jeličić, M.-L.; Klarić, D.A.; Nigović, B.; Turk, N.; Klarić, I.; Mornar, A. Pharmacokinetic Profiling and Simultaneous Determination of Thiopurine Immunosuppressants and Folic Acid by Chromatographic Methods. Molecules 2019, 24, 3469. [Google Scholar] [CrossRef] [PubMed]
- Trainor, G.L. The importance of plasma protein binding in drug discovery. Expert. Opin. Drug Discov. 2007, 2, 51–64. [Google Scholar] [CrossRef]
- Martínez-Pla, J.J.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Retention–property relationships of anticonvulsant drugs by biopartitioning micellar chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Y.; Wang, S.; Chen, C.; Ye, L. Quantitative retention–activity relationship models for quinolones using biopartitioning micellar chromatography. Biomed. Chromatogr. 2008, 22, 106–114. [Google Scholar] [CrossRef]
- Tsopelas, F.; Danias, P.; Pappa, A.; Tsantili-Kakoulidou, A. Biopartitioning micellar chromatography under different conditions: Insight into the retention mechanism and the potential to model biological processes. J. Chromatogr. A 2020, 1621, 461027. [Google Scholar] [CrossRef]
- Quiñones-Torrelo, C.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Development of Predictive Retention−Activity Relationship Models of Tricyclic Antidepressants by Micellar Liquid Chromatography. J. Med. Chem. 1999, 42, 3154–3162. [Google Scholar] [CrossRef]
- Ruiz-Ángel, M.J.; Carda-Broch, S.; Torres-Lapasió, J.R.; García-Álvarez-Coque, M.C. Retention mechanisms in micellar liquid chromatography. J. Chromatogr. A 2009, 1216, 1798–1814. [Google Scholar] [CrossRef]
- Kalyankar, T.M.; Kulkarni, P.D.; Wadher, S.J.; Pekamwar, S.S. Applications of Micellar Liquid Chromatography in Bioanalysis: A Review. J. Appl. Pharm. Sci. 2014, 4, 128–134. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Jorgensen, C.; Linville, R.M.; Galea, I.; Lambden, E.; Vögele, M.; Chen, C.; Troendle, E.P.; Ruggiu, F.; Ulmschneider, M.B.; Schiøtt, B.; et al. Permeability Benchmarking: Guidelines for Comparing in Silico, in Vitro, and in Vivo Measurements. J. Chem. Inf. Model. 2025, 65, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.D.; Takahashi, L.; Lockhart, K.; Cheong, J.; Tolan, J.; Selick, H.E.; Grove, J.R. MDCK (Madin-Darby Canine Kidney) Cells: A Tool for Membrane Permeability Screening. J. Pharm. Sci. 1999, 88, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical High Throughput Screening: Parallel Artificial Membrane Permeation Assay in the Description of Passive Absorption Processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Martel, S.; Carrupt, P.-A. Parallel Artificial Membrane Permeability Assay: A New Membrane for the Fast Prediction of Passive Human Skin Permeability. J. Med. Chem. 2006, 49, 3948–3954. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood–brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Avdeef, A. Absorption and Drug Development; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wond, S.E.; Nilmeier, J.P.; Lightstone, F.C. A Method to Predict Blood-Brain Barrier Permeability of Drug-Like Compounds Using Molecular Dynamics Simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef]
- Russo, G.; Grumetto, L.; Baert, M.; Lynen, F. Comprehensive two-dimensional liquid chromatography as a biomimetic screening platform for pharmacokinetic profiling of compound libraries in early drug development. Anal. Chim. Acta 2021, 1142, 157–168. [Google Scholar] [CrossRef]
- Morimoto, J.; Miyamoto, K.; Ichikawa, Y.; Uchiyama, M.; Makishima, M.; Hashimoto, Y.; Ishikawa, M. Improvement in aqueous solubility of achiral symmetric cyclofenil by modification to a chiral asymmetric analog. Sci. Rep. 2021, 11, 12697. [Google Scholar] [CrossRef]
- Sobańska, A.W.; Brzezińska, E. Immobilized Keratin HPLC Stationary Phase—A Forgotten Model of Transdermal Absorption: To What Molecu-lar and Biological Properties Is It Relevant? Pharmaceutics 2023, 15, 1172. [Google Scholar] [CrossRef]
- Orzel, D.; Ravald, H.; Dillon, A.; Rantala, J.; Wiedmer, S.K.; Russo, G. Immobilised artificial membrane liquid chromatography vs liposome electrokinetic capillary chromatography: Suitability in drug/bio membrane partitioning studies and effectiveness in the assessment of the passage of drugs through the respiratory mucosa. J. Chromatogr. A 2024, 1734, 465286. [Google Scholar] [CrossRef] [PubMed]
- Neri, I.; MacCallum, J.; Di Lorenzo, R.; Russo, G.; Lynen, F.; Grumetto, L. Into the toxicity potential of an array of parabens by biomimetic liquid chromatography, cell viability assessments and in silico predictions. Sci. Total Environ. 2024, 917, 170461. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.Y.; Tan, W.L.; Ho, P.C.; Fang, L.J. Modeling Caco-2 permeability of drugs using immobilized artificial membrane chromatography and physicochemical descriptors. J. Chromatogr. A 2005, 1072, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Tsopelas, F.; Vallianatou, T.; Tsantili-Kakoulidou, A. The potential of immobilized artificial membrane chromatography to predict human oral absorption. Eur. J. Pharm. Sci. 2016, 81, 82–93. [Google Scholar] [CrossRef]
- Pidgeon, C.; Venkataram, U.V. Immobilized artificial membrane chromatography: Supports composed of membrane lipids. Anal. Biochem. 1989, 176, 36–47. [Google Scholar] [CrossRef]
- Valko, K.; Rava, S.; Bunally, S.; Anderson, S. Revisiting the application of immobilized artificial membrane (IAM) chromatography to estimate in vivo distribution properties of drug discovery compounds based on the model of marketed drugs. ADMET DMPK 2020, 8, 78–97. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Siakotos, A.N.; Rouser, G.; Fleischer, S. Isolation of highly purified human and bovine brain endothelial cells and nuclei and their phospholipid composition. Lipids 1969, 4, 234–239. [Google Scholar] [CrossRef]
- Martínez-Pla, J.J.; Martín-Biosca, Y.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Evaluation of the pH effect of formulations on the skin permeability of drugs by biopartitioning micellar chromatography. J. Chromatogr. A 2004, 1047, 255–262. [Google Scholar] [CrossRef]
- Martínez-Pla, J.J.; Martín-Biosca, Y.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Biopartitioning micellar chromatography to predict skin permeability. Biomed. Chromatogr. 2003, 17, 530–537. [Google Scholar] [CrossRef]
- Waters, L.J.; Shahzad, Y.; Stephenson, J. Modelling skin permeability with micellar liquid chromatography. Eur. J. Pharm. Sci. 2013, 50, 335–340. [Google Scholar] [CrossRef]
- Molero-Monfort, M.; Escuder-Gilabert, L.; Villanueva-Camañas, R.M.; Sagrado, S.; Medina-Hernández, M.J. Biopartitioning micellar chromatography: An in vitro technique for predicting human drug absorption. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 225–236. [Google Scholar] [CrossRef]
- Molero-Monfort, M.; Martín-Biosca, Y.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Micellar liquid chromatography for prediction of drug transport. J. Chromatogr. A 2000, 870, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Čudina, O.; Marković, B.; Karljiković-Rajić, K.; Vladimirov, S. Biopartitioning Micellar Chromatography-Partition Coefficient Micelle/Water as a Potential Descriptor for Hydrophobicity in Prediction of Oral Drug Absorption. Anal. Lett. 2012, 45, 677–688. [Google Scholar] [CrossRef]
- De Vrieze, M.; Janssens, P.; Szucs, R.; Van der Eycken, J.; Lynen, F. In vitro prediction of human intestinal absorption and blood–brain barrier partitioning: Development of a lipid analog for micellar liquid chromatography. Anal. Bioanal. Chem. 2015, 407, 7453–7466. [Google Scholar] [CrossRef]
- Russo, G.; Grumetto, L.; Szucs, R.; Barbato, F.; Lynen, F. Determination of in Vitro and in Silico Indexes for the Modeling of Blood–Brain Barrier Partitioning of Drugs via Micellar and Immobilized Artificial Membrane Liquid Chromatography. J. Med. Chem. 2017, 60, 3739–3754. [Google Scholar] [CrossRef]
- Ma, W.; Yang, L.; Lv, Y.; Fu, J.; Zhang, Y.; He, L. Determine equilibrium dissociation constant of drug-membrane receptor affinity using the cell membrane chromatography relative standard method. J. Chromatogr. A 2017, 1503, 12–20. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Li, J.; Liu, R.; Che, D.; He, L. Analysis of Drug Interactions with Dopamine Receptor by Frontal Analysis and Cell Membrane Chromatography. Chromatographia 2015, 78, 649–654. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, D.; Li, J.; Che, D.; Liu, R.; Zhang, J.; Zhang, Y. Interactions between histamine H1 receptor and its antagonists by using cell membrane chromatography method. J. Pharm. Pharmacol. 2015, 67, 1567–1574. [Google Scholar] [CrossRef]
- He, L.; Yang, G.; Geng, X. Enzymatic activity and chromatographic characteristics of the cell membrane immobilized on silica surface. Chin. Sci. Bull. 1999, 44, 826–831. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Geng, X. Coating and fusing cell membranes onto a silica surface and their chromatographic characteristics. Chromatographia 2001, 54, 71–76. [Google Scholar] [CrossRef]
- Li, C.; He, L. Establishment of the model of white blood cell membrane chromatography and screening of antagonizing TLR4 receptor component from Atractylodes macrocephala Koidz. Sci. China Life Sci. 2006, 49, 11. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhang, X.; Chang, R.; Li, X. Development of a Stationary Phase of Vascular Smooth Muscle Cell Membrane Chromatography and Its Chromatographic Affinity Characteristics. Chromatographia 2011, 73, 1065–1071. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, W.; Zheng, L.; Li, M.; Zhang, Y. Construction of recombinant FGFR1 containing full-length gene and its potential application. Plasmid 2010, 64, 60–67. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Zhang, Y.; He, L. An online coupled cell membrane chromatography with LC/MS method for screening compounds from Aconitum carmichaeli Debx. acting on VEGFR-2. J. Pharm. Biomed. Anal. 2010, 53, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Slon-Usakiewicz, J.J.; Ng, W.; Dai, J.-R.; Pasternak, A.; Redden, P.R. Frontal affinity chromatography with MS detection (FAC-MS) in drug discovery. Drug Discov. Today 2005, 10, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wang, S.; Zhang, T.; Ma, J.; Zhang, J.; Zhang, Y.; Lu, W.; He, H.; He, L. Recent advances in cell membrane chromatography for traditional Chinese medicines analysis. J. Pharm. Biomed. Anal. 2014, 101, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, C.; Liu, R.; Wang, N.; Lv, Y.; Dai, B.; He, L. Advances in cell membrane chromatography. J. Chromatogr. A 2021, 1639, 461916. [Google Scholar] [CrossRef]
- Reasor, M.J.; Kacew, S. Drug-Induced Phospholipidosis: Are There Functional Consequences? Exp. Biol. Med. 2001, 226, 825–830. [Google Scholar] [CrossRef]
- Halliwell, W.H. Cationic Amphiphilic Drug-Induced Phospholipidosis. Toxicol. Pathol. 1997, 25, 53–60. [Google Scholar] [CrossRef]
- Garrido, A.; Lepailleur, A.; Mignani, S.M.; Dallemagne, P.; Rochais, C. hERG toxicity assessment: Useful guidelines for drug design. Eur. J. Med. Chem. 2020, 195, 112290. [Google Scholar] [CrossRef] [PubMed]
- Iwakuma, Y.; Okamoto, H.; Hamaguchi, R.; Kuroda, Y. The Limited Contribution of the Analyte Partition to the Water-Rich Layer in Immobilized Artificial Membrane Chromatography with an Acetonitrile-Rich Binary Mobile Phase. Chromatographia 2019, 82, 1311–1320. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Bazar, D.; Brankiewicz, W.; Kapica, H.; Ciura, K.; Zalewska-Piątek, B.; Piątek, R.; Cal, K.; Mojsiewicz-Pieńkowska, K.; Sączewski, J. Development of Safirinium dyes for new applications: Fluorescent staining of bacteria, human kidney cells, and the horny layer of the epidermis. Sci. Rep. 2022, 12, 15098. [Google Scholar] [CrossRef]
- Iwakuma, Y.; Okamoto, H.; Hamaguchi, R.; Kuroda, Y. Immobilized Artificial Membrane Chromatography Using Acetonitrile-Rich Mobile Phase for Comparison of Retention Properties Between Phospholipidosis-Inducing and Non-inducing Basic Drugs. Chromatographia 2023, 86, 43–54. [Google Scholar] [CrossRef]
- Stergiopoulos, C.; Tsopelas, F.; Valko, K. Prediction of hERG inhibition of drug discovery compounds using biomimetic HPLC measurements. ADMET DMPK 2021, 9, 191–207. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, M.; Zhang, D.; Yang, L.; Yang, T.; Li, X.; Zhang, Y. Berberine inhibits the proliferation and migration of breast cancer ZR-75-30 cells by targeting Ephrin-B2. Phytomedicine 2017, 25, 45–51. [Google Scholar] [CrossRef]
- Jia, D.; Chen, X.; Cao, Y.; Wu, X.; Ding, X.; Zhang, H.; Zhang, C.; Chai, Y.; Zhu, Z. On-line comprehensive two-dimensional HepG2 cell membrane chromatographic analysis system for charactering anti-hepatoma components from rat serum after oral administration of Radix scutellariae: A strategy for rapid screening active compounds in vivo. J. Pharm. Biomed. Anal. 2016, 118, 27–33. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, N.; Ma, J.; Zhu, Y.; Wang, M.; Wang, X.; Zhang, P. A Platelet/CMC coupled with offline UPLC-QTOF-MS/MS for screening antiplatelet activity components from aqueous extract of Danshen. J. Pharm. Biomed. Anal. 2016, 117, 178–183. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jia, D.; Cao, Y.; Gao, S.; Guo, S.; Zerbe, P.; Chai, Y.; Diao, Y.; Zhang, Y. Characterization of anti-leukemia components from Indigo naturalis using comprehensive two-dimensional K562/cell membrane chromatography and in silico target identification. Sci. Rep. 2016, 6, 25491. [Google Scholar] [CrossRef]
- Wei, F.; Hu, Q.; Huang, J.; Han, S.; Wang, S. Screening active compounds from Corydalis yanhusuo by combining high expression VEGF receptor HEK293 cell membrane chromatography with HPLC-ESI-IT-TOF-MSn method. J. Pharm. Biomed. Anal. 2017, 136, 134–139. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, C.; Hou, Y.; He, H.; Huang, H.; Yang, L.; Sun, M. The human mast cell line-1 cell membrane chromatography coupled with HPLC-ESI-MS/MS method for screening potentical anaphylactic components from chuanxinlian injection. Biomed. Chromatogr. 2017, 31, e4015. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Fu, J.; Shi, X.; Yang, Z.; Han, S. Screening allergic components of Yejuhua injection using LAD2 cell membrane chromatography model online with high performance liquid chromatography-ion trap-time of flight-mass spectrum system. J. Chromatogr. B 2017, 1055–1056, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lv, Y.; Fu, J.; Jia, Q.; Han, S. A high expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled with liquid chromatography and mass spectrometry method for screening potential anaphylactoid components in kudiezi injection. J. Pharm. Biomed. Anal. 2018, 159, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Sun, W.; Zhang, L.; Fu, J.; Lv, Y.; Lin, Y.; Han, S. Screening the anti-allergic components in Saposhnikoviae Radix using high-expression Mas-related G protein-coupled receptor X2 cell membrane chromatography online coupled with liquid chromatography and mass spectrometry. J. Sep. Sci. 2019, 42, 2351–2359. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, D.; Hu, T.; Hou, Y.; Lin, Y.; He, H.; Wang, C. Anti-pseudo-allergic capacity of alkaloids screened from Uncaria rhynchophylla. New J. Chem. 2020, 44, 38–45. [Google Scholar] [CrossRef]
- Taillardat-Bertschinger, A.; Galland, A.; Carrupt, P.-A.; Testa, B. Immobilized artificial membrane liquid chromatography: Proposed guidelines for technical optimization of retention measurements. J. Chromatogr. A 2002, 953, 39–53. [Google Scholar] [CrossRef]
- Russo, G.; Grumetto, L.; Szucs, R.; Barbato, F.; Lynen, F. Screening therapeutics according to their uptake across the blood-brain barrier: A high throughput method based on immobilized artificial membrane liquid chromatography-diode-array-detection coupled to electrospray-time-of-flight mass spectrometry. Eur. J. Pharm. Biopharm. 2018, 127, 72–84. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Breiman, L. Statistical Modeling: The Two Cultures. Stat. Sci. 2001, 16, 199–215. [Google Scholar] [CrossRef]
- Bemis, G.W.; Murcko, M.A. The Properties of Known Drugs. 1. Molecular Frameworks. J. Med. Chem. 1996, 39, 2887–2893. [Google Scholar] [CrossRef]
- Liu, L.; Ozsu, M.T. Encyclopedia of Database Systems, 1st ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wang, S.; Zhang, R.; Li, X.; Cai, F.; Ma, X.; Tang, Y.; Xu, C.; Wang, L.; Ren, P.; Liu, L.; et al. Recent advances in molecular representation methods and their applications in scaffold hopping. npj Drug Discov. 2025, 2, 14. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Handbook of Molecular Descriptors; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Guyon, I.M.; Elisseeff, A. An Introduction to Variable and Feature Selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- RDKit: Open-Source Cheminformatics. Available online: https://www.rdkit.org (accessed on 19 October 2025).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Preisach, C.; Burkhardt, H.; Schmidt-Thieme, L.; Decker, R. (Eds.) Data Analysis, Machine Learning and Applications: Proceedings of the 31st Annual Conference of the Gesellschaft Für Klassifikation EV, Albert-Ludwigs-Universität Freiburg, March 7–9, 2007; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- MacQueen, J.B. Some Methods for Classification and Analysis of Multivariate Observations. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; Statistical Laboratory of the University of California: Berkeley, CA, USA, 1967; Volume 5.1, pp. 281–297. [Google Scholar]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Pearson, K.L., III. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Liu, F.T.; Ting, K.M.; Zhou, Z.-H. Isolation Forest. In Proceedings of the 2008 Eighth IEEE International Conference on Data Mining, Pisa, Italy, 15–19 December 2008; IEEE: New York, NY, USA, 2008; pp. 413–422. [Google Scholar]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the KDD’96, Second International Conference on Knowledge Discovery and Data Mining, Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Kullback, S.; Leibler, R.A. On Information and Sufficiency. Ann. Math. Stat. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- Héberger, K. Sum of ranking differences compares methods or models fairly. TrAC Trends Anal. Chem. 2010, 29, 101–109. [Google Scholar] [CrossRef]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the Dimensionality of Data with Neural Networks. Science 2006, 313, 504–507. [Google Scholar] [CrossRef]
- Breunig, M.M.; Kriegel, H.-P.; Ng, R.T.; Sander, J. LOF: Identifying density-based local outliers. ACM SIGMOD Rec. 2000, 29, 93–104. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bates, D.M.; Watts, D.G. Nonlinear Regression Analysis and Its Applications; Wiley: Hoboken, NJ, USA, 1988. [Google Scholar]
- Ciura, K.; Kovačević, S.; Pastewska, M.; Kapica, H.; Kornela, M.; Sawicki, W. Prediction of the chromatographic hydrophobicity index with immobilized artificial membrane chromatography using simple molecular descriptors and artificial neural networks. J. Chromatogr. A 2021, 1660, 462666. [Google Scholar] [CrossRef] [PubMed]
- Lindley, D.V.; Smith, A.F.M. Bayes Estimates for the Linear Model. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 1–41. [Google Scholar] [CrossRef]
- Vapnik, V.; Golowich, S.E.; Smola, A. Support vector method for function approximation, regression estimation and signal processing. In Proceedings of the 10th International Conference on Neural Information Processing Systems, Denver, CO, USA, 3–5 December 1996; MIT Press: Cambridge, MA, USA, 1996; pp. 281–287. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Huber, P.J. Robust Estimation of a Location Parameter. Ann. Math. Stat. 1964, 35, 73–101. [Google Scholar] [CrossRef]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B Stat. Methodol. 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Wold, S. Cross-Validatory Estimation of the Number of Components in Factor and Principal Components Models. Technometrics 1978, 20, 397. [Google Scholar] [CrossRef]
- Rücker, C.; Rücker, G.; Meringer, M. y-Randomization and Its Variants in QSPR/QSAR. J. Chem. Inf. Model. 2007, 47, 2345–2357. [Google Scholar] [CrossRef]
- Cox, D.R. The Regression Analysis of Binary Sequences. J. R. Stat. Soc. Ser. B Stat. Methodol. 1958, 20, 215–232. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees, 1st ed.; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Podgorelec, V.; Zorman, M. Decision Tree Learning. In Encyclopedia of Complexity and Systems Science; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–28. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- McCulloch, W.S.; Pitts, W. A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biophys. 1943, 5, 115–133. [Google Scholar] [CrossRef]
- Rosenblatt, F. The perceptron: A probabilistic model for information storage and organization in the brain. Psychol. Rev. 1958, 65, 386–408. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Vapnik, V.N. The Nature of Statistical Learning Theory, 2nd ed.; Springer: New York, NY, USA, 2000. [Google Scholar]
- Lin, T.-Y.; Goyal, P.; Girshick, R.; He, K.; Dollár, P. Focal Loss for Dense Object Detection. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; IEEE: New York, NY, USA, 2017; pp. 2999–3007. [Google Scholar]
- Ciura, K.; Ulenberg, S.; Kapica, H.; Kawczak, P.; Belka, M.; Bączek, T. Drug affinity to human serum albumin prediction by retention of cetyltrimethylammonium bromide pseudostationary phase in micellar electrokinetic chromatography and chemically advanced template search descriptors. J. Pharm. Biomed. Anal. 2020, 188, 113423. [Google Scholar] [CrossRef]
- Ciura, K. Modeling of small molecule’s affinity to phospholipids using IAM-HPLC and QSRR approach enhanced by similarity-based machine algorithms. J. Chromatogr. A 2024, 1714, 464549. [Google Scholar] [CrossRef]
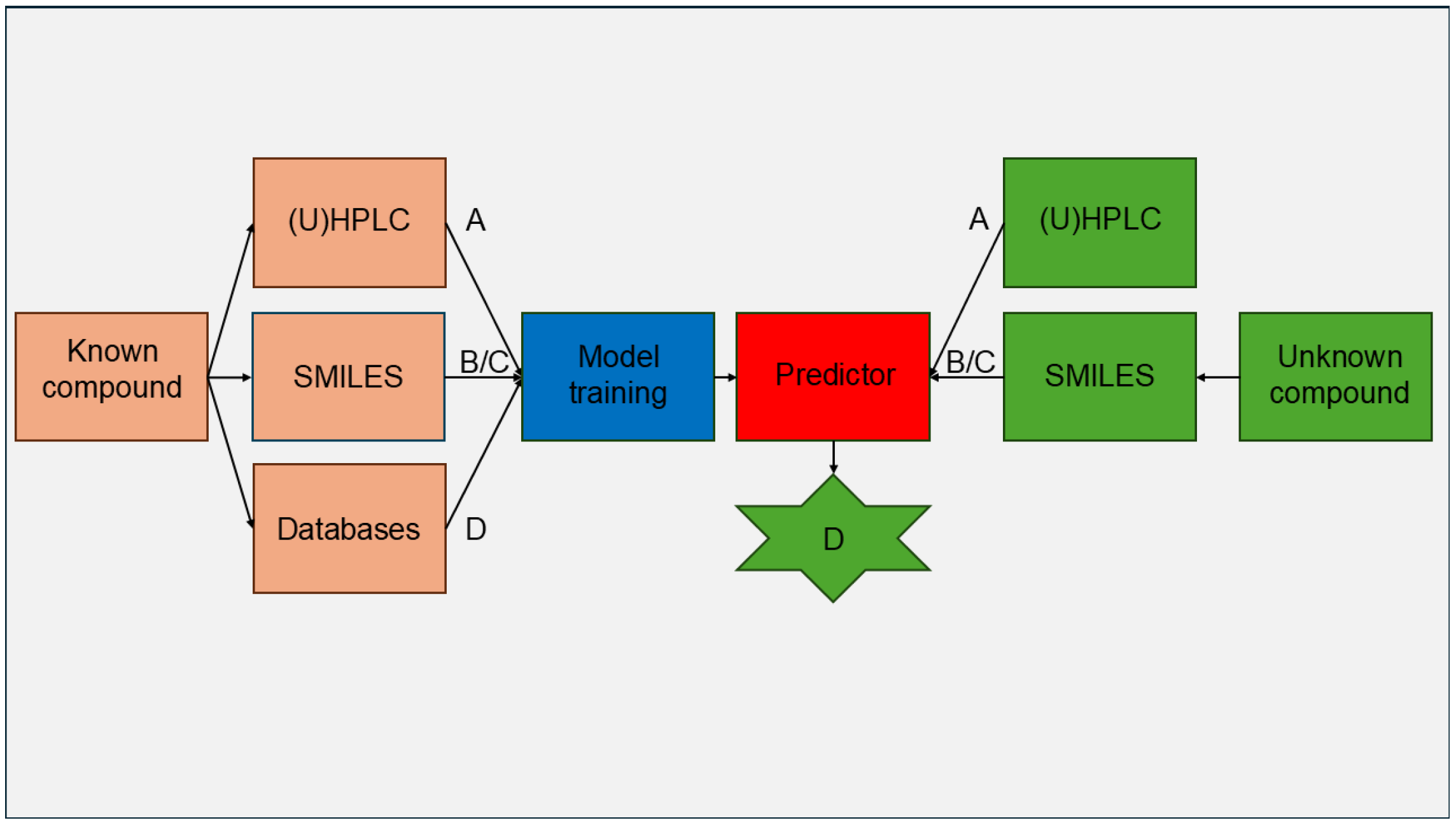
| Drug | Parameters Influenced by Lipophilicity |
|---|---|
| Absorption | Solubility Membrane Permeability |
| Distribution | Blood–Brain Barrier (BBB) permeability Volume of distribution (VD) |
| Metabolism and Excretion | Susceptibility to oxidative metabolism Half-life (t1/2) Clearance (Cl) |
| Toxicity | Drug-induced phospholipidosis (DIPL) human ether-a-go-go-related gene (hERG) toxicity |
| Method | Stationary Phase (Characteristics) | Application Area | Advantages | Disadvantage |
|---|---|---|---|---|
| IAM | Phospholipids (e.g., phosphatidylcholine) are covalently bonded to silica | Membrane permeability, BBB permeability, lipophilicity (phospholipid affinity), phospholipidosis risk, human oral absorption, protein binding | Commercially available (Regis), robust, and HTS-compatible, this is a suitable model for passive diffusion | Does not model active transport. “Type B” silica switch caused retention shifts. |
| Affinity (HSA/AGP) | Immobilised plasma proteins on a support | Plasma protein binding, chiral separations | Commercially available (Daicel), HTS-compatible, directly measures binding to key plasma proteins | Specific protein binding. Does not measure membrane permeability or lipophilicity. |
| CMC | Immobilised, intact cell membranes or whole cells (e.g., HEK 293) | Drug–membrane interactions, specificity testing | Provides the most biologically relevant model | Not commercially available, complex to prepare, lower stability and robustness, HTS-incompatible. |
| MLC | Standard RPLC phase (e.g., C18) with a micelle-containing mobile phase (e.g., SDS, CTAB) | Membrane permeability, BBB permeability, lipophilicity, human oral absorption, protein binding | Uses standard columns, cost-effective, versatile (surfactant choice alters properties) | Complex separation mechanism (dual equilibrium). Micelles may not perfectly mimic biological membranes. |
| Type of Descriptor | Definition | Examples | Application |
|---|---|---|---|
| Topological | Derived from molecular graphs and encodes information about the connectivity and branching of atoms in a molecule | Degree of branching, Molecular connectivity indices, Wiener index | Solubility Boiling point Biological activity |
| Geometrical | Encode information about the 3D shape and size of the molecule | Molecular surface area (MSA), molecular volume (MV), principal moments of inertia | Molecular interactions |
| Electrostatic | Quantify the distribution of electric charge within a molecule | Partial atomic charges, dipole moment, Eeectrostatic potential maps | Hydrogen bonding Ionic interactions. |
| Quantum | Derived from quantum mechanical calculations | HOMO-LUMO gap, ionisation potential, electron affinity | Chemical reactivity Stability Spectroscopic properties |
| Physicochemical | Represent physical and chemical properties of molecules | logP, pKa, PSA | ADME properties |
| Pharmacophoric | Represent the spatial arrangement of features in a molecule that are essential for biological activity | Hydrogen bond donors (HDo), hydrogen bond acceptors (HAc), aromatic rings | ADME properties |
| Method Category | Clustering | Dimensionality Reduction | Anomaly Detection |
|---|---|---|---|
| Loss function | Silhouette Coefficient—evaluates cluster cohesion and separation by measuring how similar points are to their own cluster compared to other clusters. Ranges from −1 to 1, where higher values indicate better-defined clusters [118]. | Kullback–Leibler Divergence—measures the difference between probability distributions of original and reduced-dimensional data. Widely used in variational autoencoders. Lower values indicate better preservation of data structure [119]. | Isolation Score—Measures how easily a point can be isolated from the rest of the data through random partitioning. Lower values indicate a higher likelihood of being an outlier [116]. |
| Evaluation metric | SRD—evaluate how different clustering algorithms rank or group similar objects [120]. Inertia measures the sum of the squared distances between each data point and its closest centroid, commonly used in k-means. Lower values indicate better-defined clusters [112]. | Reconstruction error—Quantifies the difference between the original data and its reconstruction after dimensionality reduction, significant in autoencoders. Lower values indicate better preservation of information [121]. | Local Outlier Factor (LOF) Score—Compares the local density of a point with the densities of its neighbours. Higher values indicate a more substantial likelihood of being an outlier [122]. |
| Model | Logistic Regression [134] | Decision Trees [135,136] | SVM [137] | ANN [138,139,140] |
|---|---|---|---|---|
| Strengths | Interpretable, efficient with small data | Handles non-linear data, interpretable | Effective in high-dimensional spaces | Captures complex patterns, scalable |
| Limitations | Limited to linear decision | Prone to overfitting | Computationally intensive with large data | Require large datasets, poor interpretability |
| Use Case | Binary toxicity | Rule-based ADMET screening | Drug–target interaction prediction | Multi-task toxicity |
| Loss Functions for Classification Models | Evaluation Metrics for Classification Models |
|---|---|
| Cross-entropy loss (Log Loss)—measures the difference between predicted class probabilities and true labels [141]. | Accuracy—ratio of total correct predictions (both positive and negative) out of all predictions. Best for balanced sets. |
| Hinge loss—used for margin maximisation in SVM. Penalises predictions that are on the wrong side of the decision boundary [142]. | Precision—ratio of correctly predicted positive instances out of all the cases predicted as positive. Measure how reliable an optimistic prediction is. |
| Focal loss—addresses class imbalances by focusing on complex classifiable examples. Gives small weight to easy examples [143]. | Specificity—precision, but for pessimistic predictions. |
| Recall (sensitivity)—ratio of actual positive instances that the model correctly identifies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuz, D.; Smuga, D.; Pawiński, T. Development of Prediction Capabilities for High-Throughput Screening of Physiochemical Properties by Biomimetic Chromatography. Molecules 2025, 30, 4528. https://doi.org/10.3390/molecules30234528
Tuz D, Smuga D, Pawiński T. Development of Prediction Capabilities for High-Throughput Screening of Physiochemical Properties by Biomimetic Chromatography. Molecules. 2025; 30(23):4528. https://doi.org/10.3390/molecules30234528
Chicago/Turabian StyleTuz, Damian, Damian Smuga, and Tomasz Pawiński. 2025. "Development of Prediction Capabilities for High-Throughput Screening of Physiochemical Properties by Biomimetic Chromatography" Molecules 30, no. 23: 4528. https://doi.org/10.3390/molecules30234528
APA StyleTuz, D., Smuga, D., & Pawiński, T. (2025). Development of Prediction Capabilities for High-Throughput Screening of Physiochemical Properties by Biomimetic Chromatography. Molecules, 30(23), 4528. https://doi.org/10.3390/molecules30234528





