Study on the Isolated Asphaltene Thermal Cracking from an Unconventional Oil Using Diverse Estimating Arrhenius Parameter Approaches
Abstract
1. Introduction
2. Results and Discussion
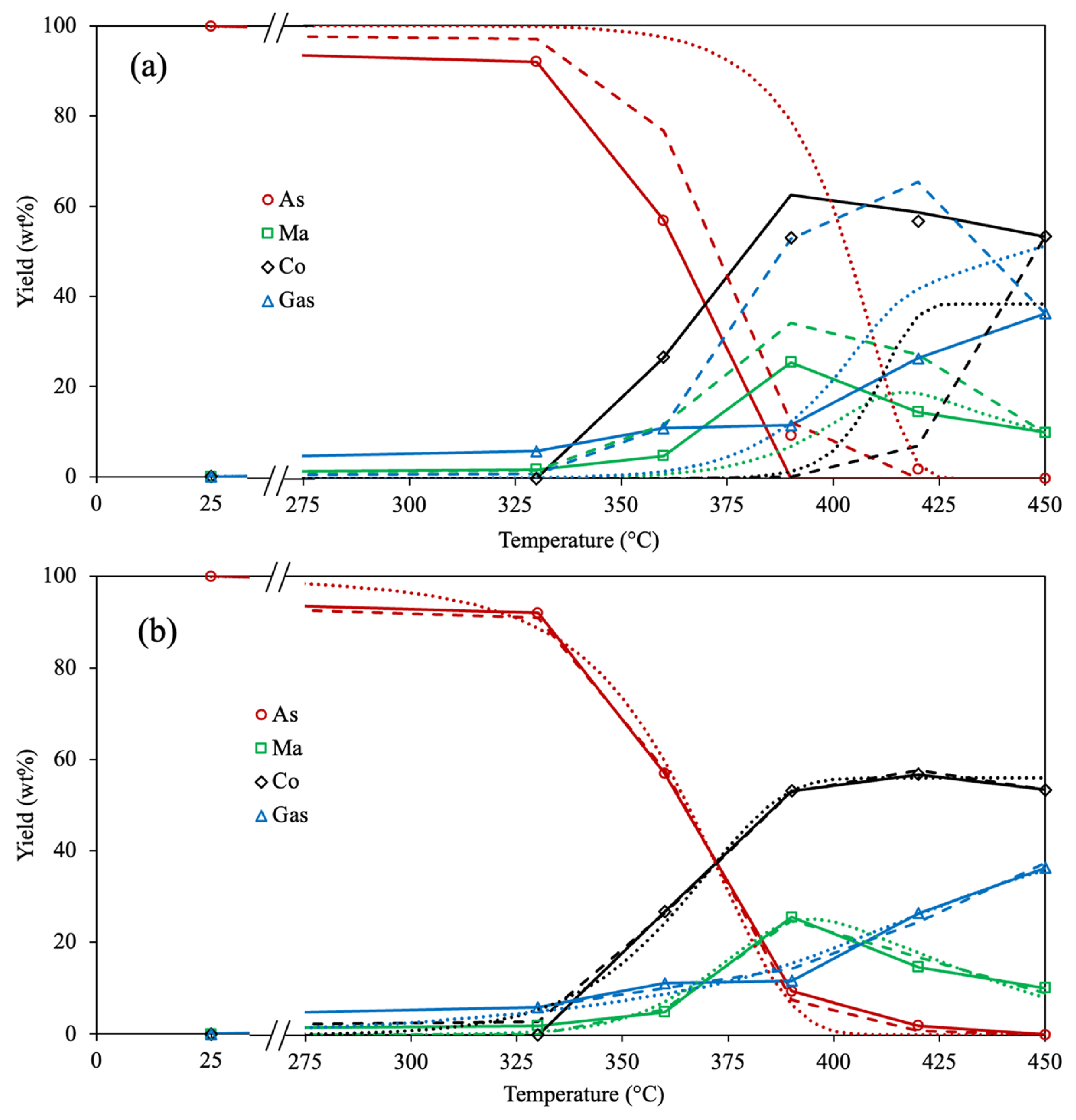
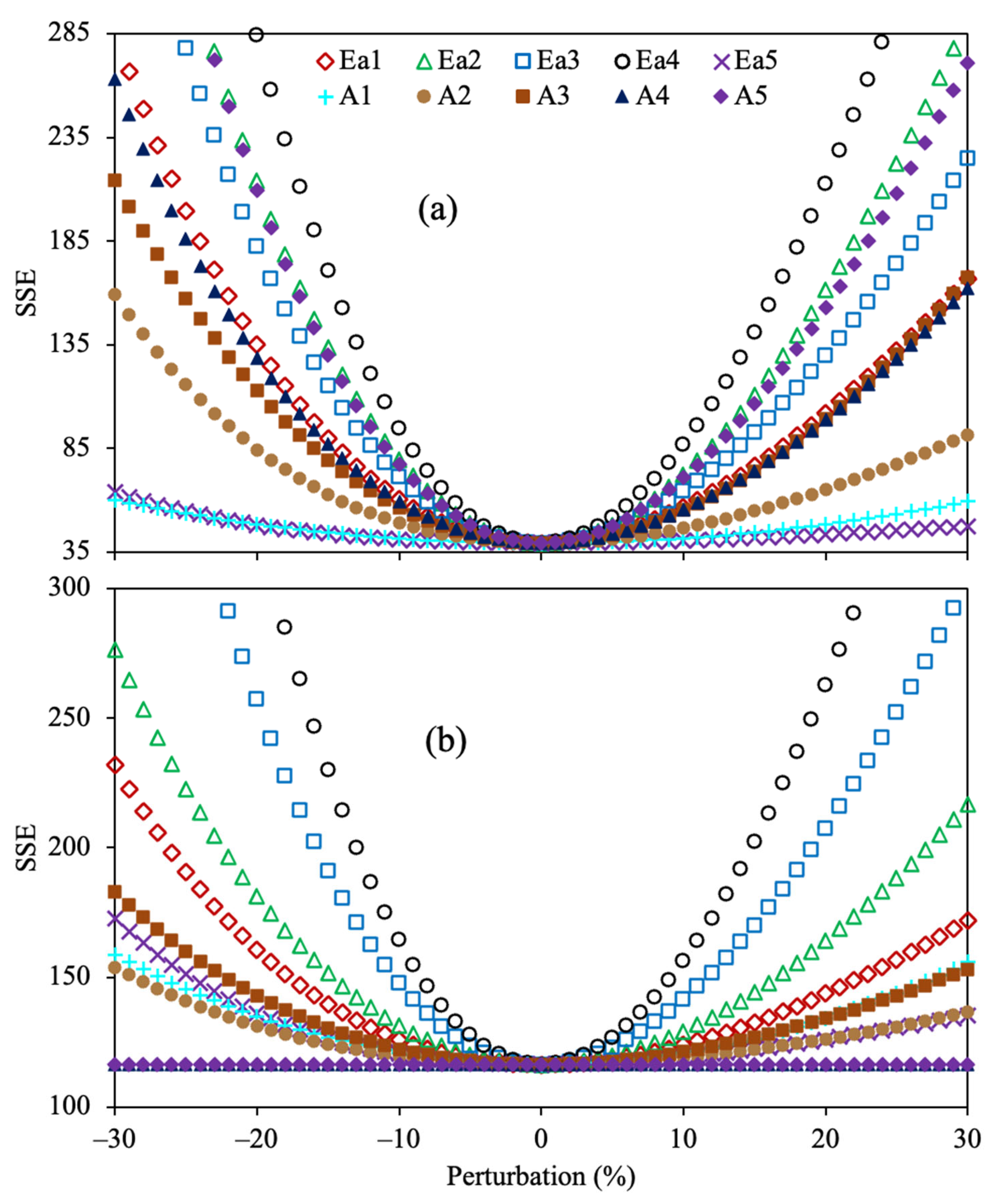
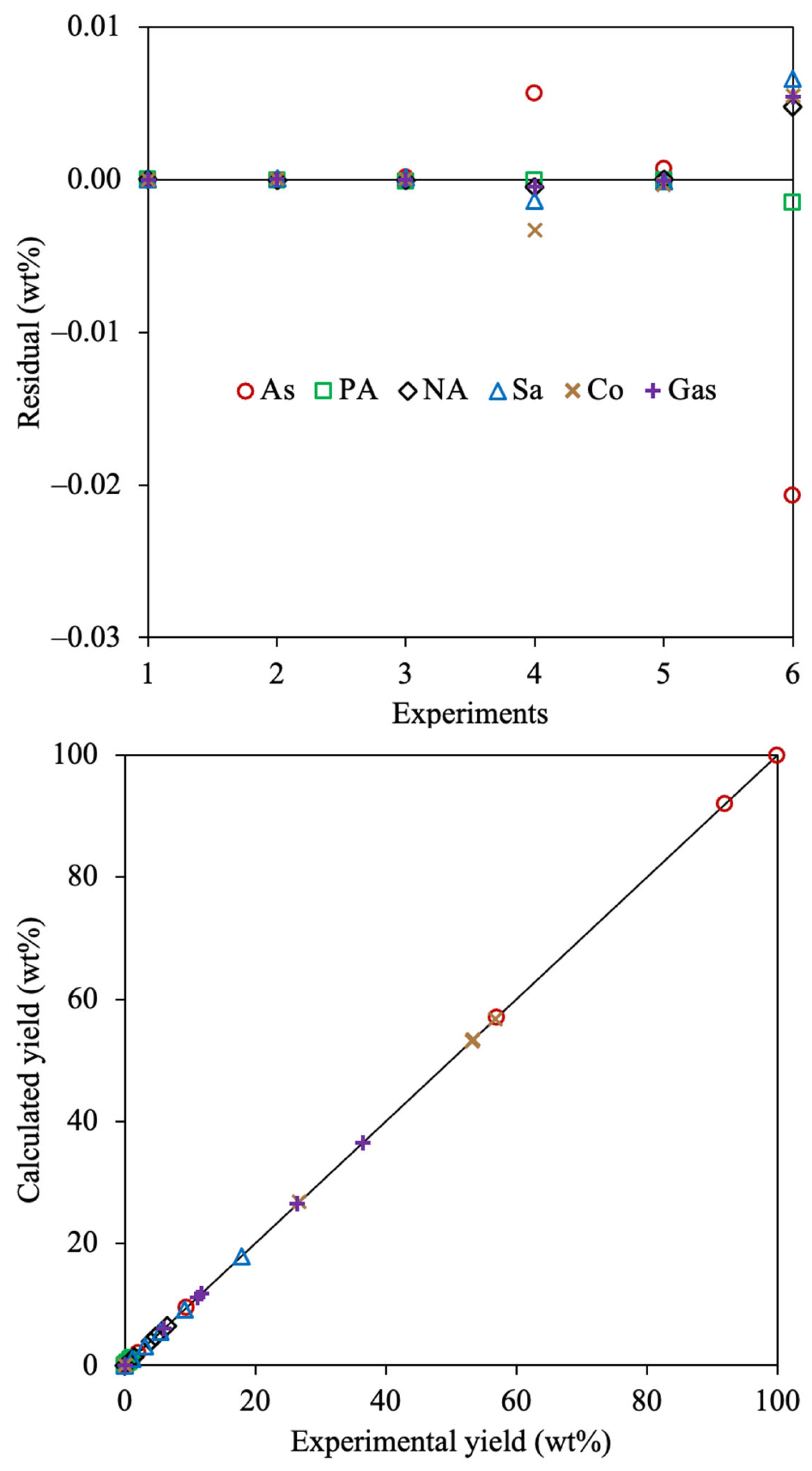
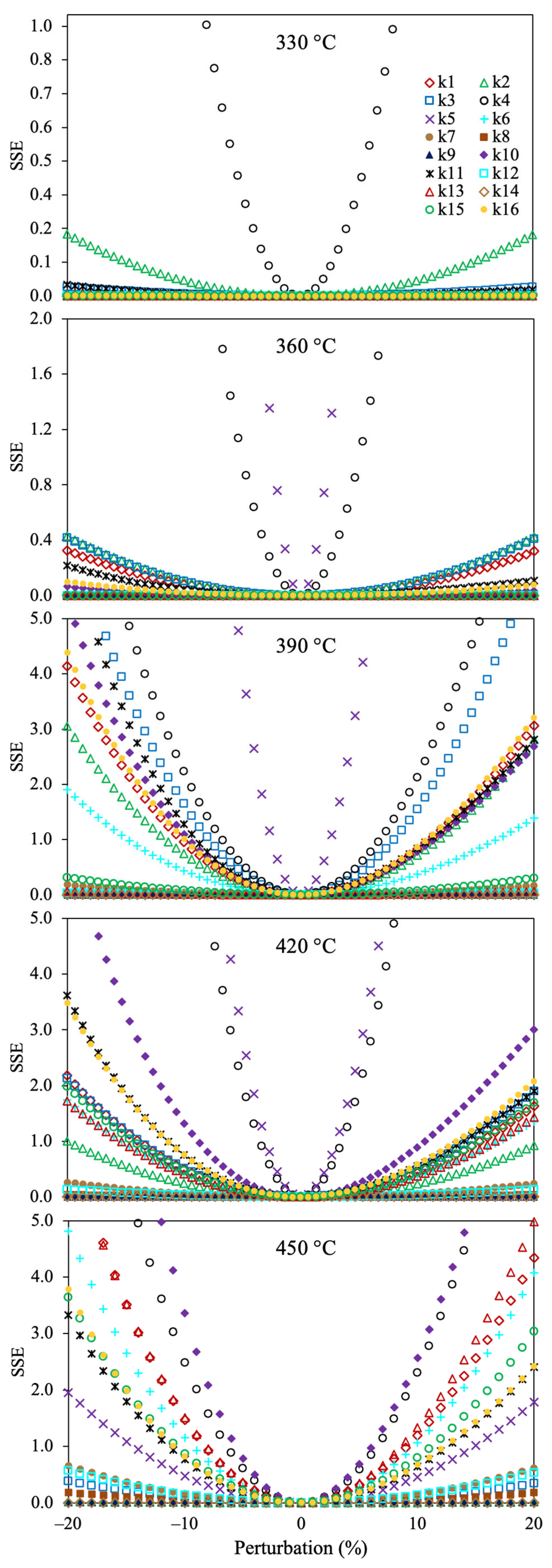
2.1. Evaluation of the Optimization Approach
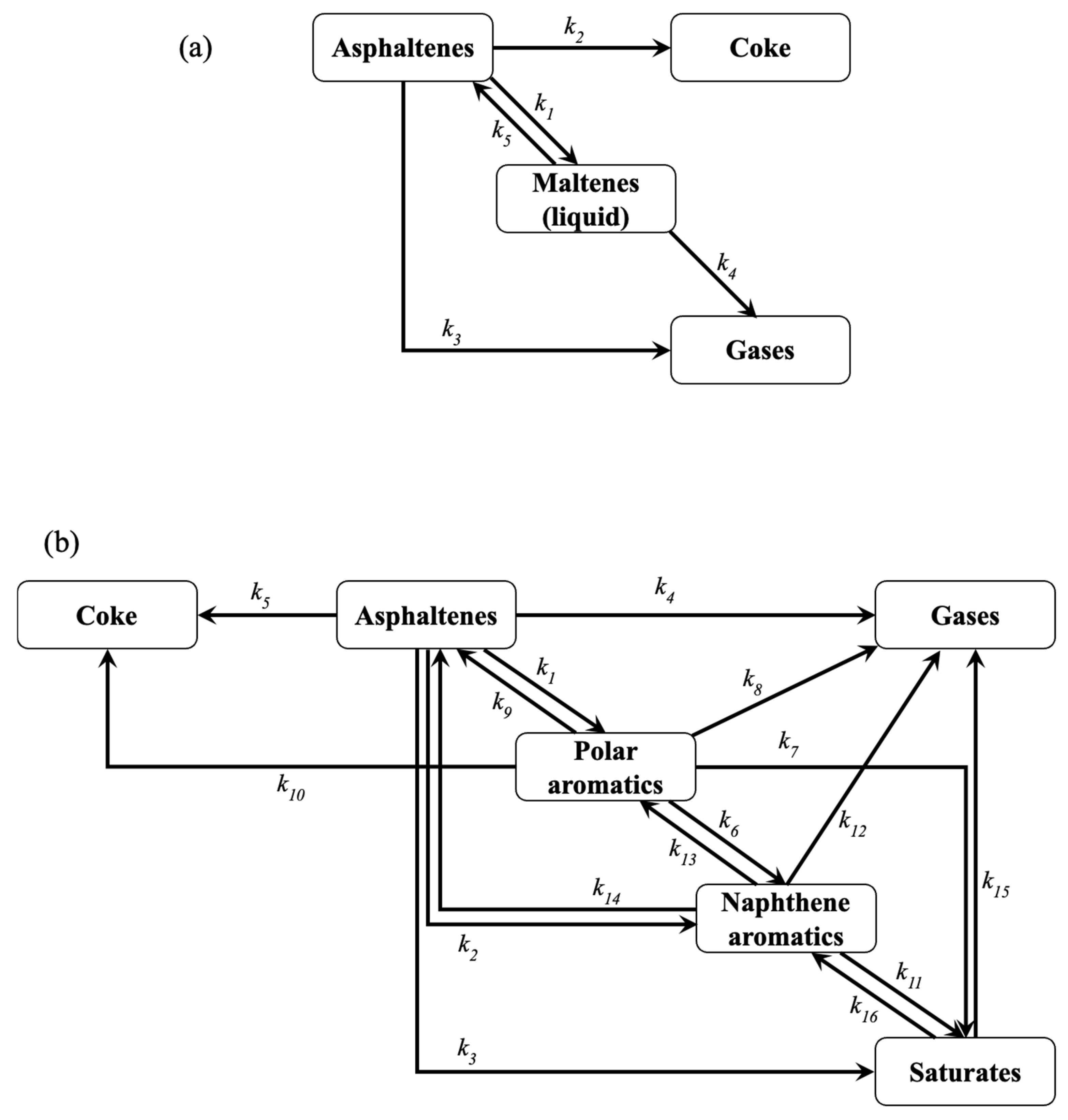
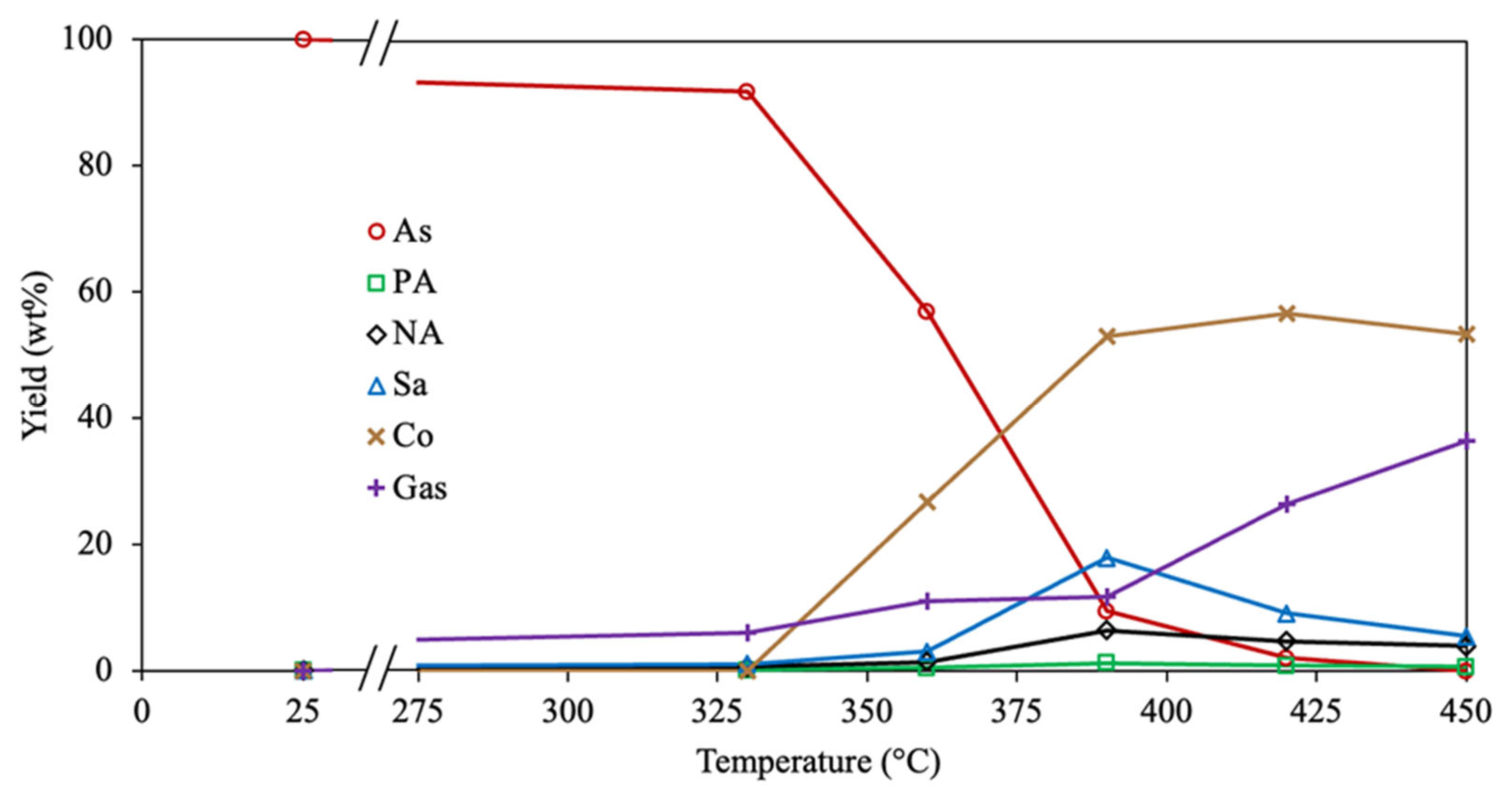
2.2. Exhaustive Reaction Scheme
3. Materials and Methods
3.1. Experimental Data
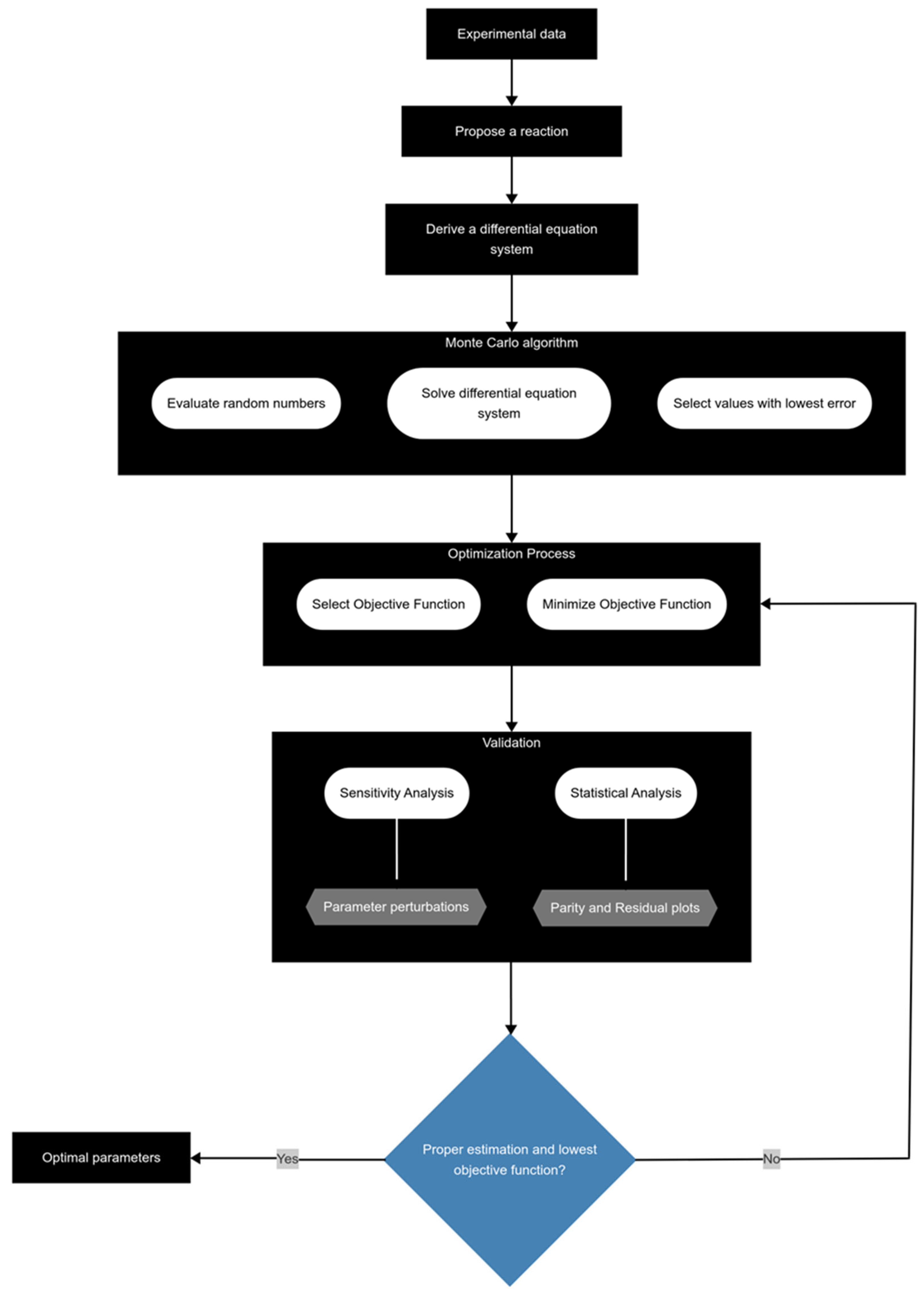
3.2. Kinetic Modeling
3.3. Parameter Estimation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ore, O.T.; Adebiyi, F.M. A Review on Current Trends and Prospects in the Pyrolysis of Heavy Oils. J. Pet. Explor. Prod. 2021, 11, 1521–1530. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhang, Y.; Zhang, W.; Qiao, P. Research on the Pyrolysis Characteristics and Kinetics of Two Typical Inferior Heavy Oils. Fuel 2022, 328, 125330. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A Review of Pyrolysis, Aquathermolysis, and Oxidation of Athabasca Bitumen. Fuel Process. Technol. 2015, 131, 270–289. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Varfolomeev, M.A.; Al-muntaser, A.; Suwaid, M.; Yuan, C.; Ancheyta, J. Chemical and Structural Changes of Resins during the Catalytic and Non-Catalytic Aquathermolysis of Heavy Crude Oils. Geoenergy Sci. Eng. 2023, 230, 212242. [Google Scholar] [CrossRef]
- Tirado, A.; Félix, G.; Al-Muntaser, A.A.; Chemam, M.S.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Molecular Asphaltene Transformations during Aquathermolysis of Heavy Crude Oil: Analysis of the Literature Data. Energy Fuels 2023, 37, 7927–7944. [Google Scholar] [CrossRef]
- Salehzadeh, M.; Husein, M.M.; Ghotbi, C.; Dabir, B.; Taghikhani, V. In-Depth Characterization of Light, Medium and Heavy Oil Asphaltenes as Well as Asphaltenes Subfractions. Fuel 2022, 324, 124525. [Google Scholar] [CrossRef]
- Zuo, P.; Qu, S.; Shen, W. Asphaltenes: Separations, Structural Analysis and Applications. J. Energy Chem. 2019, 34, 186–207. [Google Scholar] [CrossRef]
- Tirado, A.; Félix, G.; Trejo, F.; Varfolomeev, M.A.; Yuan, C.; Nurgaliev, D.K.; Sámano, V.; Ancheyta, J. Properties of Heavy and Extra-Heavy Crude Oils. In Catalytic In-Situ Upgrading of Heavy and Extra-Heavy Crude Oils; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 1–38. ISBN 978-1-119-87150-7. [Google Scholar]
- Félix, G.; Tirado, A.; Quitian, A.; Sámano, V.; Varfolomeev, M.A.; Yuan, C.; Ancheyta, J. Changes in Structural Parameters of SARA Fractions during Heavy Oil Hydrocracking Using Dispersed Catalysts. Fuel 2023, 348, 128512. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Jin, H.; Miao, Y.; Zhang, Y.; Wang, X.; Guo, L. Hydrogen Donation of Supercritical Water in Asphaltenes Upgrading by Deuterium Tracing Method. J. Supercrit. Fluids 2024, 205, 106137. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Zhou, Y.; Zheng, L.; Jin, H.; Bawaa, B.; Guo, L. Kinetic Study of Asphaltenes Phase Separation in Supercritical Water Upgrading of Heavy Oil. Fuel Process. Technol. 2023, 241, 107588. [Google Scholar] [CrossRef]
- Liu, Q.K.; Zhu, D.Q.; Tan, X.C.; Yuan, P.Q.; Cheng, Z.M.; Yuan, W.K.; Yang, J.Y. Lumped Reaction Kinetic Models for Pyrolysis of Heavy Oil in the Presence of Supercritical Water. AIChE J. 2016, 62, 207–216. [Google Scholar] [CrossRef]
- Calderón, C.J.; Félix, G.; Ancheyta, J. A Commercial Reactor Simulation for the Slurry-Phase Hydrocracking of Heavy Oil with a Mineral Catalyst Using Distillation Lumps and SARA-Based Kinetic Models. Ind. Eng. Chem. Res. 2024, 63, 17814–17823. [Google Scholar] [CrossRef]
- Chehadeh, D.; Ma, X.; Al Bazzaz, H. Recent Progress in Hydrotreating Kinetics and Modeling of Heavy Oil and Residue: A Review. Fuel 2023, 334, 126404. [Google Scholar] [CrossRef]
- Soto-Azuara, L.A.; Ramírez-López, R.; del Carmen Monterrubio-Badillo, M.; Elizalde, I. Mathematical Modeling of the Hydrocracking Kinetics of a Heavy Oil Fraction Using the Discrete Lumping Approach: The Effect of the Variation of the Lump Number. React. Kinet. Mech. Catal. 2022, 135, 655–667. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Varfolomeev, M.A.; Ancheyta, J. Effect of Reaction Pathways on the Kinetic Modeling of Aquathermolysis of Heavy Oils. Ind. Eng. Chem. Res. 2024, 63, 17836–17846. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Varfolomeev, M.A.; Soto-Robles, C.A.; Lugo-Medina, E.; Ancheyta, J. Comparison of Traditional and Sequential Approaches for Estimation of Kinetic Parameters of Heavy Oil Upgrading. Energy Fuels 2024, 38, 8044–8061. [Google Scholar] [CrossRef]
- Raghavan, A.; He, P.; Ghoniem, A.F. Inference of Reaction Kinetics for Supercritical Water Heavy Oil Upgrading with a Two-Phase Stirred Reactor Model. AIChE J. 2022, 68, e17488. [Google Scholar] [CrossRef]
- Browning, B.; Alvarez, P.; Jansen, T.; Lacroix, M.; Geantet, C.; Tayakout-Fayolle, M. A Review of Thermal Cracking, Hydrocracking, and Slurry Phase Hydroconversion Kinetic Parameters in Lumped Models for Upgrading Heavy Oils. Energy Fuels 2021, 35, 15360–15380. [Google Scholar] [CrossRef]
- Gray, M.R. Upgrading Oilsands Bitumen and Heavy Oil, 1st ed.; The University of Alberta Press: Edmonton, AB, Canada, 2015; ISBN 978-1-77212-022-6. [Google Scholar]
- Rahimi, P.M.; Gentzis, T. The Chemistry of Bitumen and Heavy Oil Processing. In Practical Advances in Petroleum Processing; Springer: New York, NY, USA, 2006; pp. 597–634. ISBN 978-0-387-25789-1. [Google Scholar]
- Colleoni, E.; Guida, P.; Samaras, V.G.; Frassoldati, A.; Faravelli, T.; Roberts, W.L. Chemical Kinetics of SARA Fractions Pyrolysis: Resins. J. Anal. Appl. Pyrolysis 2024, 177, 106281. [Google Scholar] [CrossRef]
- Kaminski, T.; Husein, M.M. Kinetic Modelling of Thermal Cracking of Arabian Atmospheric and Vacuum Residue. Fuel Process. Technol. 2019, 189, 89–97. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Lababidi, H.M.S.; Al-Rabiah, H. Thermal Cracking Kinetics of Kuwaiti Vacuum Residues in Eureka Process. Fuel 2013, 103, 923–931. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, M.M.; Saxena, A.K.; Kumar, S. Reaction Pathways and Product Yields in Mild Thermal Cracking of Vacuum Residues: A Multi-Lump Kinetic Model. Chem. Eng. J. 2005, 108, 239–248. [Google Scholar] [CrossRef]
- Souza, B.M.; Travalloni, L.; Da Silva, M.A.P. Kinetic Modeling of the Thermal Cracking of a Brazilian Vacuum Residue. Energy Fuels 2015, 29, 3024–3031. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, X.; Pu, W.; He, M.; Yang, Y. Kinetic Modeling for Upgrading of Heavy Crude Oil via Thermal Cracking in Porous Media: SARA Fractions and Gas Compositions. Fuel 2024, 371, 132087. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Liu, Z.; Meng, X. Nine-Lump Kinetic Study of Catalytic Pyrolysis of Gas Oils Derived from Canadian Synthetic Crude Oil. Int. J. Chem. Eng. 2016, 2016, 9148925. [Google Scholar] [CrossRef]
- Xiang-Hai, M.; Chun-Ming, X.; Li, L.; Jin-Sen, G. Studies on the Kinetics of Heavy Oil Catalytic Pyrolysis. Ind. Eng. Chem. Res. 2003, 42, 6012–6019. [Google Scholar] [CrossRef]
- Martínez, M.T.; Benito, A.M.; Callejas, M.A. Thermal Cracking of Coal Residues: Kinetics of Asphaltene Decomposition. Fuel 1997, 76, 871–877. [Google Scholar] [CrossRef]
- Chen, X.F.; Da, Z.J.; Gong, J.H.; Zhang, H.J.; Zhu, Y.K.; Yang, J.Y.; Yuan, P.Q.; Yuan, W.K. Demetallization of Heavy Oil through Pyrolysis: A Reaction Kinetics Analysis. AIChE J. 2021, 67, e17086. [Google Scholar] [CrossRef]
- Yasar, M.; Trauth, D.M.; Klein, M.T. Asphaltene and Resid Pyrolysis. 2. The Effect of Reaction Environment on Pathways and Selectivities. Energy Fuels 2001, 15, 504–509. [Google Scholar] [CrossRef]
- Akmaz, S.; Deniz, C.U.; Yasar, M. Investigation of Reaction Pathways and Kinetics of Turkish Asphaltenes. Chem. Eng. Trans. 2013, 32, 871–876. [Google Scholar] [CrossRef]
- Phillips, C.R.; Haidar, N.I.; Poon, Y.C. Kinetic Models for the Thermal Cracking of Athabasca Bitumen: The Effect of the Sand Matrix. Fuel 1985, 64, 678–691. [Google Scholar] [CrossRef]
- Meng, X.; Xu, C.; Gao, J.; Li, L. Catalytic Pyrolysis of Heavy Oils: 8-Lump Kinetic Model. Appl. Catal. A Gen. 2006, 301, 32–38. [Google Scholar] [CrossRef]
- Freitag, N.P.; Exelby, D.R. A SARA- Based Model for Simulating the Pyrolysis Reactions That Occur in High-Temperature EOR Processes. J. Can. Pet. Technol. 2006, 45, 38–44. [Google Scholar] [CrossRef]
- Qu, X.; Li, Y.; Li, S.; Wang, J.; Xu, H.; Li, Z. Thermal Cracking, Aquathermolysis, and Their Upgrading Effects of Mackay River Oil Sand. J. Pet. Sci. Eng. 2021, 201, 920–4105. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A New Reaction Model for Aquathermolysis of Athabasca Bitumen. Can. J. Chem. Eng. 2013, 91, 475–482. [Google Scholar] [CrossRef]
- Savage, P.E.; Klein, M.T.; Kukes, S.G. Asphaltene Reaction Pathways. 1. Thermolysis. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 1169–1174. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, F.; Yu, Y. Effects of Reaction Time and Temperature on Carbonization in Asphaltene Pyrolysis. J. Pet. Sci. Eng. 2010, 74, 20–25. [Google Scholar] [CrossRef]
- Dhir, S.; Mahapatra, N.; Kurian, V.; Alipour, M.; Gupta, R. Single Particle Asphaltene Pyrolysis in a Drop-Tube Furnace. Energy Fuels 2016, 30, 6132–6142. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Liu, D.; Lou, B.; Li, M.; Guo, S.; Yu, R.; Wu, B.; Gong, X.; Li, G. Comparative Study of the Carbonization Process and Structural Evolution during Needle Coke Preparation from Petroleum and Coal Feedstock. J. Anal. Appl. Pyrolysis 2021, 156, 105097. [Google Scholar] [CrossRef]
- Shin, S.; Im, S.I.; Kwon, E.H.; Na, J.-G.; Nho, N.S.; Lee, K.B. Kinetic Study on the Nonisothermal Pyrolysis of Oil Sand Bitumen and Its Maltene and Asphaltene Fractions. J. Anal. Appl. Pyrolysis 2017, 124, 658–665. [Google Scholar] [CrossRef]
- Alhumaidan, F.S.; Rana, M.S.; Lababidi, H.M.S.; Hauser, A. Pyrolysis of Asphaltenes Derived from Residual Oils and Their Thermally Treated Pitch. ACS Omega 2020, 5, 24412–24421. [Google Scholar] [CrossRef]
- Akmaz, S.; Gurkaynak, M.A.; Yasar, M. The Effect of Temperature on the Molecular Structure of Raman Asphaltenes during Pyrolysis. J. Anal. Appl. Pyrolysis 2012, 96, 139–145. [Google Scholar] [CrossRef]
- Gray, M.R.; Chacón-Patiño, M.L.; Rodgers, R.P. Structure–Reactivity Relationships for Petroleum Asphaltenes. Energy Fuels 2022, 36, 4370–4380. [Google Scholar] [CrossRef]
- Neumann, A.; Chacón-Patiño, M.L.; Rodgers, R.P.; Rüger, C.P.; Zimmermann, R. Investigation of Island/Single-Core- and Archipelago/Multicore-Enriched Asphaltenes and Their Solubility Fractions by Thermal Analysis Coupled with High-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2021, 35, 3808–3824. [Google Scholar] [CrossRef]
- Rüger, C.P.; Neumann, A.; Sklorz, M.; Schwemer, T.; Zimmermann, R. Thermal Analysis Coupled to Ultrahigh Resolution Mass Spectrometry with Collision Induced Dissociation for Complex Petroleum Samples: Heavy Oil Composition and Asphaltene Precipitation Effects. Energy Fuels 2017, 31, 13144–13158. [Google Scholar] [CrossRef]
- Liu, D.; Hou, J.; Luan, H.; Pan, J.; Song, Q.; Zheng, R. Coke Yield Prediction Model for Pyrolysis and Oxidation Processes of Low-Asphaltene Heavy Oil. Energy Fuels 2019, 33, 6205–6214. [Google Scholar] [CrossRef]
- Boytsova, A.; Kondrasheva, N.; Ancheyta, J. Thermogravimetric Determination and Pyrolysis Thermodynamic Parameters of Heavy Oils and Asphaltenes. Energy Fuels 2017, 31, 10566–10575. [Google Scholar] [CrossRef]
- Poutsma, M.L. Fundamental Reactions of Free Radicals Relevant to Pyrolysis Reactions. J. Anal. Appl. Pyrolysis 2000, 54, 5–35. [Google Scholar] [CrossRef]
- Qiu, N.; Li, H.; Xu, E.; Qin, J.; Zheng, L. Temperature and Time Effects on Free Radical Concentration in Organic Matter: Evidence from Laboratory Pyrolysis Experimental and Geological Samples. Energy Explor. Exploit. 2012, 30, 311–329. [Google Scholar] [CrossRef]
- Peraza, A.; Ruette, F.; Sánchez, M. Modeling Free-Radical Reactions, Produced by Hydrocarbon Cracking, with Asphaltenes. Energy Fuels 2010, 24, 3990–3997. [Google Scholar] [CrossRef]
- Ali, S.I.; Lalji, S.M.; Haneef, J.; Khan, M.A.; Louis, C. Comprehensive Analysis of Asphaltene Stability Predictors under Different Conditions. Pet. Chem. 2021, 61, 446–454. [Google Scholar] [CrossRef]
- Félix, G.; Ancheyta, J. Regular Solution Model to Predict the Asphaltenes Flocculation and Sediments Formation during Hydrocracking of Heavy Oil. Fuel 2020, 260, 116160. [Google Scholar] [CrossRef]
- Morantes, L.R.; Percebom, A.M.; Mejía-Ospino, E. On the Molecular Basis of Aggregation and Stability of Colombian Asphaltenes and Their Subfractions. Fuel 2019, 241, 542–549. [Google Scholar] [CrossRef]
- Chen, Z.; Li, R.; Li, H. A New Vapor-Liquid-Asphaltene Three-Phase Equilibrium Computation Algorithm Based on the Free-Asphaltene Assumption. Fluid Phase Equilibria 2022, 556, 113392. [Google Scholar] [CrossRef]
- Duran, J.A.; Casas, Y.A.; Xiang, L.; Zhang, L.; Zeng, H.; Yarranton, H.W. Nature of Asphaltene Aggregates. Energy Fuels 2019, 33, 3694–3710. [Google Scholar] [CrossRef]
- Spiecker, P.M.; Gawrys, K.L.; Kilpatrick, P.K. Aggregation and Solubility Behavior of Asphaltenes and Their Subfractions. J. Colloid Interface Sci. 2003, 267, 178–193. [Google Scholar] [CrossRef]
- Ratz, S.; Savage, P.E.; Tan, L. Pyrolysis Kinetics for Long-Chain n-Alkylcyclohexanes. Ind. Eng. Chem. Res. 2001, 40, 1805–1810. [Google Scholar] [CrossRef]
- Towfighi, J.; Sadrameli, M.; Niaei, A. Coke Formation Mechanisms and Coke Inhibiting Methods in Pyrolysis Furnaces. J. Chem. Eng. Jpn. 2002, 35, 923–937. [Google Scholar] [CrossRef]
- Varga, L.; Szabó, B.; Zsély, I.G.; Zempléni, A.; Turányi, T. Numerical Investigation of the Uncertainty of Arrhenius Parameters. J. Math. Chem. 2011, 49, 1798–1809. [Google Scholar] [CrossRef]
- Félix, G.; Djimasbe, R.; Tirado, A.; Varfolomeev, M.A.; Ancheyta, J. Evaluation of the Reaction Order and Kinetic Modeling of Domanic Oil Shale Upgrading at Supercritical Water Conditions. J. Supercrit. Fluids 2025, 215, 106418. [Google Scholar] [CrossRef]
- Akmaz, S.; Yasar, M. The Temporal Variation of Asphaltene Structure during Raman Crude Oil Pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 672–679. [Google Scholar] [CrossRef]
- Zachariah, A.; Wang, L.; Yang, S.; Prasad, V.; De Klerk, A. Suppression of Coke Formation during Bitumen Pyrolysis. Energy Fuels 2013, 27, 3061–3070. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Wang, R.; He, W.; Shi, L.; Guo, X.; Chen, Z.; Ji, L.; Liu, Z. Coke Formation during Thermal Reaction of Tar from Pyrolysis of a Subbituminous Coal. Fuel Process. Technol. 2017, 155, 68–73. [Google Scholar] [CrossRef]
- Qiu, N.; Li, H.; Jin, Z.; Zhu, Y. Temperature and Time Effect on the Concentrations of Free Radicals in Coal: Evidence from Laboratory Pyrolysis Experiments. Int. J. Coal Geol. 2007, 69, 220–228. [Google Scholar] [CrossRef]
- Trejo, F.; Rana, M.S.; Ancheyta, J. Thermogravimetric Determination of Coke from Asphaltenes, Resins and Sediments and Coking Kinetics of Heavy Crude Asphaltenes. Catal. Today 2010, 150, 272–278. [Google Scholar] [CrossRef]
- Law, J.C.; Headen, T.F.; Jiménez-Serratos, G.; Boek, E.S.; Murgich, J.; Müller, E.A. Catalogue of Plausible Molecular Models for the Molecular Dynamics of Asphaltenes and Resins Obtained from Quantitative Molecular Representation. Energy Fuels 2019, 33, 9779–9795. [Google Scholar] [CrossRef]
- Ungerer, P.; Rigby, D.; Leblanc, B.; Yiannourakou, M. Sensitivity of the Aggregation Behaviour of Asphaltenes to Molecular Weight and Structure Using Molecular Dynamics. Mol. Simul. 2014, 40, 115–122. [Google Scholar] [CrossRef]
- Jiménez-Serratos, G.; Totton, T.S.; Jackson, G.; Müller, E.A. Aggregation Behavior of Model Asphaltenes Revealed from Large-Scale Coarse-Grained Molecular Simulations. J. Phys. Chem. B 2019, 123, 2380–2396. [Google Scholar] [CrossRef]
- Greenfield, M.L. Molecular Modelling and Simulation of Asphaltenes and Bituminous Materials. Int. J. Pavement Eng. 2011, 12, 325–341. [Google Scholar] [CrossRef]
- Afanasjeva, N.; González-Córdoba, A.; Palencia, M. Mechanistic Approach to Thermal Production of New Materials from Asphaltenes of Castilla Crude Oil. Processes 2020, 8, 1644. [Google Scholar] [CrossRef]
- Guida, P.; Colombo, E.; Colleoni, E.; Saxena, S.; Frassoldati, A.; Roberts, W.L.; Faravelli, T. Chemical Kinetics of Asphaltene Pyrolysis. Energy Fuels 2021, 35, 8672–8684. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, G.; Zhang, Q.; Gao, C.; Ren, A.; Duan, M.; Gao, J. Seven-Lump Kinetic Model for Non-Catalytic Hydrogenation of Asphaltene. Energy Fuels 2017, 31, 5037–5045. [Google Scholar] [CrossRef]
- Rhim, J.W.; Nunes, R.V.; Jones, V.A.; Swartzel, K.R. Determination of Kinetic Parameters Using Linearly Increasing Temperature. J. Food Sci. 1989, 54, 446–450. [Google Scholar] [CrossRef]
- Browning, B.; Pitault, I.; Couenne, F.; Jansen, T.; Lacroix, M.; Alvarez, P.; Tayakout-Fayolle, M. Distributed Lump Kinetic Modeling for Slurry Phase Vacuum Residue Hydroconversion. Chem. Eng. J. 2019, 377, 119811. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Varfolomeev, M.A.; Yuan, C.; Ancheyta, J. Characteristic Curves Correlations to Predict SARA Composition and Gas Yields during Heavy Crude Oil Non-Catalytic Aquathermolysis. Chem. Eng. Commun. 2022, 210, 1865–1872. [Google Scholar] [CrossRef]


| Parameter | Temperature (°C) | Ea (kcal/mol) | A (1/min) | ||||
|---|---|---|---|---|---|---|---|
| 330 | 360 | 390 | 420 | 450 | |||
| Method 1 | |||||||
| k1 | 3.4112 × 10−4 ± 1.22 × 10−5 | 1.0004 × 10−3 ± 5.34 × 10−4 | 1.8978 × 10−1 ± 1.7081 | 2.7366 × 10−1 ± 1.7158 | 4.7483 × 10−1 ± 2.77 × 10−3 | 58.69 | 6.4007 × 1017 |
| k2 | 1.7083 × 10−9 ± 2.66 × 10−9 | 5.2253 × 10−3 ± 1.44 × 10−4 | 4.2844 × 10−1 ± 2.2499 | 9.7593 × 10−1 ± 2.4467 | 1.8766 ± 2.77 × 10−3 | 139.45 | 8.2484 × 1043 |
| k3 | 1.0257 × 10−3 ± 3.34 × 10−5 | 2.1566 × 10−3 ± 1.11 × 10−3 | 6.5875 × 10−2 ± 1.8688 | 4.0989 × 10−1 ± 2.1882 | 1.1606 ± 2.77 × 10−3 | 55.80 | 1.0823 × 1017 |
| k4 | 2.0747 × 10−4 ± 5.93 × 10−5 | 5.5793 × 10−4 ± 4.19 × 10−3 | 1.1112 × 10−3 ± 5.69 × 10−1 | 1.4700 × 10−3 ± 5.89 × 10−1 | 3.3770 × 10−3 ± 3.75 × 10−4 | 18.99 | 1.7564 × 103 |
| k5 | 1.5921 × 10−7 ± 5.93 × 10−5 | 4.7623 × 10−7 ± 7.29 × 10−3 | 9.5244 × 10−7 ± 4.92 × 10−1 | 1.8098 × 10−6 ± 3.74 × 10−1 | 3.5834 × 10−6 ± 5.19 × 10−6 | 21.93 | 1.5552 × 101 |
| AAE (%) | 4.25 × 10−2 | 4.96 × 10−4 | 29.45 | 25.89 | 4.88 × 10−2 | ||
| Method 2 | |||||||
| k1 | 3.1054 × 10−4 | 2.0277 × 10−3 | 1.1173 × 10−2 | 5.3110 × 10−2 | 2.2183 × 10−1 | 47.46 ± 9.47 × 105 | 4.9023 × 1013 ± 8.79 × 105 |
| k2 | 1.6630 × 10−9 | 5.5867 × 10−7 | 1.1088 × 10−4 | 1.3921 × 10−2 | 1.1704 | 147.15 ± 9.45 × 105 | 3.4505 × 1044 ± 9.49 × 105 |
| k3 | 1.7331 × 10−4 | 1.9270 × 10−3 | 1.7230 × 10−2 | 1.2745 × 10−1 | 7.9855 × 10−1 | 60.93 ± 8.52 × 105 | 2.0691 × 1018 ± 9.49 × 105 |
| k4 | 5.2900 × 10−69 | 1.3030 × 10−65 | 1.5835 × 10−62 | 1.0405 × 10−59 | 3.9912 × 10−57 | 197.54 ± 6.03 × 105 | 2.0005 × 103 ± 9.47 × 105 |
| k5 | 6.3042 × 10−8 | 1.6988 × 10−7 | 4.1849 × 10−7 | 9.5356 × 10−7 | 2.0292 × 10−6 | 25.08 ± 9.28 × 105 | 7.6812 × 101 ± 9.45 × 105 |
| AAE (%) | 23.52 | 68.42 | 128.75 | 105.14 | 6.77 × 10−2 | ||
| Method 3 | |||||||
| k1 | 3.7627 × 10−4 | 3.8251 × 10−3 | 3.1526 × 10−2 | 2.1647 × 10−1 | 1.2668 | 58.66 ± 1.16 × 107 | 6.7805 × 1017 ± 1.25 × 107 |
| k2 | 3.8453 × 10−7 | 9.5316 × 10−5 | 1.4348 × 10−2 | 1.3993 | 9.3321 × 101 | 139.45 ± 1.24 × 107 | 1.3042 × 1044 ± 1.15 × 107 |
| k3 | 6.4025 × 10−4 | 5.8169 × 10−3 | 4.3284 × 10−2 | 2.7071 × 10−1 | 1.4542 | 55.82 ± 1.20 × 107 | 1.0769 × 1017 ± 1.13 × 107 |
| k4 | 1.8256 × 10−2 | 3.1931 × 10−2 | 5.3095 × 10−2 | 8.4486 × 10−2 | 1.2935 × 10−1 | 14.14 ± 9.83 × 106 | 2.4321 × 103 ± 1.25 × 107 |
| k5 | 1.7225 × 10−7 | 4.0969 × 10−7 | 9.0092 × 10−7 | 1.8505 × 10−6 | 3.5807 × 10−6 | 21.92 ± 1.25 × 107 | 1.5052 × 101 ± 1.23 × 107 |
| AAE (%) | 123.02 | 84.46 | 227.15 | 45.07 | 37.03 |
| Parameter | Temperature (°C) | Ea (kcal/mol) | A (1/min) | ||||
|---|---|---|---|---|---|---|---|
| 330 | 360 | 390 | 420 | 450 | |||
| Method 1 | |||||||
| k1 | 3.3903 × 10−4 ± 2.20 × 10−7 | 1.0004 × 10−3 ± 1.01 × 10−5 | 3.1691 × 10−2 ± 5.01 × 10−4 | 1.1432 × 10−1 ± 8.61 × 10−4 | 2.2679 ± 0.7202 | 64.26 | 3.5839 × 1019 |
| k2 | 4.6196 × 10−9 ± 1.36 × 10−9 | 5.2253 × 10−3 ± 4.94 × 10−7 | 2.5741 × 10−2 ± 1.45 × 10−4 | 7.9198 × 10−2 ± 3.29 × 10−4 | 9.2485 × 10−1 ± 0.4162 | 121.27 | 2.9042 × 1037 |
| k3 | 1.0263 × 10−3 ± 1.55 × 10−7 | 2.1566 × 10−3 ± 7.10 × 10−6 | 4.1427 × 10−3 ± 3.63 × 10−4 | 2.5740 × 10−2 ± 7.91 × 10−4 | 1.9433 × 10−1 ± 0.6680 | 36.92 | 1.4069 × 1010 |
| k4 | 1.0059 × 10−5 ± 1.47 × 10−5 | 5.5793 × 10−4 ± 1.95 × 10−4 | 1.6102 × 10−3 ± 3.58 × 10−4 | 3.7999 × 10−3 ± 2.27 × 10−4 | 9.9379 × 10−3 ± 0.0117 | 46.16 | 1.5204 × 1012 |
| k5 | 1.6063 × 10−7 ± 1.48 × 10−6 | 4.7623 × 10−7 ± 1.95 × 10−4 | 1.8464 × 10−2 ± 3.17 × 10−4 | 2.8000 × 10−2 ± 2.01 × 10−4 | 3.7722 × 10−2 ± 4.1 × 10−5 | 104.48 | 1.0204 × 1031 |
| SSE | 4.20 × 10−9 | 8.92 × 10−8 | 3.41 × 10−5 | 1.37 × 10−7 | 1.71 × 10−2 | ||
| Method 2 | |||||||
| k1 | 6.4818 × 10−6 | 1.3261 × 10−3 | 1.6763 × 10−1 | 1.3938 | 8.0310 × 102 | 134.60 ± 16.11 | 3.8246 × 1043 ± 13.33 |
| k2 | 5.0881 × 10−4 | 5.1424 × 10−3 | 4.2158 × 10−2 | 2.8807 × 10−1 | 1.6783 | 58.51 ± 12.37 | 8.1056 × 1017 ± 7.47 |
| k3 | 1.0456 × 10−3 | 1.8830 × 10−3 | 3.2154 × 10−3 | 5.2420 × 10−3 | 8.2064 × 10−3 | 14.88 ± 10.37 | 2.5803 × 102 ± 5.32 |
| k4 | 8.9630 × 10−4 | 1.8651 × 10−3 | 3.6320 × 10−3 | 6.6763 × 10−3 | 1.1668 × 10−2 | 18.54 ± 8.18 | 4.6660 × 103 ± 18.11 |
| k5 | 1.8023 × 10−4 | 3.8287 × 10−3 | 6.1687 × 10−2 | 7.8134 × 10−1 | 8.0168 | 77.31 ± 16.47 | 1.8487 × 1024 ± 13.84 |
| SSE | 13.76 | 2.16 | 10.58 | 10.52 | 2.32 | ||
| Method 3 | |||||||
| k1 | 3.5754 × 10−3 | 4.2966 × 10−2 | 4.1231 × 10−1 | 3.2532 | 2.1626 × 101 | 62.89 ± 30.33 | 2.2002 × 1020 ± 28.87 |
| k2 | 1.7249 × 10−2 | 8.3937 × 10−2 | 3.5397 × 10−1 | 1.3179 | 4.3995 | 40.03 ± 23.83 | 5.4923 × 1012 ± 38.03 |
| k3 | 5.5058 × 10−3 | 9.1141 × 10−3 | 1.4415 × 10−2 | 2.1911 × 10−2 | 3.2168 × 10−2 | 12.75 ± 11.82 | 2.2938 × 102 ± 27.74 |
| k4 | 1.7000 × 10−2 | 3.2677 × 10−2 | 5.9206 × 10−2 | 1.0189 × 10−1 | 1.6763 × 10−1 | 16.53 ± 9.52 | 1.6598 × 104 ± 2.28 × 103 |
| k5 | 1.4490 × 10−13 | 1.5655 × 10−12 | 1.3637 × 10−11 | 9.8492 × 10−11 | 6.0374 × 10−10 | 60.20 ± 32.91 | 9.4362 × 108 ± 2.28 × 103 |
| SSE | 43.46 | 23.23 | 23.83 | 14.65 | 11.15 |
| Parameter | Temperature (°C) | Ea (kcal/mol) | A (1/min) | ||||
|---|---|---|---|---|---|---|---|
| 330 | 360 | 390 | 420 | 450 | |||
| k1 | 3.8296 × 10−5 | 4.2787 × 10−4 | 7.4244 × 10−3 | 1.0826 × 10−2 | 2.0579 × 10−1 | 59.07 | 1.0797 × 1017 |
| k2 | 2.2709 × 10−4 | 4.2123 × 10−4 | 2.8826 × 10−3 | 3.6453 × 10−3 | 1.4074 × 10−2 | 30.06 | 1.5027 × 107 |
| k3 | 7.9086 × 10−5 | 3.9068 × 10−4 | 4.1168 × 10−3 | 4.9373 × 10−3 | 5.6074 × 10−2 | 45.26 | 1.9335 × 1012 |
| k4 | 1.0212 × 10−3 | 2.1450 × 10−3 | 3.3553 × 10−3 | 1.0267 × 10−2 | 7.1234 × 10−2 | 28.61 | 1.6386 × 107 |
| k5 | - | 5.0019 × 10−3 | 1.4646 × 10−2 | 2.0360 × 10−2 | 4.3937 × 10−2 | 20.83 | 8.5888 × 104 |
| k6 | 8.7414 × 10−3 | 1.6010 × 10−2 | 3.0034 × 10−2 | 3.8788 × 10−2 | 8.6293 × 10−2 | 15.76 | 4.4239 × 103 |
| k7 | 2.1670 × 10−3 | 4.7991 × 10−3 | 9.4150 × 10−3 | 1.4715 × 10−2 | 3.0506 × 10−2 | 18.54 | 1.1564 × 104 |
| k8 | 4.5511 × 10−4 | 8.1044 × 10−4 | 1.6032 × 10−3 | 3.1944 × 10−3 | 6.3134 × 10−3 | 19.10 | 3.4302 × 103 |
| k9 | 1.4851 × 10−4 | 3.7298 × 10−4 | 7.4492 × 10−4 | 1.4927 × 10−3 | 2.9450 × 10−3 | 21.30 | 7.9311 × 103 |
| k10 | - | 3.8407 × 10−2 | 5.8946 × 10−2 | 8.9544 × 10−2 | 1.3103 × 10−1 | 12.43 | 7.4525 × 102 |
| k11 | 3.3203 × 10−2 | 5.8302 × 10−2 | 1.2149 × 10−1 | 2.0849 × 10−1 | 3.6737 × 10−1 | 17.57 | 7.3082 × 104 |
| k12 | 5.1758 × 10−4 | 7.3796 × 10−4 | 1.4569 × 10−3 | 3.2757 × 10−3 | 6.2550 × 10−3 | 18.58 | 2.2863 × 103 |
| k13 | 1.0549 × 10−3 | 1.6628 × 10−3 | 3.3985 × 10−3 | 1.9162 × 10−2 | 4.0538 × 10−2 | 27.88 | 9.1141 × 106 |
| k14 | 4.8665 × 10−5 | 1.7741 × 10−4 | 3.5495 × 10−4 | 7.3372 × 10−4 | 1.4308 × 10−3 | 23.76 | 2.3174 × 104 |
| k15 | 4.7533 × 10−4 | 8.9982 × 10−4 | 1.7477 × 10−3 | 6.5295 × 10−3 | 1.1447 × 10−2 | 24.00 | 1.9550 × 105 |
| k16 | 7.1213 × 10−3 | 2.1830 × 10−2 | 4.2320 × 10−2 | 1.1101 × 10−1 | 2.7702 × 10−1 | 25.83 | 1.6146 × 107 |
| SSE | 2.22 × 10−8 | 5.59 × 10−8 | 4.56 × 10−5 | 6.16 × 10−7 | 5.56 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Félix, G.; Tirado, A.; Varfolomeev, M.A.; Lugo-Medina, E.; Soto-Robles, C.A.; Ancheyta, J. Study on the Isolated Asphaltene Thermal Cracking from an Unconventional Oil Using Diverse Estimating Arrhenius Parameter Approaches. Molecules 2025, 30, 4468. https://doi.org/10.3390/molecules30224468
Félix G, Tirado A, Varfolomeev MA, Lugo-Medina E, Soto-Robles CA, Ancheyta J. Study on the Isolated Asphaltene Thermal Cracking from an Unconventional Oil Using Diverse Estimating Arrhenius Parameter Approaches. Molecules. 2025; 30(22):4468. https://doi.org/10.3390/molecules30224468
Chicago/Turabian StyleFélix, Guillermo, Alexis Tirado, Mikhail A. Varfolomeev, Eder Lugo-Medina, Carlos A. Soto-Robles, and Jorge Ancheyta. 2025. "Study on the Isolated Asphaltene Thermal Cracking from an Unconventional Oil Using Diverse Estimating Arrhenius Parameter Approaches" Molecules 30, no. 22: 4468. https://doi.org/10.3390/molecules30224468
APA StyleFélix, G., Tirado, A., Varfolomeev, M. A., Lugo-Medina, E., Soto-Robles, C. A., & Ancheyta, J. (2025). Study on the Isolated Asphaltene Thermal Cracking from an Unconventional Oil Using Diverse Estimating Arrhenius Parameter Approaches. Molecules, 30(22), 4468. https://doi.org/10.3390/molecules30224468










