Co(II)-Catalyzed Picolinamide-Directed C(sp3)-S Bond Formation with N-(phenylsulfanyl)succinimides
Abstract
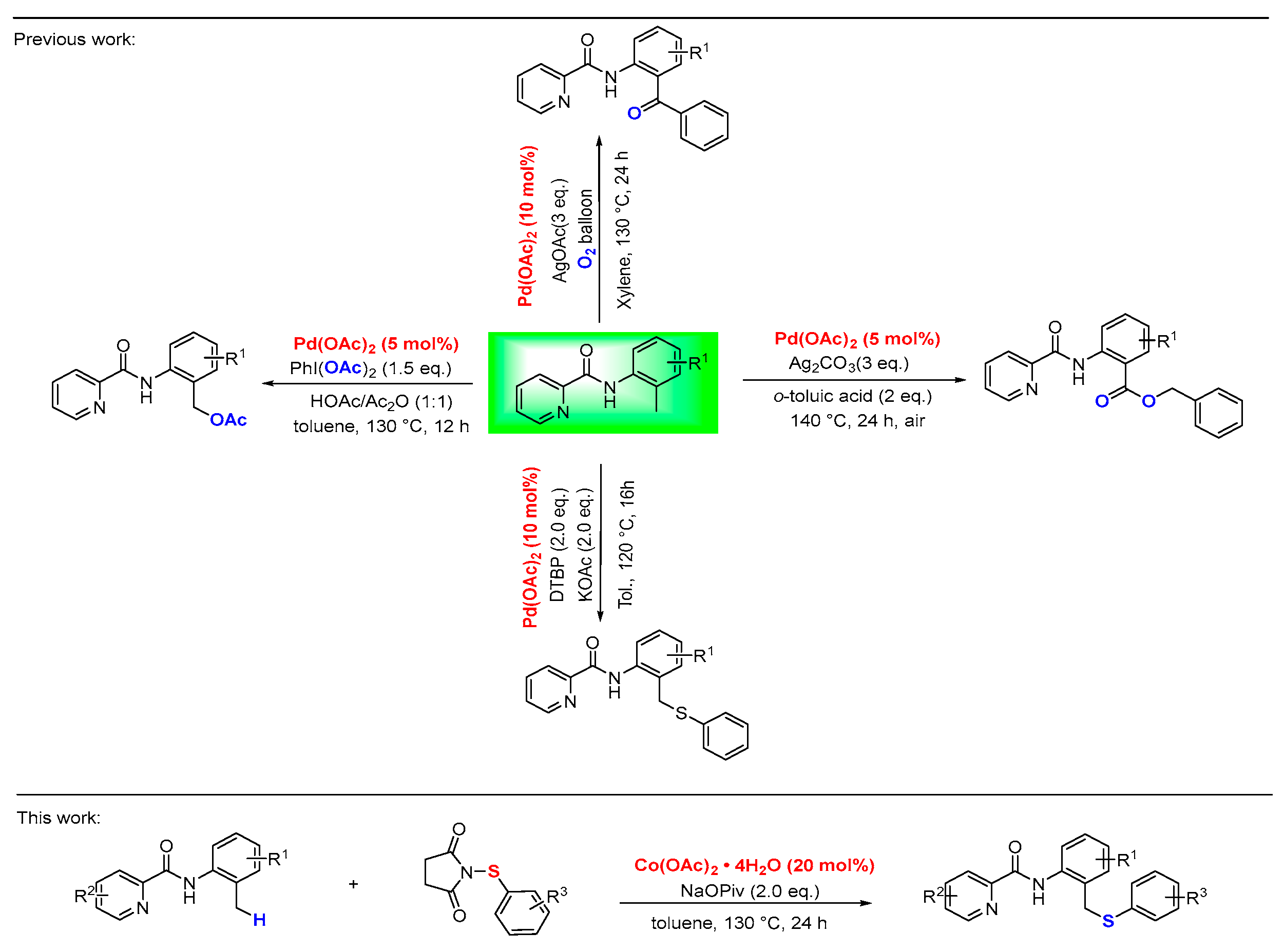
1. Introduction
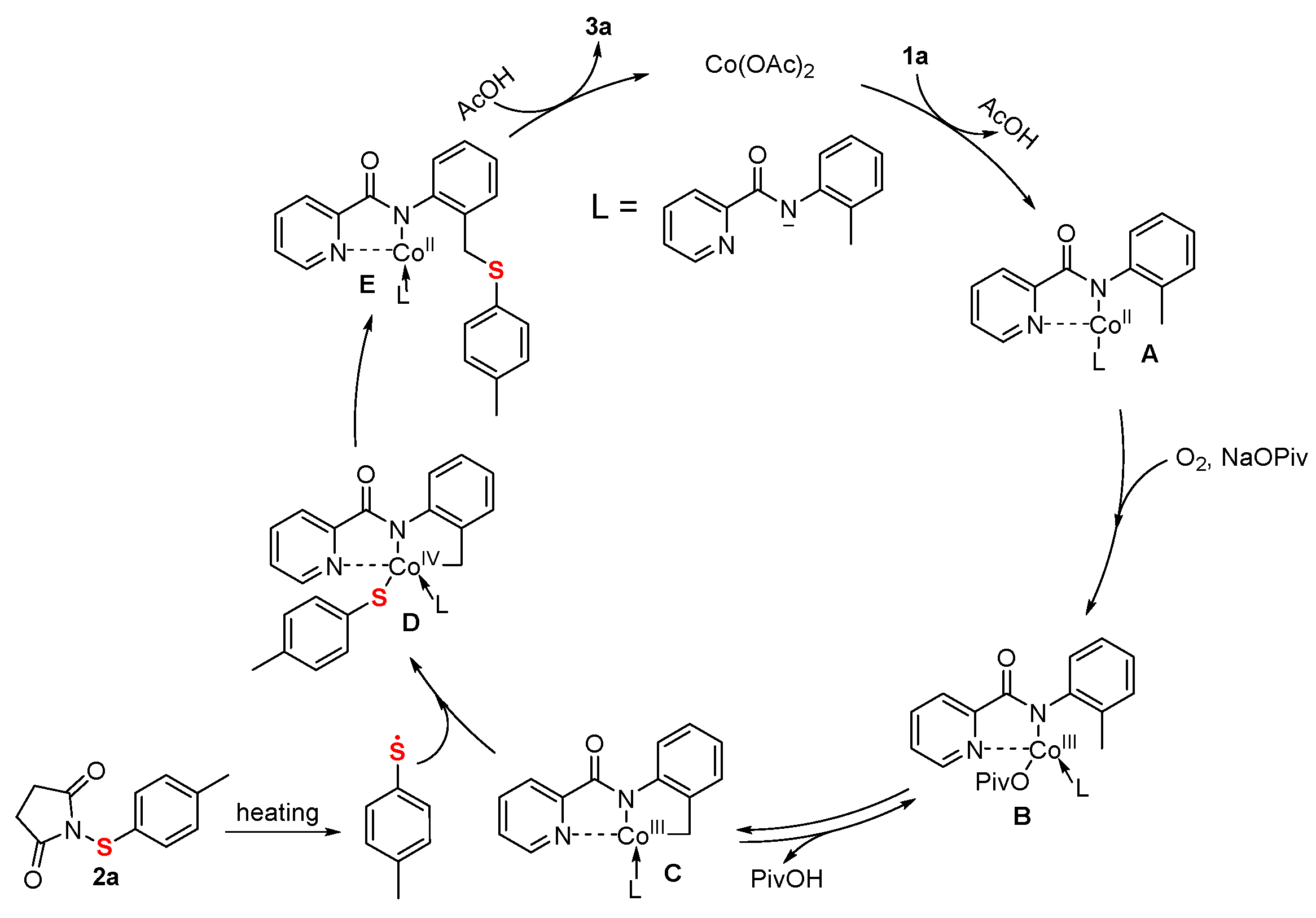
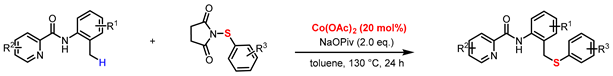
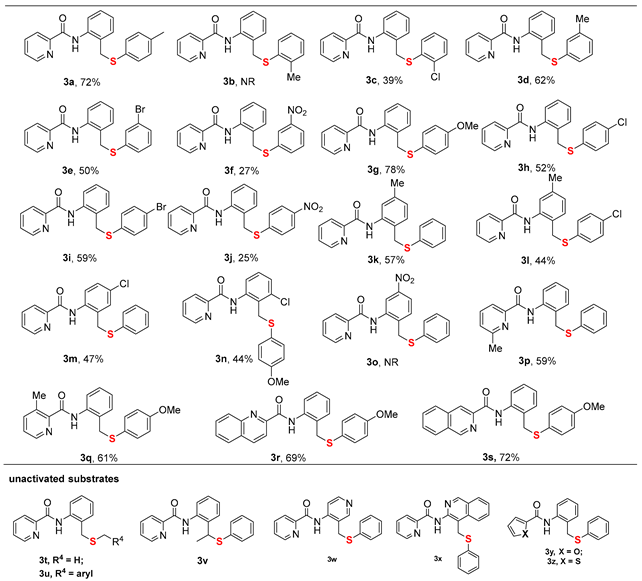
2. Results and Discussion
3. Conclusions
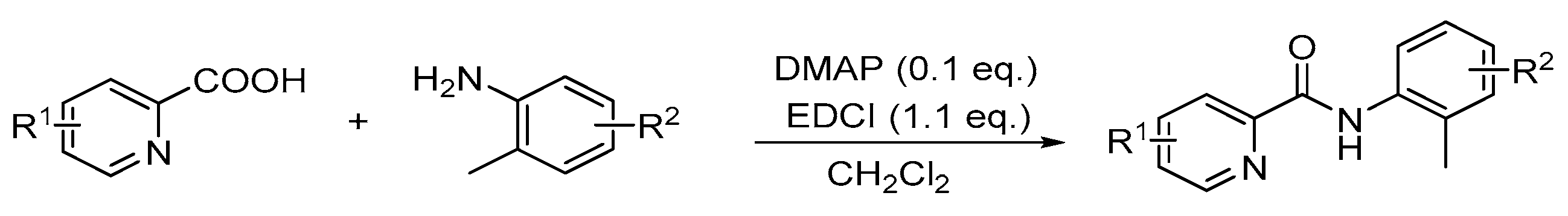
4. Experimental Section

- Preparation of N-aryl/alkylthiosuccinimide (2) [34]
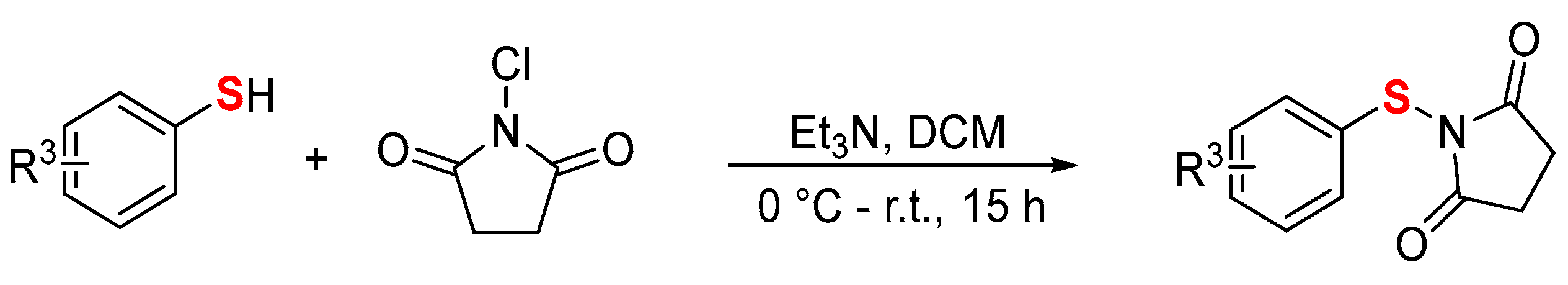
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.Y.; Qin, H.L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.; Mano, R.A.; Oliveira, D.H.; Maia, D.S.V.; Silva, W.P.; Savegnago, L.; Lenardão, E.J.; Jacob, R.G. Synthesis, antimicrobial, and antioxidant activities of chalcogen-containing nitrone derivatives from (R)-citronellal. Medicines 2017, 4, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Amlashi, D.M.; Mobini, S.; Shahedi, M.; Habibi, Z.; Bavandi, H.; Yousef, M. Biocatalytic synthesis of oxa(thia)diazole aryl thioethers. Sci. Rep. 2024, 14, 19468. [Google Scholar]
- Wang, F.; Langley, R.; Gulten, G.L.; Dover, G.; Besra, G.S.; Jacobs, W.R.; Sacchettini, J.C. Mechanism of thioamide drug action against tuberculosis and leprosy. J. Exp. Med. 2007, 204, 73–78. [Google Scholar] [CrossRef]
- Thomas, G.L.; Spandl, R.J.; Glansdorp, F.G.; Welch, M.; Bender, A.; Cockfield, J.; Lindsay, J.A.; Bryant, C.; Brown, D.F.J.; Loiseleur, O.; et al. Anti-MRSA agent discovery using diversity-oriented synthesis. Angew. Chem. Int. Ed. 2008, 47, 2808–2812. [Google Scholar] [CrossRef]
- Woo, C.M.; Gholap, S.L.; Herzon, S.B. Insights into lomaiviticin biosynthesis. isolation and structure elucidation of (-)-homoseongomycin. J. Nat. Prod. 2013, 76, 1238–1241. [Google Scholar] [CrossRef]
- Ma, N.N.; Hu, X.B.; Wu, Y.S.; Zheng, Y.W.; Ma, M.T.; Chu, X.Q.; Xu, H.; Luo, H.Q.; Shen, Z.L. Nickel-catalyzed direct cross-coupling of aryl thioether with aryl bromide. Org. Lett. 2023, 25, 1771–1775. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Luz, E.Q.; Seckler, D.; Araújo, J.S.; Angst, L.; Lima, D.B.; Rios, E.A.M.; Ribeiro, R.R.; Rampona, D.S. Fe(III)-catalyzed direct C3 chalcogenylation of indole: The effect of iodide ions. Tetrahedron 2019, 75, 1258–1266. [Google Scholar] [CrossRef]
- Nalbandian, C.J.; Miller, E.M.; Toenjes, S.T.; Gustafson, J.L. A conjugate lewis base-brønsted acid catalyst for the sulfenylation of nitrogen containing heterocycles under mild conditions. Chem. Commun. 2017, 53, 1494–1497. [Google Scholar] [CrossRef]
- Schlosser, K.M.; Krasutsky, A.P.; Hamilton, H.W.; Reed, J.E.; Sexton, K. A highly efficient procedure for 3-sulfenylation of ondole-2-carboxylates. Org. Lett. 2004, 6, 819–821. [Google Scholar] [CrossRef]
- Tang, Q.; Song, D.G.; Zhang, K.L.; Mao, W.H.; Zhao, X.H.; Du, D.; Ling, F.; Zhong, W.H. Development of an imidazole-based N,N-bidentate ligand for the manganese catalyzed direct coupling of nitriles with alcohols. RSC Adv. 2024, 14, 12978–12982. [Google Scholar] [CrossRef]
- Li, M.Y.; Eisen, M.S.; Cai, Z.G. Robust cobalt catalysts with N,N-bidentate aldimine imidazolidine-2-imine/guanidine ancillary ligand for isoprene polymerization. J. Catal. 2024, 435, 115581. [Google Scholar] [CrossRef]
- Wu, W.R.; Zhao, X.F.; Chen, G.; Liu, L.J.; Li, Y.L.; Chen, T.; James, T.D.; Liu, Y.X. Overlooked potential of N,N-bidentate directing-groups in Ni-catalyzed C-H functionalization of benzamides. Chem. Commun. 2023, 59, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Pati, B.V.; Sagara, P.S.; Ghosh, A.; Adhikari, G.K.D.; Ravikumar, P.C. Ruthenium-catalyzed regioselective C(sp2)-H activation/annulation of N-(7-Azaindole)amides with 1,3-diynes using N-amino-7-azaindole as the N,N-bidentate directing group. J. Org. Chem. 2021, 86, 9428–9443. [Google Scholar] [CrossRef] [PubMed]
- Bag, R.; Sharma, N.K. Pd-catalyzed picolinamide-directed late-stage chalcogenation of tryptophan-containing peptides. J. Org. Chem. 2023, 88, 15666–15686. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zeng, L.L.; Fei, Q.Y.; Ge, Y.X.; Huang, R.H.; Chen, F.E. Recent advances in remote C(sp3)–H functionalization via chelating group-assisted metal-catalyzed chain-walking reaction. Angew. Chem. Int. Ed. 2019, 58, 5633–5638. [Google Scholar] [CrossRef]
- Bhavyesh, D.; Soliya, S.; Konakanchi, R.; Begari, E.; Ashalu, K.C.; Naveen, T. The recent advances in iron-catalyzed C(sp3)-H functionalization. Chem. Asian J. 2024, 19, e202301056. [Google Scholar] [CrossRef]
- Sheng, T.; Zhuang, Z.; Zhao, Z.H.; Hoque, M.E.; Yu, J.-Q. Copper-catalyzed γ-C(sp3)-H lactamization and iminolactonization. Angew. Chem. 2025, 64, e202416634. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.-Y.; He, G.; Nack, W.A.; Chen, G. Palladium-catalyzed picolinamide-directed acetoxylation of unactivated γ-C(sp3)-H bonds of alkylamines. Chin. Chem. Lett. 2024, 35, 109647. [Google Scholar] [CrossRef]
- Xie, Y.J.; Yang, Y.Z.; Huang, L.H.; Zhang, X.B.; Zhang, Y.H. Pd-catalyzed arylation/oxidation of benzylic C-H bond. Org. Lett. 2012, 14, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Yu, M.; Zhang, J.T.; Liu, Z.X.; Zhang, Y.H. Synthesis of benzyl esters via functionalization of multiple C–H bonds by palladium catalysis. Org. Lett. 2015, 17, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Yao, J.Z.; Wu, Z.H.; Liu, Z.X.; Zhang, Y.H. Palladium-catalyzed oxidative acetoxylation of benzylic C-H bond using bidentate auxiliary. J. Org. Chem. 2013, 78, 10821–10831. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Yin, W.Y.; Zhang, Y.; Zhang, Y.N.; Huang, Y. Palladium catalyzed acetoxylation of benzylic C–H bonds using a bidentate picolinamide directing group. Org. Biomol. Chem. 2014, 12, 1405–1411. [Google Scholar] [CrossRef]
- Wang, K.; Hou, J.H.; Zhang, C.; Cheng, K.; Bai, R.R.; Xie, Y.Y. Palladium-catalyzed picolinamide-directed benzylic C(sp3)-H chalcogenation with diaryl disulfides and diphenyl diselenide. Adv. Synth. Catal. 2020, 362, 2947–2952. [Google Scholar] [CrossRef]
- Yang, T.X.; Zhang, Y.B.; Dou, Y.C.; Yang, D.D.; Niu, J.L. Earth-abundant cobalt-catalyzed enantioselective C-H functionalizations. Sci. China Chem. 2025, 65, 1–21. [Google Scholar] [CrossRef]
- Meher, N.K.; Kumar, P.; Geetharani, V.K. Cobalt-catalyzed regioselective 1,2-hydroboration of N-heteroarenes. Org. Lett. 2023, 25, 87–92. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Homölle, S.L.; Oliveira, J.C.A.; Ackermann, L. Enantioselective cobaltaphotoredox-catalyzed C-H activation. J. Am. Chem. Soc. 2024, 146, 24105–24113. [Google Scholar] [CrossRef]
- Saha, A.; Ramesh, E.; Sahoo, A.K. Brønsted acid promoted sulfenylacyloxylation of alkynes: Access to 4-sulfenylisocoumarins. Adv. Synth. Catal. 2022, 364, 3496–3500. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, J.J. Cobalt-catalyzed direct C-H thiolation of aromatic amides with disulfides: Application to the synthesis of quetiapine. Org. Lett. 2018, 20, 6490–6493. [Google Scholar] [CrossRef]
- Münchow, T.; Pandit, N.K.; Dana, S.; Boos, P.; Peters, S.E.; Boucat, J.; Liu, Y.R.; Scheremetjew, A.; Ackermann, L. Enantioselective C-H annulations enabled by either nickel- or cobalt-electrocatalysed C-H activation for catalyst-controlled chemodivergence. Nat. Catal. 2025, 8, 257–269. [Google Scholar] [CrossRef]
- Lan, J.Y.; Xie, H.S.; Lu, X.X.; Deng, Y.F.; Jiang, H.F.; Zeng, W. Co(II)-catalyzed regioselective cross-dehydrogenative coupling of aryl C–H bonds with carboxylic acids. Org. Lett. 2017, 19, 4279–4282. [Google Scholar] [CrossRef]
- Wu, H.X.; Guo, W.J.; Daniel, S.; Li, Y.; Liu, C.; Zeng, Z. Fluoride-catalyzed esterification of amides. Chem. Eur. J. 2018, 24, 3444–3447. [Google Scholar] [CrossRef]
- Eitzinger, A.; Otevrel, J.; Haider, V.; Macchia, A.; Massa, A.; Faust, K.; Spingler, B.; Berkessel, A.; Waser, M. Enantioselective bifunctional ammonium salt-catalyzed syntheses of 3-CF3S-, 3-RS-, and 3-F-substituted isoindolinones. Adv. Synth. Catal. 2014, 363, 1955–1962. [Google Scholar] [CrossRef]

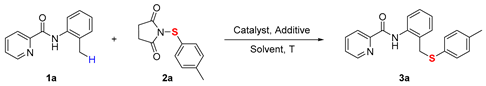 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Additive | Solvent | Yield [%] b |
| 1 | Co(OAc)2·4H2O | KOAc | Toluene | 57 |
| 2 | CoBr2 | KOAc | Toluene | 48 |
| 3 | Co(acac)2 | KOAc | Toluene | trace |
| 4 | NiCl2 | KOAc | Toluene | N.R |
| 5 | CuBr2 | KOAc | Toluene | N.R. |
| 6 | Cu(OAc)2 | KOAc | Toluene | N.R. |
| 7 | Fe(OAc)2 | KOAc | Toluene | trace |
| 8 | - | KOAc | Toluene | N.R. |
| 9 | Co(OAc)2·4H2O | NaOAc | Toluene | 47 |
| 10 | Co(OAc)2·4H2O | Na2CO3 | Toluene | 39 |
| 11 | Co(OAc)2·4H2O | K2CO3 | Toluene | 33 |
| 12 | Co(OAc)2·4H2O | NaOPiv | Toluene | 72 |
| 13 | Co(OAc)2·4H2O | - | Toluene | 42 |
| 14 | Co(OAc)2·4H2O | NaOPiv | p-xylene | 58 |
| 15 | Co(OAc)2·4H2O | NaOPiv | DMSO | 43 |
| 16 | Co(OAc)2·4H2O | NaOPiv | DMF | 48 |
| 17 | Co(OAc)2·4H2O | NaOPiv | Mesitylene | 61 |
| 18 | Co(OAc)2·4H2O | NaOPiv | Toluene | 60 c |
| 19 | Co(OAc)2·4H2O | NaOPiv | Toluene | 13 d |
| 20 | Co(OAc)2·4H2O | NaOPiv | Toluene | 76 e |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Zhou, S.; Luo, J.; Wang, G.; Wang, K. Co(II)-Catalyzed Picolinamide-Directed C(sp3)-S Bond Formation with N-(phenylsulfanyl)succinimides. Molecules 2025, 30, 4462. https://doi.org/10.3390/molecules30224462
Qin J, Zhou S, Luo J, Wang G, Wang K. Co(II)-Catalyzed Picolinamide-Directed C(sp3)-S Bond Formation with N-(phenylsulfanyl)succinimides. Molecules. 2025; 30(22):4462. https://doi.org/10.3390/molecules30224462
Chicago/Turabian StyleQin, Jinjing, Shaodong Zhou, Jinwen Luo, Guodong Wang, and Kai Wang. 2025. "Co(II)-Catalyzed Picolinamide-Directed C(sp3)-S Bond Formation with N-(phenylsulfanyl)succinimides" Molecules 30, no. 22: 4462. https://doi.org/10.3390/molecules30224462
APA StyleQin, J., Zhou, S., Luo, J., Wang, G., & Wang, K. (2025). Co(II)-Catalyzed Picolinamide-Directed C(sp3)-S Bond Formation with N-(phenylsulfanyl)succinimides. Molecules, 30(22), 4462. https://doi.org/10.3390/molecules30224462







