Covalent Docking to the Active Sites of Thiamine Diphosphate-Dependent Enzymes
Abstract
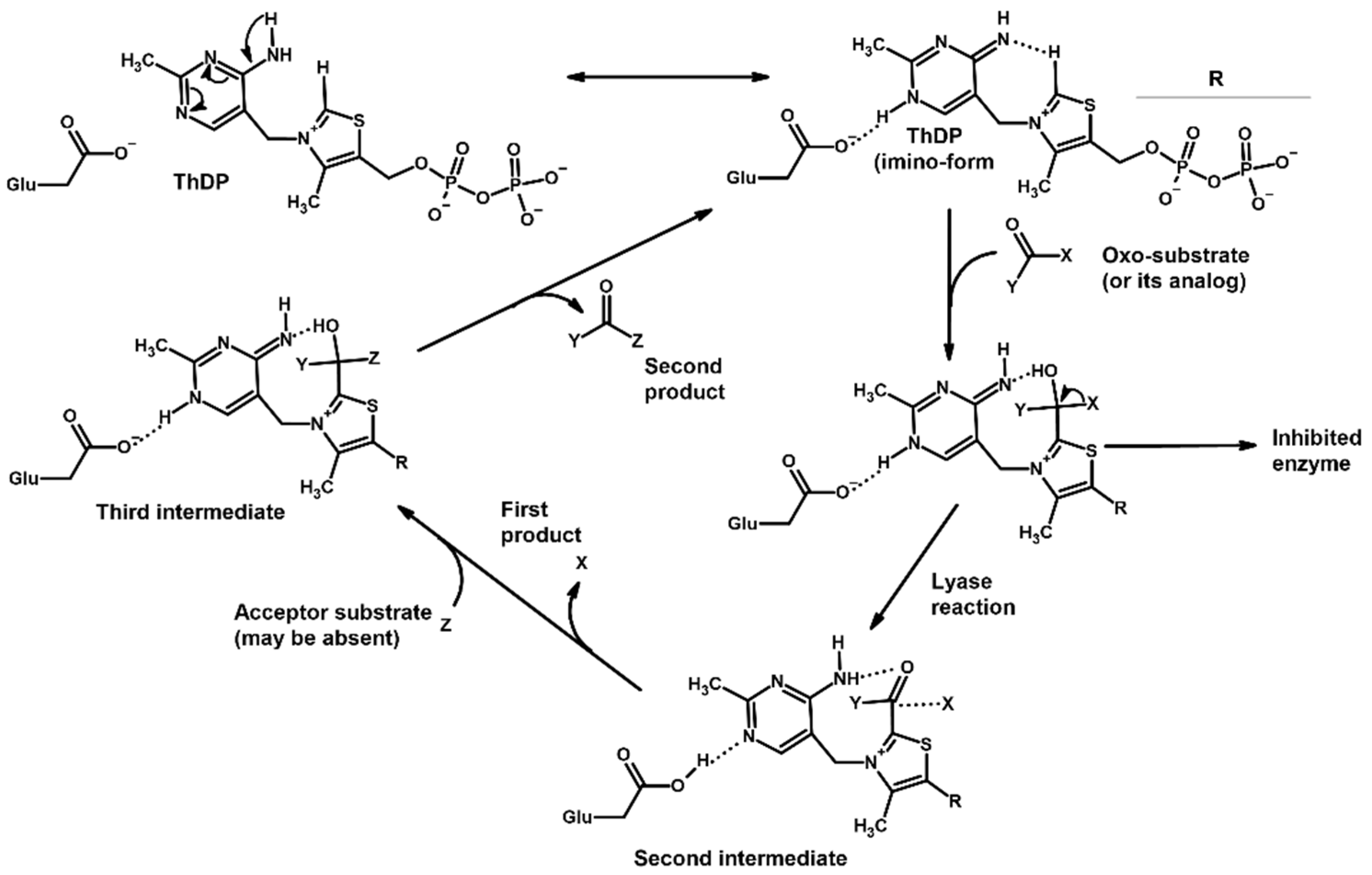
1. Introduction
2. Results
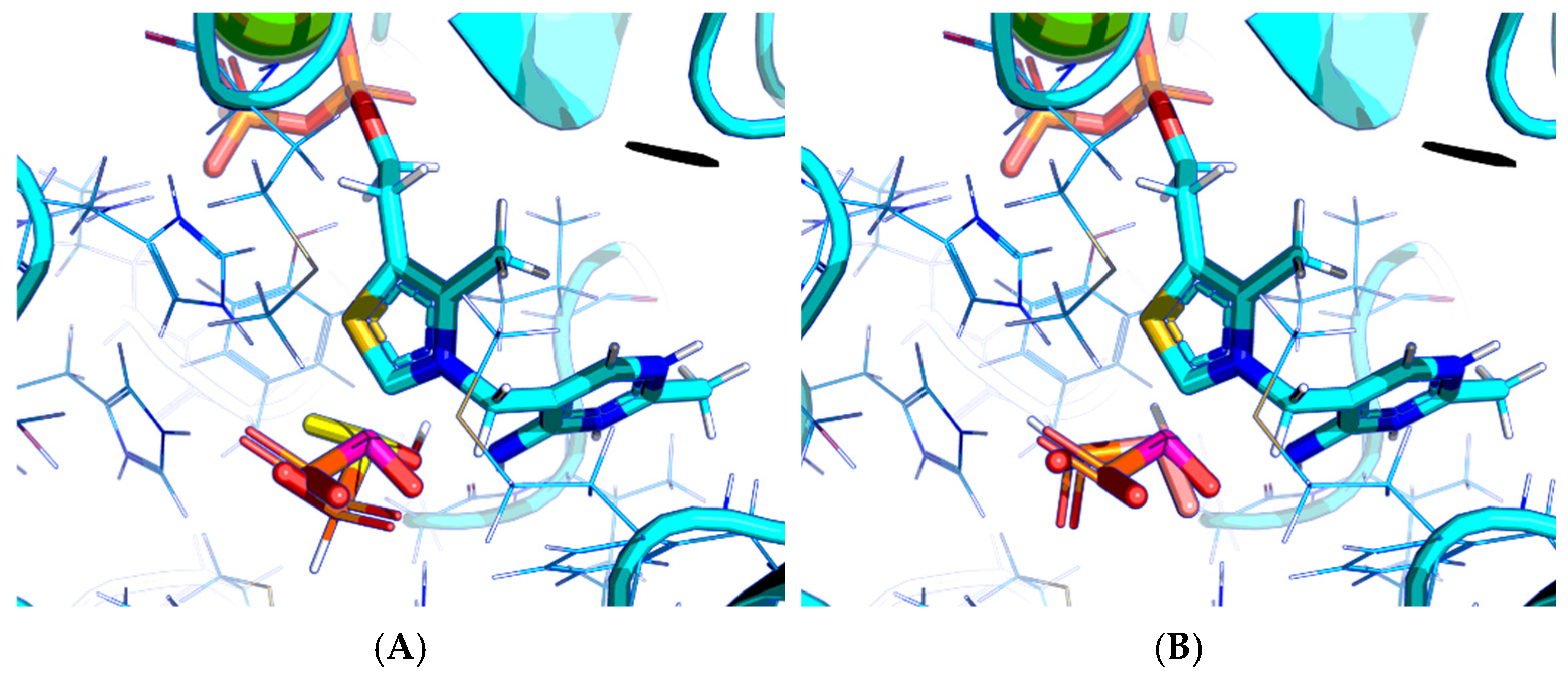
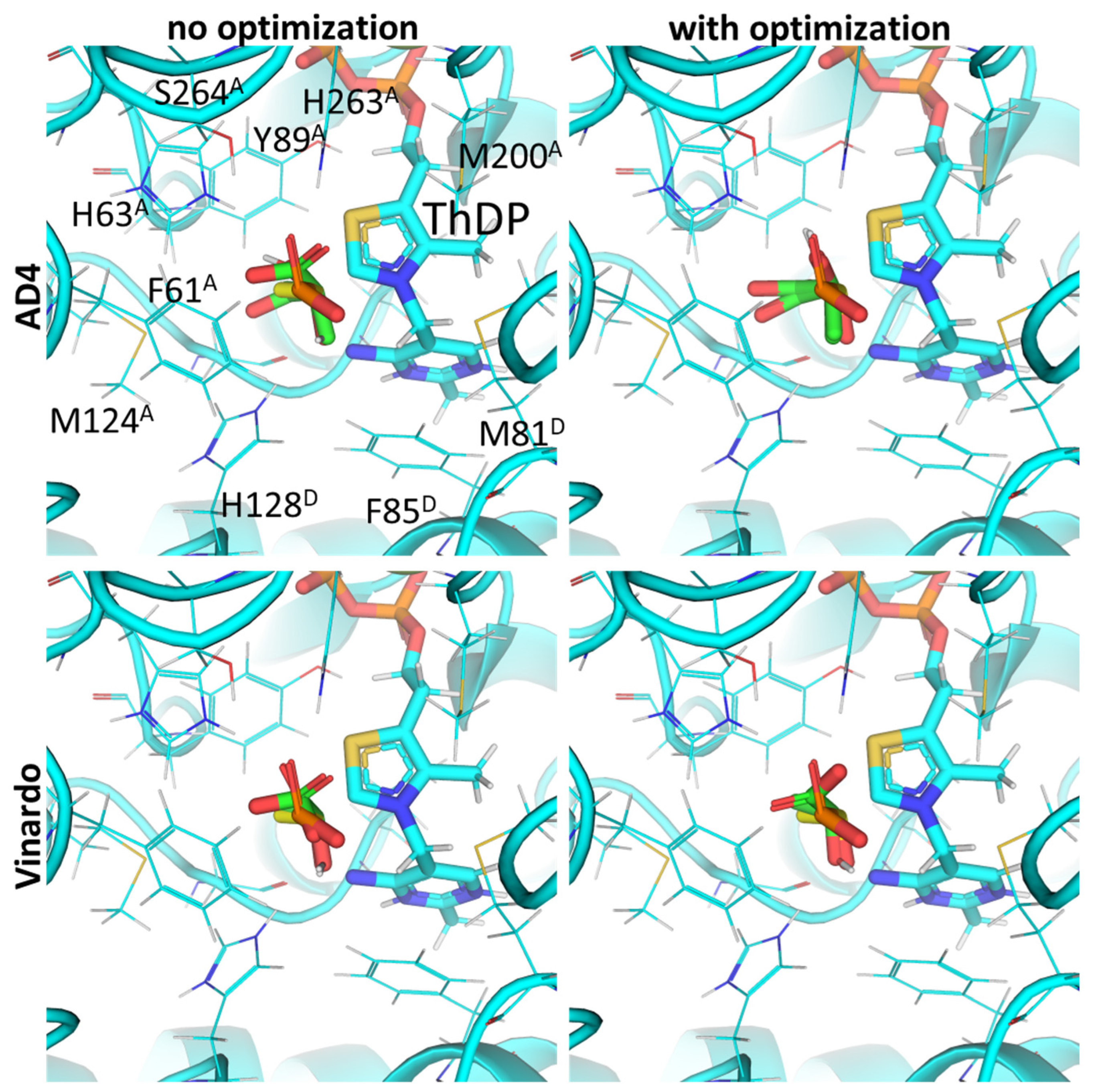
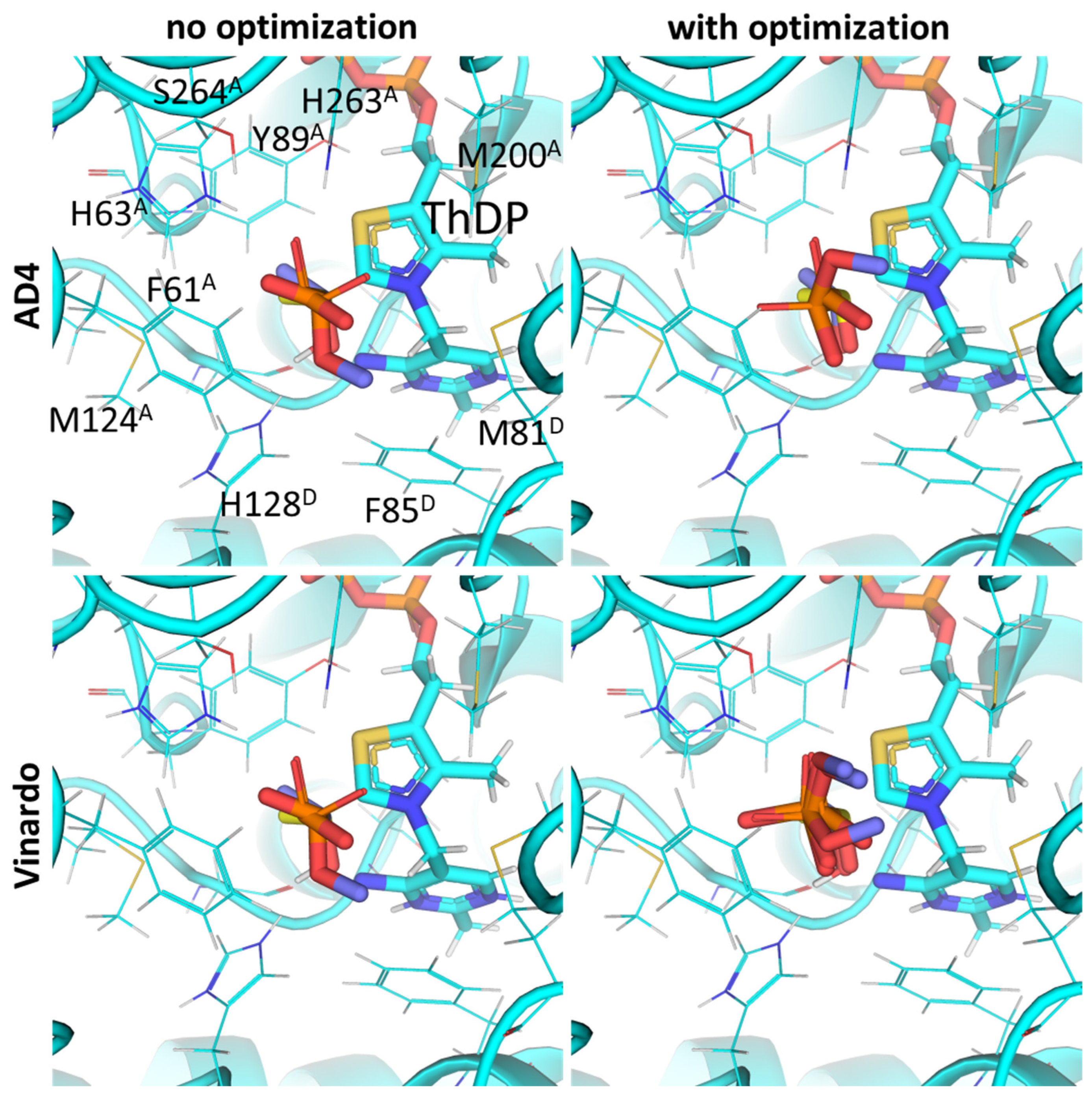
2.1. Using Pyruvate Dehydrogenase in a Complex with Thiamine Diphosphate and Acetyl Phosphinate to Validate the Docking Setup
2.2. Comparison of the Binding Efficiency of the PDHC Ligands In Silico and In Vitro
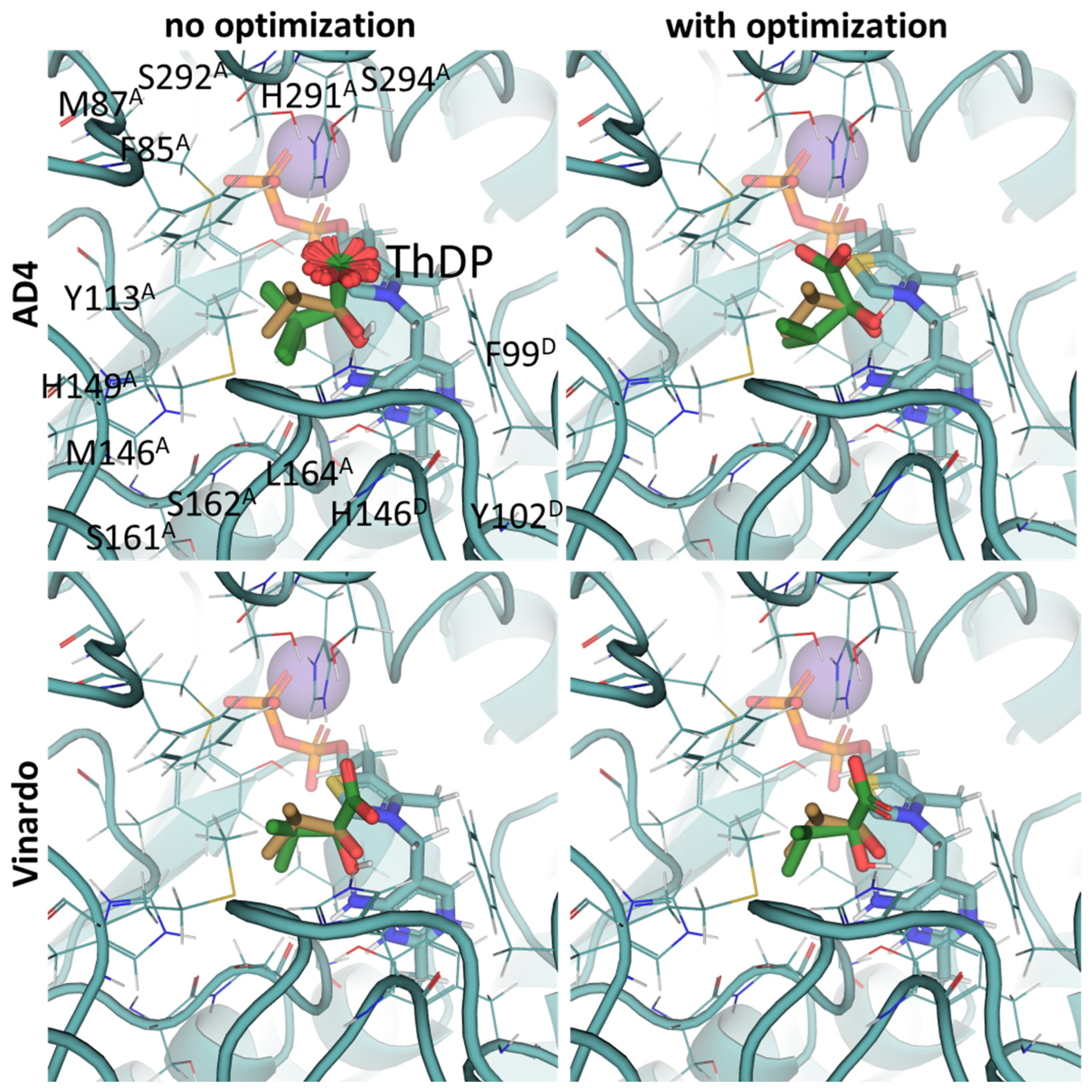
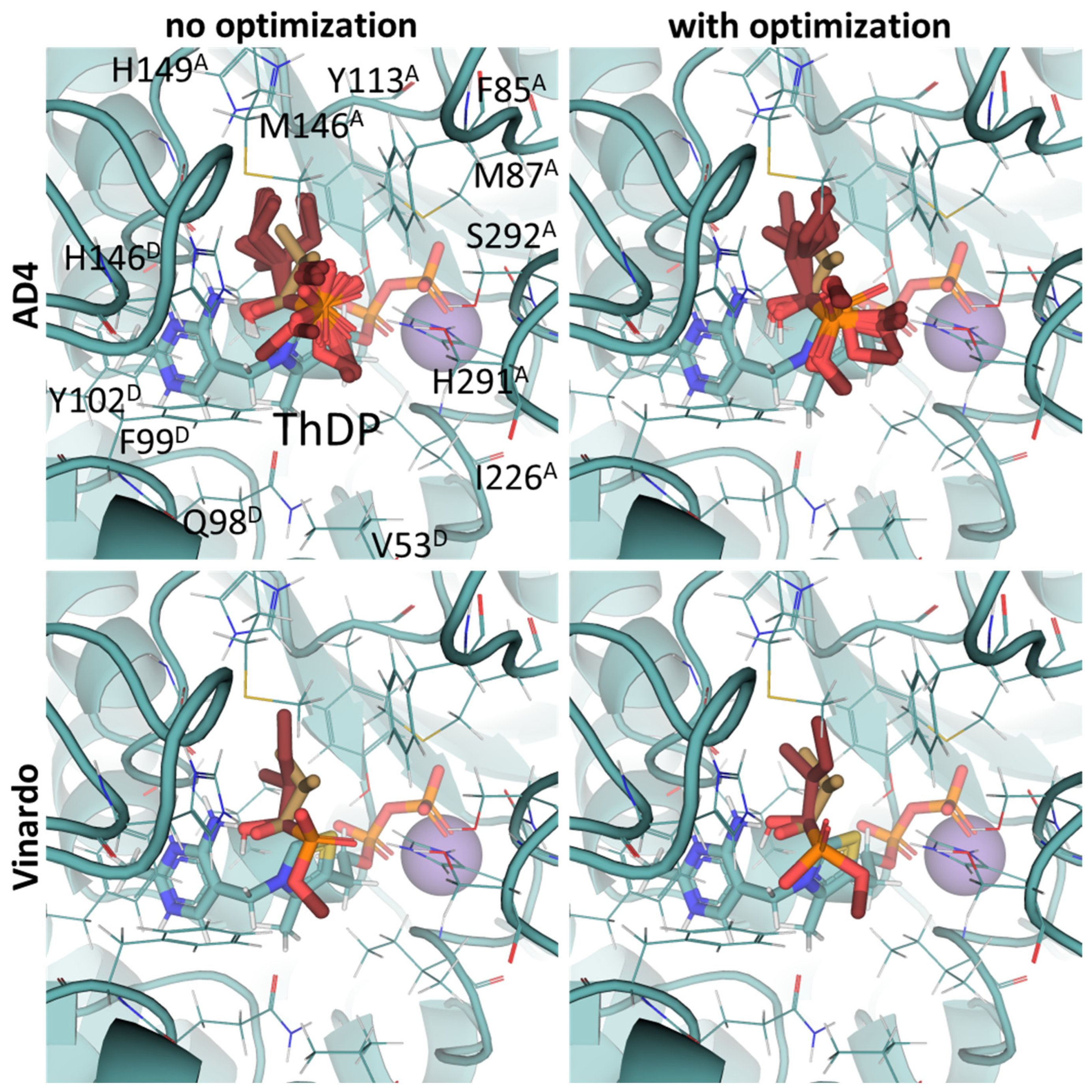
2.3. UFF Optimization Partially Improves Predictions Based on Vinardo but Not AD4 Scoring
2.4. Using Flexible Residue Setup Does Not Allow for Better Prediction of Ligand Binding to PDH
2.5. BCDH Shows Preference in Binding of Esterified vs. Non-Esterified Ligands Similarly to PDH
2.6. Docking of ω-Carboxylated Ligands Favors Binding of De-Esterified Substrate Analogs
3. Discussion
3.1. Using Covalent vs. Conventional Docking for Modeling Ligand Binding to ThDP-Dependent Enzymes
3.2. Influence of Hydrophobic Regions in Substrate Binding Sites of PDH and BCDH, but Not OGDH, on Enzyme Substrate and Inhibitor Specificity
3.3. Further Directions
4. Materials and Methods
4.1. Ligand Structures Preparation
4.2. Target Structures Preparation
4.3. Docking Procedure
- C2 atom of ThDP must be bound to a specific hydroxylated carbon atom (corresponding to a reactive carbonyl atom) of a ligand, indicated by ‘[C;H2]([O])’ (glyoxylate), ‘[C@H]([O;H1])([C;H2][O;H1])’ (xylulose-5-phosphate) or ‘[C@H]([O])’ (all the rest ligands) SMARTS patterns;
- docking box is autogenerated using bound ligand coordinates from the initial protein structure with default buffer space;
- reactive atom of a ligand should be fixed in specified coordinates, chosen from the initially bound ligand from protein structure template;
- hydrogen atoms should not be added, as those were added previously to both ligand and protein structures;
- exhaustiveness parameter should be as high as possible;
- different scoring functions (--scoring <name>), recalculations with convolutional neural networks (--cnn_scoring <name>) and UFF optimizations of bond angles and lengths of bound ligands (--covalent_optimize_lig) were used in some of the runs as specified in the text;
- when flexible side chain setup is used, only residues within 5.5 Å from an initially bound template were chosen (--flexdist 5.5); in that case ThDP itself is not considered as flexible residue. Note, this can be changed via exact specification of flexible residues (--flexres A:61,A:63,A:89,A:124,A:138,A:200,A:263,A:264,D:37,D:81,D:85,D:128,E:401);
- when flexible side chain setup is used, the high number of ligand and amino acid conformations were clustered based on RMSD values (--min_rmsd_filter 2).

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OGDH | 2-oxoglutarate dehydrogenase, or α-ketoglutarate dehydrogenase |
| TKT | Transketolase |
| PDH | pyruvate dehydrogenase |
| ThDP | thiamine diphosphate, or thiamine pyrophosphate, or cocarboxylase |
| BCDH | branched-chain 2-oxo acid dehydrogenase |
| HACL | 2-hydroxyacyl-CoA-lyase |
| AHAS | acetohydroxyacid synthase |
| AcPH | acetyl phosphinate |
| AcMePH | acetyl (methyl) phosphinate |
| AcP | acetyl phosphonate |
| AcPMe | methyl acetyl phosphonate |
| AcPMe2 | dimethyl acetyl phosphonate |
| AcPEt | ethyl acetyl phosphonate |
| SP | succinyl phosphonate |
| PMSP | phosphonomethyl succinyl phosphonate |
| PDSP | phosphonodimethyl succinyl phosphonate |
| PESP | phosphonoethyl succinyl phosphonate |
| CESP | carboxyethyl succinyl phosphonate |
| DESP | diethyl succinyl phosphonate |
| TESP | triethyl succinyl phosphonate |
| MBP | 2-methylbutyryl phosphonate |
| MBPMe | methyl 2-methylbutyryl phosphonate |
| MBPMe2 | dimethyl 2-methylbutyryl phosphonate |
| MBPEt2 | diethyl 2-methylbutyryl phosphonate |
| GP | glutaryl phosphonate |
| PLP | pyridoxal-5′-phosphate |
| WSL | Windows Subsystem for Linux |
References
- Du, Q.; Wang, H.; Xie, J. Thiamin (vitamin B1) biosynthesis and regulation: A rich source of antimicrobial drug targets? Int. J. Biol. Sci. 2011, 7, 41–52. [Google Scholar] [CrossRef]
- Makarchikov, A.F.; Wins, P.; Bettendorff, L. Biochemical and medical aspects of vitamin B1 research. Neurochem. Int. 2025, 185, 105962. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A. Thiamine in plants: Aspects of its metabolism and functions. Phytochemistry 2010, 71, 1615–1624. [Google Scholar] [CrossRef]
- Bunik, V. Vitamin-Dependent Complexes of 2-Oxo Acid Dehydrogenases: Structure, Function, Regulation and Medical Implications; Nova Science Publishers: New York, NY, USA, 2017; p. 203. [Google Scholar]
- Artiukhov, A.V.; Graf, A.V.; Bunik, V.I. Directed Regulation of Multienzyme Complexes of 2-Oxo Acid Dehydrogenases Using Phosphonate and Phosphinate Analogs of 2-Oxo Acids. Biochem. Biokhimiia 2016, 81, 1498–1521. [Google Scholar] [CrossRef]
- Paxton, R.; Scislowski, P.W.; Davis, E.J.; Harris, R.A. Role of branched-chain 2-oxo acid dehydrogenase and pyruvate dehydrogenase in 2-oxobutyrate metabolism. Biochem. J. 1986, 234, 295–303. [Google Scholar] [CrossRef]
- Tretter, L.; Adam-Vizi, V. Alpha-ketoglutarate dehydrogenase: A target and generator of oxidative stress. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M.; McCormack, J.G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980, 119, 1–8. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Anderson, J.L.; Denton, R.M. Studies on the regulation of the human E1 subunit of the 2-oxoglutarate dehydrogenase complex, including the identification of a novel calcium-binding site. Biochem. J. 2014, 459, 369–381. [Google Scholar] [CrossRef]
- Szabo, E.; Nagy, B.; Czajlik, A.; Komlodi, T.; Ozohanics, O.; Tretter, L.; Ambrus, A. Mitochondrial Alpha-Keto Acid Dehydrogenase Complexes: Recent Developments on Structure and Function in Health and Disease. Macromol. Protein Complexes V Struct. Funct. 2024, 104, 295–381. [Google Scholar] [CrossRef]
- Leandro, J.; Dodatko, T.; Aten, J.; Nemeria, N.S.; Zhang, X.; Jordan, F.; Hendrickson, R.C.; Sanchez, R.; Yu, C.; DeVita, R.J.; et al. DHTKD1 and OGDH display substrate overlap in cultured cells and form a hybrid 2-oxo acid dehydrogenase complex in vivo. Human. Mol. Genet. 2020, 29, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Leandro, J.; Khamrui, S.; Wang, H.; Suebsuwong, C.; Nemeria, N.S.; Huynh, K.; Moustakim, M.; Secor, C.; Wang, M.; Dodatko, T.; et al. Inhibition and Crystal Structure of the Human DHTKD1-Thiamin Diphosphate Complex. ACS Chem. Biol. 2020, 15, 2041–2047. [Google Scholar] [CrossRef]
- Nemeria, N.S.; Gerfen, G.; Nareddy, P.R.; Yang, L.; Zhang, X.; Szostak, M.; Jordan, F. The mitochondrial 2-oxoadipate and 2-oxoglutarate dehydrogenase complexes share their E2 and E3 components for their function and both generate reactive oxygen species. Free Radic. Biol. Med. 2018, 115, 136–145. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Grabarska, A.; Gumbarewicz, E.; Aleshin, V.A.; Kahne, T.; Obata, T.; Kazantsev, A.V.; Lukashev, N.V.; Stepulak, A.; Fernie, A.R.; et al. Synthetic analogues of 2-oxo acids discriminate metabolic contribution of the 2-oxoglutarate and 2-oxoadipate dehydrogenases in mammalian cells and tissues. Sci. Rep. 2020, 10, 1886. [Google Scholar] [CrossRef]
- Boyko, A.I.; Karlina, I.S.; Zavileyskiy, L.G.; Aleshin, V.A.; Artiukhov, A.V.; Kaehne, T.; Ksenofontov, A.L.; Ryabov, S.I.; Graf, A.V.; Tramonti, A.; et al. Delayed Impact of 2-Oxoadipate Dehydrogenase Inhibition on the Rat Brain Metabolism Is Linked to Protein Glutarylation. Front. Med. 2022, 9, 896263. [Google Scholar] [CrossRef]
- Wagner, G.R.; Bhatt, D.P.; O’Connell, T.M.; Thompson, J.W.; Dubois, L.G.; Backos, D.S.; Yang, H.; Mitchell, G.A.; Ilkayeva, O.R.; Stevens, R.D.; et al. A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation. Cell Metab. 2017, 25, 823–837 e828. [Google Scholar] [CrossRef]
- Ono, K.; Hakozaki, M.; Kimura, A.; Kochi, H. Purification, resolution, and reconstitution of rat liver branched-chain alpha-keto acid dehydrogenase complex. J. Biochem. 1987, 101, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Yeaman, S.J. Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex. Biochem. J. 1986, 237, 621–623. [Google Scholar] [CrossRef]
- Kochetov, G.A.; Solovjeva, O.N. Structure and functioning mechanism of transketolase. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 1608–1618. [Google Scholar] [CrossRef]
- Kitamura, T.; Seki, N.; Kihara, A. Phytosphingosine degradation pathway includes fatty acid alpha-oxidation reactions in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2017, 114, E2616–E2623. [Google Scholar] [CrossRef]
- Mori, K.; Naganuma, T.; Kihara, A. Role of 2-hydroxy acyl-CoA lyase HACL2 in odd-chain fatty acid production via alpha-oxidation in vivo. Mol. Biol. Cell 2023, 34, ar85. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Mkrtchyan, G.V.; Bunik, V.I. Mechanisms of Non-coenzyme Action of Thiamine: Protein Targets and Medical Significance. Biochem. Biokhimiia 2019, 84, 829–850. [Google Scholar] [CrossRef]
- Boyko, A.I.; Artiukhov, A.V.; Kaehne, T.; di Salvo, M.L.; Bonaccorsi di Patti, M.C.; Contestabile, R.; Tramonti, A.; Bunik, V.I. Isoforms of the DHTKD1-Encoded 2-Oxoadipate Dehydrogenase, Identified in Animal Tissues, Are not Observed upon the Human DHTKD1 Expression in Bacterial or Yeast Systems. Biochem. Biokhimiia 2020, 85, 920–929. [Google Scholar] [CrossRef]
- Bunik, V.I.; Tylicki, A.; Lukashev, N.V. Thiamin diphosphate-dependent enzymes: From enzymology to metabolic regulation, drug design and disease models. FEBS J. 2013, 280, 6412–6442. [Google Scholar] [CrossRef]
- Bunik, V.I.; Aleshin, V.A. Analysis of the Protein Binding Sites for Thiamin and Its Derivatives to Elucidate the Molecular Mechanisms of the Noncoenzyme Action of Thiamin (Vitamin B1). In Studies in Natural Products Chemistry; Hess, A., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2017; Volume 53, pp. 375–429. [Google Scholar]
- Artiukhov, A.V.; Kazantsev, A.V.; Lukashev, N.V.; Bellinzoni, M.; Bunik, V.I. Selective Inhibition of 2-Oxoglutarate and 2-Oxoadipate Dehydrogenases by the Phosphonate Analogs of Their 2-Oxo Acid Substrates. Front. Chem. 2021, 8, 596187. [Google Scholar] [CrossRef]
- Sousa, S.F.; Ribeiro, A.J.; Coimbra, J.T.; Neves, R.P.; Martins, S.A.; Moorthy, N.S.; Fernandes, P.A.; Ramos, M.J. Protein-ligand docking in the new millennium—A retrospective of 10 years in the field. Curr. Med. Chem. 2013, 20, 2296–2314. [Google Scholar] [CrossRef]
- Ai, Y.; Yu, L.; Tan, X.; Chai, X.; Liu, S. Discovery of Covalent Ligands via Noncovalent Docking by Dissecting Covalent Docking Based on a “Steric-Clashes Alleviating Receptor (SCAR)” Strategy. J. Chem. Inf. Model. 2016, 56, 1563–1575. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Chakraborty, A.; Vijayasree, V.; Das, S.; Sivamani, Y.; Elayaperumal, S. Overview of drugs and drug targets. In Biochemical and Molecular Pharmacology in Drug Discovery; Rudrapal, M., Egbuna, C., Cho, W.C., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2024; pp. 45–69. [Google Scholar] [CrossRef]
- Kumalo, H.M.; Bhakat, S.; Soliman, M.E. Theory and applications of covalent docking in drug discovery: Merits and pitfalls. Molecules 2015, 20, 1984–2000. [Google Scholar] [CrossRef] [PubMed]
- Boike, L.; Henning, N.J.; Nomura, D.K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef]
- London, N.; Miller, R.M.; Krishnan, S.; Uchida, K.; Irwin, J.J.; Eidam, O.; Gibold, L.; Cimermancic, P.; Bonnet, R.; Shoichet, B.K.; et al. Covalent docking of large libraries for the discovery of chemical probes. Nat. Chem. Biol. 2014, 10, 1066–1072. [Google Scholar] [CrossRef]
- Peng, H.; Wang, T.; Xie, P.; Chen, T.; He, H.W.; Wan, J. Molecular docking and three-dimensional quantitative structure-activity relationship studies on the binding modes of herbicidal 1-(substituted phenoxyacetoxy)alkylphosphonates to the E1 component of pyruvate dehydrogenase. J. Agric. Food Chem. 2007, 55, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Boateng, R.A.; Tastan Bishop, O.; Musyoka, T.M. Characterisation of plasmodial transketolases and identification of potential inhibitors: An in silico study. Malar. J. 2020, 19, 442. [Google Scholar] [CrossRef] [PubMed]
- He, H.W.; Peng, H.; Tan, X.S. Environmentally Friendly Alkylphosphonate Herbicides, 1st ed.; Chemical Industry Press: Beijing, China, 2014. [Google Scholar] [CrossRef]
- Johnston, M.L.; Bonett, E.M.; DeColli, A.A.; Freel Meyers, C.L. Antibacterial Target DXP Synthase Catalyzes the Cleavage of d-Xylulose 5-Phosphate: A Study of Ketose Phosphate Binding and Ketol Transfer Reaction. Biochemistry 2022, 61, 1810–1823. [Google Scholar] [CrossRef] [PubMed]
- Whitley, M.J.; Arjunan, P.; Nemeria, N.S.; Korotchkina, L.G.; Park, Y.H.; Patel, M.S.; Jordan, F.; Furey, W. Pyruvate dehydrogenase complex deficiency is linked to regulatory loop disorder in the alphaV138M variant of human pyruvate dehydrogenase. J. Biol. Chem. 2018, 293, 13204–13213. [Google Scholar] [CrossRef]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead finder: An approach to improve accuracy of protein-ligand docking, binding energy estimation, and virtual screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, P.A.; Forli, S.; Goodsell, D.S.; Olson, A.J.; Sanner, M.F. AutoDockFR: Advances in Protein-Ligand Docking with Explicitly Specified Binding Site Flexibility. PLoS Comput. Biol. 2015, 11, e1004586. [Google Scholar] [CrossRef]
- Dominguez, C.; Boelens, R.; Bonvin, A.M. HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 2003, 125, 1731–1737. [Google Scholar] [CrossRef]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef]
- McNutt, A.T.; Li, Y.; Meli, R.; Aggarwal, R.; Koes, D.R. GNINA 1.3: The next increment in molecular docking with deep learning. J. Cheminform. 2025, 17, 28. [Google Scholar] [CrossRef]
- McNutt, A.T.; Francoeur, P.; Aggarwal, R.; Masuda, T.; Meli, R.; Ragoza, M.; Sunseri, J.; Koes, D.R. GNINA 1.0: Molecular docking with deep learning. J. Cheminform. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Hailes, H.C.; Rother, D.; Muller, M.; Westphal, R.; Ward, J.M.; Pleiss, J.; Vogel, C.; Pohl, M. Engineering stereoselectivity of ThDP-dependent enzymes. FEBS J. 2013, 280, 6374–6394. [Google Scholar] [CrossRef]
- Wang, J.C.; Lin, J.H.; Chen, C.M.; Perryman, A.L.; Olson, A.J. Robust scoring functions for protein-ligand interactions with quantum chemical charge models. J. Chem. Inf. Model. 2011, 51, 2528–2537. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Quiroga, R.; Villarreal, M.A. Vinardo: A Scoring Function Based on Autodock Vina Improves Scoring, Docking, and Virtual Screening. PLoS ONE 2016, 11, e0155183. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Behrens, C.; Eubel, H.; Braun, H.P.; Peterhansel, C. A possible role for the chloroplast pyruvate dehydrogenase complex in plant glycolate and glyoxylate metabolism. Phytochemistry 2013, 95, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Bisswanger, H. Substrate specificity of the pyruvate dehydrogenase complex from Escherichia coli. J. Biol. Chem. 1981, 256, 815–822. [Google Scholar] [CrossRef]
- Bunik, V.I.; Artiukhov, A.; Kazantsev, A.; Goncalves, R.; Daloso, D.; Oppermann, H.; Kulakovskaya, E.; Lukashev, N.; Fernie, A.; Brand, M.; et al. Specific inhibition by synthetic analogs of pyruvate reveals that the pyruvate dehydrogenase reaction is essential for metabolism and viability of glioblastoma cells. Oncotarget 2015, 6, 40036–40052. [Google Scholar] [CrossRef]
- Schonbrunn-Hanebeck, E.; Laber, B.; Amrhein, N. Slow-binding inhibition of the Escherichia coli pyruvate dehydrogenase multienzyme complex by acetylphosphinate. Biochemistry 1990, 29, 4880–4885. [Google Scholar] [CrossRef]
- Nemeria, N.S.; Korotchkina, L.G.; Chakraborty, S.; Patel, M.S.; Jordan, F. Acetylphosphinate is the most potent mechanism-based substrate-like inhibitor of both the human and Escherichia coli pyruvate dehydrogenase components of the pyruvate dehydrogenase complex. Bioorganic Chem. 2006, 34, 362–379. [Google Scholar] [CrossRef]
- Baillie, A.C.; Wright, K.; Wright, B.J.; Earnshaw, C.G. Inhibitors of pyruvate dehydrogenase as herbicides. Pestic. Biochem. Physiol. 1988, 30, 103–112. [Google Scholar] [CrossRef]
- Kluger, R.; Pike, D.C. Active site generated analogues of reactive intermediates in enzymic reactions. Potent inhibition of pyruvate dehydrogenase by a phosphonate analogue of pyruvate1. J. Am. Chem. Soc. 1977, 99, 4504–4506. [Google Scholar] [CrossRef]
- Dixon, H.B.; Giddens, R.A.; Harrison, R.A.; Henderson, C.E.; Norris, W.E.; Parker, D.M.; Perham, R.N.; Slater, P.; Sparkes, M.J. A synthesis of acylphosphonic acids and of 1-aminoalkylphosphonic acids: The action of pyruvate dehydrogenase and lactate dehydrogenase on acetylphosphonic acid. J. Enzym. Inhib. 1991, 5, 111–117. [Google Scholar] [CrossRef]
- Ambrose, M.C.; Perham, R.N. Spin-label study of the mobility of enzyme-bound lipoic acid in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem. J. 1976, 155, 429–432. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Aleshin, V.A.; Karlina, I.S.; Kazantsev, A.V.; Sibiryakina, D.A.; Ksenofontov, A.L.; Lukashev, N.V.; Graf, A.V.; Bunik, V.I. Phosphonate Inhibitors of Pyruvate Dehydrogenase Perturb Homeostasis of Amino Acids and Protein Succinylation in the Brain. Int. J. Mol. Sci. 2022, 23, 13186. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.A.; Kluger, R.; Pike, D.C.; Gennis, R.B. Phosphonate analogues of pyruvate. Probes of substrate binding to pyruvate oxidase and other thiamin pyrophosphate-dependent decarboxylases. Biochim. Biophys. Acta (BBA)-Enzymol. 1980, 613, 10–17. [Google Scholar] [CrossRef]
- Smith, J.M.; Vierling, R.J.; Meyers, C.F. Selective inhibition of E. coli 1-deoxy-D-xylulose-5-phosphate synthase by acetylphosphonates. Medchemcomm 2012, 3, 65–67. [Google Scholar] [CrossRef]
- Chen, H.; Cross, A.C.; Thakkar, A.; Xu, H.; Li, A.; Paull, D.; Noggle, S.A.; Kruger, L.; Denton, T.T.; Gibson, G.E. Selective linkage of mitochondrial enzymes to intracellular calcium stores differs between human-induced pluripotent stem cells, neural stem cells, and neurons. J. Neurochem. 2021, 156, 867–879. [Google Scholar] [CrossRef]
- Kluger, R.; Wasserstein, P. Hydrolysis of acetyl dimethyl phosphate, a reactive acyl phosphate. Biochemistry 1972, 11, 1544–1546. [Google Scholar] [CrossRef]
- Kluger, R.; Pike, D.C.; Chin, J. Kinetics and mechanism of the reaction of dimethyl acetylphosphonate with water. Expulsion of a phosphonate ester from a carbonyl hydrate. Can. J. Chem. 1978, 65, 1792. [Google Scholar] [CrossRef]
- Pettit, F.H.; Yeaman, S.J.; Reed, L.J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc. Natl. Acad. Sci. USA 1978, 75, 4881–4885. [Google Scholar] [CrossRef] [PubMed]
- Wendel, U.; Langenbeck, U.; Seakins, J.W. Interrelation between the metabolism of L-isoleucine and L-allo-isoleucine in patients with maple syrup urine disease. Pediatr. Res. 1989, 25, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Parker, P.J.; Randle, P.J. Partial purification and properties of branched-chain 2-oxo acid dehydrogenase of ox liver. Biochem. J. 1978, 171, 751–757. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Artiukhov, A.V.; Oppermann, H.; Kazantsev, A.V.; Lukashev, N.V.; Bunik, V.I. Mitochondrial Impairment May Increase Cellular NAD(P)H: Resazurin Oxidoreductase Activity, Perturbing the NAD(P)H-Based Viability Assays. Cells 2015, 4, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Gao, Y.; Zhou, D.; Ma, X.; Chen, H.; Xu, Y.; Yang, W.; Yu, X. Structural basis for the activity and regulation of human alpha-ketoglutarate dehydrogenase revealed by Cryo-EM. Biochem. Biophys. Res. Commun. 2022, 602, 120–126. [Google Scholar] [CrossRef]
- Jiahui, W.; Xiang, Y.; Youhuan, Z.; Xiaomin, M.; Yuanzhu, G.; Dejian, Z.; Jie, W.; Yinkun, F.; Shi, F.; Juncheng, S.; et al. The mitochondrial DNAJC co-chaperone TCAIM reduces alpha-ketoglutarate dehydrogenase protein levels to regulate metabolism. Mol. Cell 2025, 85, 638–651 e639. [Google Scholar] [CrossRef]
- Nemeria, N.S.; Gerfen, G.; Guevara, E.; Nareddy, P.R.; Szostak, M.; Jordan, F. The human Krebs cycle 2-oxoglutarate dehydrogenase complex creates an additional source of superoxide/hydrogen peroxide from 2-oxoadipate as alternative substrate. Free Radic. Biol. Med. 2017, 108, 644–654. [Google Scholar] [CrossRef]
- Bunik, V.I.; Pavlova, O.G. Inactivation of alpha-ketoglutarate dehydrogenase during oxidative decarboxylation of alpha-ketoadipic acid. FEBS Lett. 1993, 323, 166–170. [Google Scholar] [CrossRef]
- Bunik, V.I.; Pavlova, O.G. Inactivation of alpha-ketoglutarate dehydrogenase during its enzymatic reaction. Biochemistry. Biokhimiia 1997, 62, 973–982. [Google Scholar]
- Bunik, V.I.; Pavlova, O.G. Inhibition of pigeon breast muscle alpha-ketoglutarate dehydrogenase by structural analogs of alpha-ketoglutarate. Biochem. Biokhimiia 1997, 62, 1012–1020. [Google Scholar]
- Bunik, V.I.; Sievers, C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur. J. Biochem. 2002, 269, 5004–5015. [Google Scholar] [CrossRef] [PubMed]
- Kabysheva, M.S.; Storozhevykh, T.P.; Pinelis, V.G.; Bunik, V.I. Synthetic regulators of the 2-oxoglutarate oxidative decarboxylation alleviate the glutamate excitotoxicity in cerebellar granule neurons. Biochem. Pharmacol. 2009, 77, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.I.; Denton, T.T.; Xu, H.; Thompson, C.M.; Cooper, A.J.; Gibson, G.E. Phosphonate analogues of alpha-ketoglutarate inhibit the activity of the alpha-ketoglutarate dehydrogenase complex isolated from brain and in cultured cells. Biochemistry 2005, 44, 10552–10561. [Google Scholar] [CrossRef]
- Bunik, V.I.; Kabysheva, M.S.; Klimuk, E.I.; Storozhevykh, T.P.; Pinelis, V.G. Phosphono analogues of 2-oxoglutarate protect cerebellar granule neurons upon glutamate excitotoxicity. Ann. N. Y. Acad. Sci. 2009, 1171, 521–529. [Google Scholar] [CrossRef]
- Biryukov, A.I.; Bunik, V.I.; Zhukov, Y.N.; Khurs, E.N.; Khomutov, R.M. Succinyl phosphonate inhibits alpha-ketoglutarate oxidative decarboxylation, catalyzed by alpha-ketoglutarate dehydrogenase complexes from E. coli and pigeon breast muscle. FEBS Lett. 1996, 382, 167–170. [Google Scholar] [CrossRef]
- Bunik, V.I.; Biryukov, A.I.; Zhukov Yu, N. Inhibition of pigeon breast muscle alpha-ketoglutarate dehydrogenase by phosphonate analogues of alpha-ketoglutarate. FEBS Lett. 1992, 303, 197–201. [Google Scholar]
- Li, Z.M.; Hu, Z.; Wang, X.; Chen, S.; Yu, W.; Liu, J.; Li, Z. Biochemical and Structural Insights into a Thiamine Diphosphate-Dependent alpha-Ketoglutarate Decarboxylase from Cyanobacterium Microcystis aeruginosa NIES-843. Int. J. Mol. Sci. 2023, 24, 12198. [Google Scholar] [CrossRef]
- Payongsri, P.; Steadman, D.; Hailes, H.C.; Dalby, P.A. Second generation engineering of transketolase for polar aromatic aldehyde substrates. Enzym. Microb. Technol. 2015, 71, 45–52. [Google Scholar] [CrossRef]
- Georges, R.N.; Ballut, L.; Octobre, G.; Comte, A.; Hecquet, L.; Charmantray, F.; Doumeche, B. Structural determination and kinetic analysis of the transketolase from Vibrio vulnificus reveal unexpected cooperative behavior. Protein Sci. 2024, 33, e4884. [Google Scholar] [CrossRef]
- Lanza, L.; Bjarnesen, D.; Cakar, M.M.; Rizzo, A.; Pandya, A.; Aranda, C.; Blazevic, Z.F.; Muller, M. Engineering of the Thiamine Diphosphate-Dependent JanthE for the Synthesis of Tertiary Alcohols. Chemistry 2025, 31, e202500890. [Google Scholar] [CrossRef]
- Westphal, R.; Waltzer, S.; Mackfeld, U.; Widmann, M.; Pleiss, J.; Beigi, M.; Muller, M.; Rother, D.; Pohl, M. (S)-Selective MenD variants from Escherichia coli provide access to new functionalized chiral alpha-hydroxy ketones. Chem. Commun. 2013, 49, 2061–2063. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Ho, T.C.S.; Fathoni, I.; Pope, R.; Saliba, K.J.; Leeper, F.J. Inhibition of Thiamine Diphosphate-Dependent Enzymes by Triazole-Based Thiamine Analogues. ACS Med. Chem. Lett. 2023, 14, 621–628. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Ho, T.C.S.; Irfan, R.; Hamid, R.A.A.; Rudge, E.S.; Iqbal, A.; Turner, A.; Hirsch, A.K.H.; Leeper, F.J. Design of thiamine analogues for inhibition of thiamine diphosphate (ThDP)-dependent enzymes: Systematic investigation through Scaffold-Hopping and C2-Functionalisation. Bioorganic Chem. 2023, 138, 106602. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.Y.; Ho, T.C.S.; Parle, D.R.; Leeper, F.J. Furan-based inhibitors of pyruvate dehydrogenase: SAR study, biochemical evaluation and computational analysis. Org. Biomol. Chem. 2023, 21, 1755–1763. [Google Scholar] [CrossRef]
- Obiol-Pardo, C.; Alcarraz-Vizan, G.; Cascante, M.; Rubio-Martinez, J. Diphenyl urea derivatives as inhibitors of transketolase: A structure-based virtual screening. PLoS ONE 2012, 7, e32276. [Google Scholar] [CrossRef]
- He, H.; Xia, H.; Xia, Q.; Ren, Y.; He, H. Design and optimization of N-acylhydrazone pyrimidine derivatives as E. coli PDHc E1 inhibitors: Structure-activity relationship analysis, biological evaluation and molecular docking study. Bioorganic Med. Chem. 2017, 25, 5652–5661. [Google Scholar] [CrossRef]
- He, J.B.; Ren, Y.L.; Sun, Q.S.; You, G.Y.; Zhang, L.; Zou, P.; Feng, L.L.; Wan, J.; He, H.W. Design, synthesis and molecular docking of amide and urea derivatives as Escherichia coli PDHc-E1 inhibitors. Bioorganic Med. Chem. 2014, 22, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Li, C.; Cao, S.; Li, J.; Li, D.; Du, Z.; Li, P.; Wang, J.; Dong, H.; Yang, D.; et al. Discovery of Indole-3-acetamide Derivatives as Potent Transketolase-Inhibiting-Based Herbicidal Leads. J. Agric. Food Chem. 2025, 73, 18119–18130. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.Y.; Ho, T.C.S.; Fathoni, I.; Hamid, R.; Hirsch, A.K.H.; Saliba, K.J.; Leeper, F.J. Evaluation of ketoclomazone and its analogues as inhibitors of 1-deoxy-d-xylulose 5-phosphate synthases and other thiamine diphosphate (ThDP)-dependent enzymes. RSC Med. Chem. 2024, 15, 1773–1781. [Google Scholar] [CrossRef]
- He, J.; He, H.; Cai, M.; Zhao, F.; He, H. Insight into the halogen bonding between PA-1 ligand and pyruvate dehydrogenase complex E1 component by crystal structure, DFT calculation, and molecular docking. J. Mol. Struct. 2020, 1199, 126991. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, A.R.; Saqib, U.; Misra, P.C.; Siddiqi, M.I.; Saxena, J.K. Identification of potential P. falciparum transketolase inhibitors: Pharmacophore design, in silico screening and docking studies. J. Biophys. Chem. 2010, 1, 96–104. [Google Scholar] [CrossRef]
- Fathoni, I.; Ho, T.C.S.; Chan, A.H.Y.; Leeper, F.J.; Matuschewski, K.; Saliba, K.J. Identification and characterization of thiamine analogs with antiplasmodial activity. Antimicrob. Agents Chemother. 2024, 68, e01096-01024. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Zhang, D.; Zheng, X.; Lin, B.; Huang, Y.; Liao, Y.; Deng, Z.; Kong, L.; You, D. Biosynthesis of the benzylpyrrolidine precursor in anisomycin by a unique ThDP-dependent enzyme. Synth. Syst. Biotechnol. 2025, 10, 76–85. [Google Scholar] [CrossRef]
- Wang, Y.E.; Yang, D.; Dai, L.; Huo, J.; Chen, L.; Kang, Z.; Mao, J.; Zhang, J. Design, Synthesis, Herbicidal Activity, and Molecular Docking Study of 2-Thioether-5-(Thienyl/Pyridyl)-1,3,4-Oxadiazoles as Potent Transketolase Inhibitors. J. Agric. Food Chem. 2022, 70, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Mazumder, M.H.; Chowdhury, A.S.; Datta, A.; Khan, M.A. Molecular-docking study of malaria drug target enzyme transketolase in Plasmodium falciparum 3D7 portends the novel approach to its treatment. Source Code Biol. Med. 2015, 10, 7. [Google Scholar] [CrossRef]
- Gushchina, I.V.; Nilov, D.K.; Shcherbakova, T.A.; Baldin, S.M.; Švedas, V.K. Search for Inhibitors of Mycobacterium tuberculosis Transketolase in a Series of Sulfo-Substituted Compounds. Acta Naturae 2023, 15, 81–83. [Google Scholar] [CrossRef]
- Zhu, D.; Johannsen, S.; Masini, T.; Simonin, C.; Haupenthal, J.; Illarionov, B.; Andreas, A.; Awale, M.; Gierse, R.M.; van der Laan, T.; et al. Discovery of novel drug-like antitubercular hits targeting the MEP pathway enzyme DXPS by strategic application of ligand-based virtual screening. Chem. Sci. 2022, 13, 10686–10698. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, K.; Joshi, N.; Alavala, R.R. Acetohydroxyacid Synthase (AHAS) Inhibitors as Antitubercular Agents: Insights From Molecular Docking and Dynamics Simulations. Chem. Biodivers. 2025, 22, e202402631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Xiao, Y.J.; Li, Y.H.; Ma, Y.; Li, Z.M. Identification of some novel AHAS inhibitors via molecular docking and virtual screening approach. Bioorganic Med. Chem. 2007, 15, 374–380. [Google Scholar] [CrossRef]
- He, Y.Z.; Li, Y.X.; Zhu, X.L.; Xi, Z.; Niu, C.; Wan, J.; Zhang, L.; Yang, G.F. Rational design based on bioactive conformation analysis of pyrimidinylbenzoates as acetohydroxyacid synthase inhibitors by integrating molecular docking, CoMFA, CoMSIA, and DFT calculations. J. Chem. Inf. Model. 2007, 47, 2335–2344. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Karu, K.; Dalby, P.A. Two-substrate enzyme engineering using small libraries that combine the substrate preferences from two different variant lineages. Sci. Rep. 2024, 14, 1287. [Google Scholar] [CrossRef]
- Klei, H.E.; Moriarty, N.W.; Echols, N.; Terwilliger, T.C.; Baldwin, E.T.; Pokross, M.; Posy, S.; Adams, P.D. Ligand placement based on prior structures: The guided ligand-replacement method. Biol. Crystallogr. 2014, 70, 134–143. [Google Scholar] [CrossRef]
- Brody, S.I.; Buonomo, J.A.; Orimoloye, M.O.; Jia, Z.; Sharma, S.; Brown, C.D.; Baughn, A.D.; Aldrich, C.C. A Nucleophilic Activity-Based Probe Enables Profiling of PLP-Dependent Enzymes. Chembiochem 2023, 24, e202200669. [Google Scholar] [CrossRef] [PubMed]
- de Chiara, C.; Homsak, M.; Prosser, G.A.; Douglas, H.L.; Garza-Garcia, A.; Kelly, G.; Purkiss, A.G.; Tate, E.W.; de Carvalho, L.P.S. D-Cycloserine destruction by alanine racemase and the limit of irreversible inhibition. Nat. Chem. Biol. 2020, 16, 686–694. [Google Scholar] [CrossRef]
- Fu, M.; Silverman, R.B. Isolation and characterization of the product of inactivation of gamma-aminobutyric acid aminotransferase by gabaculine. Bioorganic Med. Chem. 1999, 7, 1581–1590. [Google Scholar] [CrossRef]
- Silverman, R.B. Design and Mechanism of GABA Aminotransferase Inactivators. Treatments for Epilepsies and Addictions. Chem. Rev. 2018, 118, 4037–4070. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Lu, L.; Ackermann, B.; Bey, P.; Pegg, A.E. Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites. J. Biol. Chem. 1992, 267, 150–158. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, Y. Eflornithine for treatment of high-risk neuroblastoma. Trends Pharmacol. Sci. 2024, 45, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Marchand, J.; Torreilles, J.; Guerin, M.C.; Descomps, B.; Crastes de Paulet, A.; Gabriel, M.; Larcher, D. Studies of covalent adducts of NAD(P) and enolizable ketones as specific glutamate dehydrogenase inhibitors. Biochimie 1982, 64, 203–209. [Google Scholar] [CrossRef]
- Everse, J.; Zoll, E.C.; Kahan, L.; Kaplan, N.O. Addition products of diphosphopyridine nucleotides with substrates of pyridine nucleotide-linked dehydrogenases. Bioorganic Chem. 1971, 1, 207–233. [Google Scholar] [CrossRef]
- Campbell, A.C.; Becker, D.F.; Gates, K.S.; Tanner, J.J. Covalent Modification of the Flavin in Proline Dehydrogenase by Thiazolidine-2-Carboxylate. ACS Chem. Biol. 2020, 15, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Ruetz, M.; Campanello, G.C.; Purchal, M.; Shen, H.; McDevitt, L.; Gouda, H.; Wakabayashi, S.; Zhu, J.; Rubin, E.J.; Warncke, K.; et al. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 2019, 366, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, K.E.; Manta, B.; Himo, F. A quantum chemical study of the omega-transaminase reaction mechanism. Org. Biomol. Chem. 2015, 13, 8453–8464. [Google Scholar] [CrossRef] [PubMed]
- Aevarsson, A.; Chuang, J.L.; Wynn, R.M.; Turley, S.; Chuang, D.T.; Hol, W.G. Crystal structure of human branched-chain alpha-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease. Structure 2000, 8, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.I.; Degtyarev, D. Structure-function relationships in the 2-oxo acid dehydrogenase family: Substrate-specific signatures and functional predictions for the 2-oxoglutarate dehydrogenase-like proteins. Proteins 2008, 71, 874–890. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nemeria, N.S.; Zhang, X.; Nareddy, P.R.; Szostak, M.; Farinas, E.; Jordan, F. Engineering 2-oxoglutarate dehydrogenase to a 2-oxo aliphatic dehydrogenase complex by optimizing consecutive components. AIChE J. 2019, 66, e16769. [Google Scholar] [CrossRef]
- Nemeria, N.S.; Nagy, B.; Sanchez, R.; Zhang, X.; Leandro, J.; Ambrus, A.; Houten, S.M.; Jordan, F. Functional Versatility of the Human 2-Oxoadipate Dehydrogenase in the L-Lysine Degradation Pathway toward Its Non-Cognate Substrate 2-Oxopimelic Acid. Int. J. Mol. Sci. 2022, 23, 8213. [Google Scholar] [CrossRef]
- Denton, R.M.; Pullen, T.J.; Armstrong, C.T.; Heesom, K.J.; Rutter, G.A. Calcium-insensitive splice variants of mammalian E1 subunit of 2-oxoglutarate dehydrogenase complex with tissue-specific patterns of expression. Biochem. J. 2016, 473, 1165–1178. [Google Scholar] [CrossRef]
- Bezerra, G.A.; Foster, W.R.; Bailey, H.J.; Hicks, K.G.; Sauer, S.W.; Dimitrov, B.; McCorvie, T.J.; Okun, J.G.; Rutter, J.; Kolker, S.; et al. Crystal structure and interaction studies of human DHTKD1 provide insight into a mitochondrial megacomplex in lysine catabolism. IUCrJ 2020, 7, 693–706. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nemeria, N.; Shim, Y.; Zhang, X.; Guevara, E.L.; Patel, H.; Farinas, E.T.; Jordan, F. Engineering the 2-Oxoglutarate Dehydrogenase Complex to Understand Catalysis and Alter Substrate Recognition. Reactions 2022, 3, 139–159. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Mear, L.; Zhong, W.; Digre, A.; Katona, B.; Sjostedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef] [PubMed]
- Sjostedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef] [PubMed]
- Pinson, A.; Xing, L.; Namba, T.; Kalebic, N.; Peters, J.; Oegema, C.E.; Traikov, S.; Reppe, K.; Riesenberg, S.; Maricic, T.; et al. Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals. Science 2022, 377, eabl6422. [Google Scholar] [CrossRef]
- Hao, S.; Meng, Q.; Sun, H.; Li, Y.; Li, Y.; Gu, L.; Liu, B.; Zhang, Y.; Zhou, H.; Xu, Z.; et al. The role of transketolase in human cancer progression and therapy. Biomed. Pharmacother. 2022, 154, 113607. [Google Scholar] [CrossRef]
- Zahn, M.; Konig, G.; Pham, H.V.C.; Seroka, B.; Lazny, R.; Yang, G.; Ouerfelli, O.; Lotowski, Z.; Rohwerder, T. Mechanistic details of the actinobacterial lyase-catalyzed degradation reaction of 2-hydroxyisobutyryl-CoA. J. Biol. Chem. 2022, 298, 101522. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.H.; Gade, P.; Nattermann, M.; Maltseva, N.; Endres, M.; Chen, J.; Wichmann, P.; Hu, Y.; Marchal, D.G.; et al. Revealing reaction intermediates in one-carbon elongation by thiamine diphosphate/CoA-dependent enzyme family. Commun. Chem. 2024, 7, 160. [Google Scholar] [CrossRef]
- Goullieux, M.; Zoete, V.; Rohrig, U.F. Two-Step Covalent Docking with Attracting Cavities. J. Chem. Inf. Model. 2023, 63, 7847–7859. [Google Scholar] [CrossRef]
- Wen, C.; Yan, X.; Gu, Q.; Du, J.; Wu, D.; Lu, Y.; Zhou, H.; Xu, J. Systematic Studies on the Protocol and Criteria for Selecting a Covalent Docking Tool. Molecules 2019, 24, 2183. [Google Scholar] [CrossRef] [PubMed]
- Lie, M.A.; Celik, L.; Jorgensen, K.A.; Schiott, B. Cofactor activation and substrate binding in pyruvate decarboxylase. Insights into the reaction mechanism from molecular dynamics simulations. Biochemistry 2005, 44, 14792–14806. [Google Scholar] [CrossRef]
- Li, K.; Fang, S.; Zhang, X.; Wei, X.; Wu, P.; Zheng, R.; Liu, L.; Zhang, H. Effects of Environmental Stresses on Synthesis of 2-Phenylethanol and IAA by Enterobacter sp. CGMCC 5087. Microorganisms 2024, 12, 663. [Google Scholar] [CrossRef]
- Friedemann, R.; Tittman, K.; Golbik, R.; Hübner, G. DFT studies on key intermediates in thiamin catalysis. Int. J. Quantum Chem. 2004, 99, 109–114. [Google Scholar] [CrossRef]
- Mansoorabadi, S.O.; Seravalli, J.; Furdui, C.; Krymov, V.; Gerfen, G.J.; Begley, T.P.; Melnick, J.; Ragsdale, S.W.; Reed, G.H. EPR spectroscopic and computational characterization of the hydroxyethylidene-thiamine pyrophosphate radical intermediate of pyruvate:ferredoxin oxidoreductase. Biochemistry 2006, 45, 7122–7131. [Google Scholar] [CrossRef]
- Sgrignani, J.; Chen, J.; Alimonti, A.; Cavalli, A. How phosphorylation influences E1 subunit pyruvate dehydrogenase: A computational study. Sci. Rep. 2018, 8, 14683. [Google Scholar] [CrossRef]
- Wang, J.; Dong, H.; Li, S.; He, H. Theoretical study toward understanding the catalytic mechanism of pyruvate decarboxylase. J. Phys. Chem. B 2005, 109, 18664–18672. [Google Scholar] [CrossRef] [PubMed]
- Jana, G.; Jimenez, V.; Villa-Freixa, J.; Prat-Resina, X.; Delgado, E.; Alderete, J. Computational study on the carboligation reaction of acetohidroxyacid synthase: New approach on the role of the HEThDP-intermediate. Proteins 2010, 78, 1774–1788. [Google Scholar] [CrossRef]
- Sanchez, L.; Jana, G.A.; Delgado, E.J. A QM/MM study on the reaction pathway leading to 2-aceto-2-hydroxybutyrate in the catalytic cycle of AHAS. J. Comput. Chem. 2014, 35, 488–494. [Google Scholar] [CrossRef]
- Jaña, G.; Jiménez, V.; Villà-Freixa, J.; Prat-Resina, X.; Delgado, E.; Alderete, J.B. A QM/MM study on the last two steps of the catalytic cycle of acetohydroxyacid synthase. Comput. Theor. Chem. 2011, 966, 159–166. [Google Scholar] [CrossRef]
- Topal, K.G.; Atilgan, C.; Demir, A.S.; Aviyente, V. Understanding the mode of action of ThDP in benzoylformate decarboxylase. Biopolymers 2010, 93, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Rabe von Pappenheim, F.; Aldeghi, M.; Shome, B.; Begley, T.; de Groot, B.L.; Tittmann, K. Structural basis for antibiotic action of the B1 antivitamin 2′-methoxy-thiamine. Nat. Chem. Biol. 2020, 16, 1237–1245. [Google Scholar] [CrossRef]
- Uranga, J.; Rabe von Pappenheim, F.; Tittmann, K.; Mata, R.A. Dynamic Protonation States Underlie Carbene Formation in ThDP-Dependent Enzymes: A Theoretical Study. J. Phys. Chem. B 2023, 127, 9423–9432. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Y.; Liu, C. Theoretical studies on the common catalytic mechanism of transketolase by using simplified models. J. Mol. Graph. Model. 2013, 39, 23–28. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Y. Theoretical study of the catalytic mechanism of E1 subunit of pyruvate dehydrogenase multienzyme complex from Bacillus stearothermophilus. Biochemistry 2013, 52, 8079–8093. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y. Computational studies on the catalytic mechanism of phosphoketolase. Comput. Theor. Chem. 2013, 1025, 1–7. [Google Scholar] [CrossRef]
- Planas, F.; Sheng, X.; McLeish, M.J.; Himo, F. A Theoretical Study of the Benzoylformate Decarboxylase Reaction Mechanism. Front. Chem. 2018, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Planas, F.; McLeish, M.J.; Himo, F. Computational Study of Enantioselective Carboligation Catalyzed by Benzoylformate Decarboxylase. ACS Catal. 2019, 9, 5657–5667. [Google Scholar] [CrossRef]
- Planas, F.; McLeish, M.J.; Himo, F. Enzymatic Stetter Reaction: Computational Study of the Reaction Mechanism of MenD. ACS Catal. 2021, 11, 12355–12366. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, J.; Yang, G.F.; Zhan, C.G. Computational determination of fundamental pathway and activation barriers for acetohydroxyacid synthase-catalyzed condensation reactions of alpha-keto acids. J. Comput. Chem. 2010, 31, 1592–1602. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Y.; Zhang, R. A theoretical study of the catalytic mechanism of oxalyl-CoA decarboxylase, an enzyme for treating urolithiasis. RSC Adv. 2014, 4, 35777–35788. [Google Scholar] [CrossRef]
- Hou, Q.; Gao, J.; Liu, Y.; Liu, C. A QM/MM study on the catalytic mechanism of pyruvate decarboxylase. Theor. Chem. Acc. 2012, 131, 1280. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, X.; Hou, Q.; Liu, Y. Theoretical investigation on the dissociation of (R)-benzoin catalyzed by benzaldehyde lyase. Int. J. Quantum Chem. 2024, 114, 375–382. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y. QM/MM study on the catalytic mechanism of cyclohexane-1,2-dione hydrolase (CDH). Theor. Chem. Acc. 2014, 133, 1442. [Google Scholar] [CrossRef]
- Amara, P.; Fdez Galvan, I.; Fontecilla-Camps, J.C.; Field, M.J. The enamine intermediate may not be universal to thiamine catalysis. Angew. Chem. 2007, 46, 9019–9022. [Google Scholar] [CrossRef]
- Assary, R.S.; Broadbelt, L.J. Computational screening of novel thiamine-catalyzed decarboxylation reactions of 2-keto acids. Bioprocess. Biosyst. Eng. 2011, 34, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Du, H.; Zhang, X.; Gu, S.; Cai, H.; Kang, Y.; Pan, P.; Zhao, Q.; Hou, T. CarsiDock-Cov: A deep learning-guided approach for automated covalent docking and screening. Acta Pharm. Sin. B 2025, 15, 5758–5771. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Machius, M.; Chuang, J.L.; Wynn, R.M.; Chuang, D.T. The two active sites in human branched-chain alpha-keto acid dehydrogenase operate independently without an obligatory alternating-site mechanism. J. Biol. Chem. 2007, 282, 11904–11913. [Google Scholar] [CrossRef]
- Ludtke, S.; Neumann, P.; Erixon, K.M.; Leeper, F.; Kluger, R.; Ficner, R.; Tittmann, K. Sub-angstrom-resolution crystallography reveals physical distortions that enhance reactivity of a covalent enzymatic intermediate. Nat. Chem. 2013, 5, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Bellinzoni, M.; Wehenkel, A.; O’Hare, H.M.; Alzari, P.M. Functional plasticity and allosteric regulation of alpha-ketoglutarate decarboxylase in central mycobacterial metabolism. Chem. Biol. 2011, 18, 1011–1020. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Wagner, T.; Boyko, A.; Alzari, P.M.; Bunik, V.I.; Bellinzoni, M. Conformational transitions in the active site of mycobacterial 2-oxoglutarate dehydrogenase upon binding phosphonate analogues of 2-oxoglutarate: From a Michaelis-like complex to ThDP adducts. J. Struct. Biol. 2019, 208, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Laber, B.; Amrhein, N. Metabolism of 1-aminoethylphosphinate generates acetylphosphinate, a potent inhibitor of pyruvate dehydrogenase. Biochem. J. 1987, 248, 351–358. [Google Scholar]




| Type of Ligand | Actual Ligand | Ligand for Docking in Gnina |
|---|---|---|
| Substrates | Pyruvate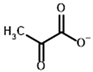 | (S)-Lactate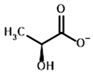 |
2-Oxobutyrate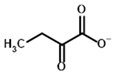 | (S)-2-hydroxybutyrate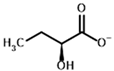 | |
Glyoxylate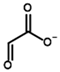 | Glycolate | |
| Inhibitors | Acetyl phosphinate (AcPH)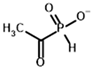 | [(R)-1-Hydroxyethyl] phosphinate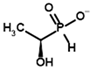 |
Acetyl (methyl) phosphinate (AcMePH)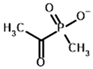 | [(R)-1-Hydroxyethyl] (methyl) phosphinate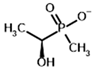 | |
Acetyl phosphonate (AcP)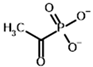 | [(R)-1-Hydroxyethyl] phosphonate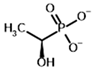 | |
Methyl acetyl phosphonate (AcPMe)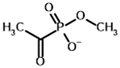 | Methyl [(R)-1-hydroxyethyl] phosphonate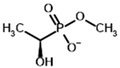 | |
Dimethyl acetyl phosphonate (AcPMe2)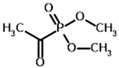 | Dimethyl [(R)-1-hydroxyethyl] phosphonate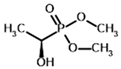 | |
Ethyl acetyl phosphonate (AcPEt)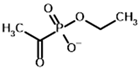 | Ethyl [(R)-1-hydroxyethyl] phosphonate |
| Enzyme Target | Type of Ligand | Name of the Ligand (Oxo-From) | Calculated Affinities, kcal/mol | |||
|---|---|---|---|---|---|---|
| Without Ligand Optimization | With Ligand Optimization | |||||
| AD4 Scoring | Vinardo | AD4 Scoring | Vinardo | |||
| Pyruvate dehydrogenase (PDH, EC 1.2.4.1) | Substrates | Pyruvate with flexible residues | −6.98 −12.44 | −2.21 −2.84 | −11.44 −16.35 | −2.73 −3.09 |
| 2-Oxobutyrate with flexible residues | −13.21 −16.40 | −1.71 −2.86 | −16.17 −18.14 | −2.85 −3.80 | ||
| Glyoxylate with flexible residues | −9.14 −12.13 | −1.57 −2.55 | −9.26 −13.49 | −1.61 −2.69 | ||
| Inhibitors | Acetyl phosphinate (AcPH) with flexible residues | −10.03 −11.75 | −2.28 −2.74 | −11.50 −17.19 | −2.83 −3.39 | |
| Acetyl (methyl) phosphinate (AcMePH) with flexible residues | −1.60 −11.69 | −2.22 −2.50 | −4.44 −14.08 | −2.48 −3.57 | ||
| Acetyl phosphonate (AcP) with flexible residues | 9.28 −10.98 | −2.15 −2.73 | −4.99 −15.59 | −0.84 −3.02 | ||
| Methyl acetyl phosphonate (AcPMe) with flexible residues | 5.77 −15.12 | −2.73 −3.20 | 9.19 −11.48 | −1.63 −2.81 | ||
| Dimethyl acetyl phosphonate (AcPMe2) with flexible residues | 4.03 −7.73 | −1.76 −3.14 | −6.51 −20.95 | −1.11 −3.23 | ||
| Ethyl acetyl phosphonate (AcPEt) with flexible residues | 2.65 −16.54 | −3.31 −3.89 | 3.19 −20.32 | −2.38 −3.96 | ||
| Branched-chain 2-oxo acid dehydrogenase (BCDH, EC 1.2.4.4) | Substrates | Pyruvate | −4.84 | −1.85 | −10.18 | −2.71 |
| 2-Oxobutyrate | −2.96 | −0.75 | −12.31 | −2.71 | ||
| 2-Oxoisovalerate | −8.57 | −2.23 | −13.89 | −2.45 | ||
| 2-Oxoisocaproate | −8.29 | −2.18 | −18.44 | −2.23 | ||
| (S)-3-Methyl-2-oxovalerate (R)-3-Methyl-2-oxovalerate (allo-isomer) | −5.97 −10.09 | −1.84 −1.67 | −10.16 −18.12 | −2.03 −2.52 | ||
| Inhibitors | Isobutyryl phosphonate | −1.60 | −0.77 | 7.32 | −0.04 | |
| Methyl isobutyryl phosphonate | −10.17 | −2.27 | −17.47 | −3.17 | ||
| Dimethyl isobutyryl phosphonate | −10.77 | −2.88 | −20.04 | −3.06 | ||
| Isovaleryl phosphonate | 15.28 | 2.25 | −17.44 | −2.56 | ||
| Methyl isovaleryl phosphonate | −8.86 | −2.12 | −14.39 | −2.98 | ||
| Dimethyl isovaleryl phosphonate | −8.93 | −2.89 | −25.37 | −2.90 | ||
| (S)-2-Methylbutyryl phosphonate (MBP) (R)-2-Methylbutyryl phosphonate | −3.41 −4.25 | −1.76 −1.53 | −19.33 −0.63 | −3.72 −0.01 | ||
| Methyl (S)-2-methylbutyryl phosphonate (MBPMe) Methyl (R)-2-methylbutyryl phosphonate | −7.03 −6.73 | −2.05 −1.21 | −17.7 −24.29 | −1.47 −3.44 | ||
| Dimethyl (S)-2-methylbutyryl phosphonate (MBPMe2) Dimethyl (R)-2-methylbutyryl phosphonate | −6.77 −7.25 | −2.82 −0.95 | −17.65 −19.23 | −3.01 −0.40 | ||
| Diethyl (S)-2-methylbutyryl phosphonate (MBPEt2) Diethyl (R)-2-methylbutyryl phosphonate | −8.33 −12.58 | −2.29 −1.95 | −23.21 −30.05 | −3.73 −3.15 | ||
| 2-Oxoglutarate dehydrogenase (OGDH, EC 1.2.4.2) | Substrates | 2-Oxoglutarate | −24.75 | −4.67 | −25.19 | −4.65 |
| 2-Oxoadipate | −28.96 | −5.11 | −29.52 | −5.12 | ||
| Inhibitors | Succinyl phosphonate (SP) | −25.56 | −5.40 | −26.82 | −5.09 | |
| Phosphonomethyl succinyl phosphonate (PMSP) | −24.47 | −4.69 | −29.76 | −5.05 | ||
| Phosphonodimethyl succinyl phosphonate (PDSP) | −12.22 | −2.72 | −26.06 | −2.89 | ||
| Phosphonoethyl succinyl phosphonate (PESP) | −28.31 | −5.65 | −30.71 | −5.30 | ||
| Carboxyethyl succinyl phosphonate (CESP) | −35.02 | −6.34 | −34.76 | −5.57 | ||
| Diethyl succinyl phosphonate (DESP) | −28.99 | −4.23 | −35.22 | −4.14 | ||
| Triethyl succinyl phosphonate (TESP) | −7.77 | −1.62 | −36.40 | −1.98 | ||
| Glutaryl phosphonate (GP) | −24.30 | −4.61 | −29.98 | −5.30 | ||
| Transketolase (TKT, EC 2.2.2.1) | Substrate | D-xylulose-5-phosphate | −29.20 | −6.90 | −28.67 | −6.46 |
| Type of Ligand | Actual Ligand | Ligand for Docking in Gnina |
|---|---|---|
| Substrates | 2-Oxoisovalerate | (S)-2-Hydroxyisovalerate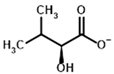 |
2-Oxoisocaproate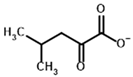 | (S)-2-Hydroxyisocaproate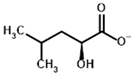 | |
3-Methyl-2-oxovalerate | (2S)-2-Hydroxy-3-methylvalerate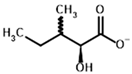 | |
| Inhibitors | Methyl 2-methylbutyryl phosphonate (MBPMe) 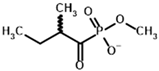 | Methyl [(1R)-1-hydroxy-2-methylpropyl] phosphonate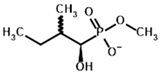 |
| Diethyl 2-methylbutyryl phosphonate (MBPEt2) 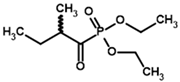 | Diethyl [(1R)-1-hydroxy-2-methylpropyl] phosphonate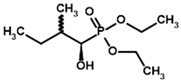 |
| Type of Ligand | Actual Ligand | Ligand for Docking in Gnina |
|---|---|---|
| Substrates | 2-Oxoglutarate | (S)-2-Hydroxyglutarate |
2-Oxoadipate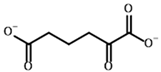 | (S)-2-Hydroxyadipate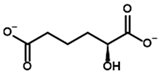 | |
| Inhibitors | Succinyl phosphonate (SP) | (R)-1-Hydroxy-3-carboxypropyl phosphonate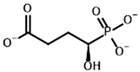 |
| Phosphonomethyl succinyl phosphonate (PMSP) 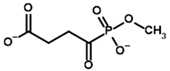 | Methyl [(R)-1-Hydroxy-3-carboxypropyl] phosphonate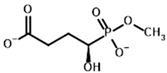 | |
| Phosphonodimethyl succinyl phosphonate (PDSP)  | Dimethyl [(R)-1-Hydroxy-3-carboxypropyl] phosphonate | |
| Phosphonoethyl succinyl phosphonate (PESP) 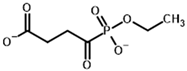 | Ethyl [(R)-1-Hydroxy-3-carboxypropyl] phosphonate 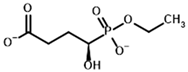 | |
| Carboxyethyl succinyl phosphonate (CESP) 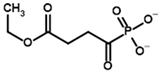 | (R)-1-Hydroxy-3-ethylcarboxypropyl phosphonate 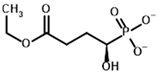 | |
| Diethyl succinyl phosphonate (DESP) 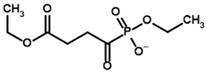 | Ethyl (R)-1-Hydroxy-3-ethylcarboxypropyl phosphonate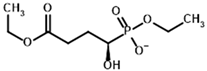 | |
| Triethyl succinyl phosphonate (TESP) 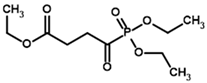 | Diethyl (R)-1-Hydroxy-3-ethylcarboxypropyl phosphonate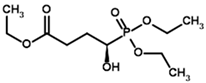 | |
Glutaryl phosphonate (GP)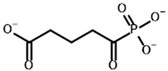 | (R)-1-Hydroxy-4-carboxybutyryl phosphonate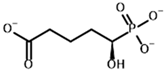 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artiukhov, A.V.; Aleshin, V.A. Covalent Docking to the Active Sites of Thiamine Diphosphate-Dependent Enzymes. Molecules 2025, 30, 4427. https://doi.org/10.3390/molecules30224427
Artiukhov AV, Aleshin VA. Covalent Docking to the Active Sites of Thiamine Diphosphate-Dependent Enzymes. Molecules. 2025; 30(22):4427. https://doi.org/10.3390/molecules30224427
Chicago/Turabian StyleArtiukhov, Artem V., and Vasily A. Aleshin. 2025. "Covalent Docking to the Active Sites of Thiamine Diphosphate-Dependent Enzymes" Molecules 30, no. 22: 4427. https://doi.org/10.3390/molecules30224427
APA StyleArtiukhov, A. V., & Aleshin, V. A. (2025). Covalent Docking to the Active Sites of Thiamine Diphosphate-Dependent Enzymes. Molecules, 30(22), 4427. https://doi.org/10.3390/molecules30224427






