Structural Characterization and In Vitro Hypoglycemic Activity of a Polysaccharides Obtained from Fructus arctii
Abstract
1. Introduction
2. Results
2.1. Structural and Physicochemical Features of FAP-W
2.2. Structural Characterization of FAP-W
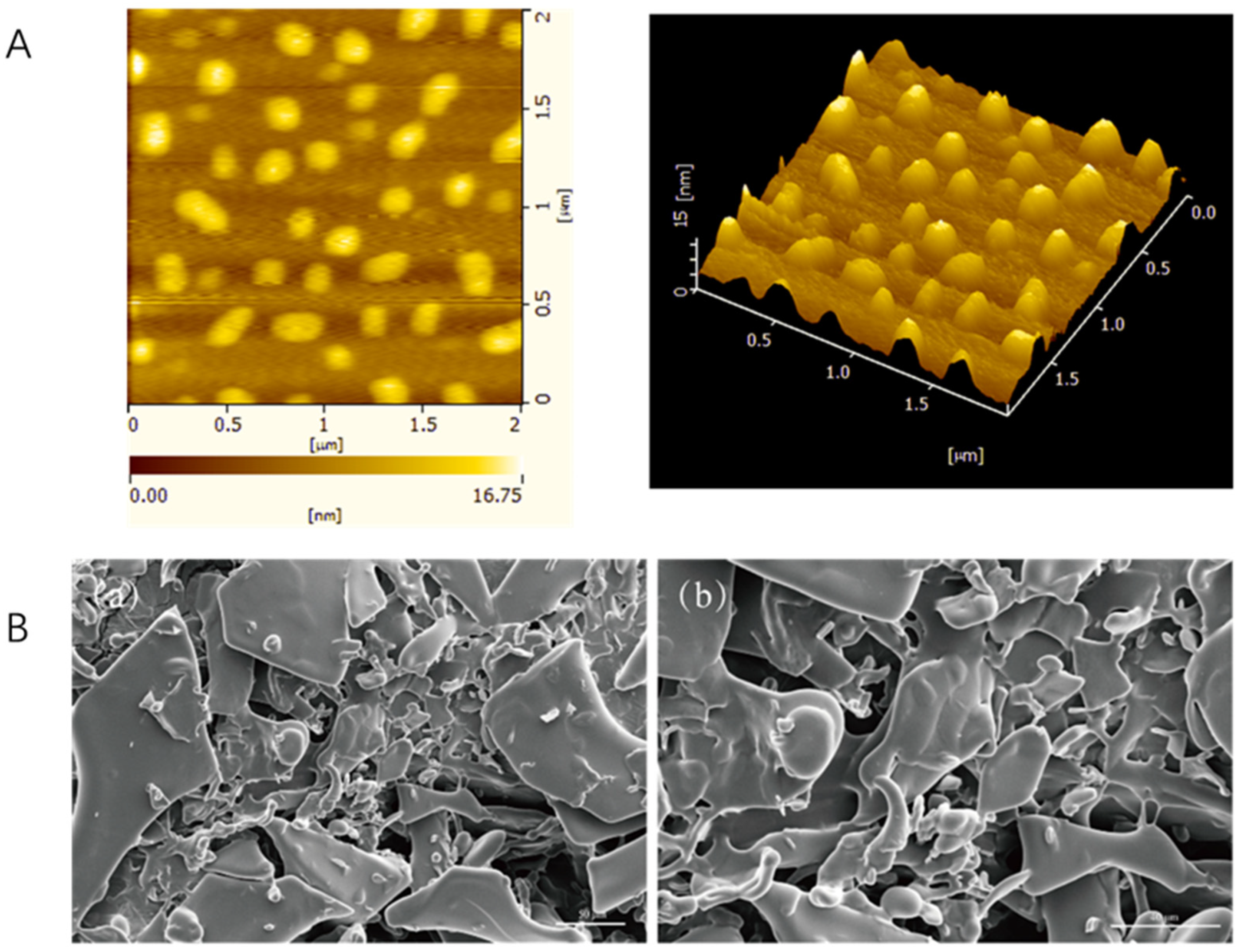
2.2.1. Atomic Force Microscope (AFM)
2.2.2. Scanning Electron Microscope (SEM)
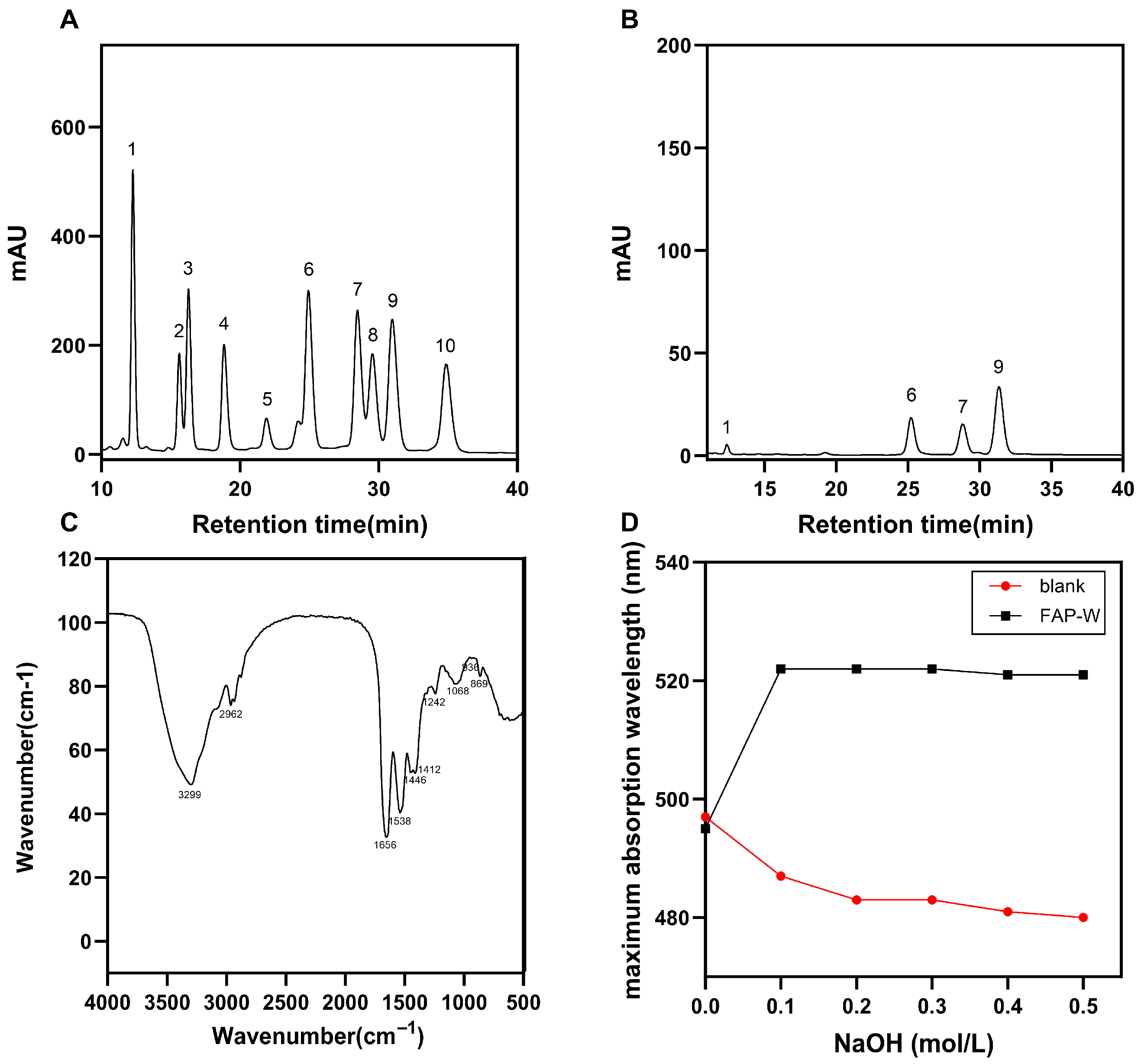
2.2.3. Molecular Weight
2.2.4. Monosaccharide Composition Analysis
2.2.5. Fourier Infrared Spectroscopy (FT-IR) Spectroscopy Analysis
2.2.6. Congo Red Test Analysis
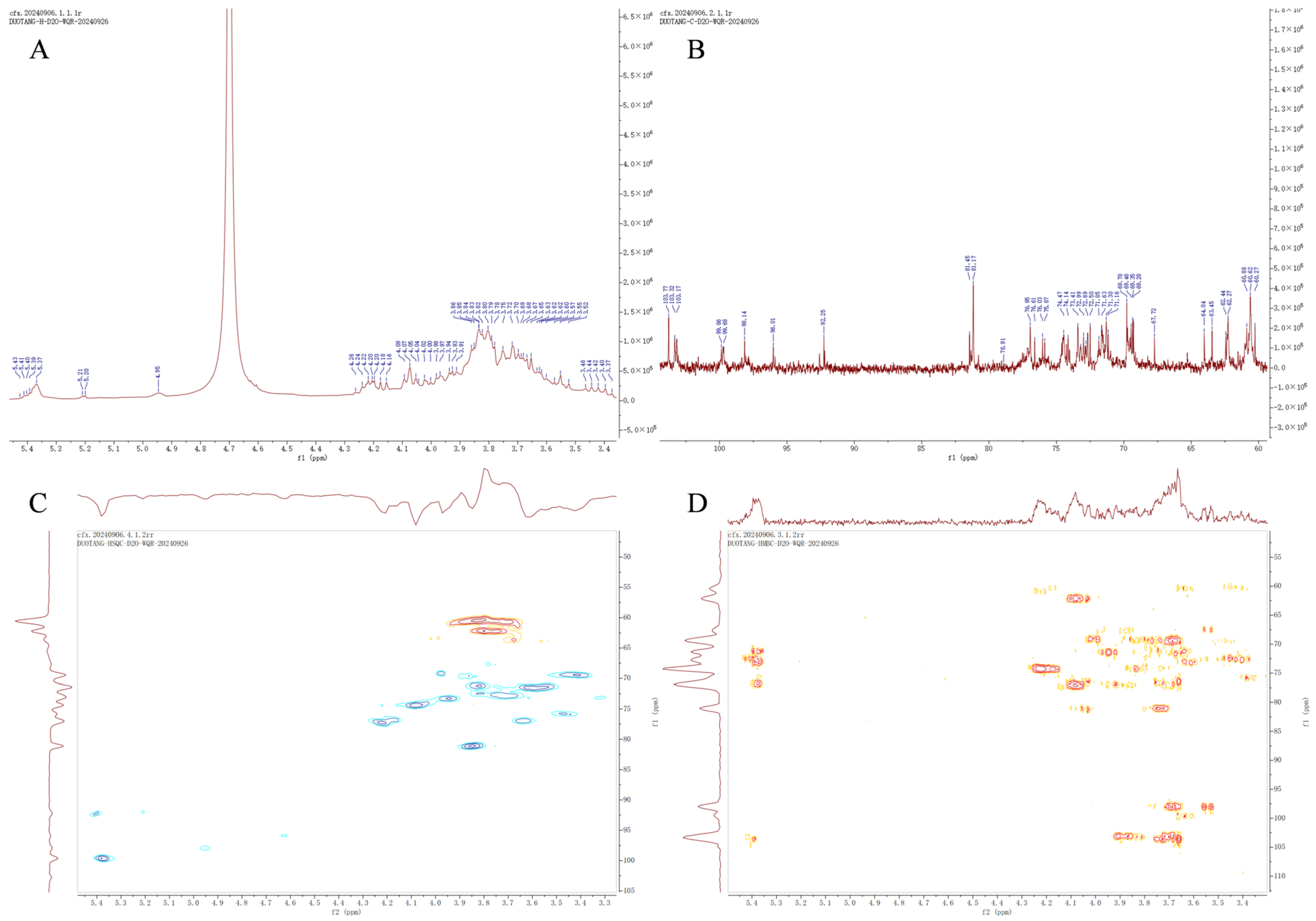
2.2.7. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.3. Hypoglycemic Activity of FAP-W in IR-HepG2 Cells
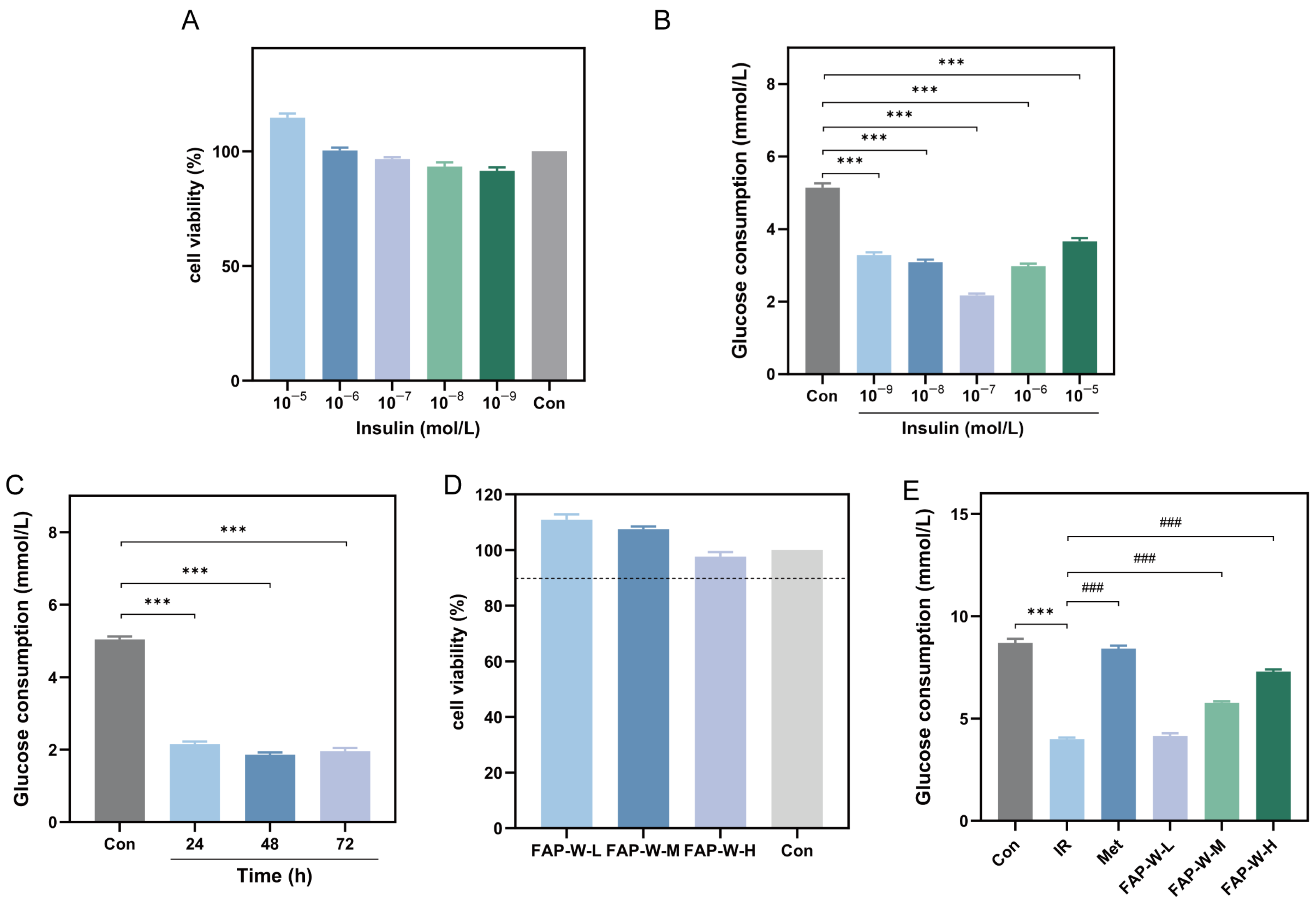
2.3.1. Validation of the Insulin Resistance Model
2.3.2. Effect of FAP-W on Cell Survival Rate and Glucose Consumption
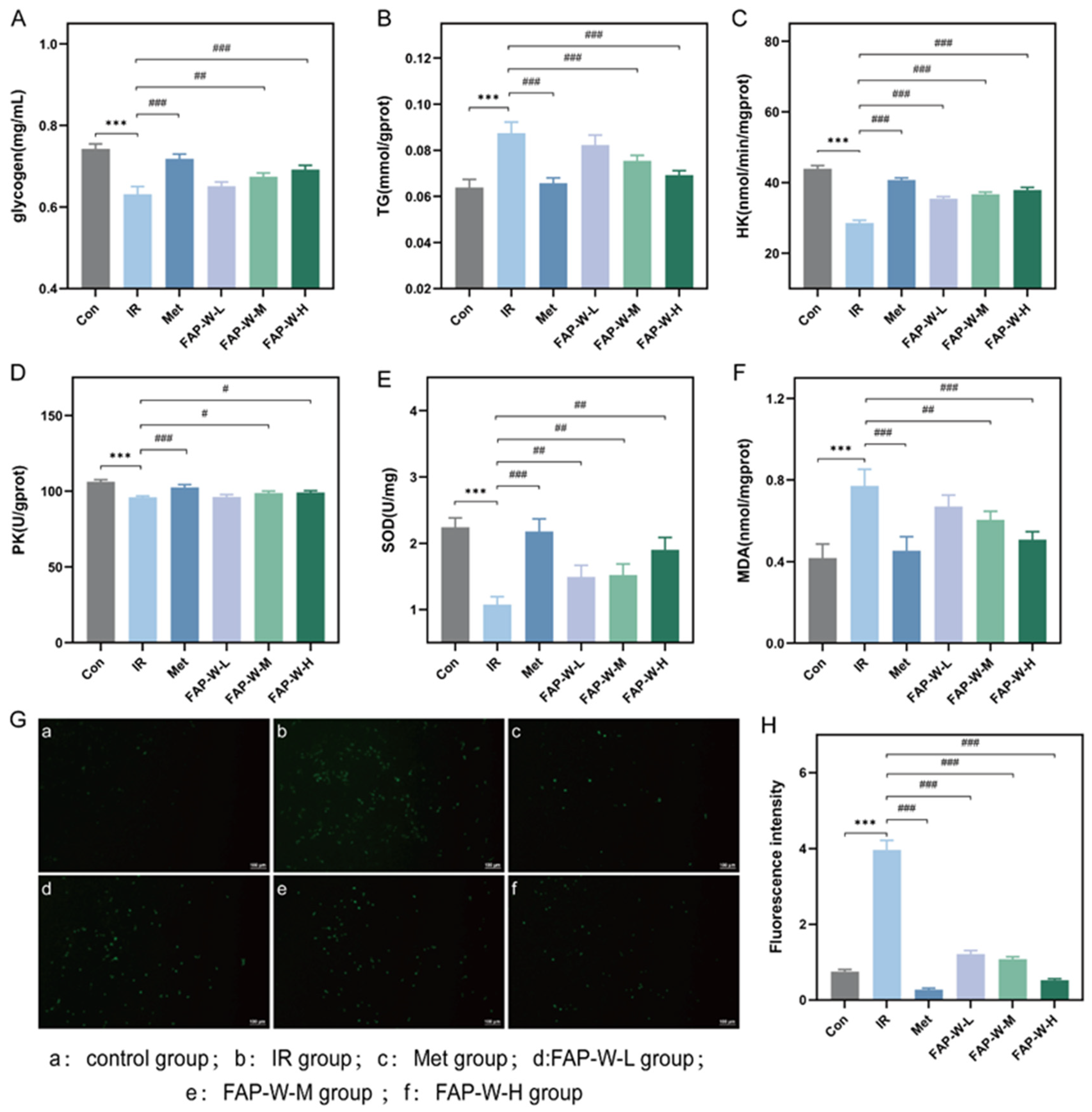
2.3.3. Glycogen and Triglyceride Content (TG) Content
2.3.4. Hexokinase (HK) and Pyruvate Kinase (PK) Activity
2.3.5. Effect of FAP-W on Oxidative Damage in the IR-HepG2 Model
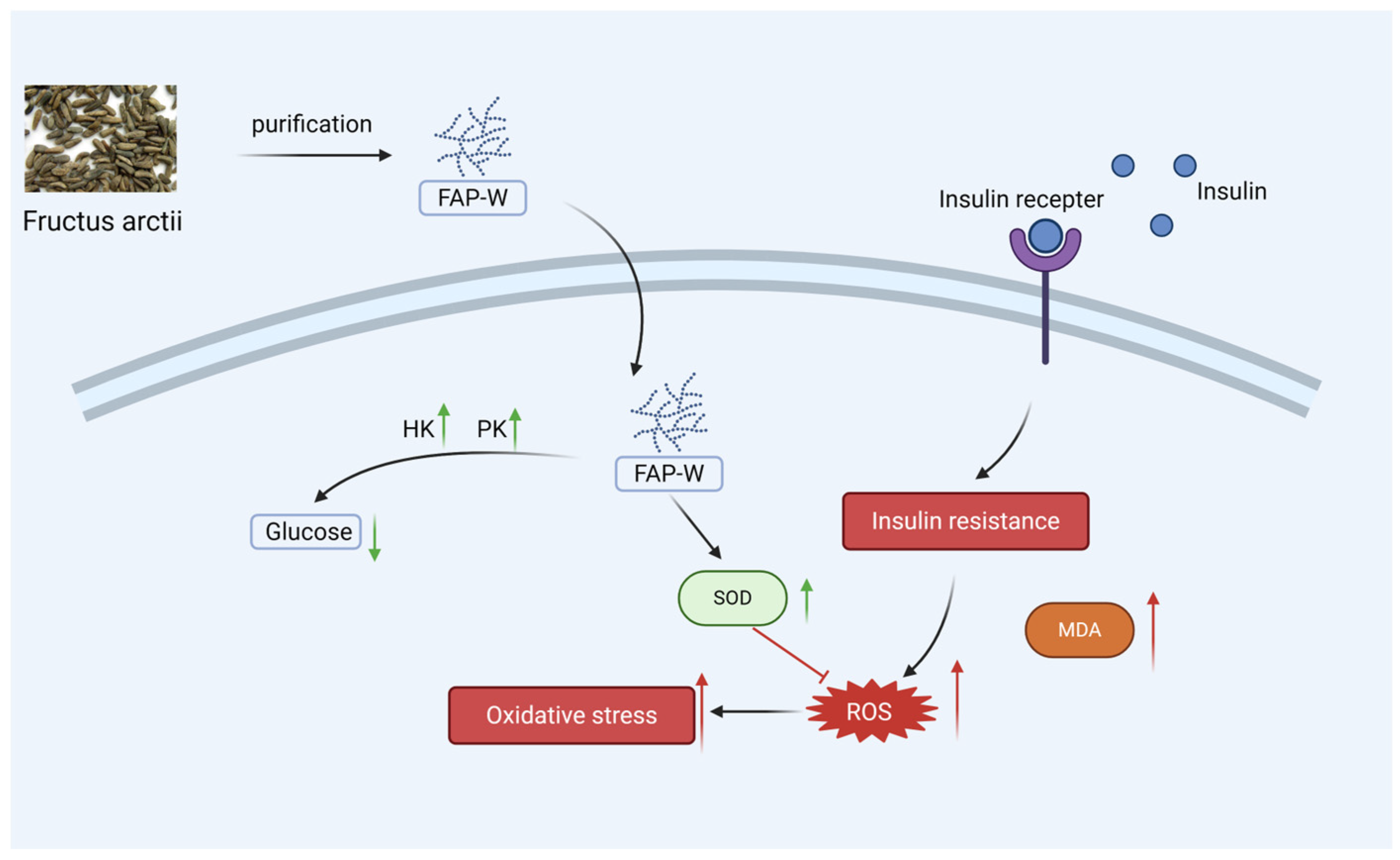
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction and Physicochemical Characterization of FAP-W
4.2.1. Extraction of FAP-W
4.2.2. Physicochemical Characterization of FAP-W
4.2.3. High-Performance Gel Permeation Chromatography (HPLC)
4.3. Structural Characterization
4.3.1. Atomic Force Microscope
4.3.2. Scanning Electron Microscope
4.3.3. Analysis of Monosaccharide Constitution
4.3.4. UV–Vis Absorption Spectra
4.3.5. FT-IR Spectroscopy
4.3.6. Congo Red Test
4.3.7. NMR Spectroscopy
4.4. Assays of Hypoglycemic In Vitro
4.4.1. Construction of an Insulin Resistance Model
4.4.2. Cytotoxicity Assay of FAP-W
4.4.3. Glucose Depletion Assay of FAP-W
4.4.4. Glucose Metabolism in IR-HepG2 Cells
4.4.5. Lipid Metabolism in IR-HepG2 Cells
4.4.6. Oxidative Damage in IR-HepG2 Cells
4.5. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alasvand Zarasvand, S.; Ogawa, S.; Nestor, B.; Bridges, W.; Haley-Zitlin, V. Effects of Herbal Tea (Non-Camellia sinensis) on Glucose Homeostasis and Serum Lipids in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutr. Rev. 2025, 83, e1128–e1145. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.; Son, J.I.; Rhee, S.Y.; Kim, D.-Y.; Chon, S.; Lim, H.; Woo, J.-T. Dietary glutamic acid and aspartic acid as biomarkers for predicting diabetic retinopathy. Sci. Rep. 2021, 11, 7244. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, M.; Gao, X.; Wang, S.; Fu, C.; Zeng, J.; He, X. Phocea, Pseudoflavonifractor and Lactobacillus intestinalis: Three Potential Biomarkers of Gut Microbiota That Affect Progression and Complications of Obesity-Induced Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 835–850. [Google Scholar] [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef]
- Yang, M.-H.; Yang, Y.; Zhou, X.; Chen, H.-G. Advances in polysaccharides of natural source of anti-diabetes effect and mechanism. Mol. Biol. Rep. 2024, 51, 101. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.J.; Wang, Y.; Chen, S.Y.; Yang, Z.M. Astragalus Polysaccharides Alleviate Type 2 Diabetic Rats by Reversing the Expressions of Sweet Taste Receptors and Genes Related to Glycolipid Metabolism in Liver. Front. Pharmacol. 2022, 13, 916603. [Google Scholar] [CrossRef]
- Zhu, L.; Ye, C.; Hu, B.; Xia, H.; Bian, Q.; Liu, Y.; Kong, M.; Zhou, S.; Liu, H. Regulation of gut microbiota and intestinal metabolites by Poria cocos oligosaccharides improves glycolipid metabolism disturbance in high-fat diet-fed mice. J. Nutr. Biochem. 2022, 107, 109019. [Google Scholar] [CrossRef]
- Liang, J.; Xu, R.; Zong, K.; Yu, N.; Wu, Z.; Wu, H.; Zhou, A. Structural analysis and anti-obesity effect of Polygonatum cyrtonema polysaccharide against obesity induced by high-fat diet in mice. Int. J. Food Sci. Technol. 2021, 56, 4473–4483. [Google Scholar] [CrossRef]
- Gong, P.; Guo, Y.; Chen, X.; Cui, D.; Wang, M.; Yang, W.; Chen, F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules 2022, 27, 4192. [Google Scholar] [CrossRef]
- Nam, Y.K.; Kim, M.H.; Ha, I.J.; Yang, W.M. Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 2359. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Qiao, Y.; Bai, X.; Zhu, W.; Li, Z.; Zheng, Z. Immunomodulatory effects of inulin-type fructans from Arctium lappa L. by targeting gut microbiota and their metabolites. Food Chem. 2025, 467, 142308. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Jiang, M.; Zhang, B.; Chen, Q.; Zhang, C.; Zheng, Z.; Qiao, X. Visualizing the Spatial Distribution of Arctium lappa L. Root Components by MALDI-TOF Mass Spectrometry Imaging. Foods 2022, 11, 3957. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Wu, E.; Li, Q.; Xiang, L.; Qi, J. Arctigenin from Fructus arctii Exhibits Antiaging Effects via Autophagy Induction, Antioxidative Stress, and Increase in Telomerase Activity in Yeast. Antioxidants 2024, 13, 684. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Y.; Yang, H.; Liu, T.; Han, T.; Dai, W.; Cen, J.; Ouyang, F.; Chen, J.; Liu, J.; et al. Effects of fermented Arctium lappa L. root by Lactobacillus casei on hyperlipidemic mice. Front. Pharmacol. 2024, 15, 1447077. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Mun, J.-G.; Jeon, H.D.; Yoon, D.H.; Choi, B.-M.; Kee, J.-Y.; Hong, S.-H. The Extract of Arctium lappa L. Fruit (Arctii Fructus) Improves Cancer-Induced Cachexia by Inhibiting Weight Loss of Skeletal Muscle and Adipose Tissue. Nutrients 2020, 12, 3195. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Chen, H.; Li, H.; Yang, X.; Guo, X.; Zhang, Y.; Ma, J.; Yang, J.; Ma, S. Extraction, structural characterization, and antioxidant activity of polysaccharides derived from Arctium lappa L. Front. Nutr. 2023, 10, 1149137. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Bai, X.; Zhu, W.; Ali, I.; Ma, C.; Zheng, Z.; Qiao, X. Integrated Mechanism of Immune Response Modulation by Arctium lappa L. Fructans Based on Microbiome and Metabolomics Technologies. J. Agric. Food Chem. 2024, 72, 10981–10994. [Google Scholar] [CrossRef]
- de Souza, A.R.C.; de Oliveira, T.L.; Fontana, P.D.; Carneiro, M.C.; Corazza, M.L.; de Messias Reason, I.J.; Bavia, L. Phytochemicals and Biological Activities of Burdock (Arctium lappa L.) Extracts: A Review. Chem. Biodivers. 2022, 19, e202200615. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, Z.; Dong, H.; Ma, C.; Qiao, Y.; Zheng, Z. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: A comparison. Int. J. Biol. Macromol. 2021, 182, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.C.; Eun, J.B. Mechanistic insights on burdock (Arctium lappa L.) extract effects on diabetes mellitus. Food Sci. Biotechnol. 2022, 31, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, J.; Kuang, J.; He, Z.; Zong, Y.; Qi, L.; Du, R.; Ji, Y.; Zhao, K. Preparation, characterization, bioactivity, and safety evaluation of PEI-modified PLGA nanoparticles loaded with polysaccharide from Cordyceps militaris. Adv. Compos. Hybrid. Mater. 2024, 8, 93. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhang, X.; Zhang, F.; Linhardt, R.J. Structural and immunological studies on the polysaccharide from spores of a medicinal entomogenous fungus Paecilomyces cicadae. Carbohydr. Polym. 2021, 254, 117462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, F.; Guo, Y.; Ji, H.; Zhang, W.; Mao, G.; Feng, W.; Chen, Y.; Yang, L.; Wu, X. Structural characterization of a novel Schisandra polysaccharides and nutritional intervention in immunotoxicity to PCBs. Carbohydr. Polym. 2021, 258, 117380. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.; Yu, W.; Shi, C.; Zhang, X.; Yu, H.; Ma, F. Enhanced konjac glucomannan hydrolysis by lytic polysaccharide monooxygenases and generating prebiotic oligosaccharides. Carbohydr. Polym. 2021, 253, 117241. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Gu, S.; Pan, L.; Sun, H.; Gong, E.; Zhu, Z.; Wen, T.; Daba, G.M.; Elkhateeb, W.A. Structure analysis and antioxidant activity of polysaccharide-iron (III) from Cordyceps militaris mycelia. Int. J. Biol. Macromol. 2021, 178, 170–179. [Google Scholar] [CrossRef]
- Wang, D.; Yin, H.B.; Xu, L.; Zhang, Y.; Li, M. Structural characterization and in vitro antioxidant, hypoglycemic and uric acid-lowering effects of burdock root polysaccharides. Chin. J. Tradit. Chin. Med. 2024, 42, 66–71. [Google Scholar] [CrossRef]
- Xiaole, C.; Wenwen, L.; Qiang, C.; Lingyu, L. Structure analysis and anti-inflammatory activity evaluation of neutral polysaccharides from Arctium lappa L. Sci. Technol. Food Ind. 2023, 44, 45–54. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Caleffi, E.R.; Krausová, G.; Hyršlová, I.; Paredes, L.L.; dos Santos, M.M.; Sassaki, G.L.; Gonçalves, R.A.; de Oliveira, A.J. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots. Int. J. Biol. Macromol. 2015, 80, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Hou, Y.; Zhang, P.; Tan, M. Plant polysaccharides: Sources, structures, and antidiabetic effects. Curr. Opin. Food Sci. 2023, 51, 101013. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Shang, X.; Hu, G.; Zhang, M.; Zuo, J.; Wang, M.; Wu, J.; Lv, Q.; Zhou, Y.; Shao, T.; Wang, G. Therapeutic potential of polysaccharides in type 2 diabetes mellitus via multi-target modulation of gut microbiota. Chin. Herb. Med. 2025, in press. [Google Scholar] [CrossRef]
- Fan, X.; Li, K.; Qin, X.; Li, Z.; Du, Y. Advances in the Preparation and Bioactivity of Polysaccharides From Medicinal Plants With Different Molecular Weights: A Review. Chem. Biodivers. 2025, 22, e03031. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, T.; Li, S.; Liu, J.; Li, M.; Lu, J.; Zhang, M.; Chen, H. Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships. Pharmaceuticals 2024, 17, 456. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, L.; Xiao, Z.; Li, Y.; Ma, J.; Ding, J.; Yang, J. Comparative Study on the Structural Properties and Bioactivities of Three Different Molecular Weights of Lycium barbarum Polysaccharides. Molecules 2023, 28, 701. [Google Scholar] [CrossRef]
- Zhong, Q.-W.; Zhou, T.-S.; Qiu, W.-H.; Wang, Y.-K.; Xu, Q.-L.; Ke, S.-Z.; Wang, S.-J.; Jin, W.-H.; Chen, J.-W.; Zhang, H.-W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef]
- Newman, P.P.; Schmitt, B.L.; Maurmann, R.M.; Pence, B.D. Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging. Molecules 2025, 30, 1178. [Google Scholar] [CrossRef]
- Jansen, L.M.; Hendriks, V.C.A.; Bentlage, H.; Ranoux, A.; Raaijmakers, H.W.C.; Boltje, T.J. The Industrial Application Potential of Sugar Beet Pulp Derived Monosaccharides d-Galacturonic Acid and l-Arabinose. ChemBioChem 2024, 25, e202400521. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Hexokinase-2-Linked Glycolytic Overload and Unscheduled Glycolysis—Driver of Insulin Resistance and Development of Vascular Complications of Diabetes. Int. J. Mol. Sci. 2022, 23, 2165. [Google Scholar] [CrossRef]
- Murao, N.; Morikawa, R.; Seino, Y.; Shimomura, K.; Maejima, Y.; Ohno, T.; Yokoi, N.; Yamada, Y.; Suzuki, A. Pyruvate kinase modulates the link between β-cell fructose metabolism and insulin secretion. FASEB J. 2025, 39, e70500. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front. Nutr. 2023, 10, 1125831. [Google Scholar] [CrossRef]
- Le, B.; Anh, P.-T.-N.; Yang, S.-H. Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells. Polymers 2021, 13, 1780. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Yan, W.; Wei, L.; Xiong, X.; Tian, Y.; Lu, Q.; Mu, L. Structural characterization and hypoglycemic activity of a polysaccharide from Imperatae Rhizoma. Int. J. Biol. Macromol. 2025, 308, 142654. [Google Scholar] [CrossRef]
- Jiang, Y.-y.; Yu, J.; Li, Y.-b.; Wang, L.; Hu, L.; Zhang, L.; Zhou, Y.-h. Extraction and antioxidant activities of polysaccharides from roots of Arctium lappa L. Int. J. Biol. Macromol. 2019, 123, 531–538. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Batieno, B.J.; Fatokun, C.; Boukar, O. A High Plant Density and the Split Application of Chemical Fertilizer Increased the Grain and Protein Content of Cowpea (Vigna unguiculata) in Burkina Faso, West Africa. Agriculture 2022, 12, 199. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yin, Y.; Xiao, S.; Zhang, R.; Yang, Y.; Li, L.; Xu, H.; Zhang, X.; Hu, P. Chemical structure elucidation and functional activities comparison of two polysaccharides purified from Citrus reticulata Blanco peels. Chem. Biol. Technol. Agric. 2024, 11, 37. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhang, R.G.; Deng, H.; Liu, X.; Wang, Y. Comparison of pulp quality characteristics and antioxidant activity of rambutan fruits at different maturity. Mod. Food Sci. 2021, 37, 138–144, 293. [Google Scholar] [CrossRef]
- Gong, P.; Wang, M.; Guo, Y.; Long, H.; Wang, Z.; Cui, D.; Yao, W.; Yang, W.; Chen, F.; Xie, J. Structure Characterization, In Vitro Antioxidant and Anti-Tumor Activity of Sulfated Polysaccharide from Siraitia grosvenorii. Foods 2023, 12, 2133. [Google Scholar] [CrossRef]
- Teng, S.; Zhang, Y.; Jin, X.; Zhu, Y.; Li, L.; Huang, X.; Wang, D.; Lin, Z. Structure and hepatoprotective activity of Usp10/NF-κB/Nrf2 pathway-related Morchella esculenta polysaccharide. Carbohydr. Polym. 2023, 303, 120453. [Google Scholar] [CrossRef]
- Chang, S.; Chen, Y.; Qiu, H.; Zhu, B.; You, L.; Cheung, P.C.K. The isolation, structure characterizations and anti-photoaging activities of sulfated polysaccharides isolated from Sargassum fusiforme. Chem. Biol. Technol. Agric. 2024, 11, 64. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Qu, Y.; Guo, X.; Yang, X.; Cui, X.; Wang, C. Structural characterization of a novel polysaccharide from Panax notoginseng residue and its immunomodulatory activity on bone marrow dendritic cells. Int. J. Biol. Macromol. 2020, 161, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wu, X.; Han, G.; Sun, L.; Zheng, C.; Hou, H.; Xu, B.B.; El-Bahy, Z.M.; Qian, C.; Kallel, M.; et al. Phyllostachys nigra (Lodd. ex Lindl.) derived polysaccharide with enhanced glycolipid metabolism regulation and mice gut microbiome. Int. J. Biol. Macromol. 2024, 257, 128588. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, W.; Liu, J.; Kang, X.; Yan, J.; Zhang, T.; Yang, L.; Shen, L.; Liu, D. The glucotoxicity protecting effect of honokiol in human hepatocytes via directly activating AMPK. Front. Nutr. 2022, 9, 1043009. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Gao, J.; Long, H.; Gao, H.; Yang, W.; Wang, J.; Li, N.; Zhao, Y.; Liu, H.; Chen, F. Structural Characterization and In Vitro Hypoglycemic Activity of a Polysaccharides Obtained from Fructus arctii. Molecules 2025, 30, 4403. https://doi.org/10.3390/molecules30224403
Gong P, Gao J, Long H, Gao H, Yang W, Wang J, Li N, Zhao Y, Liu H, Chen F. Structural Characterization and In Vitro Hypoglycemic Activity of a Polysaccharides Obtained from Fructus arctii. Molecules. 2025; 30(22):4403. https://doi.org/10.3390/molecules30224403
Chicago/Turabian StyleGong, Pin, Jiawei Gao, Hui Long, Haotian Gao, Wenjuan Yang, Jing Wang, Nan Li, Yanni Zhao, Huan Liu, and Fuxin Chen. 2025. "Structural Characterization and In Vitro Hypoglycemic Activity of a Polysaccharides Obtained from Fructus arctii" Molecules 30, no. 22: 4403. https://doi.org/10.3390/molecules30224403
APA StyleGong, P., Gao, J., Long, H., Gao, H., Yang, W., Wang, J., Li, N., Zhao, Y., Liu, H., & Chen, F. (2025). Structural Characterization and In Vitro Hypoglycemic Activity of a Polysaccharides Obtained from Fructus arctii. Molecules, 30(22), 4403. https://doi.org/10.3390/molecules30224403




