Preparation and Characterization of Polydatin–Chitosan Nanocapsules for Enhanced Drug Delivery Efficacy
Abstract
1. Introduction
2. Results
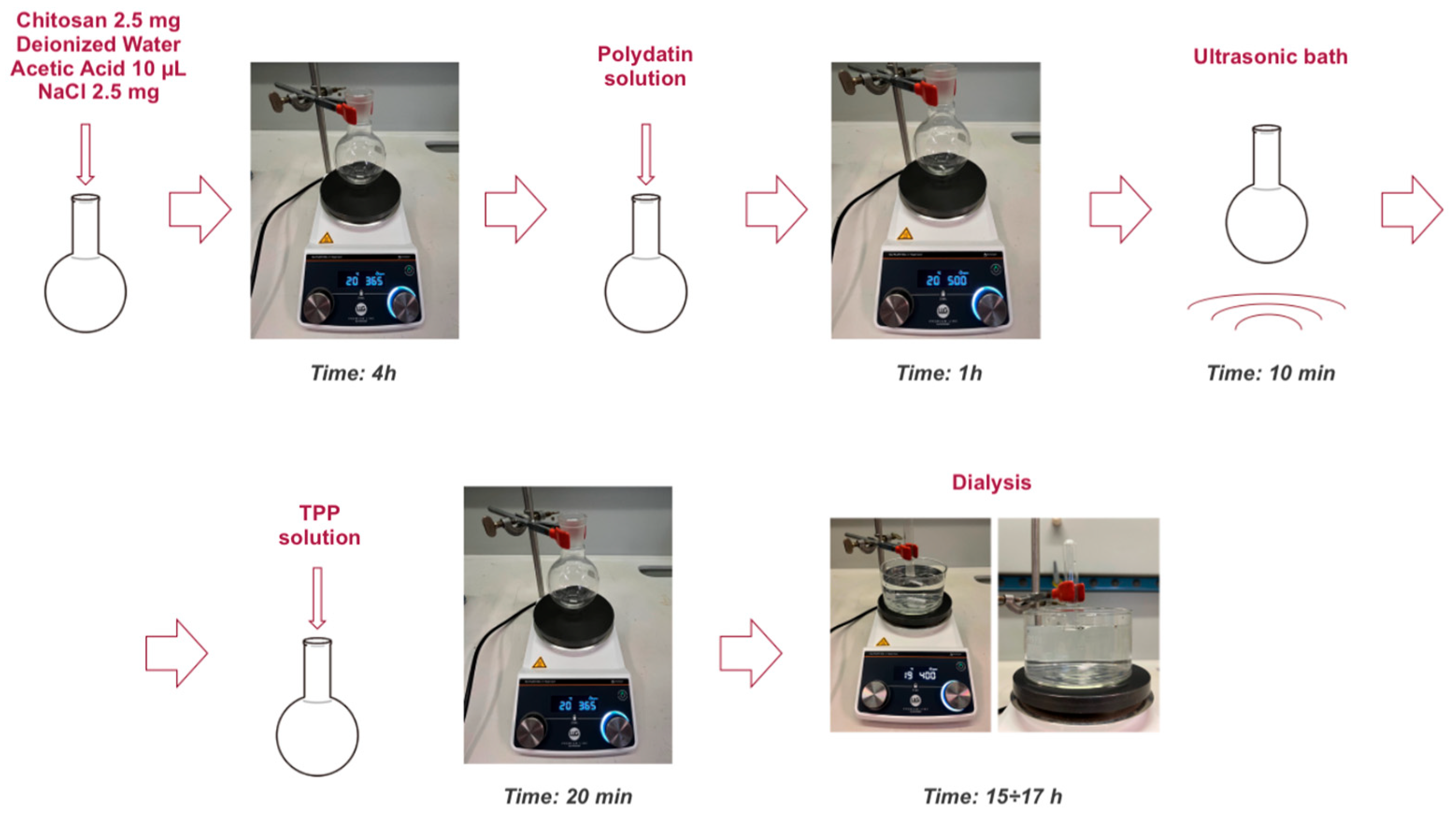
2.1. NPs Preparation
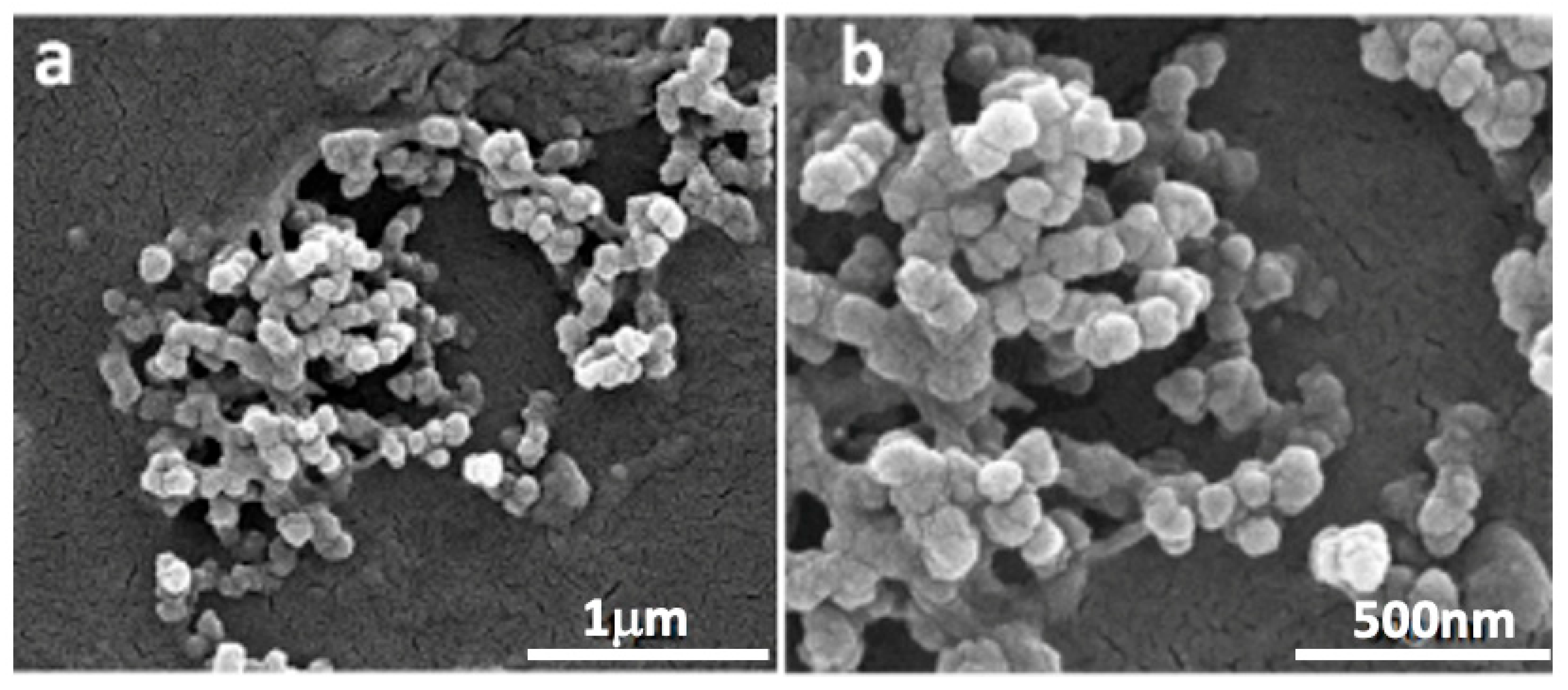
2.2. PD-CS-NPs Characterization
2.3. Evaluation of Antiproliferative Activity
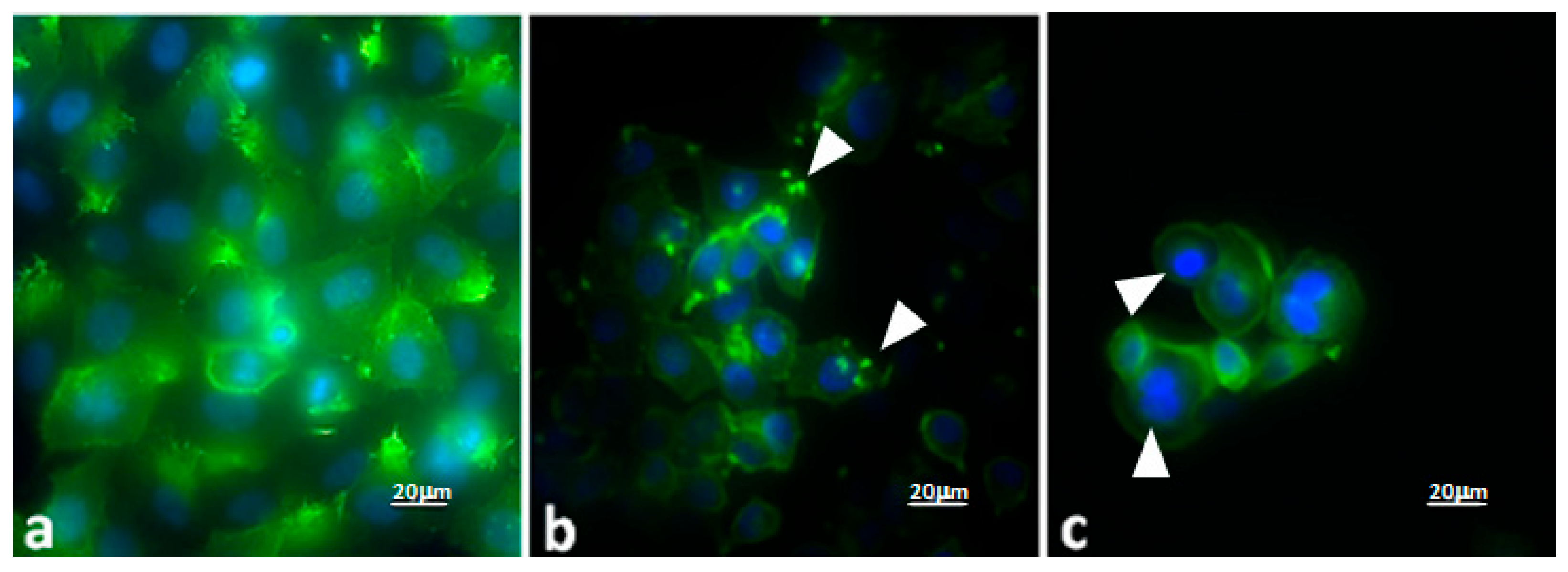
Evaluation of the Effects of PD–CS-NPs on the Actin Network and Morphology of SKBR3 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Nanoparticles Preparation
- -
- Chitosan: To obtain a final concentration of 0.5 mg/mL, 2.5 mg of the polymer was weighed.
- -
- Salt (NaCl): The same final concentration of 0.5 mg/mL was maintained, so 2.5 mg of salt was weighed.
- -
- TPP: A weight ratio of 6:1 between chitosan and TPP was selected. Applying the ratio 6:1 = 2.5:x, the calculated value was x = 0.0005 g, corresponding to 0.5 mg of TPP. The TPP was then dissolved in 700 μL of deionized water to obtain a solution with a concentration of 0.7 mg/mL.
- -
- Polydatin (PD): A final concentration of 0.5 mM was chosen. This concentration was determined based on a solubility study of polydatin in water, which showed that, at the same pH, higher concentrations were not possible. To prepare the solution, a volume of 2.5 mL of PD solution in deionized water at 1 mM concentration and pH 4.8 was prepared. Considering that the final total volume would be 5 mL, the concentration would decrease by half, reaching the desired final concentration. The solution was prepared by adding 2.465 mL of deionized water to 0.001 g of polydatin and sonicating the mixture in an ultrasonic bath for 30 min at 40 °C. Subsequently, 0.035 mL of a 1 mM acetic acid solution in deionized water was added to adjust the pH to 4.8 and reach the final volume of 2.5 mL. The preparation was completed with a second sonication step for 10 min at 25 °C.
4.3. Nanoparticles Characterization
4.3.1. Scanning Electron Microscopy
4.3.2. Dynamic and Dielectrophoretic Light Scattering
4.3.3. Evaluation of Encapsulation Efficiency
4.4. Cell Cultures
4.5. Assessment on PD-CS-NPs Activity
4.5.1. MTT Assay
4.5.2. Cellular Vitality
4.5.3. Statistical Analysis
4.5.4. Fluorescence Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Res. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef]
- Fabris, S.; Momo, F.; Ravagnan, G.; Stevanato, R. Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys. Chem. 2008, 135, 76–83. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cimmino, A.; Gioia, M.; Clementi, M.E.; Faraoni, I.; Marini, S.; Ciaccio, C. Polydatin-Induced Shift of Redox Balance and Its Anti-Cancer Impact on Human Osteosarcoma Cells. Curr. Issues Mol. Biol. 2025, 47, 21. [Google Scholar] [CrossRef]
- Malla, R.; Surepalli, N.; Farran, B.; Malhotra, S.V.; Nagaraju, G.P. Reactive oxygen species (ROS): Critical roles in breast tumor microenvironment. Crit. Rev. Oncol. Hematol. 2021, 160, 103285. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Sharma, K.; Jena, L.; Kaur, P.; Singh, S.; Munshi, A. Mitochondrial bioenergetics of breast cancer. Mitochondrion 2024, 79, 101951. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Ma, Y.; Wang, X.; Feng, Q.; Zhang, Z.; Wang, S.; Zhang, H.; Lu, X.; Xu, Y.; Zhao, E.; et al. Polydatin Inhibits Cell Viability, Migration, and Invasion Through Suppressing the c-Myc Expression in Human Cervical Cancer. Front. Cell. Dev. Biol. 2021, 12, 587218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhuang, Z.; Meng, Q.; Jiao, Y.; Xu, J.; Fan, S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol. Lett. 2014, 7, 295–301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, S.; Zhao, Q.; Liu, S.; Kuang, J.; Zhang, J.; Onga, A.; Shen, Y.; Wang, J.; Sui, H.; Ni, L.; et al. Polydatin, a potential NOX5 agonist, synergistically enhances antitumor activity of cisplatin by stimulating oxidative stress in nonsmall cell lung cancer. Int. J. Oncol. 2024, 65, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, Y.; Zhan, X.; Zhang, B.; Chen, Y.; Liu, C.; Yu, L. Polydatin Exerts an Antitumor Effect Through Regulating the miR-382/PD-L1 Axis in Colorectal Cancer. Cancer Biother. Radiopharm. 2020, 35, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bang, T.H.; Park, B.S.; Kang, H.M.; Kim, J.H.; Kim, I.R. Polydatin, a Glycoside of Resveratrol, Induces Apoptosis and Inhibits Metastasis Oral Squamous Cell Carcinoma Cells In Vitro. Pharmaceuticals 2021, 14, 902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdel-Moneim, A.; El-Shahawy, A.; Yousef, A.I.; Abd El-Twab, S.M.; Elden, Z.E.; Taha, M. Novel Polydatin-Loaded Chitosan Nanoparticles for Safe and Efficient Type 2 Diabetes Therapy: In Silico, In Vitro and In Vivo Approaches. Int. J. Biol. Macromol. 2020, 154, 1496–1504. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Tripathy, A.; Pahal, S.; Mudakavi, R.J.; Raichur, A.M.; Varma, M.M.; Sen, P. Impact of Bioinspired Nanotopography on the Antibacterial and Antibiofilm Efficacy of Chitosan. Biomacromolecules 2018, 19, 1340–1346. [Google Scholar] [CrossRef]
- Je, H.J.; Kim, E.S.; Lee, J.S.; Lee, H.G. Release Properties and cellular uptake in Caco-2 cells of size-controlled chitosan nanoparticles. J. Agric. Food Chem. 2017, 65, 10899–10906. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, T.M.; Di, L. Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10827. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed]
- Barbos, M.; Vale, N.; Costa, F.M.T.A.; Martins, M.C.L.; Gomes, P. Tethering antimicrobial peptides onto chitosan: Optimization of azide-alkyne “click” reaction conditions. Carbohydr. Polym. 2017, 165, 384–393. [Google Scholar] [CrossRef]
- Layek, B.; Lipp, L.; Singh, J. Cell Penetrating Peptide Conjugated Chitosan for Enhanced Delivery of Nucleic Acid. Int. J. Mol. Sci. 2015, 16, 28912–28930. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Shirazi, A.N.; El-Meligy, M.G.; El-Ziaty, A.K.; Nagieb, Z.A.; Parang, K.; Tiwari, R.K. Design, synthesis, and evaluation of chitosan conjugated GGRGDSK peptides as a cancer cell-targeting molecular transporter. Int. J. Biol. Macromol. 2016, 87, 611–622. [Google Scholar] [CrossRef]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan-based nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Perez, J.; Peniche, H. Chitosan-based self-assembled nanoparticles in drug delivery. Preprints 2018, 2, 0012. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication, and developments in the last decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.H.; Thanh, T.L.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan nanoparticles-based ionic gelation method: A promising candidate for plant disease management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Galaly, S.R.; Mohamed, H.M.; Mostafa, F.; Abdel-Moneim, A. Polydatin Nanoparticles Attenuate Oxidative Stress and Histopathological Changes in Streptozotocin Model of Diabetic Nephropathy: Targeting Nrf2/HO-1/NF-Κβ Signaling Pathways. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 99. [Google Scholar] [CrossRef]
- Alnagar, A.N.; Motawea, A.; Zaghloul, R.A.; Eldesoqui, M.; Abu Hashim, I.I. A Novel Facile and Efficient Prophylaxis Avenue of Chitosan Oligosaccharide/PLGA Based Polydatin Loaded Nanoparticles Against Bleomycin-Induced Lung Inflammation in Experimental Rat Model. AAPS PharmSciTech 2025, 26, 35. [Google Scholar] [CrossRef]
- Su, Z.; Tang, Y.; Li, G.; Zhu, J.; He, Y.; Zhang, J.; Zhang, F.; Lin, Y.; Liu, B.; Cai, X. Polydatin and chitosan-silver co-loaded nanocomplexes for synergistic treatment of rheumatoid arthritis via repolarizing macrophages and inducing apoptosis of fibroblast-like synoviocytes. Mater. Des. 2024, 245, 113287. [Google Scholar] [CrossRef]
- Abd El-Hameed, A.M.; Yousef, A.I.; Abd El-Twab, S.M.; El-Shahawy, A.A.G.; Abdel-Moneim, A. Hepatoprotective Effects of Polydatin-Loaded Chitosan Nanoparticles in Diabetic Rats: Modulation of Glucose Metabolism, Oxidative Stress, and Inflammation Biomarkers. Biochemistry 2021, 86, 179–189. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Arkan, E.; Kiani, A.; Hosseini, S.Z.; Abbaszadeh, F.; Fakhri, S. Protective Effects of Polydatin Amphiphilic Chitosan Nanocarriers against an Aluminum Chloride-Induced Model of Alzheimer’s Disease in Rats: Relevance to Its Anti-Inflammatory and Antioxidant Effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 7605–7624. [Google Scholar] [CrossRef]
- Brugnoli, B.; Mariano, A.; Simonis, B.; Bombelli, C.; Sennato, S.; Piozzi, A.; Taresco, V.; Chauhan, V.M.; Howdle, S.M.; d’Abusco, A.S.; et al. Self-Assembled Chitosan-Sodium Usnate Drug Delivery Nanosystems: Synthesis, Characterization, Stability Studies, in Vitro Cytotoxicity and in Vivo Biocompatibility against 143 B Cells. Carbohydr. Polym. Technol. Appl. 2023, 6, 100373. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Mariadoss, A.V.A.; Ramachandran, V.; Shalini, V.; Agilan, B.; Sangeetha, C.C.; Balu, P.; Kotakadi, V.S.; Karthikkumar, V.; Ernest, D. Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation. Antioxidants 2019, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Samra, Y.A.; Abdelghany, A.M.; Zaghloul, R.A. Polydatin gold nanoparticles potentiate antitumor effect of doxorubicin in Ehrlich ascites carcinoma-bearing mice. J. Biochem. Mol. Toxicol. 2021, 35, 22869. [Google Scholar] [CrossRef] [PubMed]
- Sichetti, M.; Giuseffi, M.; Giglio, E.; Marino, G.; Mecca, M. Effect of Natural Polyphenols on Breast Cancer Chemoprevention and Treatment. Mol. Nutr. Food Res. 2025, 69, e70055. [Google Scholar] [CrossRef] [PubMed]
- Donia, T.; Ali, E.M.M.; Kalantan, A.A.; Alzahrani, F.A.; Eid, T.M.; Khamis, A.A. Synergistic anticancer efficacy of polydatin and sorafenib against the MCF-7 breast cancer cell line via inhibiting of PI3K/AKT/mTOR pathway and reducing resistance to treatment. Biochem. Biophys. Res. Commun. 2024, 739, 150972. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.M.; Eldin, Z.E.; Taha, M.; Elbeltagi, S. Preparation, characterization, and anticancer evaluation of polydatin conjugated with zinc MOF and encapsulated by liponiosomes as a potential nanotool-induce apoptosis. J. Mol. Struct. 2024, 1315, 138982. [Google Scholar] [CrossRef]
- Koppel, D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: The method of cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Grabowski, E.; Morrison, I. Particle size distributions from analysis of quasi-elastic light-scattering data. In Measurement of Suspended Particles by Quasi-Elastic Light Scattering; Dahneke, B.E., Ed.; Wiley: Hoboken, NJ, USA, 1983. [Google Scholar]
- Giorcelli, A.; Sparvoli, F.; Mattivi, F.; Tava, A.; Balestrazzi, A.; Vrhovsek, U.; Calligari, P.; Bollini, R.; Confalonieri, M. Expression of the Stilbene Synthase (StSy) Gene from Grapevine in Transgenic White Poplar Results in High Accumulation of the Antioxidant Resveratrol Glucosides. Transgenic Res. 2004, 13, 203–214. [Google Scholar] [CrossRef]
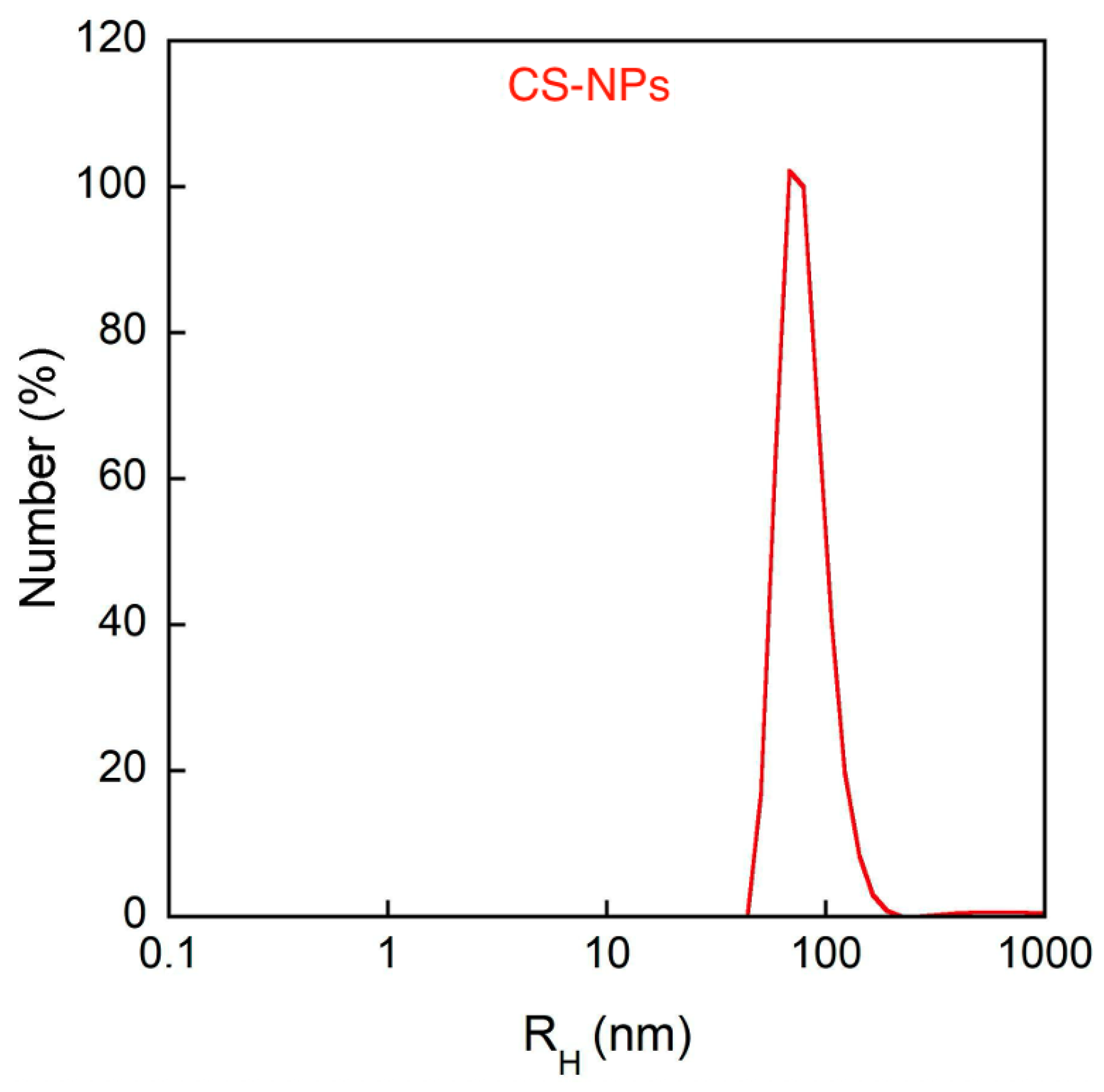
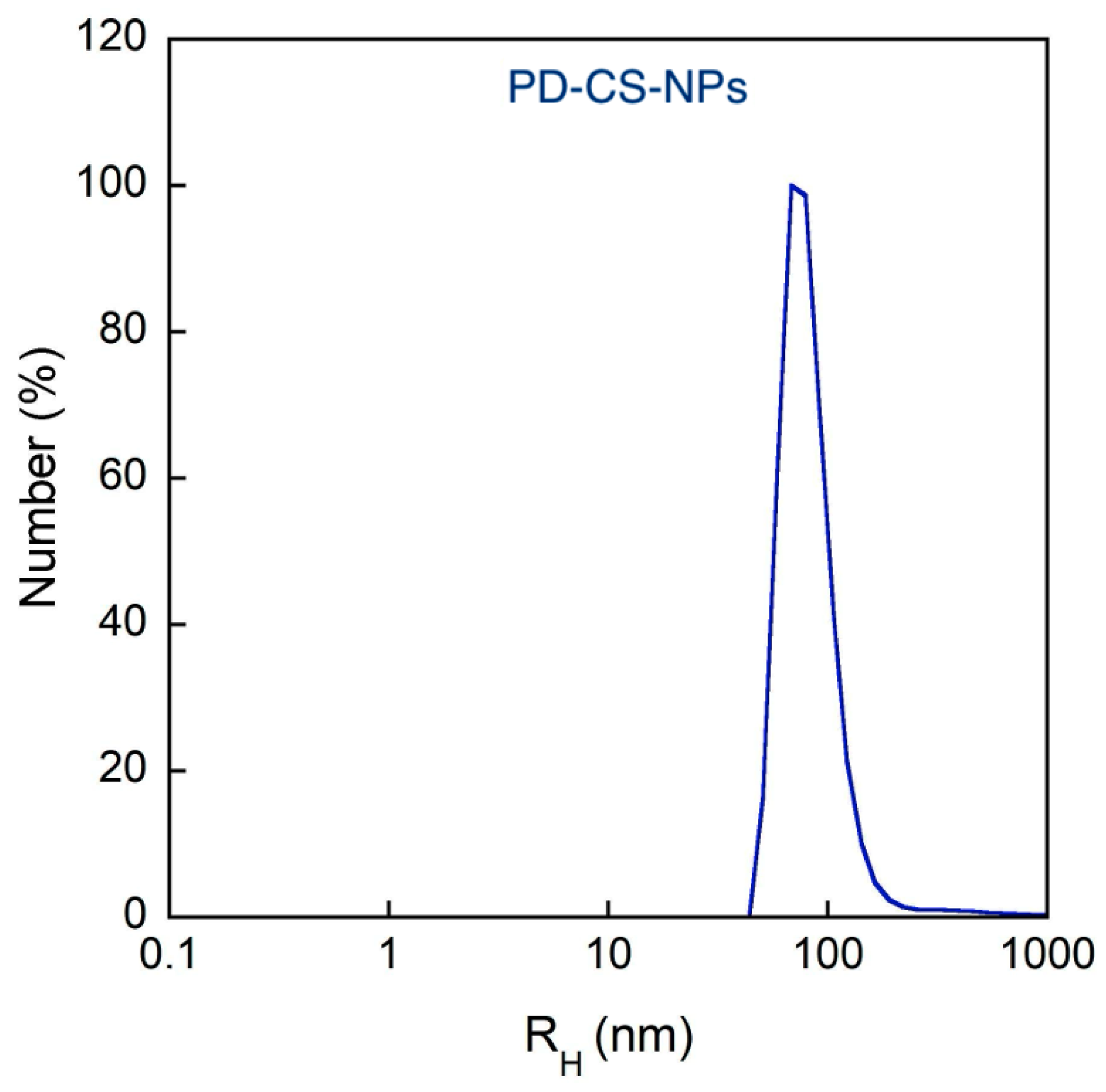


| Sample | RH [nm] (Cumulants) | PDI (Cumulants) | ζ-Pot [mV] | EE% |
|---|---|---|---|---|
| CS-NPs | 207 ± 8 | 0.65 ± 0.07 | 48 ± 1 | - |
| PD-CS-NPs | 68 ± 5 | 0.44 ± 0.08 | 5 ± 2 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichil, D.; Migani, S.; Colone, M.; Severini, L.; Sennato, S.; Bozzuto, G.; Patrizi, A.; Bombelli, C.; Ravagnan, G.; Stringaro, A.; et al. Preparation and Characterization of Polydatin–Chitosan Nanocapsules for Enhanced Drug Delivery Efficacy. Molecules 2025, 30, 4400. https://doi.org/10.3390/molecules30224400
Nichil D, Migani S, Colone M, Severini L, Sennato S, Bozzuto G, Patrizi A, Bombelli C, Ravagnan G, Stringaro A, et al. Preparation and Characterization of Polydatin–Chitosan Nanocapsules for Enhanced Drug Delivery Efficacy. Molecules. 2025; 30(22):4400. https://doi.org/10.3390/molecules30224400
Chicago/Turabian StyleNichil, Donato, Sofia Migani, Marisa Colone, Leonardo Severini, Simona Sennato, Giuseppina Bozzuto, Aurora Patrizi, Cecilia Bombelli, Giampietro Ravagnan, Annarita Stringaro, and et al. 2025. "Preparation and Characterization of Polydatin–Chitosan Nanocapsules for Enhanced Drug Delivery Efficacy" Molecules 30, no. 22: 4400. https://doi.org/10.3390/molecules30224400
APA StyleNichil, D., Migani, S., Colone, M., Severini, L., Sennato, S., Bozzuto, G., Patrizi, A., Bombelli, C., Ravagnan, G., Stringaro, A., & Mattiello, L. (2025). Preparation and Characterization of Polydatin–Chitosan Nanocapsules for Enhanced Drug Delivery Efficacy. Molecules, 30(22), 4400. https://doi.org/10.3390/molecules30224400











