Phenolic Profile and Antioxidant Activity of Fractions of Procyanidin-Rich Hawthorn (Crataegus monogyna Jacq.) Bark Extract Separated by Low-Pressure Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Fractionation of Purified Hawthorn Bark Extract and Initial Fraction Characterization
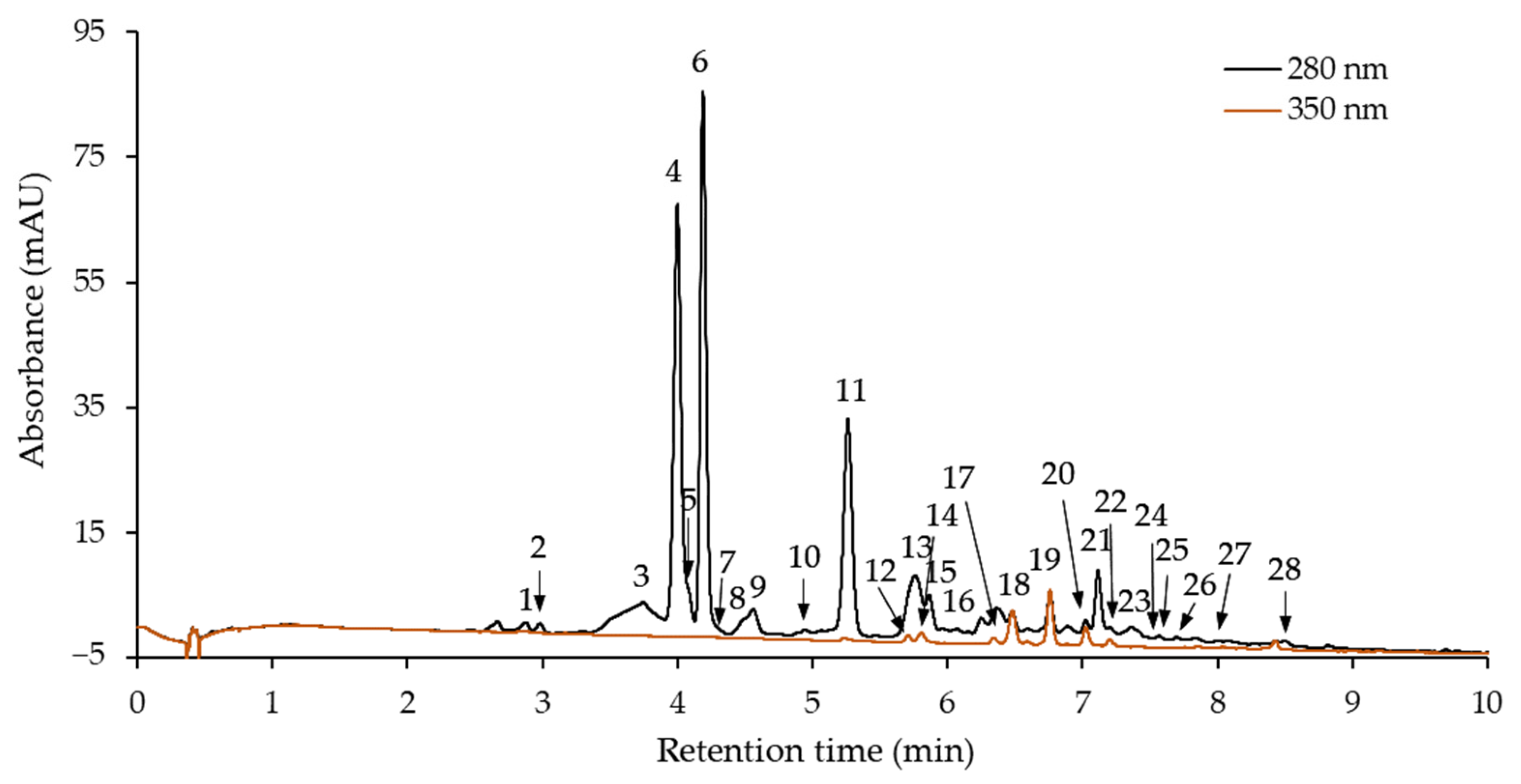
2.2. Phenolic Profile of Purified Hawthorn Bark Extract and Its Fractions
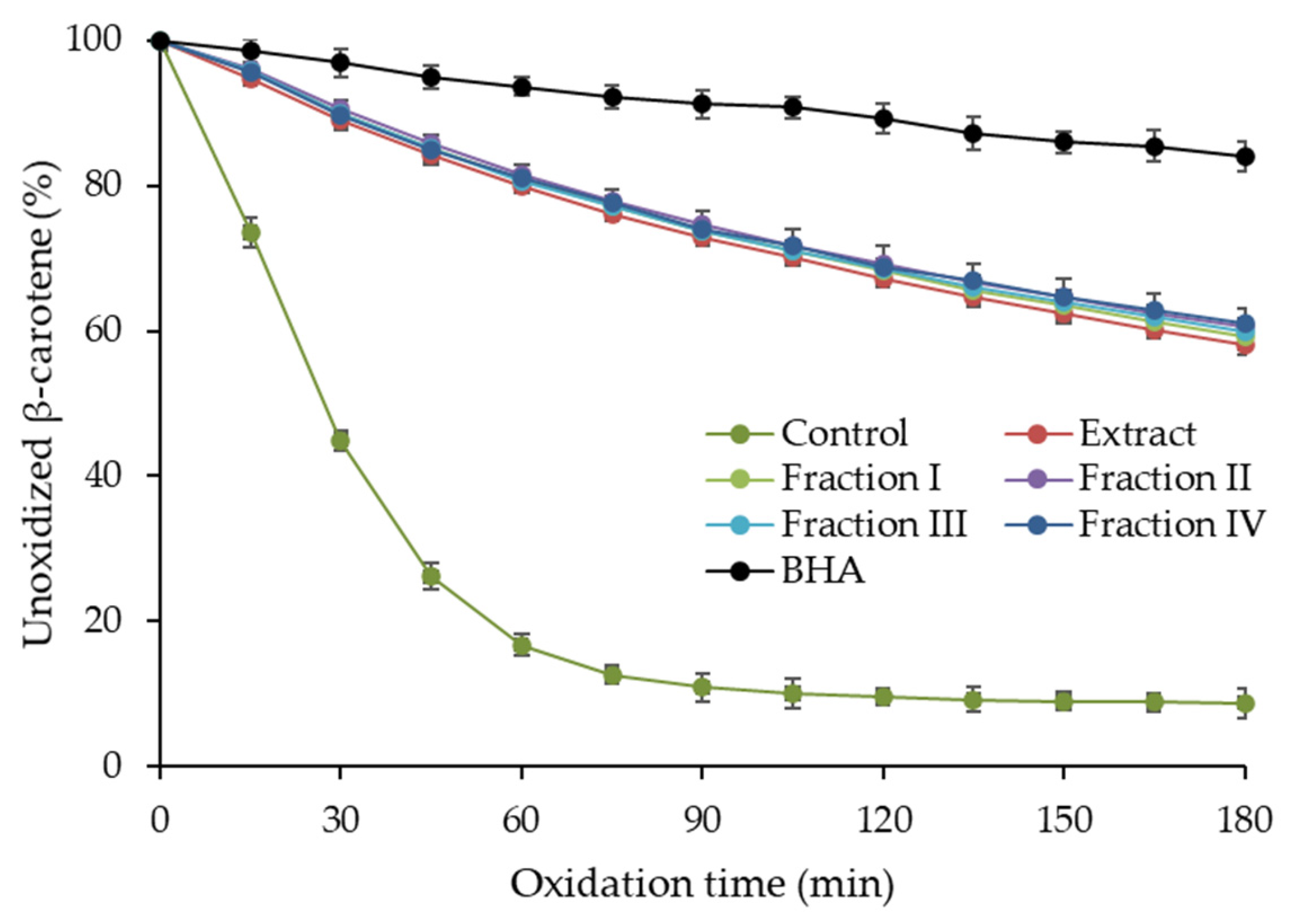
2.3. Antioxidant Activity of Purified Hawthorn Bark Extract and Its Fractions
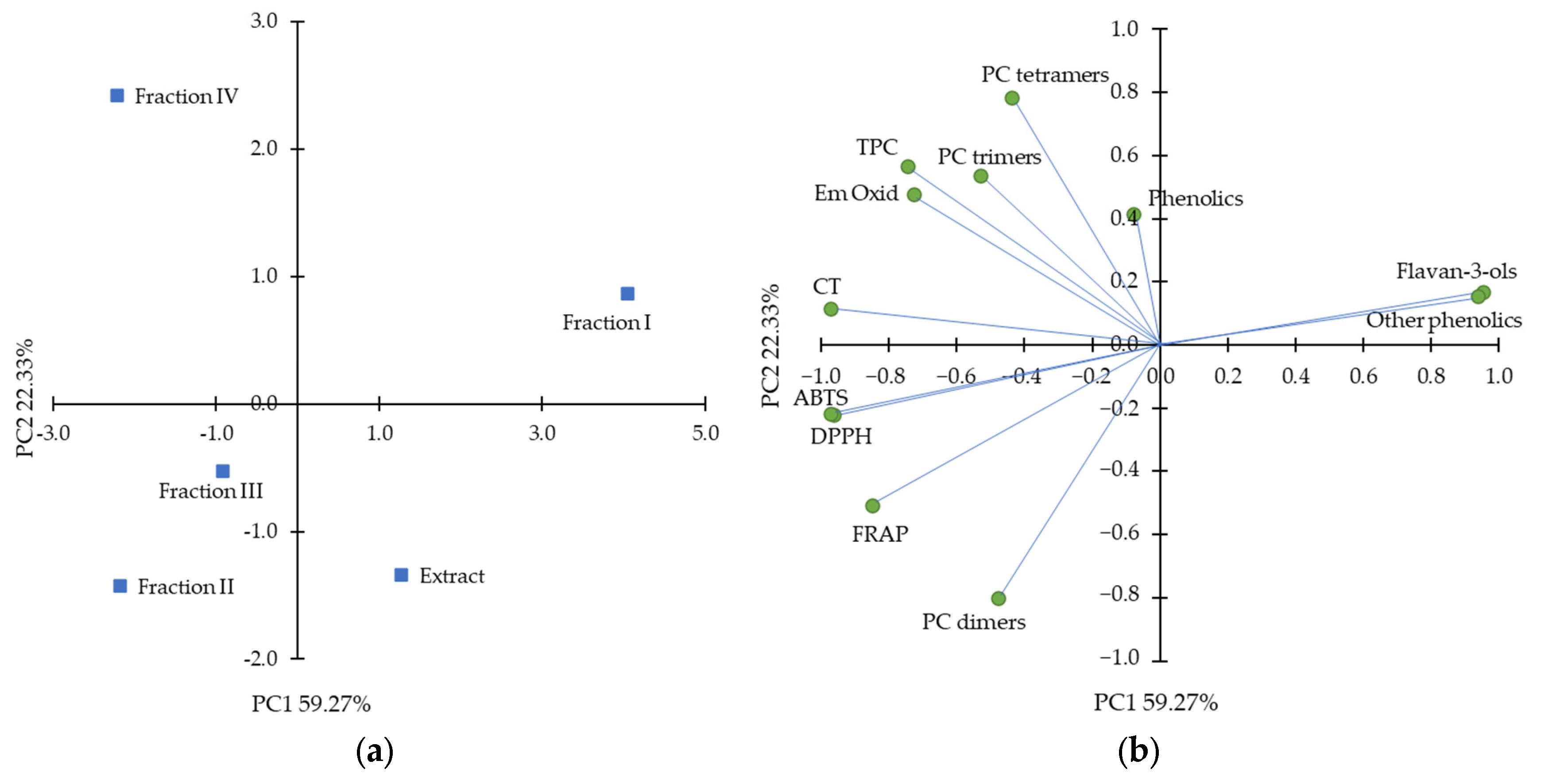
2.4. Overall Estimation of Results Using Principal Component Analysis
3. Materials and Methods
3.1. Material, Chemicals, and Reagents
3.2. Extraction of Hawthorn Bark and Purification of Crude Extract
3.3. Fractionation of Purified Hawthorn Bark Extract
3.4. Determination of Total Phenolic Content
3.5. Determination of Condensed Tannin Content
3.6. Identification and Quantification of Phenolic Compounds
3.7. Determination of ABTS Radical Cation Scavenging Activity
3.8. Determination of DPPH Radical Scavenging Activity
3.9. Determination of Ferric-Reducing Antioxidant Power
3.10. Oxidation of β-Carotene-Linoleic Acid Emulsion
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA, Agricultural Research Service, National Plant Germplasm System. Germplasm Resources Information Network (GRIN Taxonomy); National Germplasm Resources Laboratory: Beltsville, MD, USA, 2025. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=12122 (accessed on 10 September 2025).
- Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae. Molecules 2021, 26, 7266. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Q.; Ran, K.; Jiao, M.; Fu, X.; Han, Z.; Liu, C.; Li, N. The Nutritional and Bioactive Components, Potential Health Function and Comprehensive Utilization of Hawthorn: A Review. Food Front. 2025, 6, 2108–2128. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Effect of Crataegus Usage in Cardiovascular Disease Prevention: An Evidence-Based Approach. Evid. Based Complement. Altern. Med. 2013, 2013, 49363. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Mssillou, I.; Boukhira, S.; Bichara, M.D.; El Abdali, Y.; de Azevedo, R.G.; Mohamed, C.; Slighoua, M.; Conte, R.; Kiokias, S.; et al. Phenolic Composition of Crataegus monogyna Jacq. Extract and Its Anti-Inflammatory, Hepatoprotective, and Antileukemia Effects. Pharmaceuticals 2024, 17, 786. [Google Scholar] [CrossRef]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-Inflammatory, Gastroprotective, Free-Radical-Scavenging, and Antimicrobial Activities of Hawthorn Berries Ethanol Extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef]
- Szikora, Z.; Mátyus, R.O.; Szabó, B.V.; Csupor, D.; Tóth, B. Hawthorn (Crataegus spp.) Clinically Significantly Reduces Blood Pressure in Hypertension: A Meta-Analysis of Randomized Placebo-Controlled Clinical Trials. Pharmaceuticals 2025, 18, 1027. [Google Scholar] [CrossRef]
- Paul, S.; Sharma, S.; Paliwal, S.K.; Kasture, S. Role of Crataegus oxyacantha (Hawthorn) on Scopolamine Induced Memory Deficit and Monoamine Mediated Behaviour in Rats. Orient. Pharm. Exp. Med. 2017, 17, 315–324. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Albu, C.; Alecu, A.; Seciu-Grama, A.M.; Radu, G.L. Antioxidant and Antidiabetic Activity of Cornus mas L. and Crataegus monogyna Fruit Extracts. Molecules 2024, 29, 3595. [Google Scholar] [CrossRef]
- Saoudi, M.; Salem, R.B.S.-B.; Ben Salem, M.; Brahmi, N.; Badraoui, R.; Nasri, M.; El Feki, A. Beneficial Effects of Crataegus oxyacantha Extract on Neurobehavioral Deficits and Brain Tissue Damages Induced by an Insecticide Mixture of Deltamethrin and Chlorpyrifos in Adult Wistar Rats. Biomed. Pharmacother. 2019, 114, 108795. [Google Scholar] [CrossRef]
- Ülger, T.T.; Oçkun, M.A.; Guzelmeric, E.; Sen, N.B.; Sipahi, H.; Özhan, Y.; Kan, Y.; Yesilada, E. Comprehensive Analysis of the Chemical and Bioactivity Profiles of Endemic Crataegus turcicus Dönmez in Comparison with Other Crataegus Species. Molecules 2023, 28, 6520. [Google Scholar] [CrossRef]
- Elango, C.; Devaraj, S.N. Immunomodulatory Effect of Hawthorn Extract in an Experimental Stroke Model. J. Neuroinflamm. 2010, 7, 97. [Google Scholar] [CrossRef]
- Goudjil, S.; Boussekine, S.; Goudjil, S.; Goudjil, H.; Yilmaz, M.A.; Ola, M.S.; Ali, A.; Cakir, O. Investigation of Algerian Crataegus monogyna Jacq Phenolic Compounds (Using LC-ESI-MS/MS Analysis, Antioxidant Activity, and Enzyme Inhibition) and Their Potential Implications for Food and Nutraceutical Applications. Antioxidants 2024, 13, 1350. [Google Scholar] [CrossRef]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic Acids Present in Hawthorn Lower Plasma Cholesterol by Inhibiting Intestinal ACAT Activity in Hamsters. Evid. Based Complement. Altern. Med. 2011, 2011, 801272. [Google Scholar] [CrossRef]
- Pavlovic, J.; Mitic, S.; Mitic, M.; Kocic, G.; Pavlovic, A.; Tosic, S. Variation in the Phenolic Compounds Profile and Antioxidant Activity in Different Parts of Hawthorn (Crataegus pentagyna Willd.) during Harvest Periods. Pol. J. Food Nutr. Sci. 2019, 69, 367–378. [Google Scholar] [CrossRef]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-Chemical, Antioxidant, and Anti-Inflammatory Properties and Stability of Hawthorn (Crataegus monogyna Jacq.) Procyanidins Microcapsules with Inulin and Maltodextrin. J. Sci. Food Agric. 2017, 97, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Żurek, N.; Świeca, M.; Kapusta, I. UPLC-ESI-TQD-MS/MS Identification and Antioxidant, Anti-Inflammatory, Anti-Diabetic, Anti-Obesity and Anticancer Properties of Polyphenolic Compounds of Hawthorn Seeds. Plant Foods Hum. Nutr. 2024, 79, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Chen, C.M.; Gao, Y.S.; Feng, H.L.; Ding, Y.M.; Shi, Y.; Zhou, H.T.; Chen, Q.X. Structural Analysis of Proanthocyanidins Isolated from Fruit Stone of Chinese Hawthorn with Potent Antityrosinase and Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 123–129. [Google Scholar] [CrossRef]
- Renda, G.; Özel, A.; Barut, B.; Korkmaz, B.; Yayli, N. In Vitro Protection by Crataegus microphylla Extracts against Oxidative Damage and Enzyme Inhibition Effects. Turk. J. Pharm. Sci. 2018, 15, 77–84. [Google Scholar] [CrossRef]
- Włoch, A.; Kapusta, I.; Bielecki, K.; Oszmiański, J.; Kleszczyńska, H. Activity of Hawthorn Leaf and Bark Extracts in Relation to Biological Membrane. J. Membr. Biol. 2013, 246, 545–556. [Google Scholar] [CrossRef]
- Oszmiański, J.; Bourzeix, M. Preparation of Catechin and Procyanidin Standards From Hawthorn (Crataegus azarolus L.) and Pine (Pinus mesogeensis fieschi) Barks. Pol. J. Food Nutr. Sci. 1995, 4, 89–96. [Google Scholar]
- Krajka-Kuźniak, V.; Paluszczak, J.; Oszmiański, J.; Baer-Dubowska, W. Hawthorn (Crataegus oxyacantha L.) Bark Extract Regulates Antioxidant Response Element (ARE)-Mediated Enzyme Expression via Nrf2 Pathway Activation in Normal Hepatocyte Cell Line. Phytother. Res. 2014, 28, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Molnar, M.; Kovač, M.J.; Pavić, V. A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents. Molecules 2024, 29, 2615. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin Complexation with Metal Ions and Its Implication on Human Health, Environment and Industry: An Overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Karamać, M.; Pegg, R.B. Limitations of the Tetramethylmurexide Assay for Investigating the Fe(II) Chelation Activity of Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 6425–6431. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant Activity of the Phenolic Compounds of Hawthorn, Pine and Skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Czubaszek, A.; Czaja, A.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kucharska, A.Z. Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts. Molecules 2021, 26, 6292. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J. Influence of Polyphenols Isolated from Scutellaria baicalensis Georgi and Crataegus oxyacantha on the Oxidative Stability of Cholesterol in Butter Stored in Various Conditions. Eur. Food Res. Technol. 2007, 224, 635–642. [Google Scholar] [CrossRef]
- Aguilera-Rodríguez, F.R.; Zamora-Perez, A.L.; Galván-Moreno, C.L.; Gutiérrez-Hernández, R.; Estrada, C.A.R.; Esparza-Ibarra, E.L.; Lazalde-Ramos, B.P. Cytotoxic and Genotoxic Evaluation of the Aqueous and Hydroalcoholic Leaf and Bark Extracts of Crataegus oxyacantha in Murine Model. Plants 2021, 10, 2217. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J.; Chen, L.; Hu, C.; Chen, P.; Xie, B.; Sun, Z. Identification of A-Series Oligomeric Procyanidins from Pericarp of Litchi Chinensis by FT-ICR-MS and LC-MS. Food Chem. 2012, 135, 31–38. [Google Scholar] [CrossRef]
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F.Y. Structural Diversity and Antioxidant Activity of Condensed Tannins Fractionated from Mangosteen Pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Meiners, A.; Hübner, F.; Esselen, M. Isolation and Characterization of Novel Oligomeric Proanthocyanidins in Chokeberries Using High-Resolution Mass Spectrometry and Investigation of Their Antioxidant Potential. Appl. Sci. 2024, 14, 7839. [Google Scholar] [CrossRef]
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant Properties of Catechins and Proanthocyanidins: Effect of Polymerisation, Galloylation and Glycosylation. Free Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef]
- Luca, S.V.; Bujor, A.; Miron, A.; Aprotosoaie, A.C.; Skalicka-Woźniak, K.; Trifan, A. Preparative Separation and Bioactivity of Oligomeric Proanthocyanidins. Phytochem. Rev. 2020, 19, 1093–1140. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Chai, W.; Wu, Y.; Li, X.; Zeng, S.; Cheng, Y.; Jiang, W.; Pan, Q.; Xia, X.; Chen, G. Relationships between Degree of Polymerization and Activities: A Study on Condensed Tannins from the Bark of Ficus altissima. Int. J. Biol. Macromol. 2024, 274, 133306. [Google Scholar] [CrossRef]
- Chen, X.; Song, H.; Zhou, S.; Yuan, C.; Li, J. Exploring Separation Patterns and Mechanisms of Proanthocyanidins in Grape Seeds and Pomace with Diverse Molecular Weights, Compositions, and Structures. Food Chem. X 2023, 20, 101008. [Google Scholar] [CrossRef]
- Brown, R.H.; Mueller-Harvey, I.; Zeller, W.E.; Reinhardt, L.; Stringano, E.; Gea, A.; Drake, C.; Ropiak, H.M.; Fryganas, C.; Ramsay, A.; et al. Facile Purification of Milligram to Gram Quantities of Condensed Tannins According to Mean Degree of Polymerization and Flavan-3-ol Subunit Composition. J. Agric. Food Chem. 2017, 65, 8072–8082. [Google Scholar] [CrossRef]
- Xiao, J.S.; Liu, L.; Wu, H.; Xie, B.J.; Yang, E.N.; Sun, Z. Da Rapid Preparation of Procyanidins B2 and C1 from Granny Smith Apples by Using Low Pressure Column Chromatography and Identification of Their Oligomeric Procyanidins. J. Agric. Food Chem. 2008, 56, 2096–2101. [Google Scholar] [CrossRef]
- Sui, Y.; Zheng, Y.; Li, X.; Li, S.; Xie, B.; Sun, Z. Characterization and Preparation of Oligomeric Procyanidins from Litchi chinensis Pericarp. Fitoterapia 2016, 112, 168–174. [Google Scholar] [CrossRef]
- Lis, M.; Szczypka, M.; Suszko-Pawłowska, A.; Sokół-Łętowska, A.; Kucharska, A.; Obmińska-Mrukowicz, B. Hawthorn (Crataegus monogyna) Phenolic Extract Modulates Lymphocyte Subsets and Humoral Immune Response in Mice. Planta Med. 2020, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Zhang, Z.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Liquid Chromatographic/Electrospray Ionization Mass Spectrometric Studies of Proanthocyanidins in Foods. J. Mass Spectrom. 2003, 38, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Said, R.B.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix Dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A Comprehensive Review Encompassing Structure Elucidation via Mass Spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ghozzi, I.; Fontaine, J.X.; Molinié, R.; Elboutachfaiti, R.; Akkouche, L.; Sebei, K.; Mathiron, D.; Hano, C.; Garros, L.; Choque, E.; et al. Relationship Between the Structure of the Flavone C-Glycosides of Linseed (Linum usitatissimum L.) and Their Antioxidant Activity. Molecules 2024, 29, 5829. [Google Scholar] [CrossRef]
- Gai, F.; Janiak, M.A.; Sulewska, K.; Peiretti, P.G.; Karamać, M. Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages. Molecules 2023, 28, 1807. [Google Scholar] [CrossRef]
- Sulewska, K.; Rybarczyk-Płońska, A.; Karamać, M. Antioxidant Capacity of Lentil Flour Hydrolysates Obtained with Pancreatin. Pol. J. Food Nutr. Sci. 2022, 72, 381–391. [Google Scholar] [CrossRef]
- Radman, S.; Mastelić, L.; Ljubenkov, I.; Lazarevski, S.; Politeo, O.; Podrug, R.; Prga, I.; Čorić, I.; Popović, M.; Bratinčević, M.V.; et al. Sea Fennel (Crithmum maritimum L.) Flowers as an Emerging Source of Bioactive Compounds. Pol. J. Food Nutr. Sci. 2024, 74, 221–231. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tran, V.N.; Dang, D.X.T.; Pham, T.K.O.; Tran, T.Q.N.; Ton, N.M.N.; Tran, T.T.T.; Le, V.V.M. Cellulase Treatment of Acerola Seeds and Its Effect on Physicochemical Properties and Antioxidant Potential of Dietary Fiber-Rich Cookies. Pol. J. Food Nutr. Sci. 2024, 74, 268–279. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Peiretti, P.G. Effect of the Growth Stage of False Flax (Camelina sativa L.) on the Phenolic Compound Content and Antioxidant Potential of the Aerial Part of the Plant. Pol. J. Food Nutr. Sci. 2020, 70, 189–198. [Google Scholar] [CrossRef]

| Extract/Fraction | Fraction Yield (%) | TPC (mg GAE/g) | CT Content (A500/mg) |
|---|---|---|---|
| Extract | – | 801.2 ± 11.4 d | 1.88 ± 0.08 c |
| Fraction I | 24.6 ± 1.5 a | 816.3 ± 6.5 cd | 1.58 ± 0.03 d |
| Fraction II | 11.8 ± 0.9 c | 862.8 ± 8.1 b | 2.65 ± 0.11 a |
| Fraction III | 19.0 ± 1.3 b | 826.3 ± 7.4 c | 2.21 ± 0.02 b |
| Fraction IV | 10.3 ± 1.7 c | 896.5 ± 11.1 a | 2.66 ± 0.04 a |
| Compound No. | tR (min) | λmax (nm) | [M − H]− (m/z) | Fragment Ions (m/z) | Compound |
|---|---|---|---|---|---|
| 1 | 2.88 | 278 | 577 | 425, 289 | B-type procyanidin dimer(1) |
| 2 | 2.99 | 280 | 289 | – | (+)-Catechin |
| 3 | 3.75 | 278 | 577 | 289 | B-type procyanidin dimer(2) |
| 4 | 4.01 | 278 | 577 | 289 | Procyanidin B2 |
| 5 | 4.10 | 278 | 865 | 577, 289 | B-type procyanidin trimer(1) |
| 6 | 4.19 | 280 | 289 | – | (−)-Epicatechin |
| 7 | 4.22 | 279 | 865 | 577, 289 | B-type procyanidin trimer(2) |
| 8 | 4.57 | 278 | 577 | 425, 289 | B-type procyanidin dimer(3) |
| 9 | 4.62 | 280 | 865 | 577, 575, 289 | B-type procyanidin trimer(3) |
| 10 | 4.95 | 280 | 865 | 577, 289 | B-type procyanidin trimer(4) |
| 11 | 5.27 | 280 | 865 | 577, 575, 289 | B-type procyanidin trimer(5) |
| 12 | 5.71 | 268, 348 | 447 | 285 | Isoorientin |
| 13 | 5.77 | 280 | 1153 | 865, 577, 289 | B-type procyanidin tetramer(1) |
| 14 | 5.81 | 268, 348 | 447 | 285 | Orientin |
| 15 | 5.87 | 280 | 577 | 289 | B-type procyanidin trimer(6) |
| 16 | 6.31 | 279 | 577 | 289 | B-type procyanidin dimer(4) |
| 17 | 6.48 | 268, 336 | 431 | 311, 283, 269 | Vitexin |
| 18 | 6.59 | 256, 350 | – | 301 | Quercetin derivative |
| 19 | 6.76 | 270, 338 | 431 | 311, 283, 269 | Isovitexin |
| 20 | 7.03 | 256, 355 | 463 | 301 | Quercetin 3-O-galactoside |
| 21 | 7.12 | 280 | 577 | 289 | B-type procyanidin dimer(5) |
| 22 | 7.21 | 255, 350 | 463 | 301 | Quercetin 3-O-glucoside |
| 23 | 7.37 | 280 | 865 | 577, 575, 289 | B-type procyanidin trimer(7) |
| 24 | 7.52 | 279 | 863 | 575, 289 | A-type procyanidin trimer |
| 25 | 7.57 | 278 | 575 | 289 | A-type procyanidin dimer |
| 26 | 7.75 | 280 | 1153 | 865, 577, 289 | B-type procyanidin tetramer(2) |
| 27 | 8.05 | 280 | 865 | 577, 289 | B-type procyanidin trimer(8) |
| 28 | 8.50 | 280 | 1153 | 865, 577, 289 | B-type procyanidin tetramer(3) |
| Phenolic Class | Compound | Extract | Fraction | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| Flavan-3-ol | (+)-Catechin | 2.7 ± 0.3 | 10.4 ± 0.3 | nd | nd | nd |
| (−)-Epicatechin | 211.9 ± 4.3 | 741.3 ± 13.7 | 2.5 ± 0.2 | nd | nd | |
| Σ Flavan-3-ols | 214.6 ± 4.1 | 751.7 ± 13.6 | 2.5 ± 0.2 | – | – | |
| Procyanidin dimer | Procyanidin B2 | 187.5 ± 4.4 | 14.3 ± 1.1 | 770.6 ± 49.7 | 47.3 ± 2.4 | nd |
| B-type procyanidin dimer(1) | 6.2 ± 0.5 | 5.8 ± 0.3 | 53.8 ± 2. 9 | nd | nd | |
| B-type procyanidin dimer(2) | 63.7 ± 1.4 | nd | 17.0 ± 1.1 | 278.6 ± 12.2 | nd | |
| B-type procyanidin dimer(3) | 19.8 ± 1.2 | 4.7 ± 1.5 | 7.4 ± 0.4 | 14.1 ± 1.6 | nd | |
| B-type procyanidin dimer(4) | tr | nd | 3.1 ± 0.3 | 8.2 ± 0.5 | nd | |
| B-type procyanidin dimer(5) | 24.4 ± 1.7 | nd | 4.0 ± 0.2 | 86.7 ± 3.8 | nd | |
| A-type procyanidin dimer | 1.1 ± 0.1 | nd | nd | 4.9 ± 0.4 | nd | |
| Σ Procyanidin dimers | 302.7 ± 6.9 | 24.8 ± 2.7 | 855.9 ± 53.7 | 439.8 ± 20.1 | – | |
| Procyanidin trimer | B-type procyanidin trimer(1) | tr | nd | nd | nd | 121.0 ± 16.5 |
| B-type procyanidin trimer(2) | tr | nd | nd | nd | 33.5 ± 8.3 | |
| B-type procyanidin trimer(3) | 24.1 ± 1.7 | nd | nd | 30.4 ± 1.9 | 12.8 ± 2.2 | |
| B-type procyanidin trimer(4) | 3.2 ± 0.5 | nd | nd | 9.9 ± 1.3 | 11.9 ± 1.1 | |
| B-type procyanidin trimer(5) | 100.1 ± 2.4 | nd | 3.5 ± 0.3 | 332.0 ± 8.9 | 185.4 ± 13.8 | |
| B-type procyanidin trimer(6) | 15.3 ± 1.1 | nd | nd | 5.2 ± 0.2 | 94.7 ± 3.6 | |
| B-type procyanidin trimer(7) | 7.3 ± 0.4 | nd | nd | nd | 20.5 ± 3.6 | |
| B-type procyanidin trimer(8) | tr | nd | nd | nd | 14.0 ± 0.0 | |
| A-type procyanidin trimer | tr | nd | nd | 15.8 ± 1.0 | 8.1 ± 0.1 | |
| Σ Procyanidin trimers | 150.0 ± 3.2 | – | 3.5 ± 0.3 | 393.3 ± 12.7 | 501.9 ± 20.7 | |
| Procyanidin tetramer | B-type procyanidin tetramer(1) | 45.9 ± 1.3 | nd | nd | nd | 314.4 ± 11.4 |
| B-type procyanidin tetramer(2) | tr | nd | nd | nd | 8.3 ± 0.1 | |
| B-type procyanidin tetramer(3) | 2.6 ± 0.1 | nd | nd | nd | 22.8 ± 0.9 | |
| Σ Procyanidin tetramers | 48.5 ± 1.2 | – | – | – | 345.5 ± 17.2 | |
| Other flavonoids | Isoorientin | 0.7 ± 0.0 | 2.4 ± 0.2 | nd | nd | nd |
| Isovitexin | 3.9 ± 0.1 | 14.7 ± 0.5 | nd | nd | nd | |
| Orientin | 6.5 ± 0.2 | 25.6 ± 1.2 | nd | nd | nd | |
| Vitexin | 3.1 ± 0.0 | 12.1 ± 0.3 | 0.8 ± 0.0 | nd | nd | |
| Quercetin derivative | 0.6 ± 0.0 | 1.9 ± 0.3 | nd | nd | nd | |
| Quercetin 3-O-galactoside | 10.3 ± 0.1 | 40.9 ± 2.9 | nd | nd | nd | |
| Quercetin 3-O-glucoside | 1.1 ± 0.1 | 4.4 ± 0.5 | 4.7 ± 0.4 | nd | nd | |
| Σ Other flavonoids | 26.2 ± 0.2 | 102.0 ± 5.8 | 5.5 ± 0.4 | – | – | |
| Σ Phenolics | 742.0 ± 10.7 b | 878.5 ± 21.6 a | 867.4 ± 54.5 a | 833.1 ± 33.1 a | 847.4 ± 29.4 a | |
| Extract/Fraction | ABTS•+ Scavenging Activity (mmol TE/g) | DPPH• Scavenging Activity (mmol TE/g) | FRAP (mmol Fe2+/g) |
|---|---|---|---|
| Extract | 8.48 ± 0.28 bc | 6.10 ± 0.12 b | 17.72 ± 0.81 b |
| Fraction I | 7.86 ± 0.11 c | 4.95 ± 0.10 c | 13.91 ± 0.73 c |
| Fraction II | 9.28 ± 0.35 a | 6.71 ± 0.11 a | 21.06 ± 1.15 a |
| Fraction III | 8.97 ± 0.29 ab | 6.45 ± 0.08 a | 18.27 ± 0.08 b |
| Fraction IV | 8.95 ± 0.12 ab | 6.52 ± 0.10 a | 17.67 ± 0.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamać, M.; Janiak, M.A.; Sulewska, K.; Amarowicz, R. Phenolic Profile and Antioxidant Activity of Fractions of Procyanidin-Rich Hawthorn (Crataegus monogyna Jacq.) Bark Extract Separated by Low-Pressure Liquid Chromatography. Molecules 2025, 30, 4375. https://doi.org/10.3390/molecules30224375
Karamać M, Janiak MA, Sulewska K, Amarowicz R. Phenolic Profile and Antioxidant Activity of Fractions of Procyanidin-Rich Hawthorn (Crataegus monogyna Jacq.) Bark Extract Separated by Low-Pressure Liquid Chromatography. Molecules. 2025; 30(22):4375. https://doi.org/10.3390/molecules30224375
Chicago/Turabian StyleKaramać, Magdalena, Michał A. Janiak, Katarzyna Sulewska, and Ryszard Amarowicz. 2025. "Phenolic Profile and Antioxidant Activity of Fractions of Procyanidin-Rich Hawthorn (Crataegus monogyna Jacq.) Bark Extract Separated by Low-Pressure Liquid Chromatography" Molecules 30, no. 22: 4375. https://doi.org/10.3390/molecules30224375
APA StyleKaramać, M., Janiak, M. A., Sulewska, K., & Amarowicz, R. (2025). Phenolic Profile and Antioxidant Activity of Fractions of Procyanidin-Rich Hawthorn (Crataegus monogyna Jacq.) Bark Extract Separated by Low-Pressure Liquid Chromatography. Molecules, 30(22), 4375. https://doi.org/10.3390/molecules30224375








