Antiproliferative Potential of Cobalt(II) Phenanthroline Complexes with Pyridonates
Abstract
1. Introduction
2. Results and Discussion
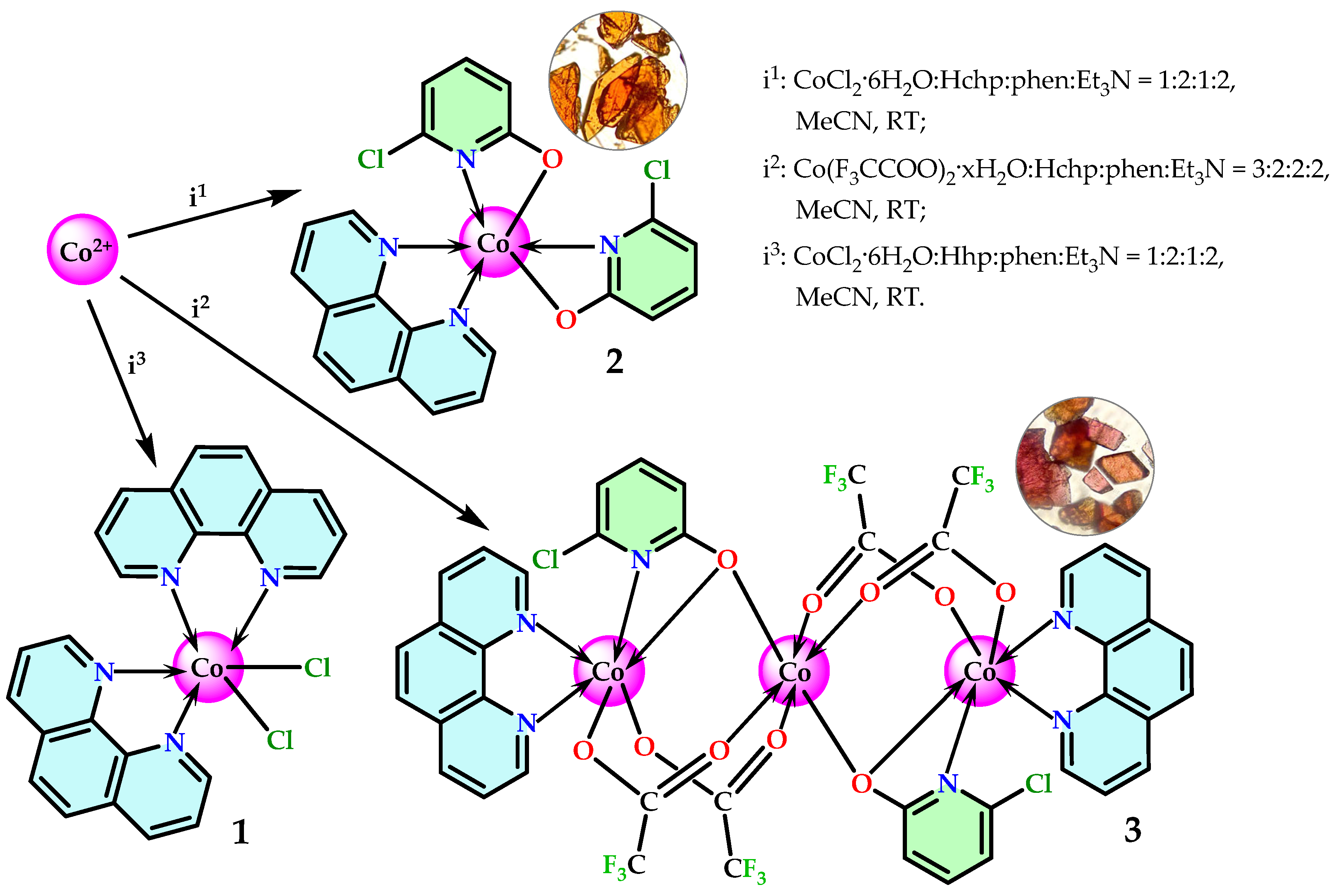
2.1. Synthesis and Characterization
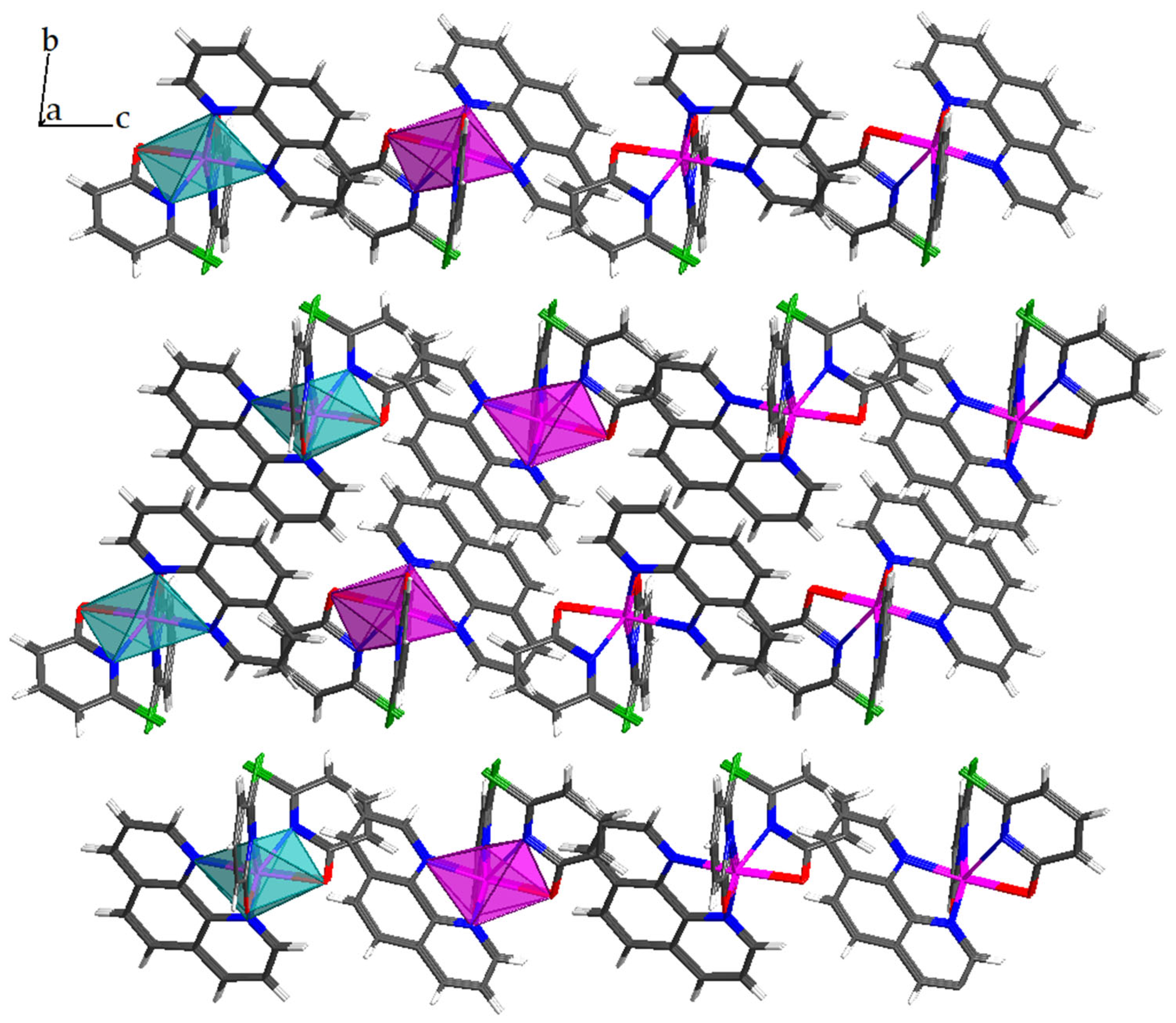
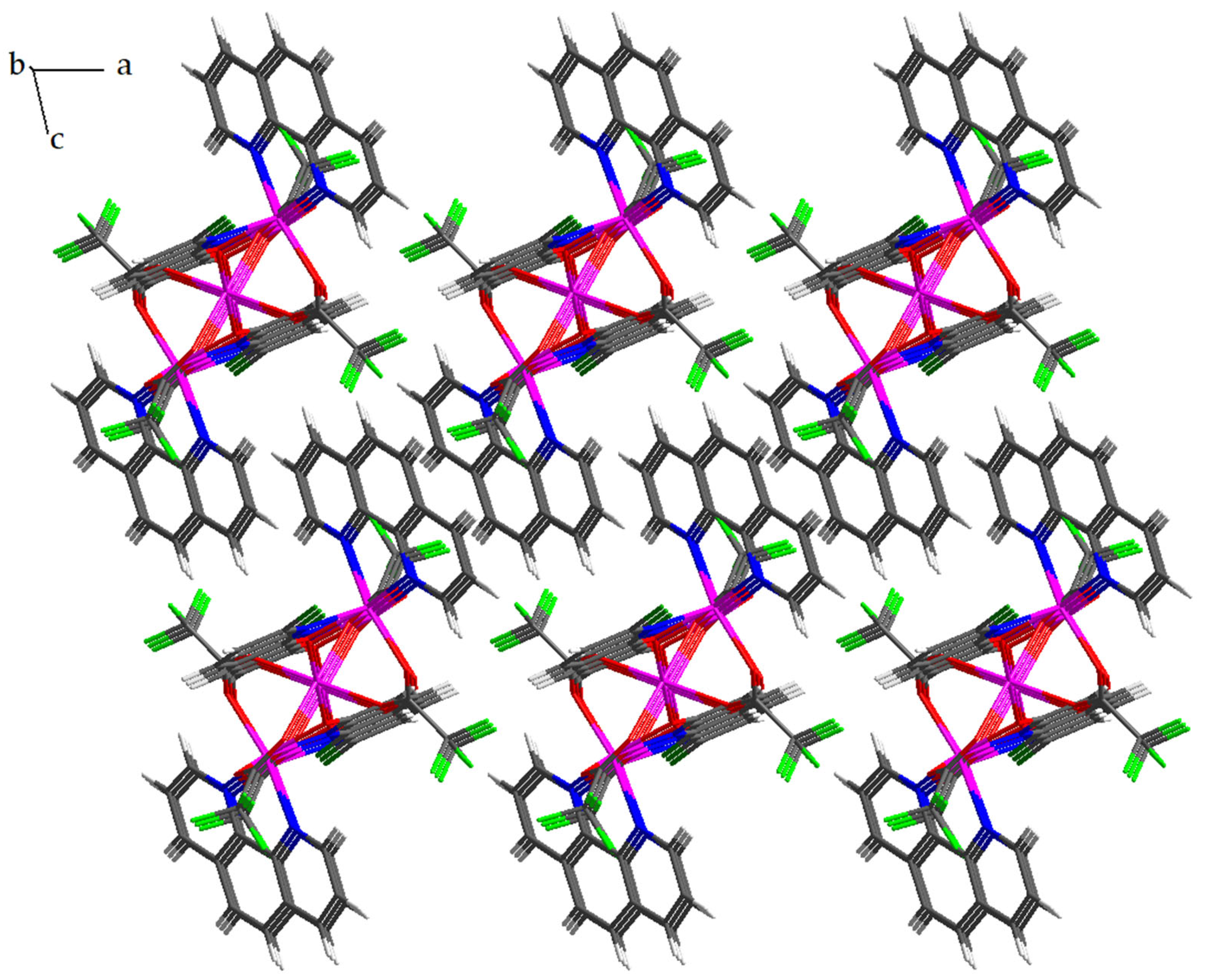
2.2. Crystal Structure
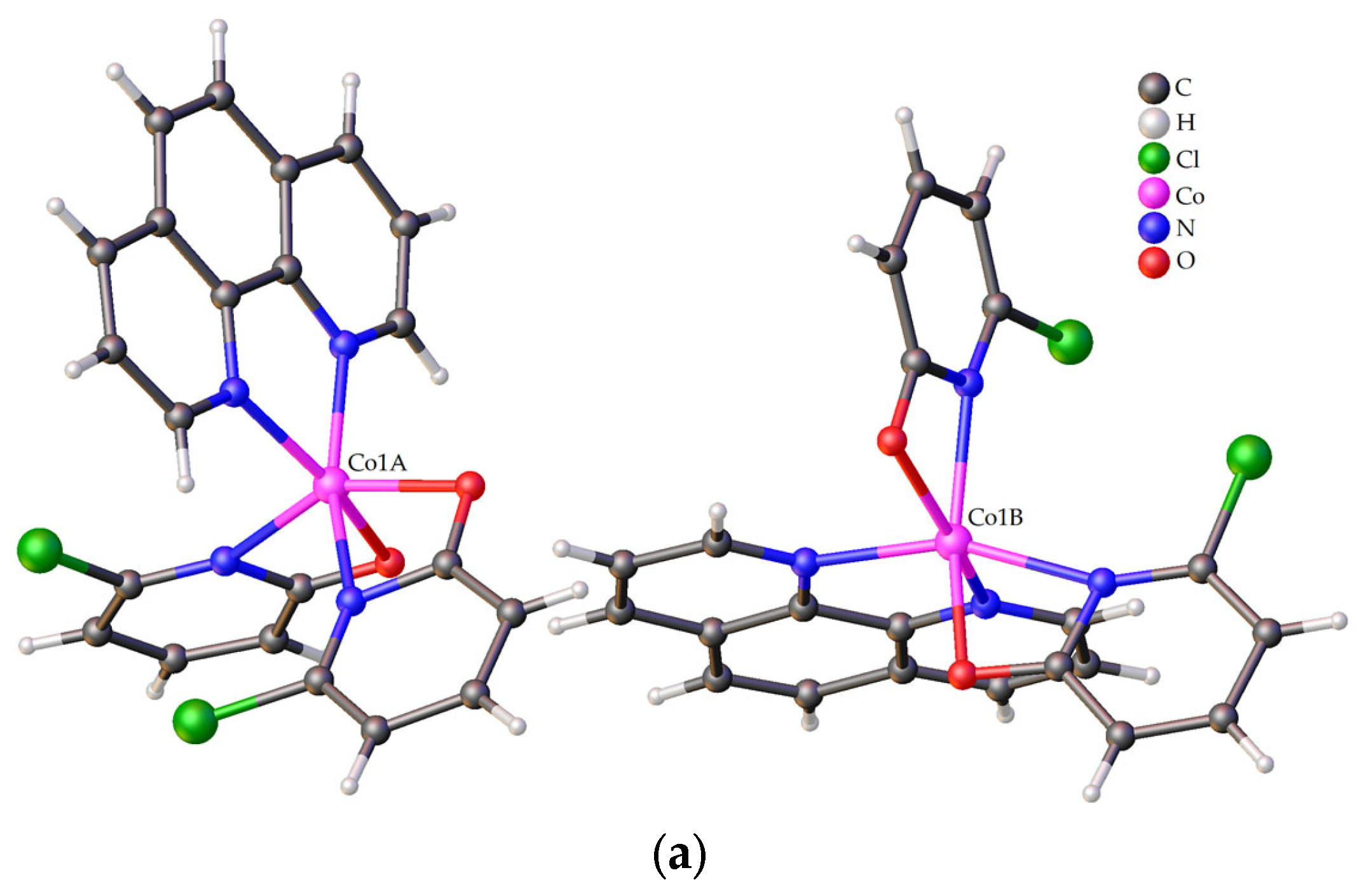
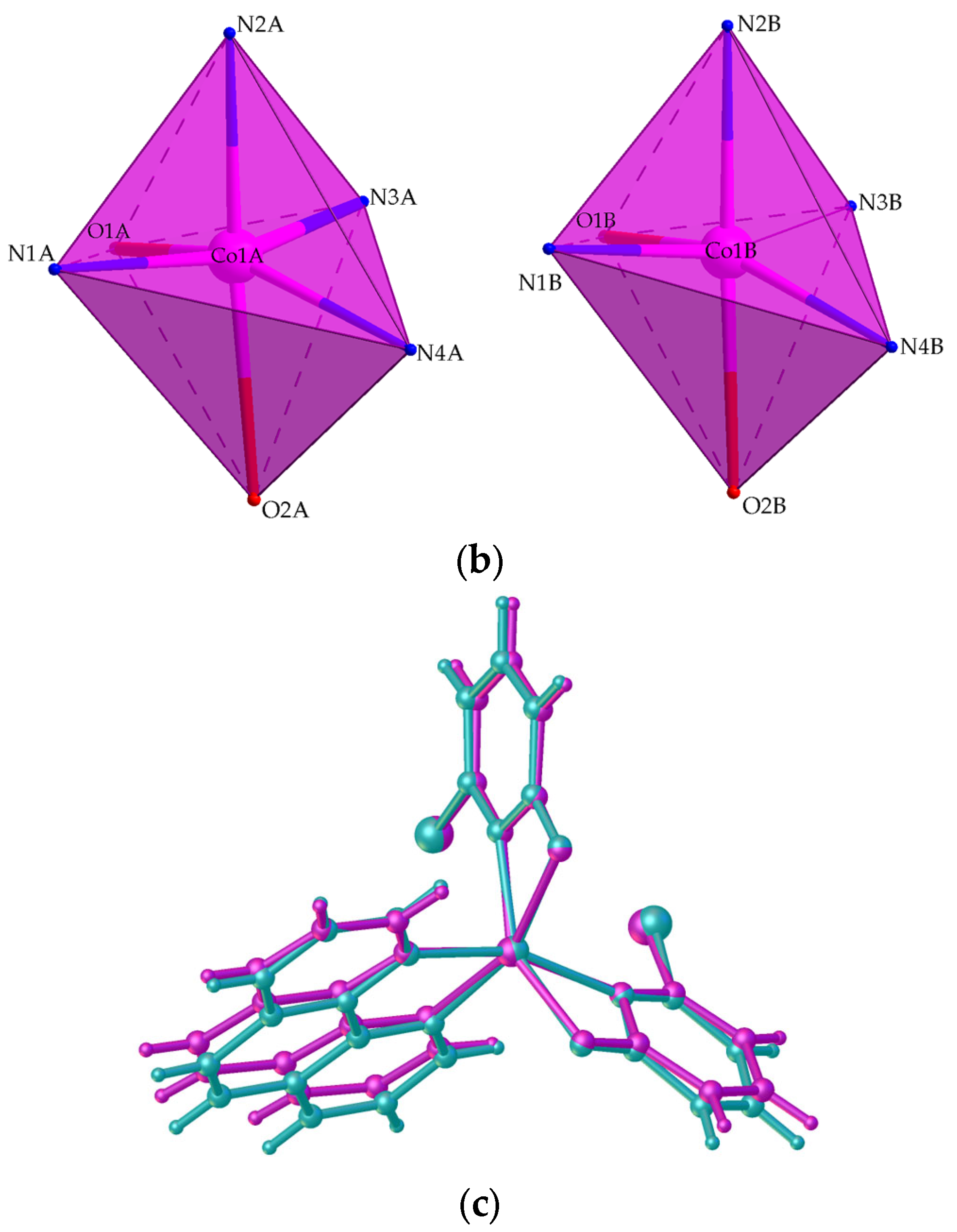
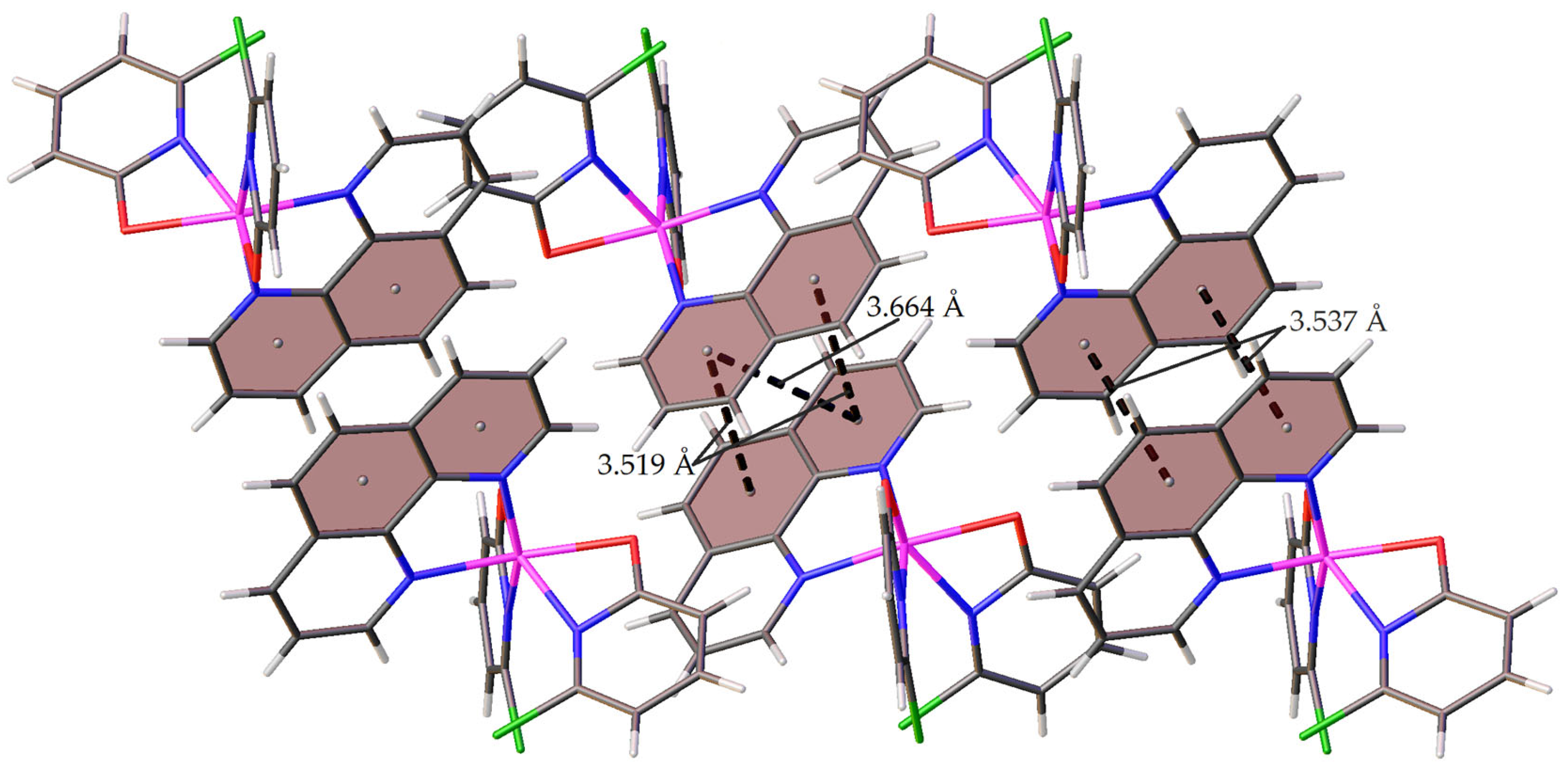
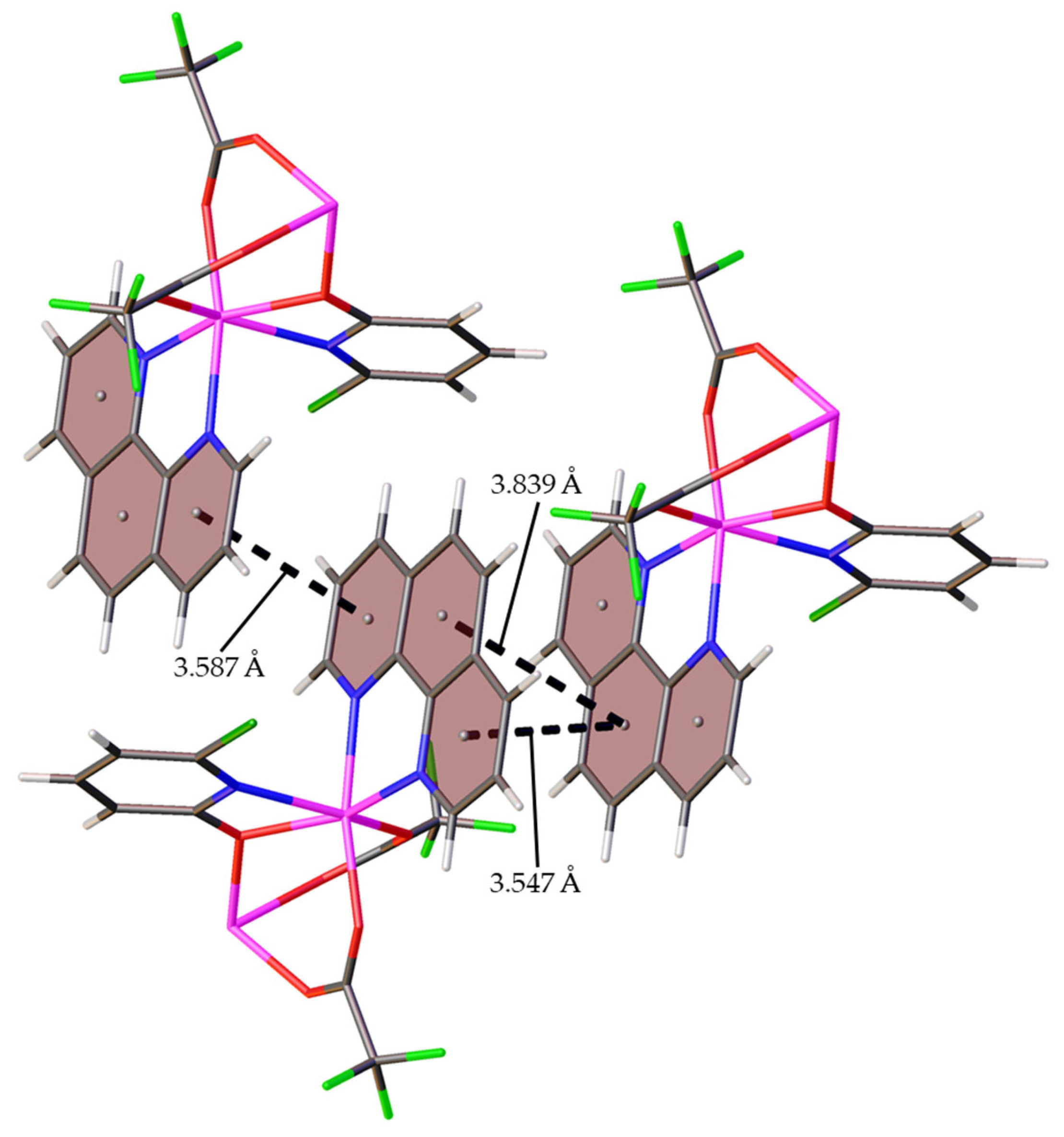
2.2.1. Crystal Structure of 1
2.2.2. Crystal Structure of 2
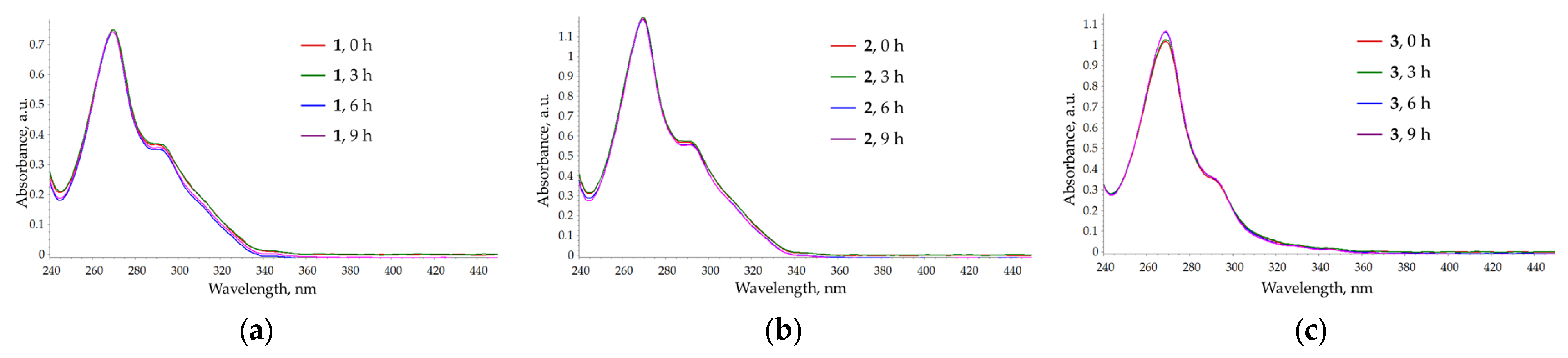
2.3. UV/Vis Absorption of Compound 1–3
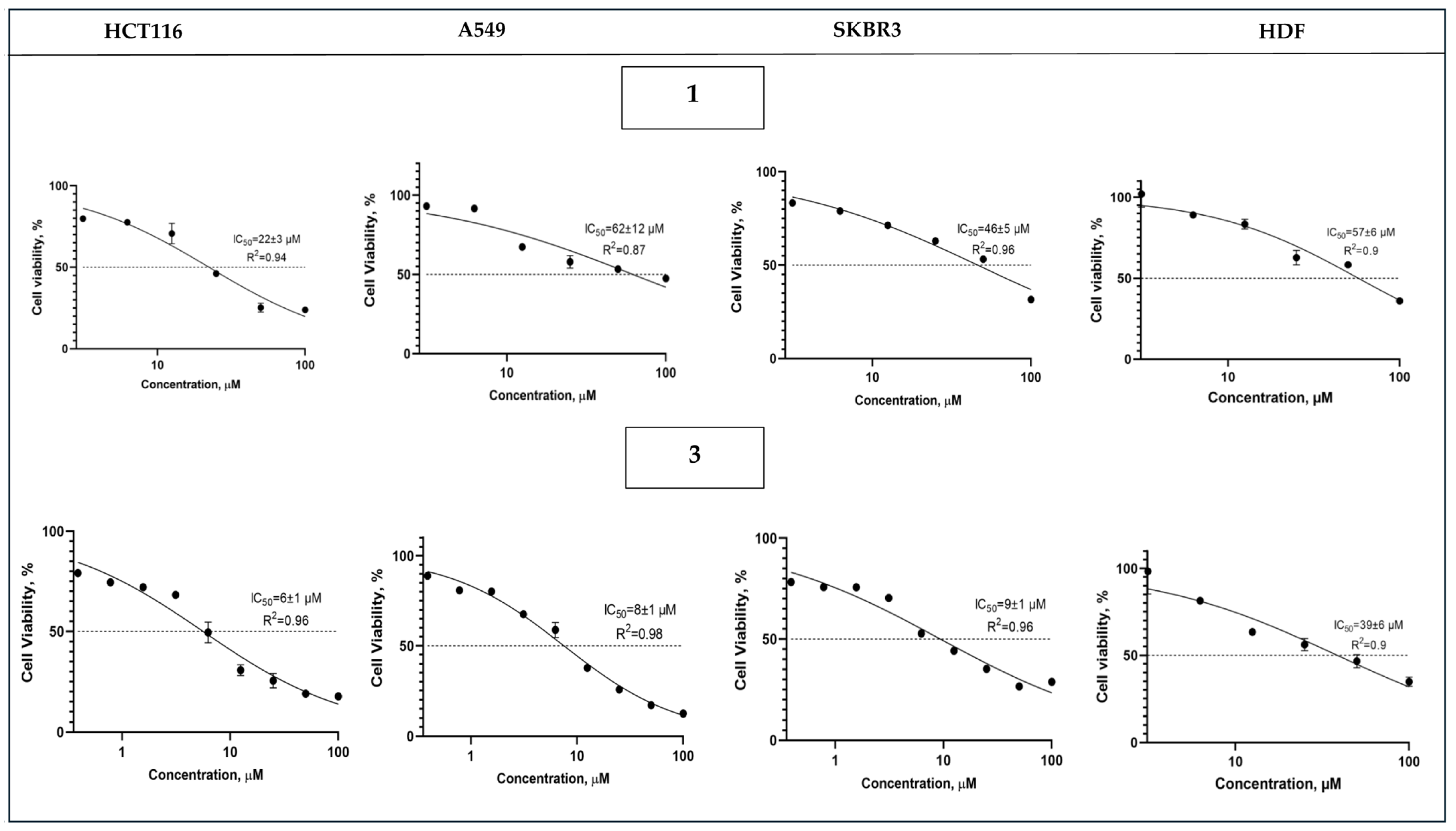
2.4. Cytotoxic Properties of 1 and 3
3. Materials and Methods
3.1. General Remarks
3.2. Biological Activity: Cell Culture and Cytotoxicity Assays
3.3. Synthesis of 1
3.4. Synthesis of 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malekshah, R.E.; Shakeri, F.; Khaleghian, A.; Salehi, M. Developing a Biopolymeric Chitosan Supported Schiff-base and Cu(II), Ni(II) and Zn(II) Complexes and Biological Evaluation as Pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc Complexes with Nitrogen Donor Ligands as Anticancer Agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef]
- Ali, B.; Iqbal, M.A. Coordination Complexes of Manganese and Their Biomedical Applications. ChemistrySelect 2017, 2, 1586–1604. [Google Scholar] [CrossRef]
- Golubeva, Y.A.; Lider, E.V. Copper(II) Complexes Based on 2,2’-Bipyridine and 1,10-Phenanthroline as Potential Objects for Developing Antitumor Drugs. J. Struct. Chem. 2024, 65, 1159–1209. [Google Scholar] [CrossRef]
- Nunes, P.; Correia, I.; Marques, F.; Matos, A.P.; Santos, M.M.C.; Azevedo, C.G.; Capelo, J.L.; Santos, H.M.; Gama, S.; Pinheiro, T.; et al. Copper Complexes with 1,10-Phenanthroline Derivatives: Underlying Factors Affecting Their Cytotoxicity. Inorg. Chem. 2020, 59, 9116–9134. [Google Scholar] [CrossRef]
- Quiñone, D.; Veiga, N.; Torres, J.; Castiglioni, J.; Bazzicalupi, C.; Bianchi, A.; Kremer, C. Synthesis, Solid-state Characterization and Solution Studies of New Phytate Compounds with Cu(II) and 1,10-Phenanthroline: Progress in the Structural Elucidation of Phytate Coordinating Ability. Dalton Trans. 2016, 45, 12156–12166. [Google Scholar] [CrossRef]
- Savinykh, P.E.; Golubeva, Y.A.; Smirnova, K.S.; Klyushova, L.S.; Berezin, A.S.; Lider, E.V. Synthesis, Crystal Structure and Cytotoxic Activity of 2,2’-Bipyridine/1,10-Phenanthroline Based Copper(II) Complexes with Diphenylphosphinic Acid. Polyhedron 2024, 261, 117141. [Google Scholar] [CrossRef]
- McCarron, P.; McCann, M.; Devereux, M.; Kavanagh, K.; Skerry, C.; Karakousis, P.C.; Aor, A.C.; Mello, T.P.; Santos, A.L.S.; Campos, D.L.; et al. Unprecedented in vitro Antitubercular Activitiy of Manganese(II) Complexes Containing 1,10-Phenanthroline and Dicarboxylate Ligands: Increased Activity, Superior Selectivity, and Lower Toxicity In Comparison to Their Copper(II) Analogs. Front. Microbiol. 2018, 9, 1432. [Google Scholar] [CrossRef]
- Almeida, J.D.C.; Silva, R.G.; Zanetti, R.D.; Moreira, M.B.; Portes, M.C.; Polloni, L.; de Vasconcelos Azevedo, F.V.P.; Von Poelhsitz, G.; Pivatto, M.; Godoy Netto, A.V.; et al. DNA Interactions, Antitubercular and Cytotoxic Activity of Heteroleptic CuII Complexes Containing 1,10-Phenanthroline. J. Mol. Struct. 2021, 1235, 130234. [Google Scholar] [CrossRef]
- Serment-Guerrero, J.; Bravo-Gomez, M.E.; Lara-Rivera, E.; Ruiz-Azuara, L. Genotoxic Assessment of the Copper Chelated Compounds Casiopeinas: Clues about Their Mechanisms of Action. J. Inorg. Biochem. 2017, 166, 68–75. [Google Scholar] [CrossRef]
- Leal García, M.; García Ortuño, L.; Ruiz-Azuara, L.; Gracia-Mora, I.; Luna-delVillar, J.; Sumano, H. Assessment of Acute Respiratory and Cardiovascular Toxicity of Casiopeinas in Anaesthetized Dogs. Basic Clin. Pharmacol. Toxicol. 2007, 101, 151–158. [Google Scholar] [CrossRef]
- Unavane, S.; Patil, R.; Syed, S.; Jain, H.K. Exploring the Therapeutic Potential of Copper and Cobalt Complexes as Anticancer Agents: A Comprehensive Review. Transit. Met. Chem. 2025, 50, 407–430. [Google Scholar] [CrossRef]
- Law, B.Y.K.; Qu, Y.Q.; Mok, S.W.F.; Liu, H.; Zeng, W.; Han, Y.; Gordillo-Martinez, F.; Chan, W.-K.; Wong, K.M.-C.; Wong, V.K.W. New Perspectives of Cobalt Tris(bipyridine) System: Anti-Cancer Effect and its Collateral Sensitivity Towards Multidrug-resistant (MDR) Cancers. Oncotarget 2017, 8, 55003–55021. [Google Scholar] [CrossRef]
- Verma, P.K.; Singh, R.K.; Kumar, S.; Shukla, A.; Kumar, S.; Gond, M.K.; Bharty, M.K.; Acharya, A. Cobalt(III) Complex Exerts Anti-cancer Effects on T Cell Lymphoma Through Induction of Cell Cycle Arrest and Promotion of Apoptosis. DARU J. Pharm. Sci. 2022, 30, 127–138. [Google Scholar] [CrossRef]
- Silva, A.; Luís, D.; Santos, S.; Silva, J.; Mendo, A.S.; Coito, L.; Silva, T.F.S.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. Biological Characterization of the Antiproliferative Potential of Co(II) and Sn(IV) Coordination Compounds in Human Cancer Cell Lines: A Comparative Proteomic Approach. Drug Metabol. Drug Interact. 2013, 28, 167–176. [Google Scholar] [CrossRef]
- Alexandersson, R. Blood and Urinary Concentrations as Estimators of Cobalt Exposure. Arch. Environ. Health Int. J. 1988, 43, 299–303. [Google Scholar] [CrossRef]
- Petit, A.; Mwale, F.; Zukor, D.J.; Catelas, I.; Antoniou, J.; Huk, O.L. Effect of Cobalt and Chromium Ions on bcl-2, bax, Caspase-3, and Caspase-8 Expression in Human U937 Macrophages. Biomaterials 2004, 25, 2013–2018. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Baravikov, D.E.; Koshenskova, K.A.; Kiskin, M.A.; Nelyubina, Y.V.; Primakov, P.V.; Voronina, Y.K.; Garaeva, V.V.; Aleshin, D.A.; Aliev, T.M.; et al. What Are the Prospects for Using Complexes of Copper(II) and Zinc(II) to Suppress the Vital Activity of Mycolicibacterium smegmatis? RSC Adv. 2022, 12, 5173–5183. [Google Scholar] [CrossRef]
- Ghosh, S.; Barve, A.C.; Kumbhar, A.A.; Kumbhar, A.S.; Puranik, V.G.; Datar, P.A.; Sonawane, U.B.; Joshi, R.R. Synthesis, Characterization, X-ray Structure and DNA Photocleavage by cis-Dichloro bis(diimine) Co(III) Complexes. J. Inorg. Biochem. 2006, 100, 331–343. [Google Scholar] [CrossRef]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1,10-Phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Hurtado Rodríguez, D.; Salinas Torres, A.; Hugo, R.; Becerra, D.; Castillo, J. Bioactive 2-Pyridone-containing Heterocycle Syntheses Using Multicomponent Reactions. RSC Adv. 2022, 12, 34965–34983. [Google Scholar] [CrossRef]
- Sangwan, S.; Yadav, N.; Kumar, R.; Chauhan, S.; Dhanda, V.; Walia, P.; Duhan, A. A Score Years’ Update in the Synthesis and Biological Evaluation of Medicinally Important 2-Pyridones. Eur. J. Med. Chem. 2022, 232, 114199. [Google Scholar] [CrossRef]
- Szyc, Ł.; Guo, J.; Yang, M.; Dreyer, J.; Tolstoy, P.M.; Nibbering, E.T.J.; Czarnik-Matusewicz, B.; Elsaesser, T.; Limbach, H.-H. The Hydrogen-Bonded 2-Pyridone Dimer Model System. 1. Combined NMR and FT-IR Spectroscopy Study. J. Phys. Chem. A 2010, 114, 7749–7760. [Google Scholar] [CrossRef]
- Petriček, S. Structural Diversity of Nickel and Manganese Chloride Complexes with Pyridin-2-One. Molecules 2020, 25, 846. [Google Scholar] [CrossRef]
- Huq, F.; Tayyem, H.; Beale, P.; Yu, J. Studies on the Activity of Three Palladium(II) Compounds of the Form: Trans-PdL2Cl2 where L=2-pydroxypyridine, 3-hydroxypyridine, and 4-hydroxypyridine. J. Inorg. Biochem. 2007, 101, 30–35. [Google Scholar] [CrossRef]
- Rajee, A.O.; Obaleye, J.A.; Louis, H.; Ayinla, S.O.; Aliyu, A.A.; Osunniran, W.A.; Lawal, A.; Mathias, G.E.; Rasaki, M.E.; Manicum, A.L.E. Ruthenium Polypyridyl Complexes with Hydroxypyridine: Experimental, DFT Studies, and in silico Antitubercular Activity Investigation. Chem. Afr. 2024, 7, 835–847. [Google Scholar] [CrossRef]
- Nikiforova, M.E.; Kayumova, D.B.; Malkerova, I.P.; Alikhanyan, A.S.; Kiskin, M.A.; Sidorov, A.A.; Khoroshilov, A.V.; Dalinger, I.L.; Starosotnikov, A.M.; Bastrakov, M.A.; et al. Structure and Thermal Characteristics of Bis(triethylammonium)-tetrakis(3,5-dinitro-2-pyridonato)cobalt(II). Russ. J. Coord. Chem. 2023, 49, 286–293. [Google Scholar] [CrossRef]
- Panja, S.; Ahsan, S.; Pal, T.; Kolb, S.; Ali, W.; Sharma, S.; Das, C.; Grover, J.; Dutta, A.; Werz, D.B.; et al. Non-directed Pd-catalysed Electrooxidative Olefination of Arenes. Chem. Sci. 2022, 13, 9432–9439. [Google Scholar] [CrossRef]
- Rosado Piquer, L.; Sañudo, E.C. Microwave Assisted Synthesis of Polynuclear Ni(II) Complexes. Polyhedron 2019, 169, 195–201. [Google Scholar] [CrossRef]
- Nikiforova, M.E.; Sidorov, A.A.; Aleksandrov, G.G.; Ikorskii, V.N.; Smolyaninov, I.V.; Okhlobystin, A.O.; Berberova, N.T.; Eremenko, I.L. Replacement of Carboxylate Bridges in Polynuclear Nickel Pivalates with 2-Hydroxy-6-methylpyridine Anions. Russ. Chem. Bull. Int. Ed. 2007, 56, 943–952. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, N.; Shi, W.; Guo, Y.; Wu, D. Crystal Structure of Diaqua-bis(μ2-6-chloropyridin-2-olato-κ3N,O:O)-tetrakis(chloropyridin-2-olato-κ1O)-bis(phenanthroline-κ2N,N′)diterbium(III), C54H38Cl6Tb2N10O8. New Cryst. Struct. 2018, 234, 221–222. [Google Scholar] [CrossRef]
- Fedulin, A.; Gupta, S.K.; Rüter, I.; Meyer, F.; von Wangelin, A.J. Polynuclear Iron(II) Pyridonates: Synthesis and Reactivity of Fe4 and Fe5 Clusters. Inorg. Chem. 2022, 61, 6149–6159. [Google Scholar] [CrossRef]
- Gálico, D.A.; Kitos, A.A.; Ovens, J.S.; Sigoli, F.A.; Murugesu, M. Lanthanide-based Molecular Cluster-Aggregates: Optical Barcoding and White-Light Emission with Nanosized {Ln20} Compounds. Angew. Chem. Int. Ed. 2021, 60, 6130–6136. [Google Scholar] [CrossRef]
- Badr, A.M.A.; Barakat, A.; Albering, J.H.; Sharaf, M.M.; Ul-Haq, Z.; Soliman, S.M. Structure, Antimicrobial Activity, Hirshfeld Analysis, and Docking Studies of Three Silver(I) Complexes-Based Pyridine Ligands. Appl. Sci. 2020, 10, 4853. [Google Scholar] [CrossRef]
- You, Z.; Zhang, L.; Shi, D.; Li, N.L. Synthesis and Crystal Structure of a Novel One-Dimensional Silver(I) Complex with High Urease Inhibitory Activity. Inorg. Chem. Commun. 2009, 12, 1231–1233. [Google Scholar] [CrossRef]
- Nikiforova, M.E.; Kiskin, M.A.; Sidorov, A.A.; Uvarova, M.A.; Eremenko, I.L. The Role of 2-hydroxypyridine in the Formation of Pivalate Zn–Gd Complexes. Russ. J. Coord. Chem. 2024, 50, 914–926. [Google Scholar] [CrossRef]
- Wagler, J.; Gericke, R. Ge–Cu-Complexes Ph(pyO)Ge(μ2-pyO)2CuCl and PhGe(μ2-pyO)4CuCl–representatives of Cu(I)→Ge(IV) and Cu(II)→Ge(IV) Dative Bond Systems. Molecules 2023, 28, 5442. [Google Scholar] [CrossRef]
- Zhou, G.; Schnack, J. Hendecanuclear [Cu6Gd5] Magnetic Cooler with High Molecular Symmetry of D3h. Chin. Chem. Lett. 2021, 32, 838–841. [Google Scholar] [CrossRef]
- Rosado Piquer, L.; Dey, S.; Castilla-Amorós, L.; Teat, S.J.; Cirera, J.; Rajaraman, G.; Sañudo, E.C. Microwave Assisted Synthesis of heterometallic 3d–4f M4Ln Complexes. Dalton Trans. 2019, 48, 12440–12450. [Google Scholar] [CrossRef]
- Blake, A.J.; Gilby, L.M.; Parsons, S.; Rawson, J.M.; Reed, D.; Solan, G.A.; Winpenny, R.E.P. Synthesis, Structural Characterisation and Nuclear Magnetic Resonance Studies of Cobalt Complexes of Pyridonate Ligands. J. Chem. Soc. Dalton Trans. 1996, 17, 3575–3581. [Google Scholar] [CrossRef]
- Hazell, A.; McGinley, J.; McKenzie, C.J. Dichlorobis(1,10-phenanthroline-N,N’)cobalt(II)–acetonitrile (1/1.5). Acta Crystallogr. Sect. C 1997, 53, 723–725. [Google Scholar] [CrossRef]
- Khan, H.Y.; Ansari, M.N.; Shadab, G.; Tabassum, S.; Arjmand, F. Evaluation of Cytotoxic Activity and Genotoxicity of Structurally Well Characterized Potent Cobalt(II) Phen-based Antitumor Drug Entities: An in vitro and in vivo Approach. Bioorg. Chem. 2019, 88, 102963. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zhang, X.; Sun, G.; Hui, J. Synthesis of Cationic Cobalt(II) Complexes and Their Efficiency as Catalysts for the Polymerization of 1,3-Butadiene. Inorg. Chim. Acta 2014, 414, 8–14. [Google Scholar] [CrossRef]
- Chen, X.; Han, S.; Wang, R.; Li, Y. Four Supramolecular Isomers of Dichloridobis(1,10-phenanthroline)cobalt(II): Synthesis, Structure Characterization and Isomerization. Acta Crystallogr. Sect. C 2016, 72, 6–13. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Liu, T. A Polymorph of Cis-dichloridobis(1,10-phenanthroline-κ2N,N′)cobalt(II). Acta Crystallogr. Sect. E 2007, 63, m1880. [Google Scholar] [CrossRef]
- Sanmartín, J.; Bermejo, M.R.; García Vázquez, J.A.; Romero, J.; Sousa, A. Direct Electrochemical Synthesis and Characterization of Cobalt and Nickel Complexes with 2-Pyridinone and 2-Pyridinemethanethiol-1-oxide. Transit. Met. Chem. 1994, 19, 209. [Google Scholar] [CrossRef]
- Perrin, D.D.; Dempsey, B.; Serjeant, E.P. pKa Prediction for Organic Acids and Bases; Chapman and Hall: London, UK, 1981; Volume 1. [Google Scholar]
- Spinner, E.; White, J.C.B. Spectral and Ionisation Constant Studies of Substituted 2-Hydroxypyridines (1,2-Dihydro-2-oxopyridines). J. Chem. Soc. B 1966, 991–995. [Google Scholar] [CrossRef]
- Sidorov, A.A.; Nikiforova, M.E.; Pakhmutova, E.V.; Aleksandrov, G.G.; Ikorskii, V.N.; Novotortsev, V.M.; Eremenko, I.L.; Moiseev, I.I. Mono- and Polynuclear CoII Complexes with 2-Hydroxy-6-methylpyridine. Russ. Chem. Bull. 2006, 55, 1920–1932. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Spinner, E.; White, J.C.B. The Spectra and Structures of the Cations and Anions of Substituted 2-Hydroxypyridines (1,2-Dihydro-2-oxopyridines). J. Chem. Soc. B 1966, 996–1001. [Google Scholar] [CrossRef]
- Deacon, G. Relationships Between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Cirera, J.; Ruiz, E.; Alvarez, S. Shape and Spin State in Four-Coordinate Transition-Metal Complexes: The Case of the d6 Configuration. Chem. Eur. J. 2006, 12, 3162–3167. [Google Scholar] [CrossRef]
- Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Yambulatov, D.S.; Kiskin, M.A.; Nelyubina, Y.V.; Primakov, P.V.; Bekker, O.B.; Levitskiy, O.A.; Magdesieva, T.V.; Imshennik, V.K.; Maksimov, Y.V.; et al. Improved in vitro Antimycobacterial Activity of Trinuclear Complexes Cobalt(II,III) and Iron(III) with 2-Furoic Acid Against Mycolicibacterium smegmatis. ChemistrySelect 2020, 5, 11837–11842. [Google Scholar] [CrossRef]
- Goldberg, A.E.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Trinuclear Zinc β-Naphthoate Complexes: Synthesis and Structure. Russ. Chem. Bull. 2011, 60, 849–854. [Google Scholar] [CrossRef]
- Dimiza, F.; Lazou, M.; Papadopoulos, A.P.; Hatzidimitriou, A.G.; Psomas, G. Manganese(II) Coordination Compounds of Carboxylate Non-Steroidal Anti-Inflammatory Drugs. J. Inorg. Biochem. 2020, 203, 110906. [Google Scholar] [CrossRef]
- Gómez, V.; Corbella, M. Versatility in the Coordination Modes of n-Chlorobenzoato Ligands: Synthesis, Structure and Magnetic Properties of Three Types of Polynuclear MnII Compounds. Eur. J. Inorg. Chem. 2009, 4471–4482. [Google Scholar] [CrossRef]
- Rardin, R.L.; Poganiuch, P.; Bino, A.; Goldberg, D.P.; Tolman, W.B.; Liu, S.; Lippard, S.J. Synthesis and Characterization of Trinuclear Iron(II) and Manganese(II) Carboxylate Complexes: Structural Trends in Low Valent Iron and Manganese Carboxylates. J. Am. Chem. Soc. 1992, 114, 5240–5249. [Google Scholar] [CrossRef]
- Cai, J.; Xu, Y.; Ng, S.W. Di-μ2-acetato-1:2κ3O,O′:O;2:3κ3O:O,O′-tetrakis(μ2-2-hydroxybenzoato)-1:2κ4O:O′;2:3κ4O:O′-bis(1,10-phenanthroline)-1κ2N,N′;3κ2N,N′-trizinc(II). Acta Crystallogr. Sect. E 2007, 63, m2940. [Google Scholar] [CrossRef]
- Koo, B.K.; Lee, U.A. Linear Trinuclear Cobalt(II) Complex with Acetate Bridging Ligand: Hexakisacetatodiphenanthrolinetricobalt(II). J. Korean Chem. Soc. 2003, 47, 667–670. [Google Scholar] [CrossRef]
- Koshenskova, K.A.; Bardina, E.E.; Makotchenko, E.V.; Kharlamova, V.Y.; Mironov, I.V.; Bekker, O.B.; Treshalina, H.M.; Sokolova, D.V.; Pokrovsky, V.S.; Borodin, E.A.; et al. Gold(III) Complexes Containing (Non)Protonated Oligopyridines–Unexpected Results in Cancer Drug Research. New J. Chem. 2025, 49, 14037–14052. [Google Scholar] [CrossRef]
- Uvarova, M.A.; Dolgushin, F.M.; Metlin, M.T.; Metlina, D.A.; Taydakov, I.V.; Sokolova, D.V.; Kasyanenko, N.A.; Komolkina, N.A.; Lutsenko, I.A.; Eremenko, I.L. Synthesis, DNA Binding and Cytotoxicity Studies of Luminescent Ln(III) Thiophencarboxylate Complexes. J. Mol. Struct. 2026, 1349, 143671. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-crystal Structure Determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]



| Bond | d, Å | |
|---|---|---|
| 1 | 2 | |
| Co–O(chp) | 2.0963(15) 2.1619(15) 2.0988(16) 2.1679(15) | 2.027(3) 2.092(3) |
| Co–N(chp) | 2.1209(17) 2.1819(17) 2.1233(17) 2.1698(18) | 2.250(4) |
| Co–N(η-phen) | 2.1201(19) 2.1091(17) 2.1136(18) 2.1245(18) | 2.091(4) 2.130(4) |
| Co–O(µ-OOCCF3) | ― | 2.087(3) 2.060(3) 2.103(3) 2.114(3) |
| Compound | OC-6 1 | TPR-6 2 | PPY-6 3 | |
|---|---|---|---|---|
| 1 | Co1A | 4.819 | 9.638 | 21.427 |
| Co1B | 5.030 | 8.918 | 20.965 | |
| 2 | Co1 | 0.090 | 16.222 | 29.264 |
| Co2 | 3.240 | 9.593 | 21.455 | |
| Compound | IC50, µM | SI a | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| HCT116 | A549 | SKBR3 | HDF | HCT116 | A549 | SKBR3 | ||
| 1 | 22 ± 3 | 62 ± 12 | 46 ± 5 | 57 ± 6 | 2.6 | 0.9 | 1.2 | This work |
| 3 | 6 ± 1 * | 8 ± 1 | 9 ± 1 | 39 ± 6 | 6.8 | 5.1 | 4.1 | This work |
| (phenH2)[AuCl4]∙NO3 | 88.9 ± 3.2 | 132.3 ± 45.4 | 112.5 ± 10.7 | 228.3 ± 13.9 | 2.6 | 1.7 | 2.0 | [64] |
| [Au(neoc)Cl3] b | 0.2 ± 0.05 | 2.60 ± 1.18 | 2.00 ± 0.56 | 35.62 ± 2.45 | 169.6 | 13.7 | 17.8 | [64] |
| [Eu2(tph)4(OAc)2(phen)2] c | 3.7 ± 0.4 | 4.0 ± 0.7 | 8.2 ± 1.2 | 7.3 ± 1.4 | 3.0 | 1.9 | 1.8 | [65] |
| Cisplatin d | 8 ± 0.5 | 5 ± 0.3 | 8 ± 0.6 | 23 ± 2 | 2.9 | 4.6 | 2.9 | This work |
| Parameters | Complex | |
|---|---|---|
| 1 | 2 | |
| Empirical formula | C22H14Cl2CoN4O2 | C42H22Cl2Co3F12N6O10 |
| Formula weight | 496.20 | 1246.34 |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a (Å) | 9.3330 (5) | 9.7217 (8) |
| b (Å) | 14.7266 (7) | 11.0191 (9) |
| c (Å) | 15.0683 (7) | 12.0031 (11) |
| α (°) | 82.8863 (16) | 69.720 (3) |
| β (°) | 89.2581 (17) | 76.610 (3) |
| γ (°) | 81.6392 (16) | 87.115 (3) |
| V (Å3) | 2033.23 (17) | 1172.70 (18) |
| Z | 4 | 1 |
| Dcalc (g∙cm−3) | 1.621 | 1.765 |
| Temperature (K) | 100 | 100 |
| μ (mm−1) | 1.135 (Mo-Kα) | 1.274 (Mo-Kα) |
| Radiation wavelength, Å | 0.71073 | 0.71073 |
| Tmin/Tmax | 0.622/0.747 | 0.680/0.737 |
| θmin–θmax, deg | 1.408–30.000 | 2.503–26.000 |
| F(000) | 1004 | 619 |
| Rint | 0.0476 | 0.0669 |
| Reflections measured | 61,178 | 15,198 |
| Independent reflections | 11,829 | 4594 |
| Observed reflections I > 2σ(I) | 9870 | 3377 |
| R1, wR2 (I > 2σ(I)) | 0.0430, 0.0785 | 0.0543, 0.1036 |
| GooF | 1.111 | 1.033 |
| Δρmin/Δρmax, (e/Å3) | 0.419/−0.449 | 1.141/−0.539 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforova, M.E.; Lutsenko, I.A.; Dolgushin, F.M.; Shmelev, M.A.; Sidorov, A.A.; Yambulatov, D.S.; Sokolova, D.V.; Pokrovsky, V.S.; Eremenko, I.L. Antiproliferative Potential of Cobalt(II) Phenanthroline Complexes with Pyridonates. Molecules 2025, 30, 4367. https://doi.org/10.3390/molecules30224367
Nikiforova ME, Lutsenko IA, Dolgushin FM, Shmelev MA, Sidorov AA, Yambulatov DS, Sokolova DV, Pokrovsky VS, Eremenko IL. Antiproliferative Potential of Cobalt(II) Phenanthroline Complexes with Pyridonates. Molecules. 2025; 30(22):4367. https://doi.org/10.3390/molecules30224367
Chicago/Turabian StyleNikiforova, Marina E., Irina A. Lutsenko, Fedor M. Dolgushin, Maxim A. Shmelev, Alexey A. Sidorov, Dmitriy S. Yambulatov, Darina V. Sokolova, Vadim S. Pokrovsky, and Igor L. Eremenko. 2025. "Antiproliferative Potential of Cobalt(II) Phenanthroline Complexes with Pyridonates" Molecules 30, no. 22: 4367. https://doi.org/10.3390/molecules30224367
APA StyleNikiforova, M. E., Lutsenko, I. A., Dolgushin, F. M., Shmelev, M. A., Sidorov, A. A., Yambulatov, D. S., Sokolova, D. V., Pokrovsky, V. S., & Eremenko, I. L. (2025). Antiproliferative Potential of Cobalt(II) Phenanthroline Complexes with Pyridonates. Molecules, 30(22), 4367. https://doi.org/10.3390/molecules30224367






