Adsorption of Algal-Derived 2-Methylisoborneol (MIB) and Dimethyl Disulfide (DMDS) onto Activated Carbon: The Role of Pore Structure and Hydrophobicity
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization Results
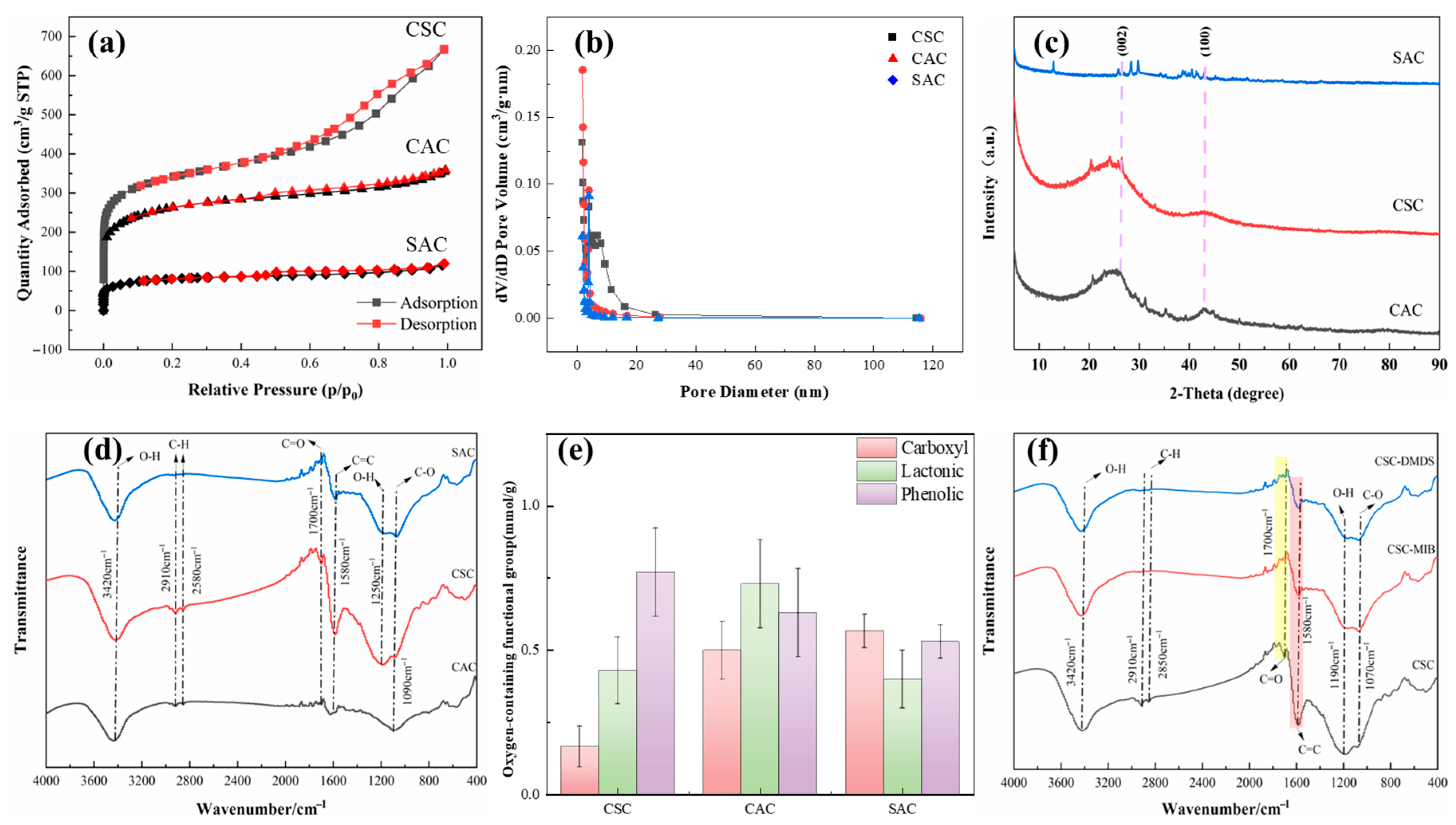
2.1.1. Microstructural Analysis
2.1.2. Specific Surface Area and Pore Volume
2.1.3. Crystal Structure
2.1.4. Surface Functional Group Type and Content
2.2. Influencing Factors
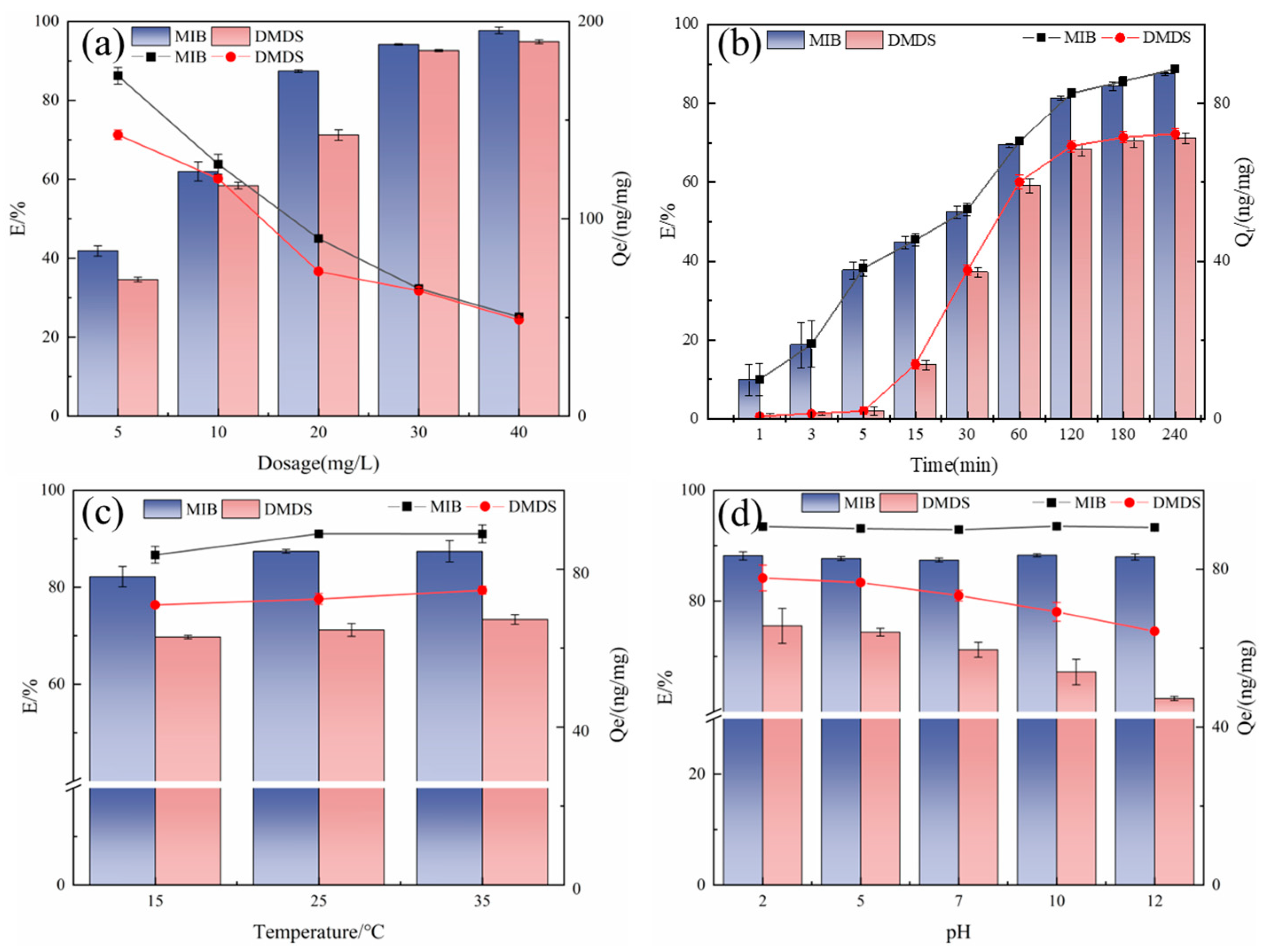
2.2.1. Adsorption Capacity Optimization
2.2.2. Dosage
2.2.3. Adsorption Time
2.2.4. Temperature
2.2.5. pH
2.3. Adsorption Process and Kinetic Analysis of Three Carbon Materials
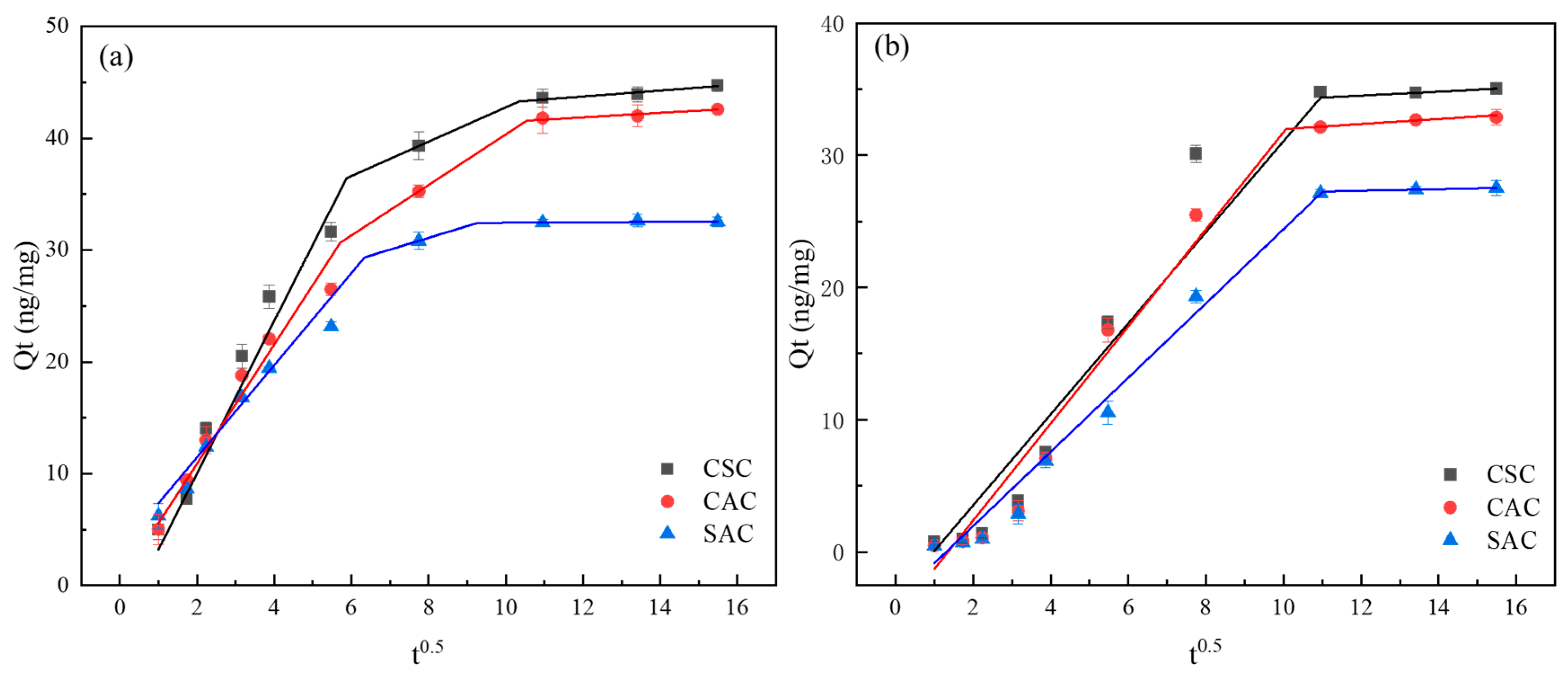
2.3.1. Adsorption Kinetics
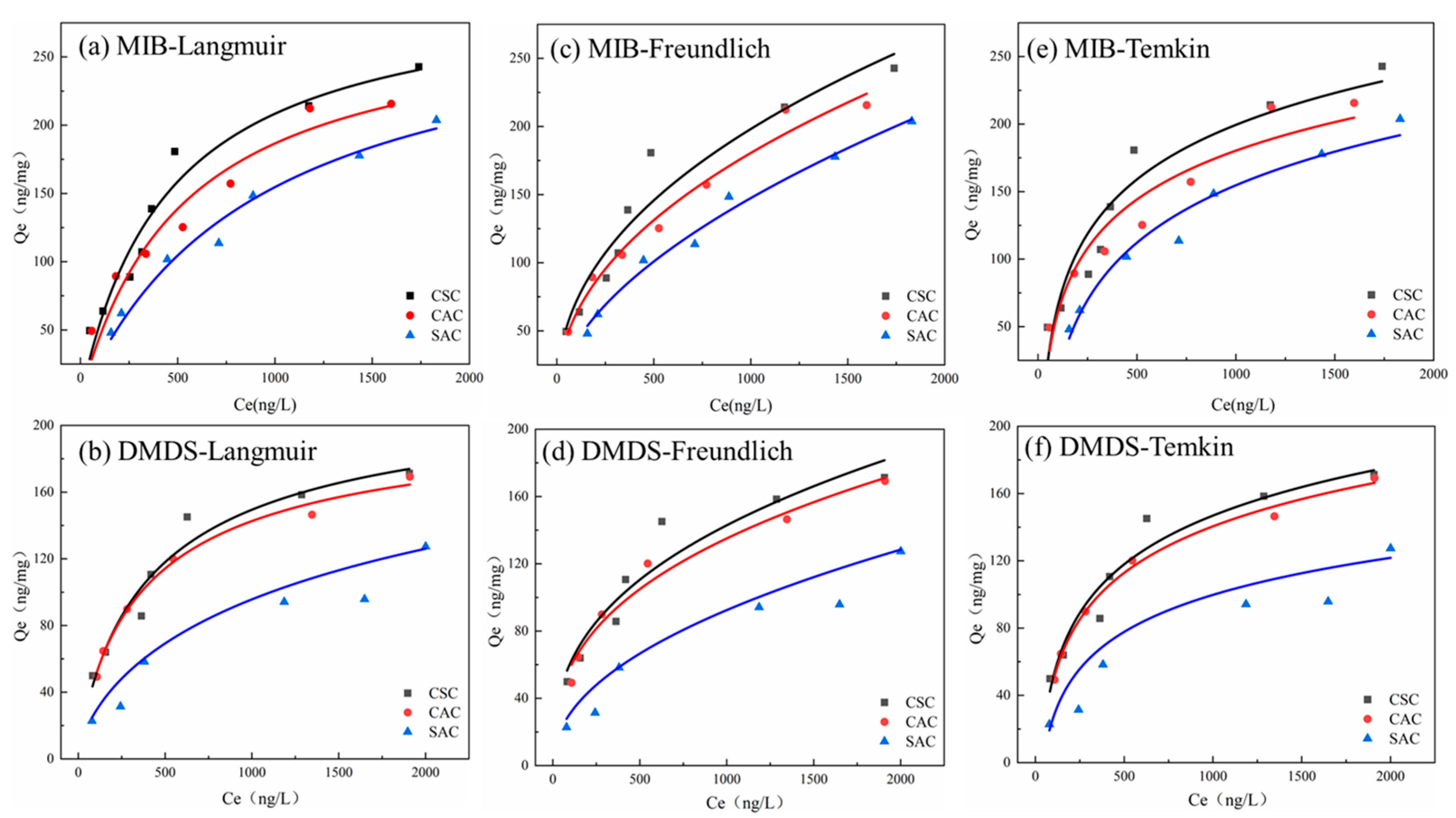
2.3.2. Adsorption Isotherms
2.3.3. Adsorption Thermodynamics
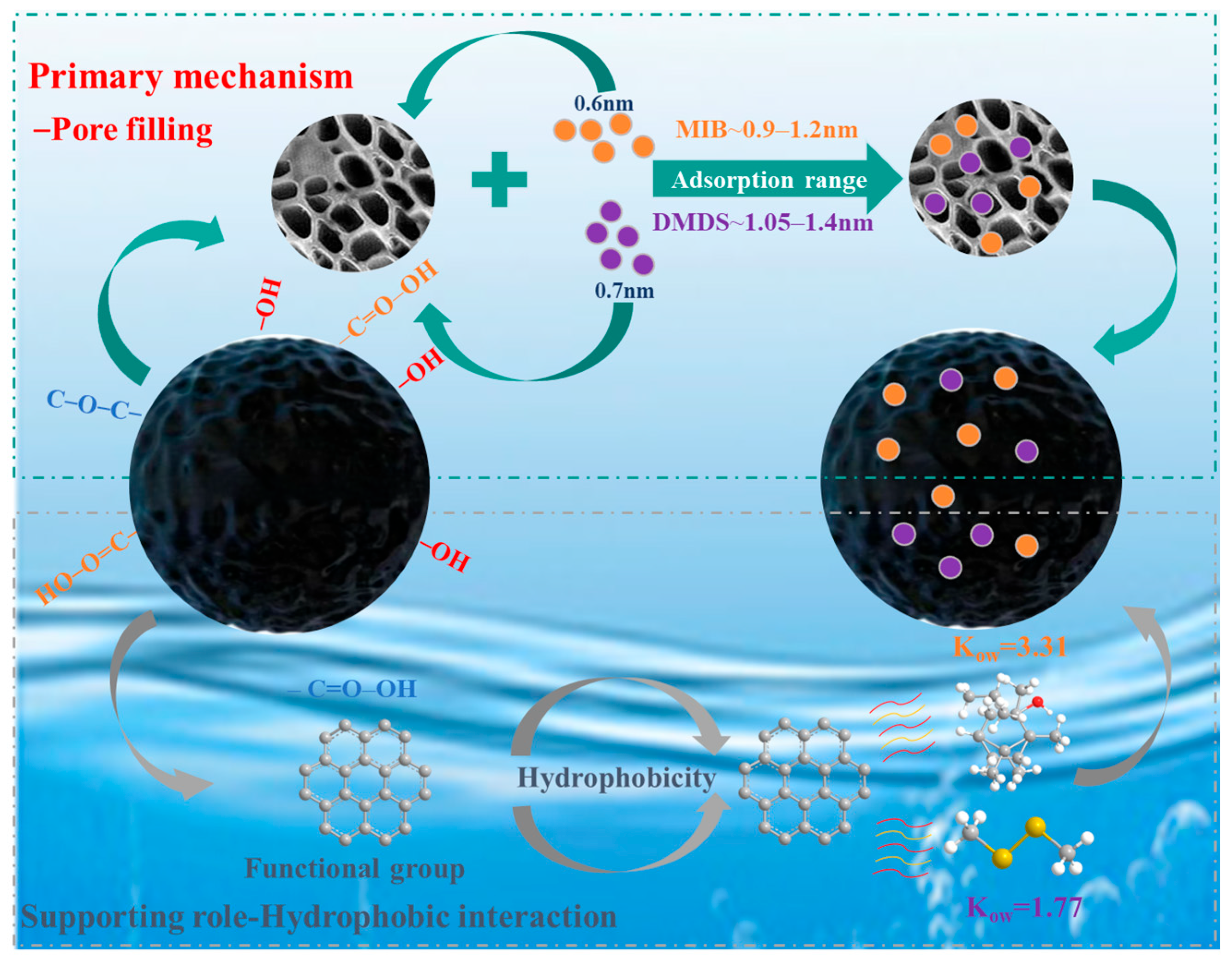
2.4. Mechanism Analysis
2.5. Regeneration
3. Experimental Materials and Methods
3.1. Experimental Materials
3.2. Adsorption Experiment
3.3. Methods of Analysis
3.3.1. Analytical Method for MIB and DMDS Quantification
3.3.2. Characterization of PAC
3.4. Reusability Evaluation of CSC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAC | powdered activated carbon |
| CAC | coal-based activated carbon |
| CSC | coconut shell-derived activated carbon |
| SAC | Sargassum activated carbon |
| MIB | 2-methylisoborneol |
| DMDS | dimethyl disulfide |
| PFO | pseudo-first-order model |
| PSO | pseudo-second-order model |
References
- Chapra, S.C.; Boehlert, B.; Fant, C.; Bierman, V.J.; Henderson, J.; Mills, D.; Mas, D.M.L.; Rennels, L.; Jantarasami, L.; Martinich, J.; et al. Climate Change Impacts on Harmful Algal Blooms in US Freshwaters: A Screening-Level Assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef]
- Li, W.; Han, Z.; Sun, D. Preparation of sludge-based activated carbon for adsorption of dimethyl sulfide and dimethyl disulfide during sludge aerobic composting. Chemosphere 2021, 279, 363–370. [Google Scholar] [CrossRef]
- Nakayama, A.; Sakamoto, A.; Matsushita, T.; Matsui, Y.; Shirasaki, N. Effects of pre, post, and simultaneous loading of natural organic matter on 2-methylisoborneol adsorption on superfine powdered activated carbon: Reversibility and external pore-blocking. Water Res. 2020, 182, 115992. [Google Scholar] [CrossRef]
- Yu, C.C.; Shi, C.F.; Tang, J.; Ji, Q.Y.; Wang, X.; Xu, X.G.; Wang, G.X. Release of taste and odour compounds during Zizania latifolia decay: A microcosm system study. Environ. Pollut. 2019, 254, 112954. [Google Scholar] [CrossRef]
- Huang, X.; Shi, B.Y.; Hao, H.T.; Su, Y.L.; Wu, B.; Jia, Z.Y.; Wang, C.M.; Wang, Q.; Yang, M.; Yu, J.W. Identifying the function of activated carbon surface chemical properties in the removability of two common odor compounds. Water Res. 2020, 178, 115797. [Google Scholar] [CrossRef]
- Son, H.; Lee, C.; Bae, S.; Kang, L. Effects of activated carbon types and service life on removal of odorous compounds: Geosmin and 2-MIB. J. Korean Soc. Environ. Eng. 2007, 29, 404–411. [Google Scholar]
- Watanabe, T.; Zaini, M.A.A.; Amano, Y.; Machida, M. Removal of 2-methylisoborneol from aqueous solution by cattle manure compost (CMC) derived activated carbons. J. Water Supply Res. Technol.-Aqua 2014, 63, 239–247. [Google Scholar] [CrossRef]
- Yu, J.W.; Yang, M.; Lin, T.F.; Guo, Z.H.; Zhang, Y.; Gu, J.N.; Zhang, S.X. Effects of surface characteristics of activated carbon on the adsorption of 2-methylisobornel (MIB) and geosmin from natural water. Sep. Purif. Technol. 2007, 56, 363–370. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakao, S.; Taniguchi, T.; Matsushita, T. Geosmin and 2-methylisoborneol removal using superfine powdered activated carbon: Shell adsorption and branched-pore kinetic model analysis and optimal particle size. Water Res. 2013, 47, 2873–2880. [Google Scholar] [CrossRef]
- Huang, X.; Geng, M.; Yang, F.; Yu, J.; Shi, B.; Yang, M. Electron Donor–Acceptor Interaction Strengthens 2-Methylisoborneol Affinity to Nitrogen-Doped Activated Carbon: A Combined Batch and Theoretical Calculation Study. ACS EST Water 2023, 3, 3035–3043. [Google Scholar] [CrossRef]
- Cui, J. Preparation of Biochar and Adsorption Performance of Olfactory Components in Black Odour Water Bodies. Master’s Thesis, Jiangnan University, Wuxi, China, 2019. [Google Scholar]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, Z.Y.; Choi, S.K.; Lu, Y.Y. Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite. Energies 2018, 11, 1502. [Google Scholar] [CrossRef]
- Rekha, A.; Srinivasan, L.; Pavithra, S.; Gomathi, T.; Sudha, P.N.; Lavanya, G.; Arumugam, N.; Almansour, A.I.; Sakkarapalayam, M.M.; Vidhya, A. Biosorption efficacy studies of Sargassum wightii and its biochar on the removal of chromium from aqueous solution. J. Taiwan Inst. Chem. Eng. 2023, 166, 105241. [Google Scholar] [CrossRef]
- Qu, W.Y.; Yuan, T.; Yin, G.J.; Xu, S.A.; Zhang, Q.; Su, H.J. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Yang, Y.X.; Sun, C.; Huang, Q.X.; Yan, J.H. Hierarchical porous structure formation mechanism in food waste component derived N-doped biochar: Application in VOCs removal. Chemosphere 2022, 291, 132702. [Google Scholar] [CrossRef]
- Lu, S.Y.; Huang, X.L.; Tang, M.H.; Peng, Y.Q.; Wang, S.C.; Makwarimba, C.P. Synthesis of N-doped hierarchical porous carbon with excellent toluene adsorption properties and its activation mechanism. Environ. Pollut. 2021, 284, 117113. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, K.; Fang, J.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem. Eng. J. 2020, 385, 123842. [Google Scholar] [CrossRef]
- Li, Z.C.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell -based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Cao, B.X.; Li, M.; Zhang, T.; Gong, T.C.; Yang, T.X.; Xi, B.D.; Lu, H.L.; Wang, Z.H. Dynamics and mechanisms of atrazine adsorption on biogas-residue biochar with citric acid modification. Sep. Purif. Technol. 2024, 337, 126151. [Google Scholar] [CrossRef]
- Ali, R.; Aslam, Z.; Shawabkeh, R.A.; Asghar, A.; Hussein, I.A. BET, FTIR, and RAMAN characterizations of activated carbon from waste oil fly ash. Turk. J. Chem. 2020, 44, 279–295. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zhao, Y.; Long, Y.Y.; Zhu, Y.; Wang, H.T.; Lu, W.J. Evaluation of the biological stability of waste during landfill stabilization by thermogravimetric analysis and Fourier transform infrared spectroscopy. Bioresour. Technol. 2011, 102, 9403–9408. [Google Scholar] [CrossRef]
- Yang, F.; Yu, J.; Wang, Q.; Wang, C.; Du, Y.; Liu, Z.; Zhang, L.; Liu, Z.; Jing, C.; Tang, J.; et al. Enhancing the Adsorption Performance of 2-Methylisoborneol by Activated Carbon by Promoting Hydrophobic Effects. ACS EST Water 2022, 2, 1789. [Google Scholar] [CrossRef]
- Newcombe, G.; Morrison, J.; Hepplewhite, C. Simultaneous adsorption of MIB and NOM onto activated carbon. I. Characterisation of the system and NOM adsorption. Carbon 2002, 40, 2135–2146. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakao, S.; Sakamoto, A.; Taniguchi, T.; Pan, L.; Matsushita, T.; Shirasaki, N. Adsorption capacities of activated carbons for geosmin and 2-methylisoborneol vary with activated carbon particle size: Effects of adsorbent and adsorbate characteristics. Water Res. 2015, 85, 95–102. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Song, M.; Wei, Y.X.; Cai, S.P.; Yu, L.; Zhong, Z.P.; Jin, B.S. Study on adsorption properties and mechanism of Pb2 + with different carbon based adsorbents. Sci. Total Environ. 2018, 618, 1416–1422. [Google Scholar] [CrossRef]
- Jerzy, C.; Jacek, J.; Mietek, J. Assessing the contribution of micropores and mesopores from nitrogen adsorption on nanoporous carbons: Application to pore size analysis. Carbon 2021, 183, 150–157. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Lan, C. Adsorption of textile dyes on Pine cone from colored wastewater: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 268, 117–125. [Google Scholar] [CrossRef]
- Ho, L.; Newcombe, G. Effect of NOM, turbidity and floc size on the PAC adsorption of MIB during alum coagulation. Water Res. 2005, 39, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, Y.F.; Nie, Z.J.; Hofmann, R. The effect of water temperature on the removal of 2-methylisoborneol and geosmin by preloaded granular activated carbon. Water Res. 2020, 183, 116065. [Google Scholar] [CrossRef]
- Wu, J.; Wan, S.; Yuan, D.; Yi, S.; Zhou, L.; Sun, L. Co-regulating the pore structure and surface chemistry of sludge-based biochar for high–performance deodorization of gaseous dimethyl disulfide. Chemosphere 2024, 364, 142992. [Google Scholar] [CrossRef]
- Xiang, Y.J.; Xu, Z.Y.; Wei, Y.Y.; Zhou, Y.Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.C.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Li, L.; Li, J.Y.; Zhu, C.W.; Yu, S.L. Study of the binding regularity and corresponding mechanism of drinking water odorous compound 2-MIB with coexisting dissolved organic matter. Chem. Eng. J. 2020, 395, 125015. [Google Scholar] [CrossRef]
- Yang, M.; Yu, J.; Li, Z.; Guo, Z.; Burch, M.; Lin, T.-F. Taihu Lake not to blame for Wuxi’s woes. Science 2008, 319, 158. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Huang, S.-C.; Juang, R.-S. Characteristics of pseudo-second-order kinetic model for liquid-phase adsorption: A mini-review. Chem. Eng. J. 2009, 151, 1–9. [Google Scholar] [CrossRef]
- Debord, J.; Harel, M.; Bollinger, J.-C.; Chu, K.H. The Elovich isotherm equation: Back to the roots and new developments. Chem. Eng. Sci. 2022, 262, 118012. [Google Scholar] [CrossRef]
- McLintock, I.S. The Elovich Equation in Chemisorption Kinetics. Nature 1967, 216, 1204–1205. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2021, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Y.; Yan, C.; Jiang, W.; Chang, C. The Langmuir monolayer adsorption model of organic matter into effective pores in activated carbon. J. Colloid Interface Sci. 2013, 389, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.L.; Ovejero, G.; Delgado, J.A.; Martinez, I. Comparison of adsorption equilibrium and kinetics of four chlorinated organics from water onto GAC. Water Res. 2002, 36, 599–608. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Sreejalekshmi, K.G.; Varghese, S.; Anirudhan, T.S. Removal of EDTA from Aqueous Solutions Using Activated Carbon Prepared from Rubber Wood Sawdust: Kinetic and Equilibrium Modeling. Clean-Soil Air Water. 2010, 38, 361–369. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Hameed, B.H.; Ahmad, A.L. Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem. Eng. J. 2007, 127, 111–119. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Kasaoka, S.; Sakata, Y.; Tanaka, E.; Naitoh, R. Design of Molecular Sieving Carbon Studies on Adsorption of Various Dyes in Liquid Phase. Nippon Kagaku Kaishi 1987, 1987, 2260–2266. [Google Scholar] [CrossRef]
- Park, M.; Wu, S.M.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.A.; Nguyen, L.T.; Glanville, T.D.; Ahn, H.; Frana, T.S.; van Leeuwen, J. Method for sampling and analysis of volatile biomarkers in process gas from aerobic digestion of poultry carcasses using time-weighted average SPME and GC-MS. Food Chem. 2017, 232, 799–807. [Google Scholar] [CrossRef]
- Asiain-Mira, R.; Zamora, P.; Monsalvo, V.; Torrente-Murciano, L. Effect of functional groups on the adsorption of urea on activated carbon. Carbon 2024, 228, 119361. [Google Scholar] [CrossRef]
- Cantarella, M.; Carroccio, S.C.; Dattilo, S.; Avolio, R.; Castaldo, R.; Puglisi, C.; Privitera, V. Molecularly imprinted polymer for selective adsorption of diclofenac from contaminated water. Chem. Eng. J. 2019, 367, 180–188. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Zango, Z.U.; Bakar, N.H.H.A.; Sambudi, N.S.; Jumbri, K.; Abdullah, N.A.F.; Kadir, E.A.; Saad, B. Adsorption of chrysene in aqueous solution onto MIL-88(Fe) and NH2-MIL-88(Fe) metal-organic frameworks: Kinetics, isotherms, thermodynamics and docking simulation studies. J. Environ. Chem. Eng. 2020, 8, 103544. [Google Scholar] [CrossRef]
- Ajmani, A.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. Hexavalent chromium adsorption on virgin, biochar, and chemically modified carbons prepared from Phanera vahlii fruit biomass: Equilibrium, kinetics, and thermodynamics approach. Environ. Sci. Pollut. Res. 2019, 26, 32137–32150. [Google Scholar] [CrossRef]
- Agarwal, A.; Kumar, A.; Gupta, P.; Tomar, R.; Singh, N.B. Cu (II) ions removal from water by charcoal obtained from marigold flower waste. Mater. Today Proc. 2019, 34, 875–879. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2018, 273, 425–434. [Google Scholar] [CrossRef]




| Materials | BET SSA (m2 g−1) | Vt (cm3 g−1) | Smeso (m2 g−1) | Vmeso (cm3 g−1) | Smicro (m2 g−1) | Vmicro (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|---|---|---|---|
| CAC | 984.9779 | 0.5595 | 337.8621 | 0.2777 | 856.3402 | 0.3710 | 2.2721 |
| CSC | 1264.6279 | 1.0317 | 524.6927 | 0.7019 | 878.3475 | 0.3630 | 3.2633 |
| SAC | 299.3159 | 0.1849 | 108.5387 | 0.0983 | 254.9459 | 0.1086 | 2.4710 |
| Compounds | Kinetic Model | Parameters | Types of AC | ||
|---|---|---|---|---|---|
| CAC | CSC | SAC | |||
| MIB | Pseudo-first-order | qe/(ng/mg) | 40.5437 | 42.8033 | 34.0526 |
| K1 (min−1) | 0.05146 | 0.06003 | 0.05953 | ||
| R2 | 0.9576 | 0.9823 | 0.9439 | ||
| Pseudo-second-order | qe/(ng/mg) | 44.9475 | 47.3695 | 37.3408 | |
| K2 | 0.00151 | 0.00162 | 0.00218 | ||
| R2 | 0.9867 | 0.9969 | 0.9780 | ||
| Elovich model | α | 6.6108 | 7.7941 | 7.6184 | |
| β | 0.1158 | 0.1109 | 0.1479 | ||
| R2 | 0.9909 | 0.9788 | 0.9848 | ||
| Weber–Morris | K1 | 5.33 | 6.81 | 4.12 | |
| C1 | 0.24 | −3.60 | 3.24 | ||
| K2 | 2.26 | 1.54 | 1.05 | ||
| C2 | 17.76 | 27.41 | 22.69 | ||
| K3 | 0.2 | 0.27 | 0.02 | ||
| C3 | 39.45 | 40.55 | 32.23 | ||
| R2 | 0.9918 | 0.9880 | 0.9702 | ||
| DMDS | Pseudo-first-order | qe/(ng/mg) | 34.1751 | 36.0123 | 29.0173 |
| K1 (min−1) | 0.0198 | 0.0203 | 0.0180 | ||
| R2 | 0.9927 | 0.9951 | 0.9939 | ||
| Pseudo-second-order | qe/(ng/mg) | 44.2102 | 45.1435 | 37.9584 | |
| K2 | 0.000395 | 0.000422 | 0.000417 | ||
| R2 | 0.9856 | 0.9885 | 0.9879 | ||
| Elovich model | α | 0.8642 | 1.0245 | 0.6795 | |
| β | 0.0750 | 0.0788 | 0.0864 | ||
| R2 | 0.9760 | 0.9798 | 0.9798 | ||
| Weber–Morris | K1 | 3.67 | 3.44 | 2.81 | |
| C1 | −4.96 | −3.36 | −3.65 | ||
| K2 | 0.19 | 0.15 | 0.06 | ||
| C2 | 30.07 | 32.70 | 26.61 | ||
| R2 | 0.9874 | 0.9915 | 0.9914 | ||
| Compounds | Isotherm Model | Parameters | Types of AC | ||
|---|---|---|---|---|---|
| CAC | CSC | SAC | |||
| MIB | Langmuir model | Q0/(ng/mg) | 285.1784 | 304.1376 | 280.6320 |
| KL (L/mg) | 0.00189 | 0.0022 | 0.00323 | ||
| RL | 0.209 | 0.185 | 0.134 | ||
| R2 | 0.9282 | 0.9428 | 0.9636 | ||
| Freundlich model | KF/(ng(1−1/n)L−1g−1) | 7.4989 | 12.9356 | 3.0294 | |
| 1/n | 0.4605 | 0.4392 | 0.562 | ||
| R2 | 0.9729 | 0.9257 | 0.9879 | ||
| Temkin model | KT (L/mg) | 0.03178 | 0.0284 | 0.01201 | |
| BT (J mol−1) | 52.1317 | 60.0906 | 62.1558 | ||
| R2 | 0.9785 | 0.9145 | 0.9585 | ||
| DMDS | Langmuir model | Q0/(ng/mg) | 209.5339 | 224.7103 | 275.2114 |
| KL (L/mg) | 0.0068 | 0.0114 | 0.0054 | ||
| RL | 0.068 | 0.079 | 0.085 | ||
| R2 | 0.9864 | 0.9320 | 0.9828 | ||
| Freundlich model | KF/(ng(1−1/n)L−1g−1) | 0.3648 | 11.0081 | 3.5039 | |
| 1/n | 0.3711 | 0.3711 | 0.4738 | ||
| R2 | 0.9661 | 0.9084 | 0.9846 | ||
| Temkin model | KT (L/mg) | 0.00338 | 0.0336 | 0.0231 | |
| BT (J mol−1) | 39.9146 | 41.8064 | 31.7653 | ||
| R2 | 0.9926 | 0.9329 | 0.9728 | ||
| Compounds | T/(K) | ΔH/(kJ·mol−1) | ΔS/(J·mol−1·K−1) | ΔG/(kJ·mol−1) |
|---|---|---|---|---|
| MIB | 288 | 8.4928 | 49.54 | −5.77 |
| 298 | −6.34 | |||
| 308 | −6.78 | |||
| DMDS | 288 | 8.8361 | 49.65 | −5.46 |
| 298 | −5.94 | |||
| 308 | −6.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhao, Y.; Guo, H.; Peng, D.; Kong, W.; Yan, F.; Zhou, S.; Li, Q.; Shen, B.; Lyu, C. Adsorption of Algal-Derived 2-Methylisoborneol (MIB) and Dimethyl Disulfide (DMDS) onto Activated Carbon: The Role of Pore Structure and Hydrophobicity. Molecules 2025, 30, 4348. https://doi.org/10.3390/molecules30224348
Zhao Y, Zhao Y, Guo H, Peng D, Kong W, Yan F, Zhou S, Li Q, Shen B, Lyu C. Adsorption of Algal-Derived 2-Methylisoborneol (MIB) and Dimethyl Disulfide (DMDS) onto Activated Carbon: The Role of Pore Structure and Hydrophobicity. Molecules. 2025; 30(22):4348. https://doi.org/10.3390/molecules30224348
Chicago/Turabian StyleZhao, Yuqin, Yulan Zhao, Hui Guo, Denghui Peng, Wenwen Kong, Fengjian Yan, Shumei Zhou, Quansheng Li, Boxiong Shen, and Chongrui Lyu. 2025. "Adsorption of Algal-Derived 2-Methylisoborneol (MIB) and Dimethyl Disulfide (DMDS) onto Activated Carbon: The Role of Pore Structure and Hydrophobicity" Molecules 30, no. 22: 4348. https://doi.org/10.3390/molecules30224348
APA StyleZhao, Y., Zhao, Y., Guo, H., Peng, D., Kong, W., Yan, F., Zhou, S., Li, Q., Shen, B., & Lyu, C. (2025). Adsorption of Algal-Derived 2-Methylisoborneol (MIB) and Dimethyl Disulfide (DMDS) onto Activated Carbon: The Role of Pore Structure and Hydrophobicity. Molecules, 30(22), 4348. https://doi.org/10.3390/molecules30224348






