Multiway Analysis of the Electrochemical Oxidation Pathway of a Lignin Using Chemometrics
Abstract
1. Introduction
2. Results
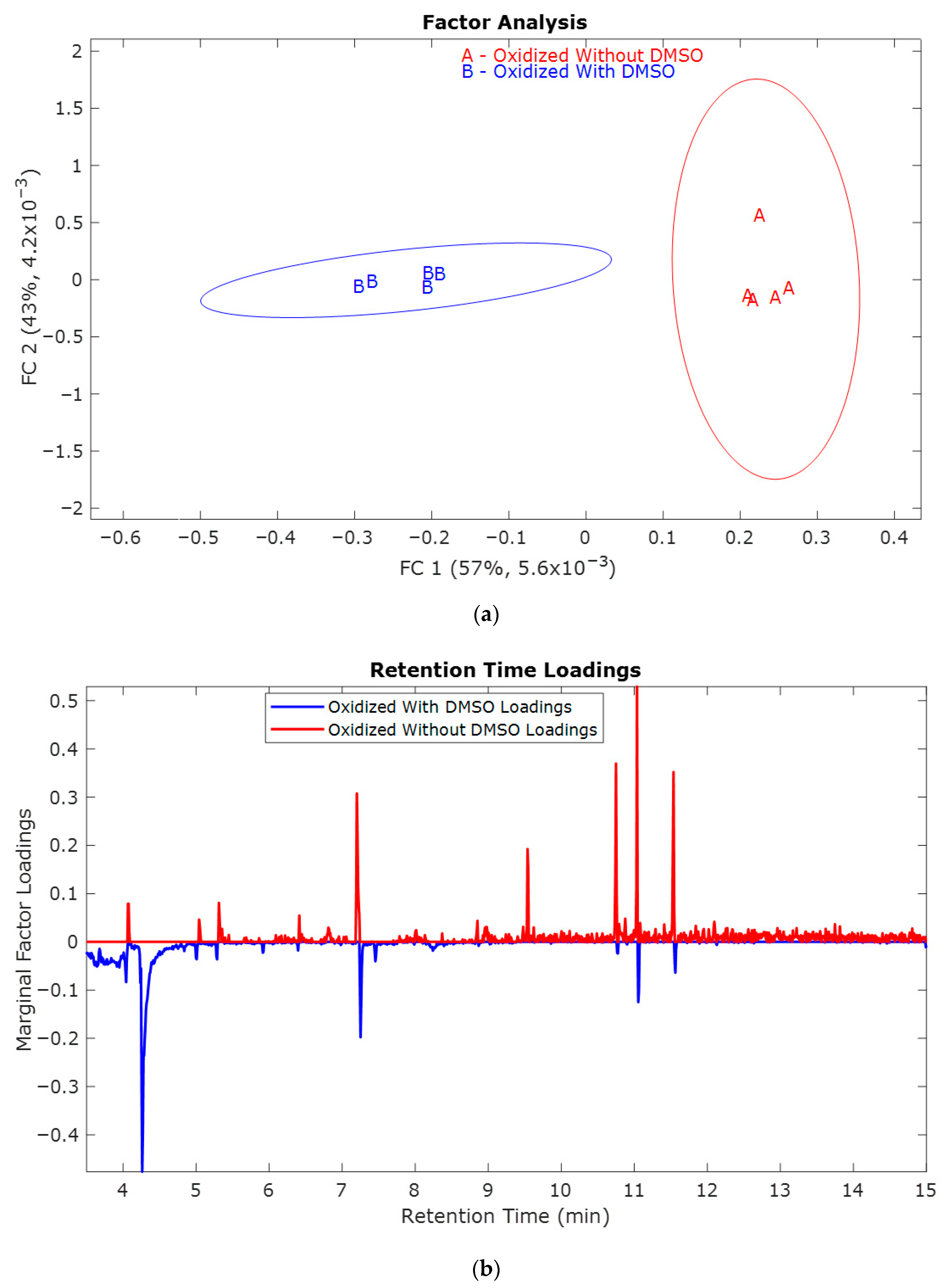
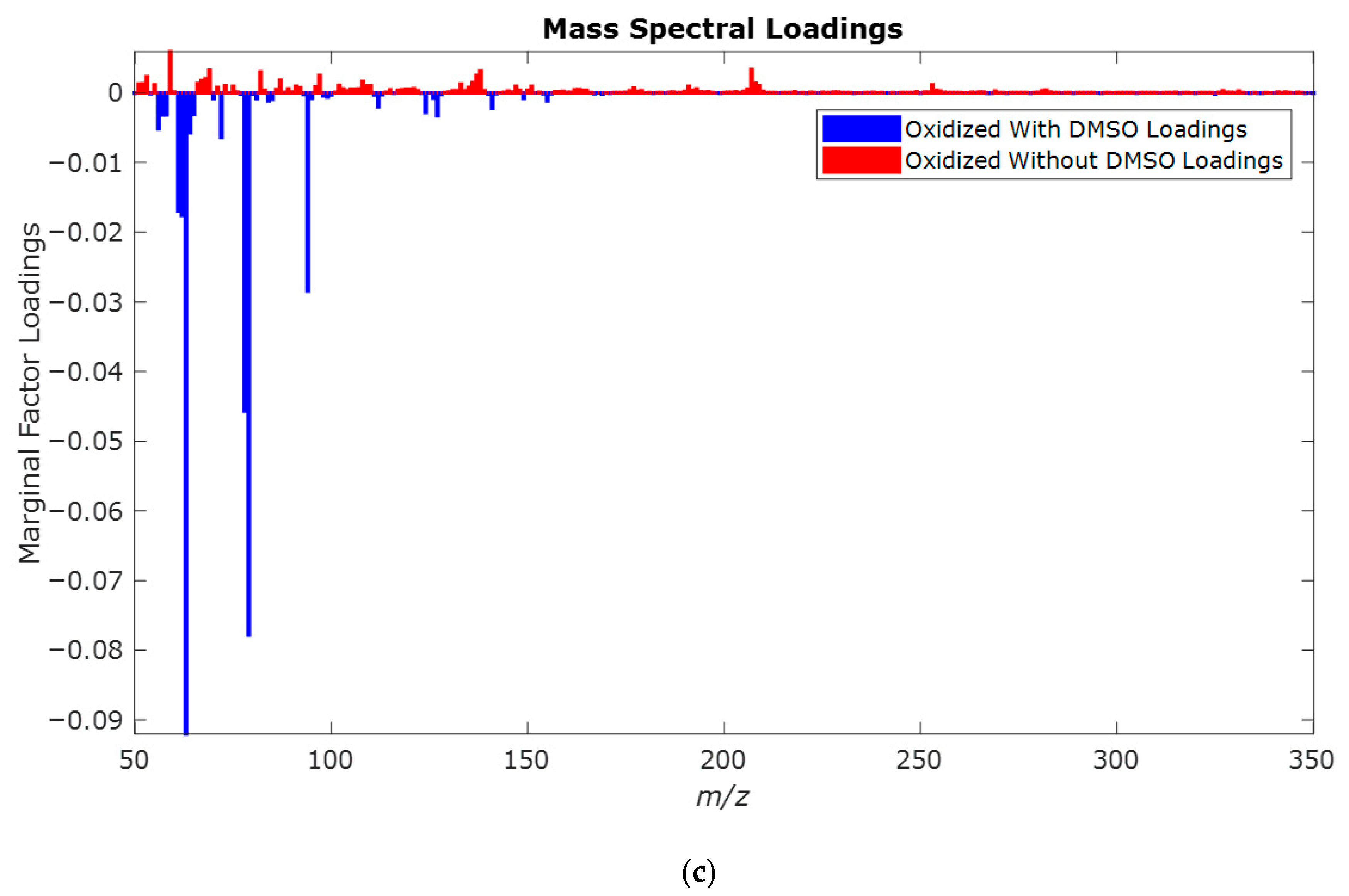
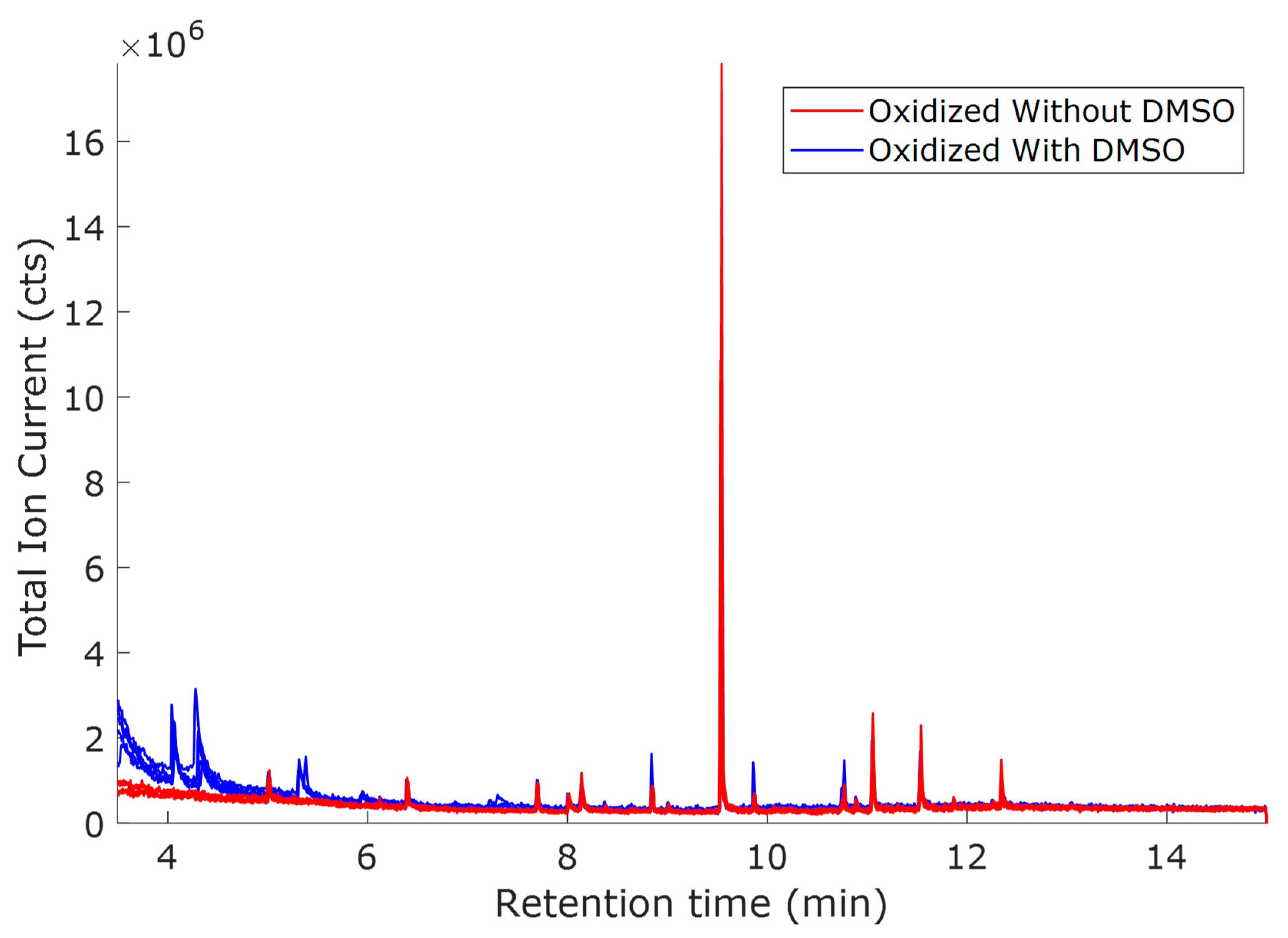
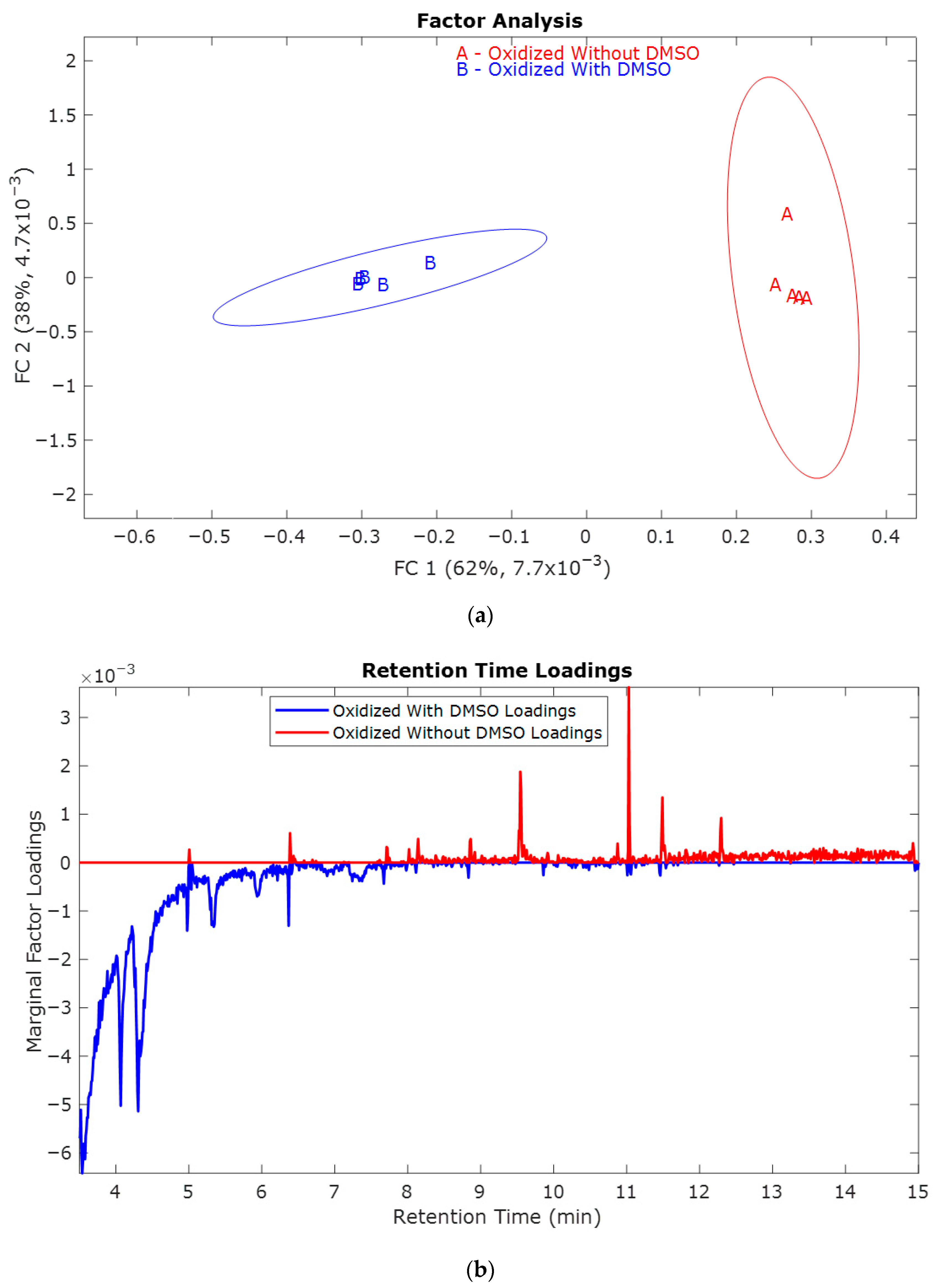
2.1. Study of the Effect of Oxidation Potential 1.0 V vs. Hg/HgO
2.2. Study of the Effect of Oxidation Potential 0.8 V vs. Hg/HgO
2.3. Study of the Effect of Oxidation Potential 0.6 V vs. Hg/HgO
3. Materials and Methods
3.1. Materials
3.2. Sample Details
3.3. Electrochemical Conversion of the Lignin
3.4. Theory
3.5. Instrumental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surrey, J. World Energy, Technology and Climate Policy Outlook 2030; Office for Official Publications of the European Communities: Luxembourg, 2003. [Google Scholar]
- Jiang, H.; Xue, A.; Wang, Z.; Xia, R.; Wang, L.; Tang, Y.; Wan, P.; Chen, Y. Electrochemical degradation of lignin by ROS. Sustain. Chem. 2020, 1, 345–360. [Google Scholar] [CrossRef]
- United Nations. Agenda 21: Programme of Action for Sustainable Development; United Nations Conference on Environment and Development (UNCED): Rio de Janeiro, Brazil, 1992; Available online: https://sustainabledevelopment.un.org/content/documents/Agenda21.pdf (accessed on 27 September 2024).
- Hammitt, J.K.; Lempert, R.J.; Schlesinger, M.E.J.N. A Sequential-Decision Strategy for Abating Climate Change. Nature 1992, 357, 315–318. [Google Scholar] [CrossRef]
- Moreno, A.; Sipponen, M.H.J.M.H. Lignin-Based Smart Materials: A Roadmap to Processing and Synthesis for Current and Future Applications. Sustain. Chem. 2020, 7, 2237–2257. [Google Scholar] [CrossRef]
- Verdía-Barbará, P.; Choudhary, H.; Nakasu, P.S.; Al-Ghatta, A.; Han, Y.; Hopson, C.; Aravena, R.I.; Mishra, D.K.; Ovejero-Pérez, A.; Simmons, B.A.; et al. Recent advances in the use of ionic liquids and deep eutectic solvents for lignocellulosic biorefineries and biobased chemical and material production. Chem. Rev. 2025, 125, 4415–4470. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Perlack, R.D.; Wright, L.L.; Turhollow, A.F.; Graham, R.L.; Stokes, B.J.; Erbach, D.C. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; U.S. Department of Energy and U.S. Department of Agriculture: Oak Ridge, TN, USA, 2005. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Green Chem. 2010, 12, 1139–1159. [Google Scholar] [CrossRef] [PubMed]
- Bateni, F.; Ghahremani, R.; Staser, J.A. Electrochemical oxidative valorization of lignin by the nanostructured PbO2/MWNTs electrocatalyst in a low-energy depolymerization process. J. Appl. Electrochem. 2020, 51, 65–78. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Phenols from lignin. Chem. Eng. Technol. 2008, 31, 736–745. [Google Scholar] [CrossRef]
- Meng, X.; Scheidemantle, B.; Li, M.; Wang, Y.-Y.; Zhao, X.; Toro-González, M.; Singh, P.; Pu, Y.; Wyman, C.E.; Ozcan, S.; et al. Synthesis, characterization, and utilization of a lignin-based adsorbent for effective removal of azo dye from aqueous solution. ACS Omega 2020, 5, 2865–2877. [Google Scholar] [CrossRef]
- Kumar, V. Pyrolysis and Gasification of Lignin and Effect of Alkali Addition. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2009. [Google Scholar]
- Khan, R.J.; Lau, C.Y.; Guan, J.; Lam, C.H.; Zhao, J.; Ji, Y.; Wang, H.; Xu, J.; Lee, D.-J.; Leu, S.-Y. Recent advances of lignin valorization techniques toward sustainable aromatics and potential benchmarks to fossil refinery products. Bioresour. Technol. 2022, 346, 126419. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.J.; González-Rodríguez, S.; Chen, X.; Lu-Chau, T.A.; Eibes, G.; Pizzi, A.; Moreira, M.T. Electrochemical oxidation of lignin for the simultaneous production of bioadhesive precursors and value-added chemicals. Electrochim. Acta 2023, 459, 142549. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, C.; Zhang, X.; Zhang, Q.; Chen, L.; Li, Y.; Wang, C.; Ma, L. Amorphous FeNi–ZrO2-catalyzed hydrodeoxygenation of lignin-derived phenolic compounds to naphthenic fuel. Chem. Eng. J. 2020, 8, 9335–9345. [Google Scholar] [CrossRef]
- He, H.; Luo, D.; Ma, H.; Xia, S. Mechanistic Effects of Solvent Systems on the Ni–Sn-Catalyzed Hydrodeoxygenation of Lignin Derivatives to Non-Oxygenates. Catal. Sci. Technol. 2022, 12, 154–166. [Google Scholar] [CrossRef]
- Chu, S.; Subrahmanyam, A.V.; Huber, G.W. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound. Green Chem. 2013, 15, 125–136. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Z.; Zhao, X.; Liu, S.; Cai, L.; Shi, S.Q.; Ni, Y. Effect of Various Microwave Absorbents on the Microwave-Assisted Lignin Depolymerization Process. ACS Sustain. Chem. Eng. 2020, 8, 16086–16090. [Google Scholar] [CrossRef]
- Zou, R.; Wang, Y.; Jiang, L.; Yu, Z.; Zhao, Y.; Wu, Q.; Dai, L.; Ke, L.; Liu, Y.; Ruan, R. Microwave-Assisted Co-Pyrolysis of Lignin and Waste Oil Catalyzed by Hierarchical ZSM-5/MCM-41 Catalyst to Produce Aromatic Hydrocarbons. Bioresour. Technol. 2019, 289, 121609. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, C.; Huang, J.; Li, L.; Jin, H. Enhancement of Depolymerization Slag Gasification in Supercritical Water and Its Gasification Performance in Fluidized Bed Reactor. Energy Fuels 2021, 168, 829–837. [Google Scholar] [CrossRef]
- Sato, T.; Furusawa, T.; Ishiyama, Y.; Sugito, H.; Miura, Y.; Sato, M.; Suzuki, N.; Itoh, N. Hydrogen Production from the Gasification of Lignin with Nickel Catalysts in Supercritical Water. Energy Fuels 2007, 32, 699–704. [Google Scholar] [CrossRef]
- Stärk, K.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. Oxidative Depolymerization of Lignin in Ionic Liquids. Green Chem. 2010, 3, 719–723. [Google Scholar] [CrossRef]
- Lange, H.; Decina, S.; Crestini, C. Oxidative Upgrade of Lignin–Recent Routes Reviewed. Polymers 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Zhu, L.; Lu, T.; Long, Y.; Ma, L. High-Quality Bio-Oil from One-Pot Catalytic Hydrocracking of Kraft Lignin over Supported Noble Metal Catalysts in Isopropanol System. Energy Fuels 2016, 212, 302–310. [Google Scholar] [CrossRef]
- Kim, Y.; Shim, J.; Choi, J.-W.; Suh, D.J.; Park, Y.-K.; Lee, U.; Choi, J.; Ha, J.-M. Continuous-Flow Production of Petroleum-Replacing Fuels from Highly Viscous Kraft Lignin Pyrolysis Oil Using Its Hydrocracked Oil as a Solvent. Renew. Energy 2020, 213, 112728. [Google Scholar] [CrossRef]
- Barbier, J.; Charon, N.; Dupassieux, N.; Loppinet-Serani, A.; Mahé, L.; Ponthus, J.; Courtiade, M.; Ducrozet, A.; Quoineaud, A.-A.; Cansell, F. Hydrothermal Conversion of Lignin Compounds. A Detailed Study of Fragmentation and Condensation Reaction Pathways. Bioresour. Technol. 2012, 46, 479–491. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Chen, Y.; Li, G.; Li, H.; Tang, Y.; Wan, P. Electrochemical Depolymerization of Lignin into Renewable Aromatic Compounds in a Non-Diaphragm Electrolytic Cell. Green Chem. 2014, 4, 29917–29924. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin Depolymerization (LDP) in Alcohol over Nickel-Based Catalysts via a Fragmentation–Hydrogenolysis Process. Energy Fuels 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Luo, J.; Liu, T.L. Electrochemical valorization of lignin: Status, challenges, and prospects. J. Bioresour. Bioprod. 2023, 8, 1–14. [Google Scholar] [CrossRef]
- Tolba, R.; Tian, M.; Wen, J.; Jiang, Z.-H.; Chen, A. Electrochemical Oxidation of Lignin at IrO2-Based Oxide Electrodes. J. Electroanal. Chem. 2010, 649, 9–15. [Google Scholar] [CrossRef]
- Bailey, A.; Brooks, H. Electrolytic Oxidation of Lignin. J. Am. Chem. Soc. 1946, 68, 445–446. [Google Scholar] [CrossRef]
- Movil-Cabrera, O.; Rodriguez-Silva, A.; Arroyo-Torres, C.; Staser, J.A. Electrochemical Conversion of Lignin to Useful Chemicals. Biomass Bioenergy 2016, 88, 89–96. [Google Scholar] [CrossRef]
- Ghahremani, R.; Staser, J.A. Electrochemical Oxidation of Lignin for the Production of Value-Added Chemicals on Ni-Co Bimetallic Electrocatalysts. Holzforschung 2018, 72, 1951–1960. [Google Scholar] [CrossRef]
- Wang, Y.-s.; Yang, F.; Liu, Z.-h. Electrocatalytic Degradation of Aspen Lignin over Pb/PbO2 Electrode in Alkali Solution. Catal. Commun. 2015, 67, 49–53. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, Q.; Shao, D.; Yang, H.; Liang, J.; Feng, J.; Yan, W. Fabrication and Characterization of PbO2 Electrode Modified with [Fe(CN)6]3− and Its Application on Electrochemical Degradation of Alkali Lignin. Electrochim. Acta 2015, 286, 509–516. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, Z.; Li, M.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. M-Gallate (M = Ni, Co) Metal–Organic Framework-Derived Ni/C and Bimetallic Ni–Co/C Catalysts for Lignin Conversion into Monophenols. ACS Sustain. Chem. Eng. 2019, 7, 12955–12963. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Sullivan, K.P.; Gogoi, P.; Deng, Y. Electrochemical Lignin Conversion. ChemSusChem 2020, 13, 4318–4343. [Google Scholar] [CrossRef]
- Garedew, M.; Lam, C.H.; Petitjean, L.; Huang, S.; Song, B.; Lin, F.; Jackson, J.E.; Saffron, C.M.; Anastas, P.T. Electrochemical Upgrading of Depolymerized Lignin: A Review of Model Compound Studies. Green Chem. 2021, 23, 2868–2899. [Google Scholar] [CrossRef]
- Lucas, F.W.S.; Grim, R.G.; Tacey, S.A.; Downes, C.A.; Hasse, J.; Roman, A.M.; Farberow, C.A.; Schaidle, J.A.; Holewinski, A. Electrochemical Routes for the Valorization of Biomass-Derived Feedstocks: From Chemistry to Application. ACS Energy Lett. 2021, 6, 1205–1270. [Google Scholar] [CrossRef]
- Yang, C.; Farmer, L.A.; Pratt, D.A.; Maldonado, S.; Stephenson, C.R.J. Mechanism of Electrochemical Generation and Decomposition of Phthalimide-N-Oxyl. J. Am. Chem. Soc. 2021, 143, 10324–10332. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Ma, L.; Duan, H. Electrocatalytic Oxidative Upgrading of Biomass Platform Chemicals: From the Aspect of Reaction Mechanism. Chem. Commun. 2022, 58, 897–907. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, M.G.A.; Gueret, R.; Chen, J.; Piątek, J.; Beele, B.; Sipponen, M.H.; Frauscher, M.; Budnyk, S.; Rodrigues, B.V.M.; Slabon, A. Electrochemical Depolymerization of Lignin in a Biomass-Based Solvent. ChemSusChem 2022, 15, e202200718. [Google Scholar] [CrossRef]
- Smith, C.; Utley, J.H.P.; Petrescu, M.; Viertler, H. Biomass Electrochemistry: Anodic Oxidation of an Organo-Solv Lignin in the Presence of Nitroaromatics. J. Electroanal. Chem. 1989, 19, 535–539. [Google Scholar] [CrossRef]
- Wang, B.; Gu, L.; Ma, H. Electrochemical Oxidation of Pulp and Paper Making Wastewater Assisted by Transition Metal Modified Kaolin. J. Hazard. Mater. 2007, 143, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Ramasamy, K.K. Electrochemical Oxidation of Lignin and Waste Plastic. ACS Appl. Energy Mater. 2020, 5, 27735–27740. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; van der Zalm, J.; Thind, S.S.; Chen, A. Electrochemical Oxidation of Lignin at Electrochemically Reduced TiO2 Nanotubes. J. Electrochem. Soc. 2020, 863, 114049. [Google Scholar] [CrossRef]
- Tian, M.; Wen, J.; MacDonald, D.; Asmussen, R.M.; Chen, A. A Novel Approach for Lignin Modification and Degradation. J. Mater. Sci. Technol. 2010, 12, 527–530. [Google Scholar] [CrossRef]
- Särkkä, H.; Vepsäläinen, M.; Sillanpää, M. Natural Organic Matter (NOM) Removal by Electrochemical Methods—A Review. J. Electroanal. Chem. 2015, 755, 100–108. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Nagao, M.; Teranishi, S. Hydrogen Production by Direct Lignin Electrolysis at Intermediate Temperatures. ChemElectroChem 2017, 4, 3032–3036. [Google Scholar] [CrossRef]
- Caravaca, A.; Garcia-Lorefice, W.E.; Gil, S.; de Lucas-Consuegra, A.; Vernoux, P. Towards a Sustainable Technology for H2 Production: Direct Lignin Electrolysis in a Continuous-Flow PEM Reactor. In Proceedings of the 25th Topical Meeting of the International Society of Electrochemistry, Toledo, Spain, 12–19 May 2019. [Google Scholar] [CrossRef]
- Movil, O.; Garlock, M.; Staser, J.A. Non-Precious Metal Nanoparticle Electrocatalysts for Electrochemical Modification of Lignin for Low-Energy and Cost-Effective Production of Hydrogen. Int. J. Hydrogen Energy 2015, 40, 4519–4530. [Google Scholar] [CrossRef]
- Prothmann, J.; Spégel, P.; Sandahl, M.; Turner, C. Identification of Lignin Oligomers in Kraft Lignin Using Ultra-High-Performance Liquid Chromatography/High-Resolution Multiple-Stage Tandem Mass Spectrometry (UHPLC/HRMSn). Anal. Bioanal. Chem. 2018, 410, 7803–7814. [Google Scholar] [CrossRef] [PubMed]
- Benar, P.; Gonçalves, A.R.; Mandelli, D.; Ferreira, M.M.C.; Schuchardt, U. Principal Component Analysis of the Hydroxymethylation of Sugarcane Lignin: A Time-Depending Study by FTIR. J. Wood Chem. Technol. 1999, 19, 151–165.56. [Google Scholar] [CrossRef]
- Fink, F.; Emmerling, F.; Falkenhagen, J. Identification and Classification of Technical Lignins by means of Principle Component Analysis and k-Nearest Neighbor Algorithm. Chemistry-Methods 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Chen, Z.; NaderiNasrabadi, M.; Staser, J.A.; Harrington, P.B. Application of Generalized Standard Addition Method and Ultraviolet Spectroscopy to Quantify Electrolytic Depolymerization of Lignin. J. Anal. Testing 2020, 4, 35–44. [Google Scholar] [CrossRef]
- Quiroz, M.A.; Reyna, S.; Martínez-Huitle, C.A.; Ferro, S.; De Battisti, A. Electrocatalytic Oxidation of p-Nitrophenol from Aqueous Solutions at Pb/PbO2 Anodes. Appl. Catal. B Environ. 2005, 59, 259–266. [Google Scholar] [CrossRef]
- Ohguri, N.; Nosaka, A.Y.; Nosaka, Y. Detection of OH Radicals as the Effect of Pt Particles in the Membrane of Polymer Electrolyte Fuel Cells. J. Power Sources 2010, 195, 4647–4652. [Google Scholar] [CrossRef]
- Stan, S.D.; Woods, J.S.; Daeschel, M.A. Investigation of the Presence of OH Radicals in Electrolyzed NaCl Solution by Electron Spin Resonance Spectroscopy. J. Agric. Food Chem. 2005, 53, 4901–4905. [Google Scholar] [CrossRef]
- Sah, G.; Chen, Z.; Staser, J.A.; Harrington, P.D.B. Multiway Analysis of the Electrochemical Oxidation Pathway of a Lignin Model Compound, Benzyl Phenyl Ether, with Chemometrics. J. Anal. Test. 2025, 9, 346–353. [Google Scholar] [CrossRef]
- NaderiNasrabadi, M.; Bateni, F.; Chen, Z.; Harrington, P.B.; Staser, J.A. Biomass-Depolarized Electrolysis. J. Electrochem. Soc. 2019, 166, E317. [Google Scholar] [CrossRef]
- Gong, R.; Gao, D.; Liu, R.; Sorsche, D.; Biskupek, J.; Kaiser, U.; Rau, S.; Streb, C. Self-Activation of a Polyoxometalate-Derived Composite Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 12671–12676. [Google Scholar] [CrossRef]
- Thanni, Q.; Sah, G.; Harrington, P.d.B. Recent Chemometric Opportunities in Criminalistics. Encycl. Anal. Chem. Appl. Theory Instrum. 2021, 1–23. [Google Scholar] [CrossRef]
- Botros, L.L.; Jablonski, J.; Chang, C.; Bergana, M.M.; Wehling, P.; Harnly, J.M.; Downey, G.; Harrington, P.B.; Potts, A.R.; Moore, J.C. Exploring Authentic Skim and Nonfat Dry Milk Powder Variance for the Development of Nontargeted Adulterant Detection Methods Using Near-Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 2013, 61, 9810–9818. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Harrington, P.d.B.; Rearden, P.; Shetty, V.; Noyola, A. A Quantitative Reliability Metric for Querying Large Database. Forensic Sci. Int. 2022, 331, 111155. [Google Scholar] [CrossRef]

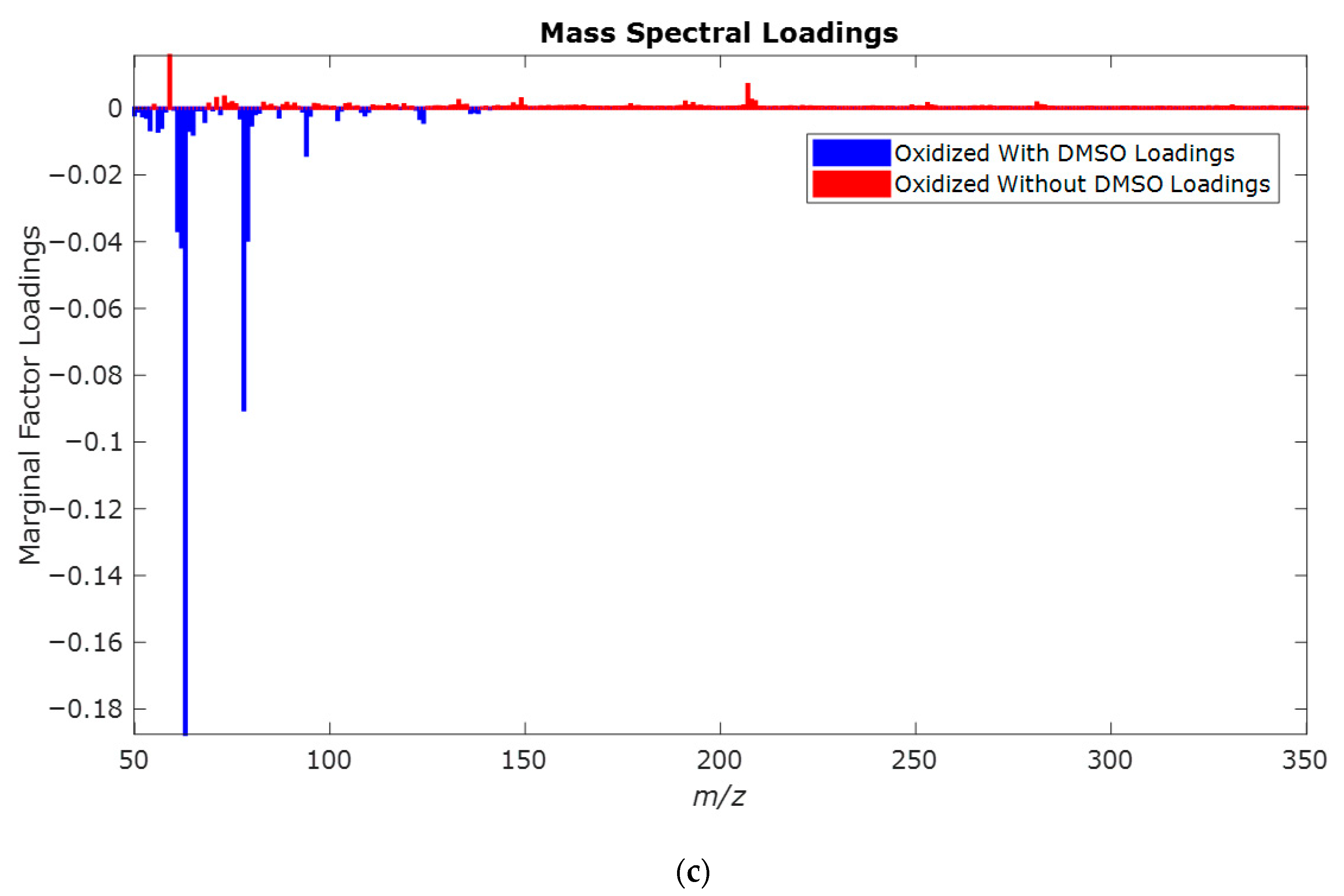
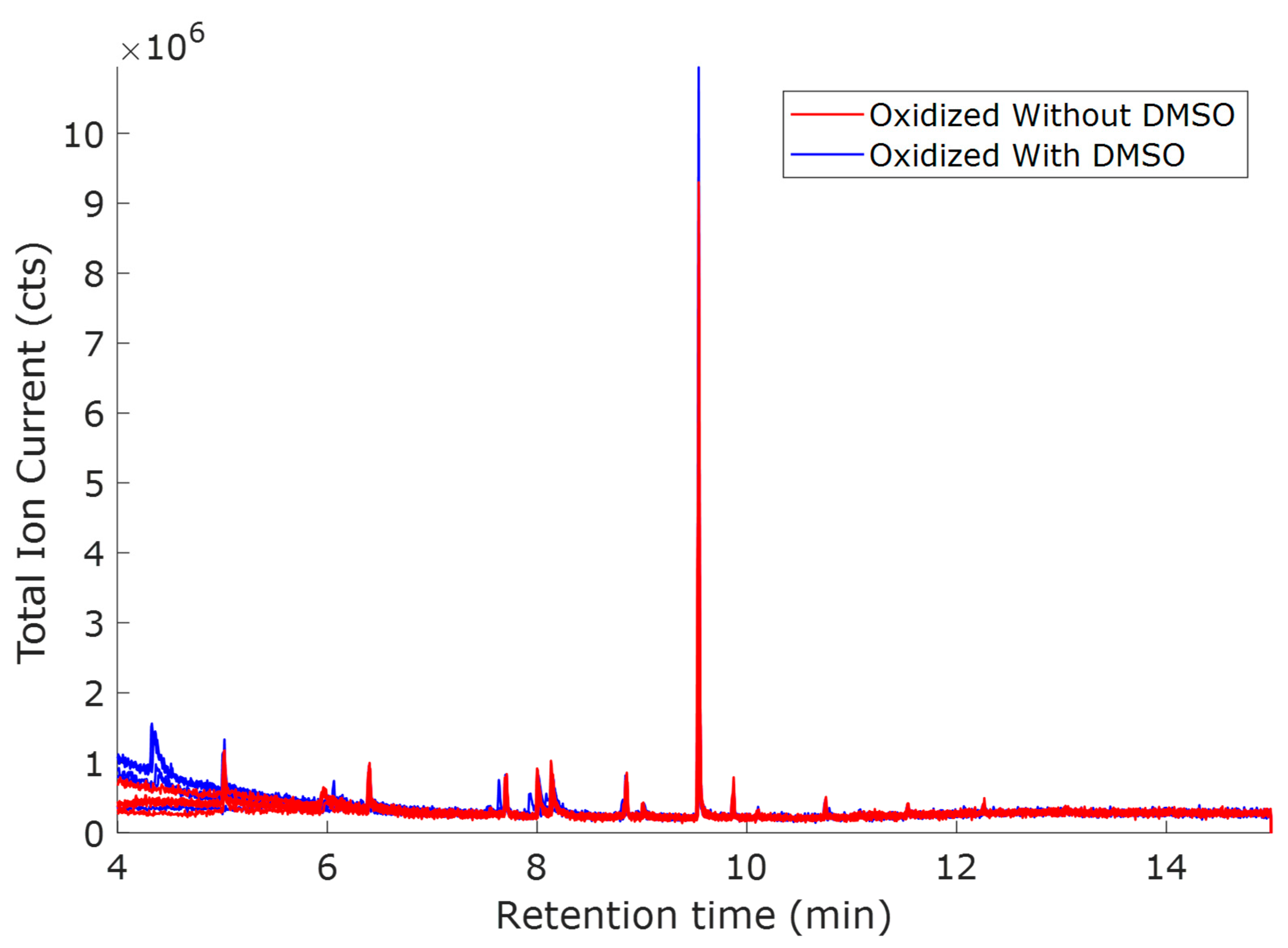
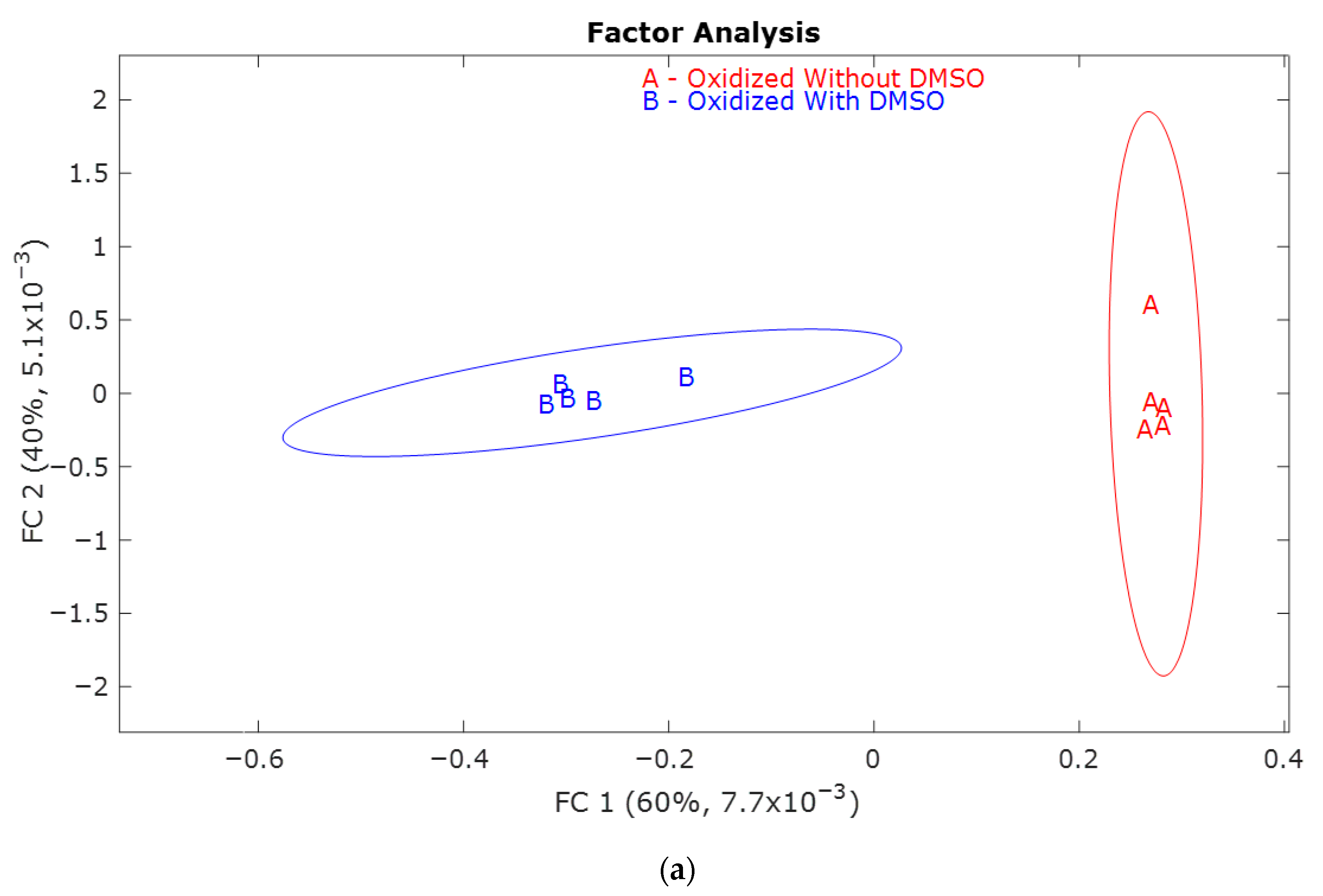
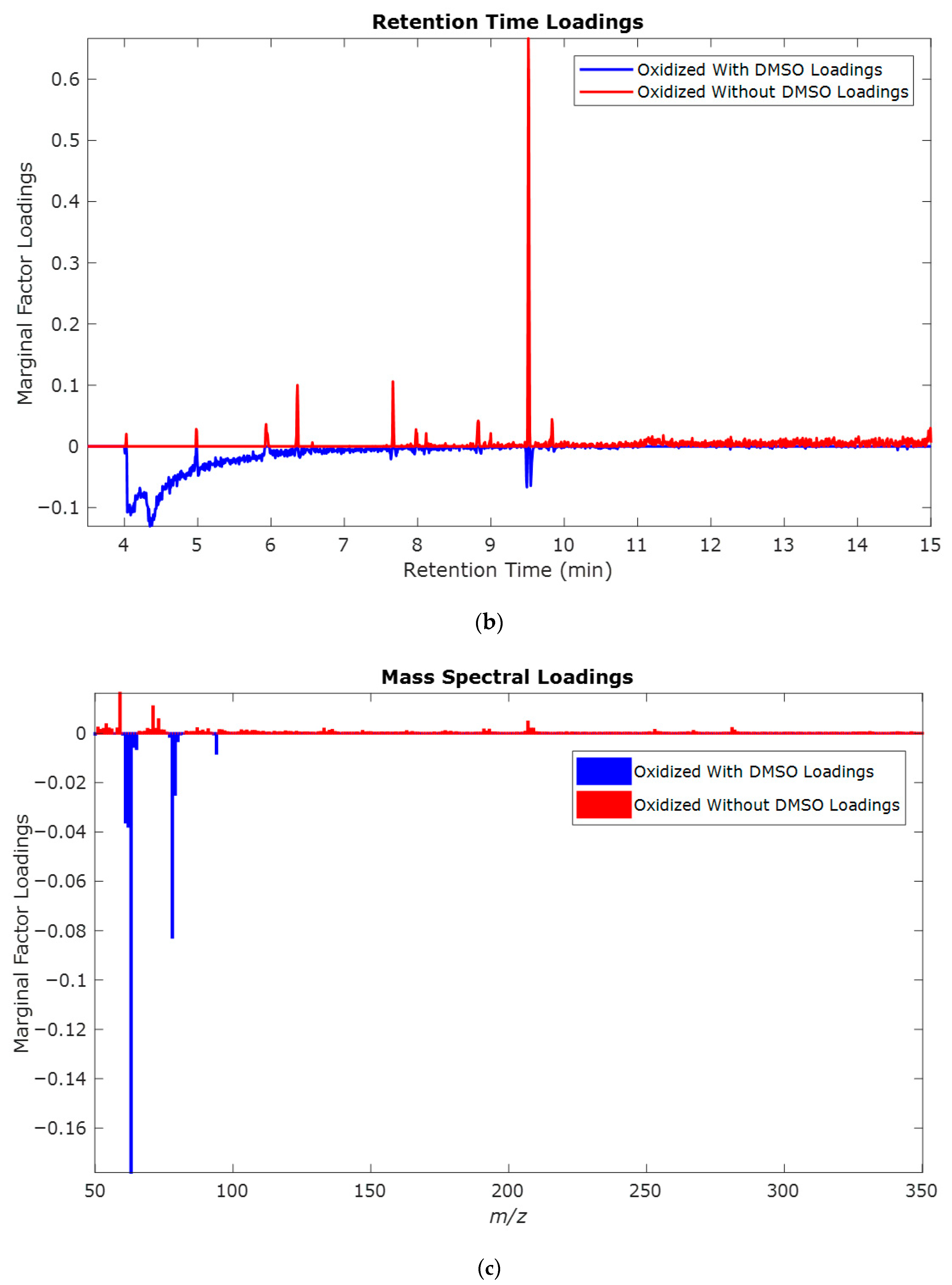
| Retention Time (min) | QRMf (%) | Similarity (0–1) | Compound Name | Structure |
|---|---|---|---|---|
| 3.67 | 1 | 0.64 | 5-Hexen-2-one, 5-methyl | 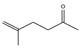 |
| 4.26 | 36 | 0.90 | Acetic acid, ethoxy-, ethyl ester | 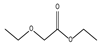 |
| 7.20 | 5 | 0.64 | Cyclohexanecarboxylic acid, -methyl ester |  |
| 8.01 | 2 | 0.93 | Propanoic acid, 2-methyl-2, 2-dimethyl-1-(2-hydroxy-1-methylethyl)-, propyl ester | 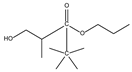 |
| 8.14 | 90 | 0.96 | Propanoic acid, 2-methyl-, 3-hydroxy-2,4,4-trimethylpentyl ester | 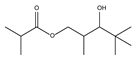 |
| 8.97 | 8 | 0.68 | 2-Oxetanone | 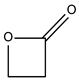 |
| 9.01 | 38 | 0.76 | Phenol, 2,6-bis(1,1-dimethylethyl)- |  |
| 9.55 | 8 | 0.98 | Furan, 2-butyltetrahydro- |  |
| 9.88 | 81 | 0.91 | Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester | 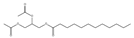 |
| 10.78 | 53 | 1.00 | Isopropyl myristate | 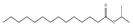 |
| 11.06 | 96 | 0.89 | 1,2-Benzenedicarboxylic acid, bis(2-methyl propyl) ester |  |
| 11.57 | 99 | 0.83 | 1,2-Benzenedicarboxylic acid, dibutyl ester |  |
| Retention Time (min) | QRMf (%) | Similarity (0–1) | Compound Name | Structure |
|---|---|---|---|---|
| 5.29 | 99 | 0.90 | Pentane 2,3-dimethyl | 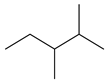 |
| 7.24 | 0.08 | 1.00 | Butanal, 4-hydroxy-3-methyl |  |
| 8.01 | 5 | 0.94 | Propanoic acid, 2-methyl-,2,2-dimethyl-1-(2-hydroxy-1-methylethyl)-, propyl ester | 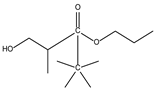 |
| 8.14 | 100 | 0.96 | Butanoic acid, 3-hydroxy-2,2-dimethyl-, ethyl ester |  |
| 8.38 | 48 | 1.00 | 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | 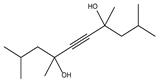 |
| 9.01 | 12 | 0.57 | Phenol, 2,6-bis(1,1-dimethylethyl)- |  |
| 9.55 | 32 | 0.98 | Furan, 2-butyltetrahydro- |  |
| 10.78 | 24 | 0.86 | Isopropyl myristate | 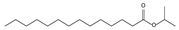 |
| 11.06 | 94 | 0.88 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 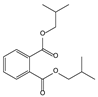 |
| 11.54 | 96 | 1.00 | 1,2-Benzenedicarboxylic acid, butyl 2-ethylhexyl ester |  |
| Retention Time (min) | QRMf % | Similarity | Compound Name | Structure |
|---|---|---|---|---|
| 8.14 | 13 | 0.70 | Propanoic acid, 2-methyl-, heptyl ester |  |
| 9.54 | 42 | 0.83 | Furan, 2-butyltetrahydro- | 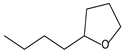 |
| 10.76 | 27 | 0.87 | 9-Octadecenoic acid (Z) | 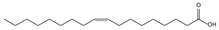 |
| 11.53 | 60 | 0.96 | 1,2-Benzenedicarboxylic acid, dibutyl ester | 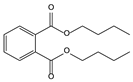 |
| Retention Time (min) | QRMf (%) | Similarity (0–1) | Compound Name | Structure |
|---|---|---|---|---|
| 5.38 | 82 | 0.86 | Cyclohexene, 1-methyl-4-(1-methylethenyl) | 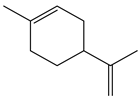 |
| 9.01 | 40 | 0.84 | Phenol, 2,4-bis(1,1-dimethylethyl)- | 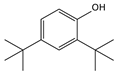 |
| 9.54 | 24 | 0.99 | Furan, 2-butyltetrahydro- | 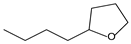 |
| 11.06 | 98 | 0.88 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 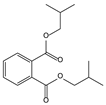 |
| Retention Time (min) | QRMf % | Similarity | Compound Name | Structure |
|---|---|---|---|---|
| 5.38 | 82 | 0.86 | Cyclohexene, 1-methyl-4-(1-methylethenyl) | 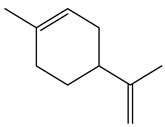 |
| 8.02 | 34 | 0.90 | 1,3-Butanediol, 2-methyl- | 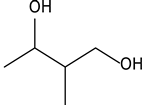 |
| 8.14 | 67 | 0.96 | Butanoic acid, 3-hydroxy-2,2-dimethyl-, hexyl ester | 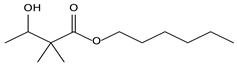 |
| 9.01 | 40 | 0.84 | Phenol, 2,4-bis-(1,1-dimethylethyl)- | 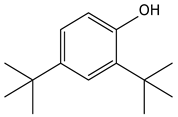 |
| 9.54 | 46 | 0.84 | Furan, 2-butyltetrahydro- |  |
| Retention Time (min) | QRMf | Similarity | Compound Name | Structure |
|---|---|---|---|---|
| 8.02 | 10 | 0.93 | Propanoic acid, 2-methyl- 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)-, propyl ester | 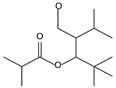 |
| 8.09 | 100 | 0.95 | Butanoic acid, 3-hydroxy-2,2-dimethyl-, hexyl ester |  |
| 9.01 | 100 | 0.58 | Phenol, 2,4-bis(1,1-dimethylethyl)- |  |
| 9.54 | 5 | 0.95 | Furan, 2-butyltetrahydro- |  |
| Sample Number | Sample Name | Oxidation Potential (V) vs. Hg/HgO | Presence (D)/Absence of DMSO |
|---|---|---|---|
| 1. | 1.0 | - | |
| 2. | 1.0 | D | |
| 3. | 0.8 | - | |
| 4. | 0.8 | D | |
| 5. | 0.6 | - | |
| 6. | 0.6 | D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, G.; Staser, J.A.; Harrington, P.B. Multiway Analysis of the Electrochemical Oxidation Pathway of a Lignin Using Chemometrics. Molecules 2025, 30, 4305. https://doi.org/10.3390/molecules30214305
Sah G, Staser JA, Harrington PB. Multiway Analysis of the Electrochemical Oxidation Pathway of a Lignin Using Chemometrics. Molecules. 2025; 30(21):4305. https://doi.org/10.3390/molecules30214305
Chicago/Turabian StyleSah, Gobind, John A. Staser, and Peter B. Harrington. 2025. "Multiway Analysis of the Electrochemical Oxidation Pathway of a Lignin Using Chemometrics" Molecules 30, no. 21: 4305. https://doi.org/10.3390/molecules30214305
APA StyleSah, G., Staser, J. A., & Harrington, P. B. (2025). Multiway Analysis of the Electrochemical Oxidation Pathway of a Lignin Using Chemometrics. Molecules, 30(21), 4305. https://doi.org/10.3390/molecules30214305










