The Evaluation of (1R,4R,7R,10R)-α,α′,α″,α‴-Tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid (DOTMA) as a Chelator for Zirconium-89
Abstract
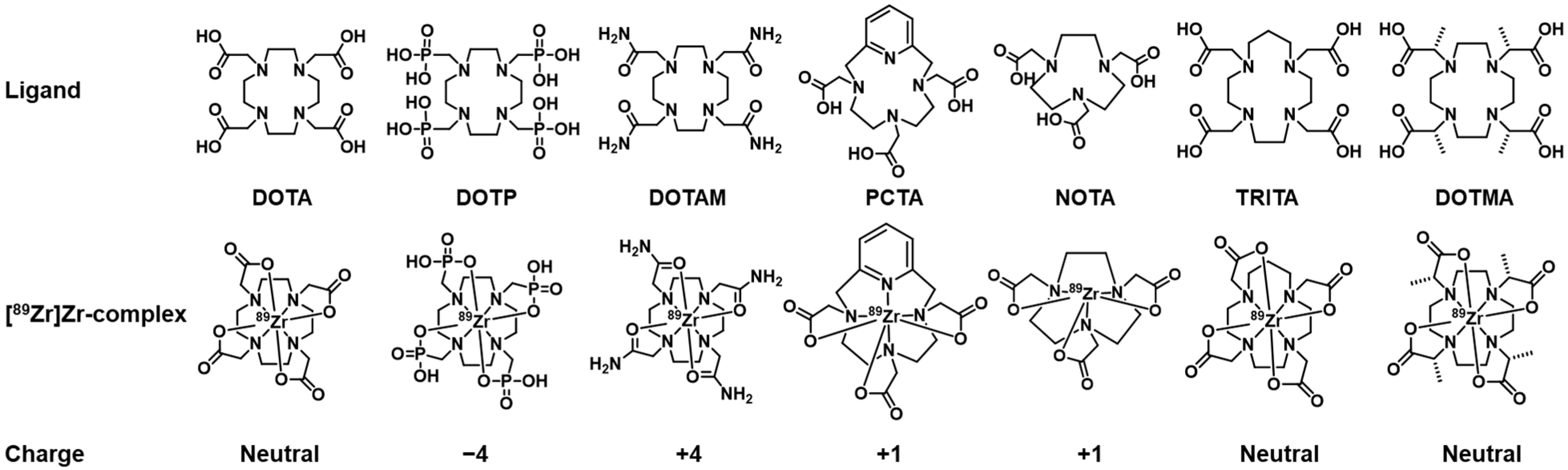
1. Introduction
2. Results and Discussion
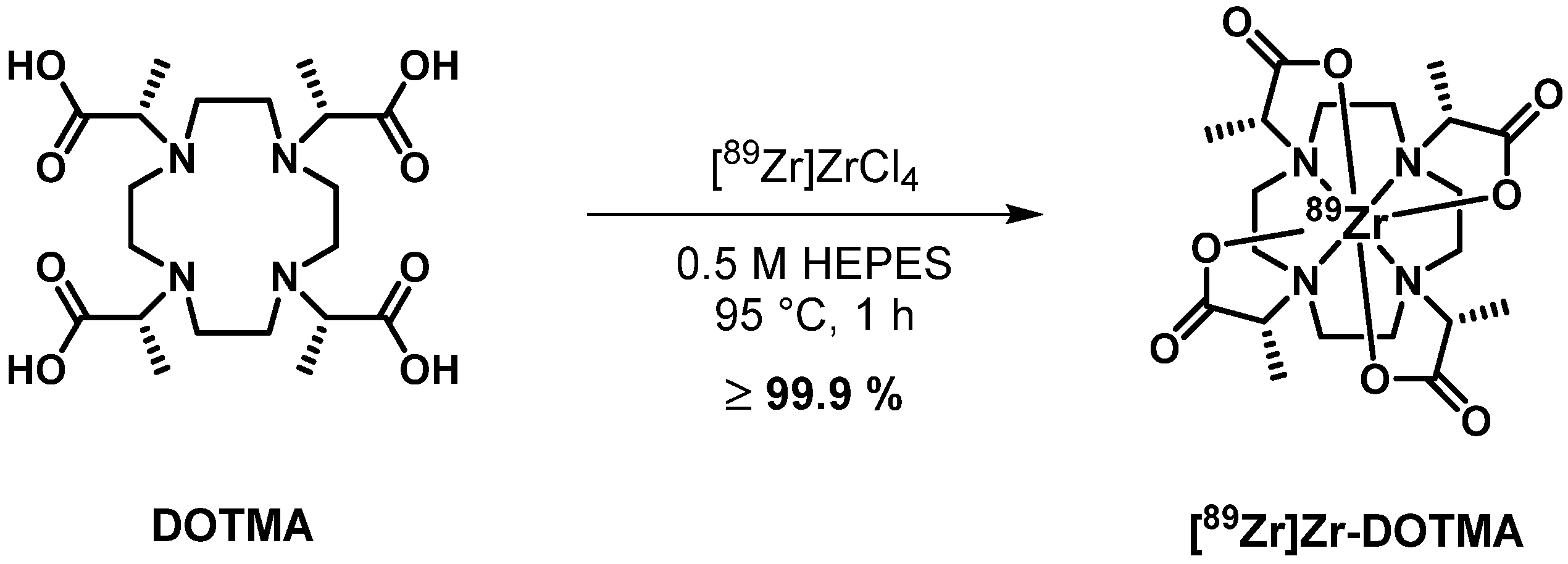
2.1. Structural Characterization of Zr-DOTMA
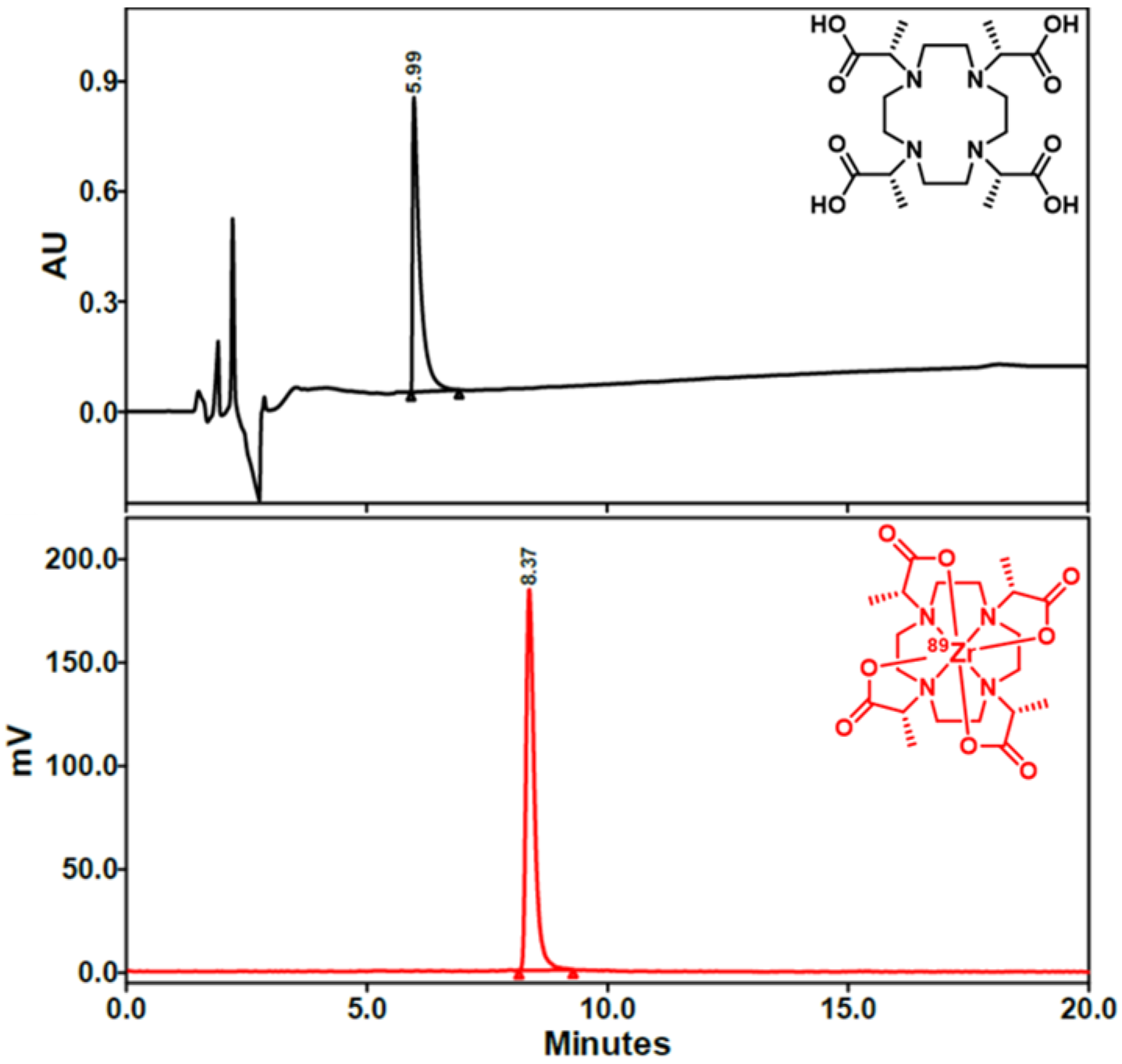
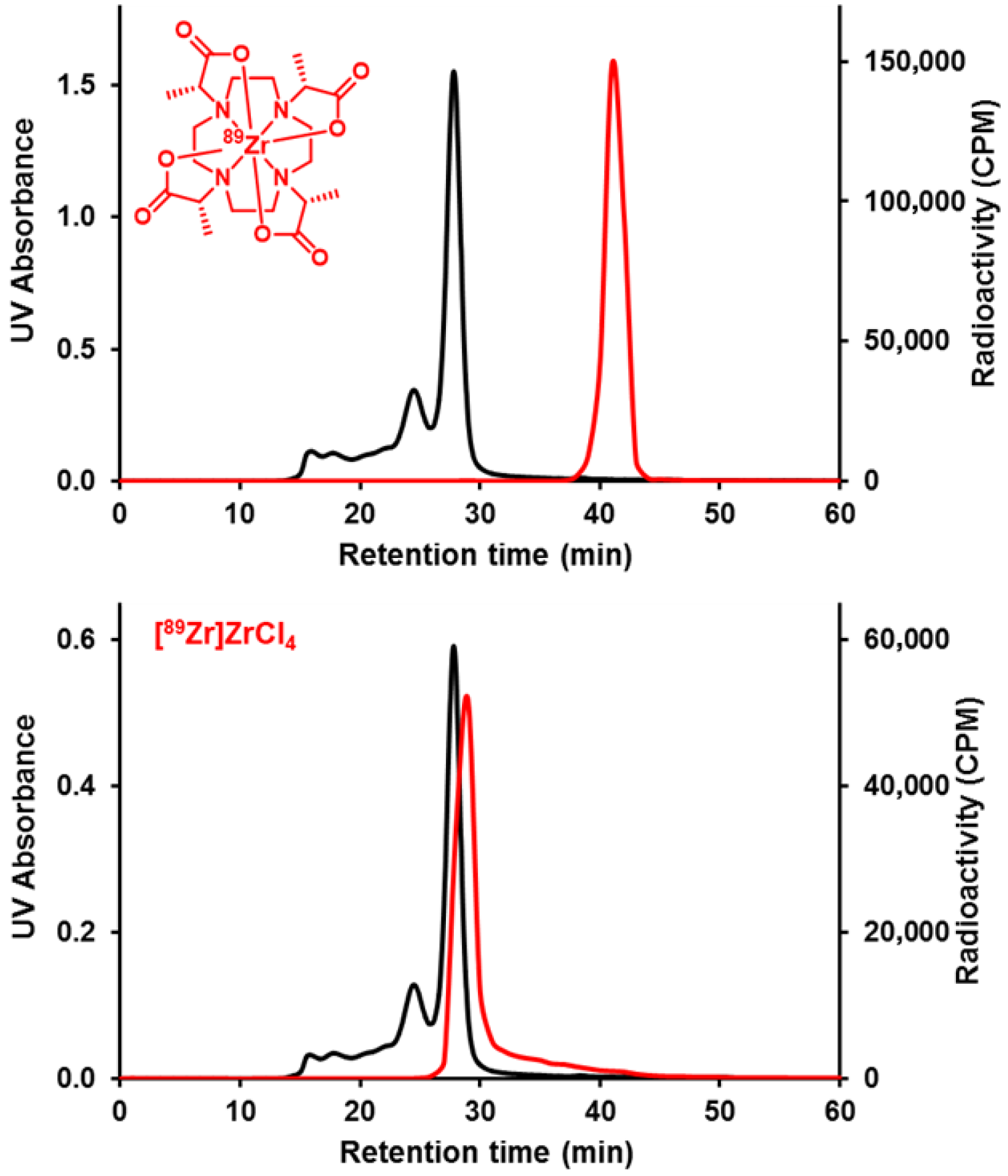
2.2. Radiochemistry
2.3. Lipophilicity (LogP) and In Vitro Stability Studies
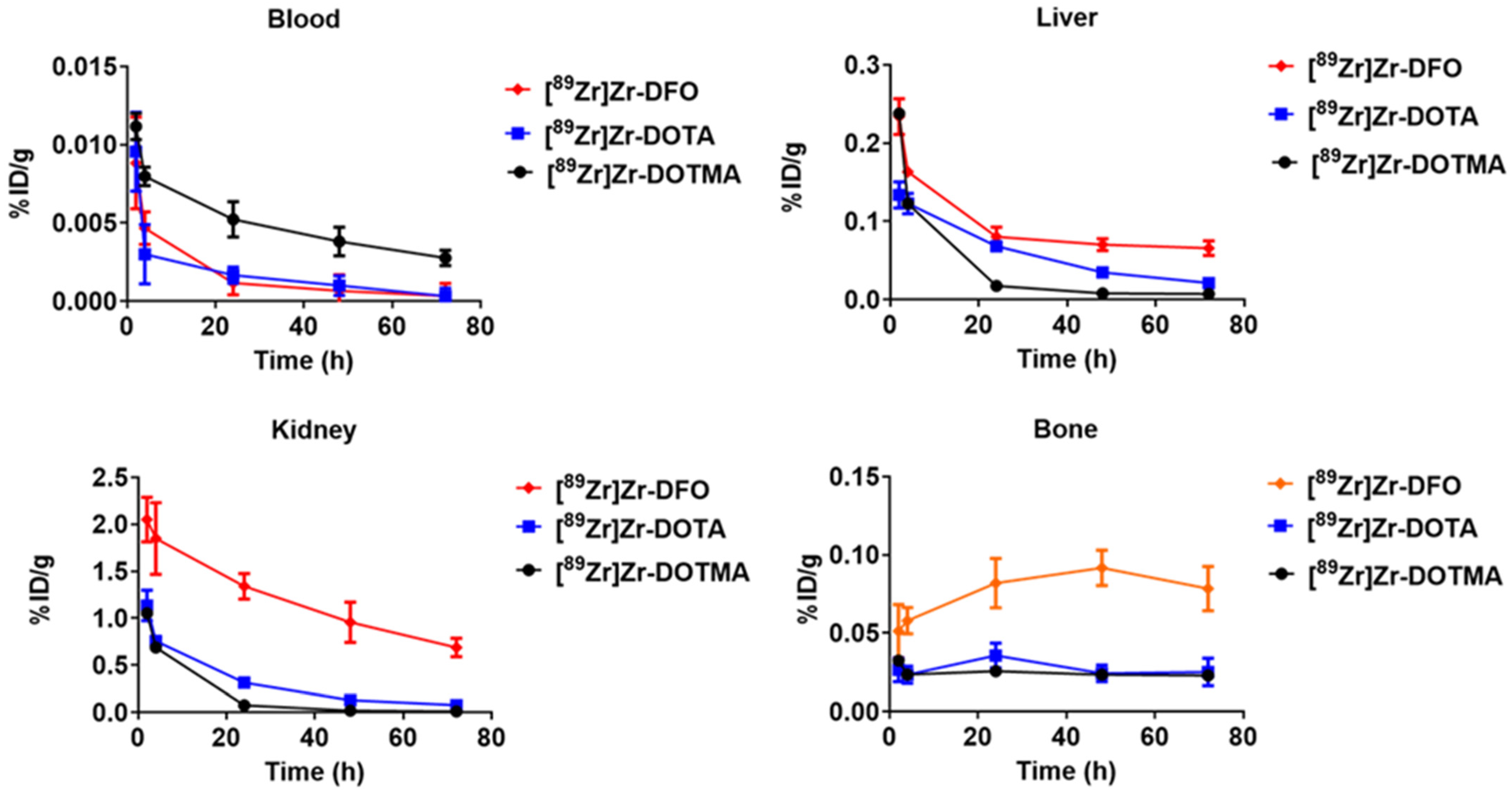
2.4. Biodistribution Studies in Non-Tumor-Bearing, Normal Mice
2.5. Limitations of the Current Research and Future Research Directions
3. Materials and Methods
3.1. Reagents and Equipment
3.2. Solid-State NMR Data Acquisition Parameters
3.3. Synthesis of Zirconium (1R,4R,7R,10R)-α,α′,α″,α‴-Tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid (Zr-DOTMA)
3.4. Density Functional Theory (DFT) Calculations and Modelling of the Zr-DOTMA Structure
3.5. Radiochemical Synthesis of [89Zr]Zr-DOTMA with [89Zr]Zr(ox)4 [10]
3.6. Preparation of [89Zr]Zr-Chloride
3.7. Radiochemical Synthesis of [89Zr]Zr-DOTMA with [89Zr]ZrCl4 [10]
3.8. Determination of Partition Coefficient (LogP) [10]
3.9. In Vitro EDTA Challenge Study [10]
3.10. In Vitro Metal Competition Study [10]
3.11. In Vitro Serum Stability Study [10]
3.12. Animal Care and Use
3.13. Biodistribution Studies
3.14. Statistical Analysis [10]
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Allott, L.; Da Pieve, C.; Meyers, J.; Spinks, T.; Ciobota, D.M.; Kramer-Marek, G.; Smith, G. Evaluation of DFO-HOPO as an octadentate chelator for zirconium-89. Chem. Commun. 2017, 53, 8529–8532. [Google Scholar] [CrossRef]
- Alnahwi, A.H.; Ait-Mohand, S.; Dumulon-Perreault, V.; Dory, Y.L.; Guerin, B. Promising Performance of 4HMS, a New Zirconium-89 Octadendate Chelator. ACS Omega 2020, 5, 10731–10739. [Google Scholar] [CrossRef]
- Bhatt, N.B.; Pandya, D.N.; Wadas, T.J. Recent Advances in Zirconium-89 Chelator Development. Molecules 2018, 23, 638. [Google Scholar] [CrossRef]
- Bhatt, N.B.; Pandya, D.N.; Xu, J.; Tatum, D.; Magda, D.; Wadas, T.J. Evaluation of macrocyclic hydroxyisophthalamide ligands as chelators for zirconium-89. PLoS ONE 2017, 12, e0178767. [Google Scholar] [CrossRef]
- Brandt, M.; Cowell, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. Radiolabelling of the octadentate chelators DFO* and oxoDFO* with zirconium-89 and gallium-68. J. Biol. Inorg. Chem. 2020, 25, 789–796. [Google Scholar] [CrossRef]
- Deri, M.A.; Ponnala, S.; Zeglis, B.M.; Pohl, G.; Dannenberg, J.J.; Lewis, J.S.; Francesconi, L.C. Alternative chelator for (8)(9)Zr radiopharmaceuticals: Radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem. 2014, 57, 4849–4860. [Google Scholar] [CrossRef]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with (8)(9)Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef]
- Kang, C.S.; Zhang, S.; Wang, H.; Liu, Y.; Ren, S.; Chen, Y.; Li, J.; Bandara, N.; Rogachev, A.Y.; Rogers, B.E.; et al. Novel Chelating Agents for Zirconium-89-Positron Emission Tomography (PET) Imaging: Synthesis, DFT Calculation, Radiolabeling, and In Vitro and In Vivo Complex Stability. ACS Omega 2022, 7, 37229–37236. [Google Scholar] [CrossRef] [PubMed]
- Khozeimeh Sarbisheh, E.; Summers, K.L.; Salih, A.K.; Cotelesage, J.J.H.; Zimmerling, A.; Pickering, I.J.; George, G.N.; Price, E.W. Radiochemical, Computational, and Spectroscopic Evaluation of High-Denticity Desferrioxamine Derivatives DFO2 and DFO2p toward an Ideal Zirconium-89 Chelate Platform. Inorg. Chem. 2023, 62, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Bhatt, N.; Yuan, H.; Day, C.S.; Ehrmann, B.M.; Wright, M.; Bierbach, U.; Wadas, T.J. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 2017, 8, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Henry, K.E.; Day, C.S.; Graves, S.A.; Nagle, V.L.; Dilling, T.R.; Sinha, A.; Ehrmann, B.M.; Bhatt, N.B.; Menda, Y.; et al. Polyazamacrocycle Ligands Facilitate (89)Zr Radiochemistry and Yield (89)Zr Complexes with Remarkable Stability. Inorg. Chem. 2020, 59, 17473–17487. [Google Scholar] [CrossRef]
- Pandya, D.N.; Pailloux, S.; Tatum, D.; Magda, D.; Wadas, T.J. Di-macrocyclic terephthalamide ligands as chelators for the PET radionuclide zirconium-89. Chem. Commun. 2015, 51, 2301–2303. [Google Scholar] [CrossRef]
- Price, E.W.; Zeglis, B.M.; Lewis, J.S.; Adam, M.J.; Orvig, C. H6phospa-trastuzumab: Bifunctional methylenephosphonate-based chelator with 89Zr, 111In and 177Lu. Dalton Trans. 2014, 43, 119–131. [Google Scholar] [CrossRef]
- Salih, A.K.; Raheem, S.J.; Garcia, M.D.; Ahiahonu, W.K.; Price, E.W. Design, Synthesis, and Evaluation of DFO-Em: A Modular Chelator with Octadentate Chelation for Optimal Zirconium-89 Radiochemistry. Inorg. Chem. 2022, 61, 20964–20976. [Google Scholar] [CrossRef] [PubMed]
- Sarbisheh, E.K.; Salih, A.K.; Raheem, S.J.; Lewis, J.S.; Price, E.W. A High-Denticity Chelator Based on Desferrioxamine for Enhanced Coordination of Zirconium-89. Inorg. Chem. 2020, 59, 11715–11727. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; He, S.; Ye, Y.; Rangger, C.; Kaeopookum, P.; Summer, D.; Haas, H.; Kremser, L.; Lindner, H.; Foster, J.; et al. Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89. Biomolecules 2019, 9, 91. [Google Scholar] [CrossRef]
- Guarrochena, X.; Kronberger, J.; Tieber, M.; Ciesielski, P.; Mindt, T.L.; Feiner, I.V.J. Straightforward Synthesis of DFO*—An Octadentate Chelator for Zirconium-89. ChemMedChem 2024, 19, e202300495. [Google Scholar] [CrossRef] [PubMed]
- Imura, R.; Ida, H.; Sasaki, I.; Ishioka, N.S.; Watanabe, S. Re-Evaluations of Zr-DFO Complex Coordination Chemistry for the Estimation of Radiochemical Yields and Chelator-to-Antibody Ratios of (89)Zr Immune-PET Tracers. Molecules 2021, 26, 4977. [Google Scholar] [CrossRef]
- Salih, A.K.; Dominguez Garcia, M.; Raheem, S.J.; Ahiahonu, W.K.; Price, E.W. DFO-Km: A Modular Chelator as a New Chemical Tool for the Construction of Zirconium-89-Based Radiopharmaceuticals. Inorg. Chem. 2023, 62, 20806–20819. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Ren, S.; Chen, Y.; Liu, D.; Li, M.; Sagastume, E.; Chong, H.S. Synthesis and evaluation of Diaza-Crown Ether-Backboned chelator containing hydroxamate groups for Zr-89 chelation chemistry. Bioorg. Med. Chem. Lett. 2022, 72, 128847. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Bhatt, N.B.; Pandya, D.N.; Rideout-Danner, S.; Gage, H.D.; Marini, F.C.; Wadas, T.J. A comprehensively revised strategy that improves the specific activity and long-term stability of clinically relevant (89)Zr-immuno-PET agents. Dalton Trans. 2018, 47, 13214–13221. [Google Scholar] [CrossRef]
- Pandya, D.N.; Bhatt, N.B.; Almaguel, F.; Rideout-Danner, S.; Gage, H.D.; Solingapuram Sai, K.K.; Wadas, T.J. (89)Zr-Chloride Can Be Used for Immuno-PET Radiochemistry Without Loss of Antigen Reactivity In Vivo. J. Nucl. Med. 2019, 60, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef]
- Prive, B.M.; Derks, Y.H.W.; Rosar, F.; Franssen, G.M.; Peters, S.M.B.; Khreish, F.; Bartholoma, M.; Maus, S.; Gotthardt, M.; Laverman, P.; et al. (89)Zr-labeled PSMA ligands for pharmacokinetic PET imaging and dosimetry of PSMA-617 and PSMA-I&T: A preclinical evaluation and first in man. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Burgard, C.; Linxweiler, J.; Wagner, M.; Ezziddin, S. Histologically Confirmed Testicular Metastasis Revealed by [(89)Zr]Zr-PSMA-617 PET/CT in a Patient with Biochemical Recurrence of Prostate Cancer and Negative Conventional PSMA PET/CT Imaging. Diagnostics 2023, 13, 1352. [Google Scholar] [CrossRef]
- Dilworth, J.R.; Pascu, S.I. The chemistry of PET imaging with zirconium-89. Chem. Soc. Rev. 2018, 47, 2554–2571. [Google Scholar] [CrossRef]
- Feiner, I.V.J.; Brandt, M.; Cowell, J.; Demuth, T.; Vugts, D.; Gasser, G.; Mindt, T.L. The Race for Hydroxamate-Based Zirconium-89 Chelators. Cancers 2021, 13, 4466. [Google Scholar] [CrossRef]
- Ikotun, O.F.; Lapi, S.E. The rise of metal radionuclides in medical imaging: Copper-64, zirconium-89 and yttrium-86. Future Med. Chem. 2011, 3, 599–621. [Google Scholar] [CrossRef]
- Jauw, Y.W.; Menke-van der Houven van Oordt, C.W.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; van Dongen, G.A. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front. Pharmacol. 2016, 7, 131. [Google Scholar] [CrossRef]
- La, M.T.; Tran, V.H.; Kim, H.K. Progress of Coordination and Utilization of Zirconium-89 for Positron Emission Tomography (PET) Studies. Nucl. Med. Mol. Imaging 2019, 53, 115–124. [Google Scholar] [CrossRef]
- van de Watering, F.C.; Rijpkema, M.; Perk, L.; Brinkmann, U.; Oyen, W.J.; Boerman, O.C. Zirconium-89 labeled antibodies: A new tool for molecular imaging in cancer patients. BioMed Res. Int. 2014, 2014, 203601. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Cai, W. PET tracers based on Zirconium-89. Curr. Radiopharm. 2011, 4, 131–139. [Google Scholar] [CrossRef]
- Adumeau, P.; Davydova, M.; Zeglis, B.M. Thiol-Reactive Bifunctional Chelators for the Creation of Site-Selectively Modified Radioimmunoconjugates with Improved Stability. Bioconjug. Chem. 2018, 29, 1364–1372. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Lewis, J.S. The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J. Vis. Exp. 2015, 96, 52521. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Carrasquillo, J.A.; Riedl, C.C.; Yeh, R.; Hatzoglou, V.; Ross, D.S.; Jhaveri, K.; Chandarlapaty, S.; Hyman, D.M.; Zeglis, B.M.; et al. Identification of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer by Using HER2-targeted (89)Zr-Pertuzumab PET/CT. Radiology 2020, 296, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Sinha, A.; Yuan, H.; Mutkus, L.; Stumpf, K.; Marini, F.C.; Wadas, T.J. Imaging of Fibroblast Activation Protein Alpha Expression in a Preclinical Mouse Model of Glioma Using Positron Emission Tomography. Molecules 2020, 25, 3672. [Google Scholar] [CrossRef] [PubMed]
- Perk, L.R.; Vosjan, M.J.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250–259. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. 89Zr-TLX250 for PET/CT Imaging of ccRCC—ZIRCON Study (89ZR-TLX250). Available online: https://www.clinicaltrials.gov/study/NCT06750419?term=telix&page=1&viewType=Table&rank=4 (accessed on 1 July 2023).
- Rosar, F.; Bartholoma, M.; Maus, S.; Prive, B.M.; Khreish, F.; Franssen, G.M.; Derks, Y.H.W.; Nagarajah, J.; Ezziddin, S. 89Zr-PSMA-617 PET/CT May Reveal Local Recurrence of Prostate Cancer Unidentified by 68Ga-PSMA-11 PET/CT. Clin. Nucl. Med. 2022, 47, 435–436. [Google Scholar] [CrossRef]
- Rosar, F.; Schaefer-Schuler, A.; Bartholoma, M.; Maus, S.; Petto, S.; Burgard, C.; Prive, B.M.; Franssen, G.M.; Derks, Y.H.W.; Nagarajah, J.; et al. [(89)Zr]Zr-PSMA-617 PET/CT in biochemical recurrence of prostate cancer: First clinical experience from a pilot study including biodistribution and dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4736–4747. [Google Scholar] [CrossRef] [PubMed]
- Clough, T.J.; Jiang, L.; Wong, K.L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Suchy, M.; Li, A.X.; Milne, M.; Bartha, R.; Hudson, R.H.E. DOTMA-based amides (DOTMAMs) as a platform for the development of PARACEST MRI contrast agents. Rsc Adv. 2016, 6, 62647–62655. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Garda, Z.; Kucera, B.E.; Tircso, G.; Young, V.G.; Woods, M. Properties, solution state behavior, and crystal structures of chelates of DOTMA. Inorg. Chem. 2011, 50, 7955–7965. [Google Scholar] [CrossRef]
- Cohen, R.; Vugts, D.J.; Stigter-van Walsum, M.; Visser, G.W.; van Dongen, G.A. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-mode fluorescence and PET imaging. Nat. Protoc. 2013, 8, 1010–1018. [Google Scholar] [CrossRef]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef]
- Deschamps-Labat, L.; Pehourcq, F.; Jagou, M.; Bannwarth, B. Relationship between lipophilicity and binding to human serum albumin of arylpropionic acid non-steroidal anti-inflammatory drugs. J. Pharm. Biomed. Anal. 1997, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kuga, N.; Shikano, N.; Takamura, N.; Nishii, R.; Yamasaki, K.; Kobayashi, M.; Nagamachi, S.; Tamura, S.; Kawai, K. Competitive displacement of serum protein binding of radiopharmaceuticals with amino acid infusion investigated with N-isopropyl-p-123I-iodoamphetamine. J. Nucl. Med. 2009, 50, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; van Duijnhoven, S.M.; Lappchen, T.; van den Bosch, S.M.; Robillard, M.S. Trans-cyclooctene tag with improved properties for tumor pretargeting with the diels-alder reaction. Mol. Pharm. 2014, 11, 3090–3096. [Google Scholar] [CrossRef]
- van Onzen, A.; Versteegen, R.M.; Hoeben, F.J.M.; Filot, I.A.W.; Rossin, R.; Zhu, T.; Wu, J.; Hudson, P.J.; Janssen, H.M.; Ten Hoeve, W.; et al. Bioorthogonal Tetrazine Carbamate Cleavage by Highly Reactive trans-Cyclooctene. J. Am. Chem. Soc. 2020, 142, 10955–10963. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Rossin, R.; ten Hoeve, W.; Janssen, H.M.; Robillard, M.S. Click to release: Instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. Engl. 2013, 52, 14112–14116. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Ten Hoeve, W.; Rossin, R.; de Geus, M.A.R.; Janssen, H.M.; Robillard, M.S. Click-to-Release from trans-Cyclooctenes: Mechanistic Insights and Expansion of Scope from Established Carbamate to Remarkable Ether Cleavage. Angew. Chem. Int. Ed. Engl. 2018, 57, 10494–10499. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Davis, C.B.; Abdel-Atti, D.; Carlin, S.D.; Chen, A.; Aggeler, R.; Agnew, B.J.; Lewis, J.S. Chemoenzymatic strategy for the synthesis of site-specifically labeled immunoconjugates for multimodal PET and optical imaging. Bioconjug. Chem. 2014, 25, 2123–2128. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Davis, C.B.; Aggeler, R.; Kang, H.C.; Chen, A.; Agnew, B.J.; Lewis, J.S. Enzyme-mediated methodology for the site-specific radiolabeling of antibodies based on catalyst-free click chemistry. Bioconjug. Chem. 2013, 24, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Emmetiere, F.; Pillarsetty, N.; Weissleder, R.; Lewis, J.S.; Reiner, T. Building Blocks for the Construction of Bioorthogonally Reactive Peptides via Solid-Phase Peptide Synthesis. ChemistryOpen 2014, 3, 48–53. [Google Scholar] [CrossRef]
- Adumeau, P.; Vivier, D.; Sharma, S.K.; Wang, J.; Zhang, T.; Chen, A.; Agnew, B.J.; Zeglis, B.M. Site-Specifically Labeled Antibody-Drug Conjugate for Simultaneous Therapy and ImmunoPET. Mol. Pharm. 2018, 15, 892–898. [Google Scholar] [CrossRef]
- van Duijnhoven, S.M.; Rossin, R.; van den Bosch, S.M.; Wheatcroft, M.P.; Hudson, P.J.; Robillard, M.S. Diabody Pretargeting with Click Chemistry In Vivo. J. Nucl. Med. 2015, 56, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Barich, D.H.; Gorman, E.M.; Zell, M.T.; Munson, E.J. 3-Methylglutaric acid as a C solid-state NMR standard. Solid. State Nucl. Mag. 2006, 30, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bertani, P.; Raya, J.; Bechinger, B. N chemical shift referencing in solid state NMR. Solid. State Nucl. Mag. 2014, 61–62, 15–18. [Google Scholar] [CrossRef] [PubMed]

| Complex | EDTA | pH | % Intact of [89Zr]Zr-Complex (n = 3) | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 1 d | 3 d | 5 d | 7 d | |||
| [89Zr]Zr-DOTMA | 100-fold | 7.0 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 |
| 5.0 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ||
| 500-fold | 7.0 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |
| 5.0 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ||
| 1000-fold | 7.0 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |
| 5.0 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ||
| Time Point | Complex | % Intact of [89Zr]Zr-Complex (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe3+ | Zn2+ | Co2+ | Cu2+ | Mg2+ | Gd3+ | Ga3+ | ||
| 0 h | [89Zr]Zr-DOTMA | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 |
| 1 d | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |
| 3 d | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |
| 5 d | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |
| 7 d | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | ≥99.99 | |
| Time Point | % Intact of [89Zr]Zr-DOTMA (n = 3) | |
|---|---|---|
| Radio-SE-HPLC | Radio-ITLC | |
| 0 h | ≥99.99 | ≥99.99 |
| 1 d | ≥99.99 | ≥99.99 |
| 3 d | ≥99.99 | ≥99.99 |
| 5 d | ≥99.99 | ≥99.99 |
| 7 d | 99.9 ± 0.1 | ≥99.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandya, D.N.; Miro, P.; Sinnwell, M.A.; Crull, G.B.; Wadas, T.J. The Evaluation of (1R,4R,7R,10R)-α,α′,α″,α‴-Tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid (DOTMA) as a Chelator for Zirconium-89. Molecules 2025, 30, 4129. https://doi.org/10.3390/molecules30204129
Pandya DN, Miro P, Sinnwell MA, Crull GB, Wadas TJ. The Evaluation of (1R,4R,7R,10R)-α,α′,α″,α‴-Tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid (DOTMA) as a Chelator for Zirconium-89. Molecules. 2025; 30(20):4129. https://doi.org/10.3390/molecules30204129
Chicago/Turabian StylePandya, Darpan N., Pere Miro, Michael A. Sinnwell, George B. Crull, and Thaddeus J. Wadas. 2025. "The Evaluation of (1R,4R,7R,10R)-α,α′,α″,α‴-Tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid (DOTMA) as a Chelator for Zirconium-89" Molecules 30, no. 20: 4129. https://doi.org/10.3390/molecules30204129
APA StylePandya, D. N., Miro, P., Sinnwell, M. A., Crull, G. B., & Wadas, T. J. (2025). The Evaluation of (1R,4R,7R,10R)-α,α′,α″,α‴-Tetramethyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic Acid (DOTMA) as a Chelator for Zirconium-89. Molecules, 30(20), 4129. https://doi.org/10.3390/molecules30204129








