USP7 at the Crossroads of Ubiquitin Signaling, Cell Cycle, and Tumorigenesis
Abstract
1. Introduction
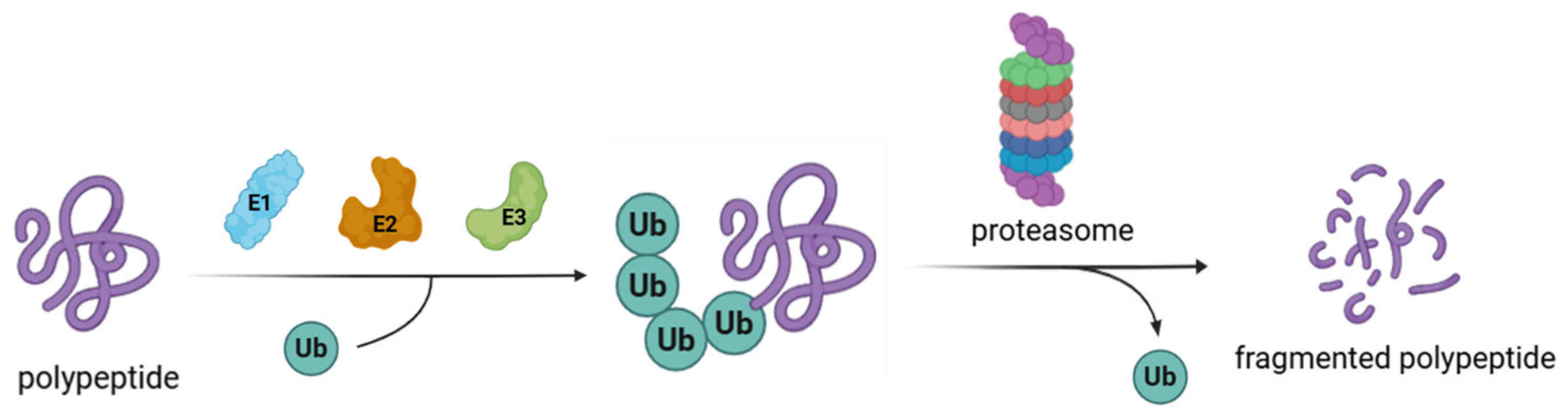
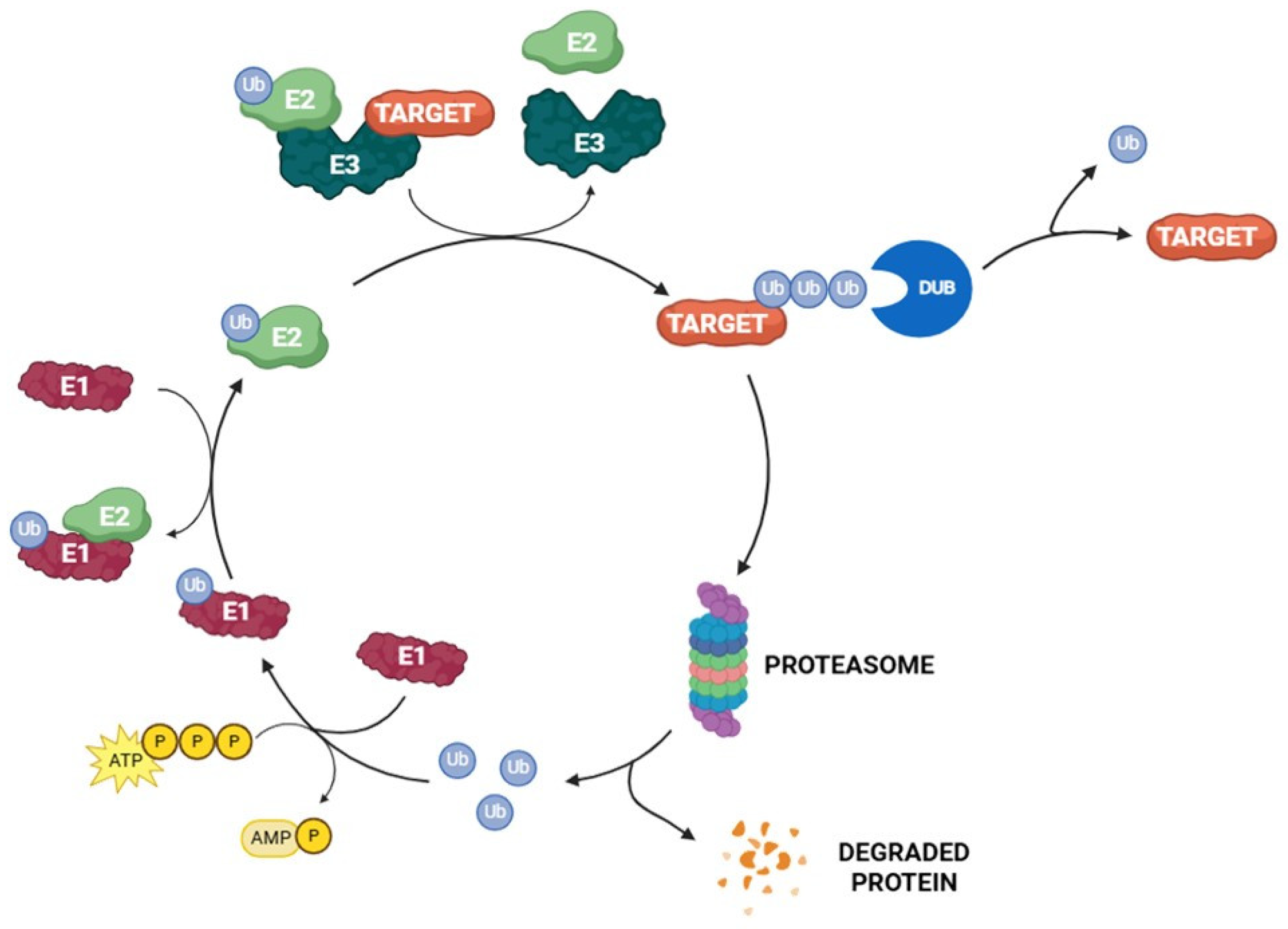
1.1. Structure and Function of the Ubiquitin–Proteasome System
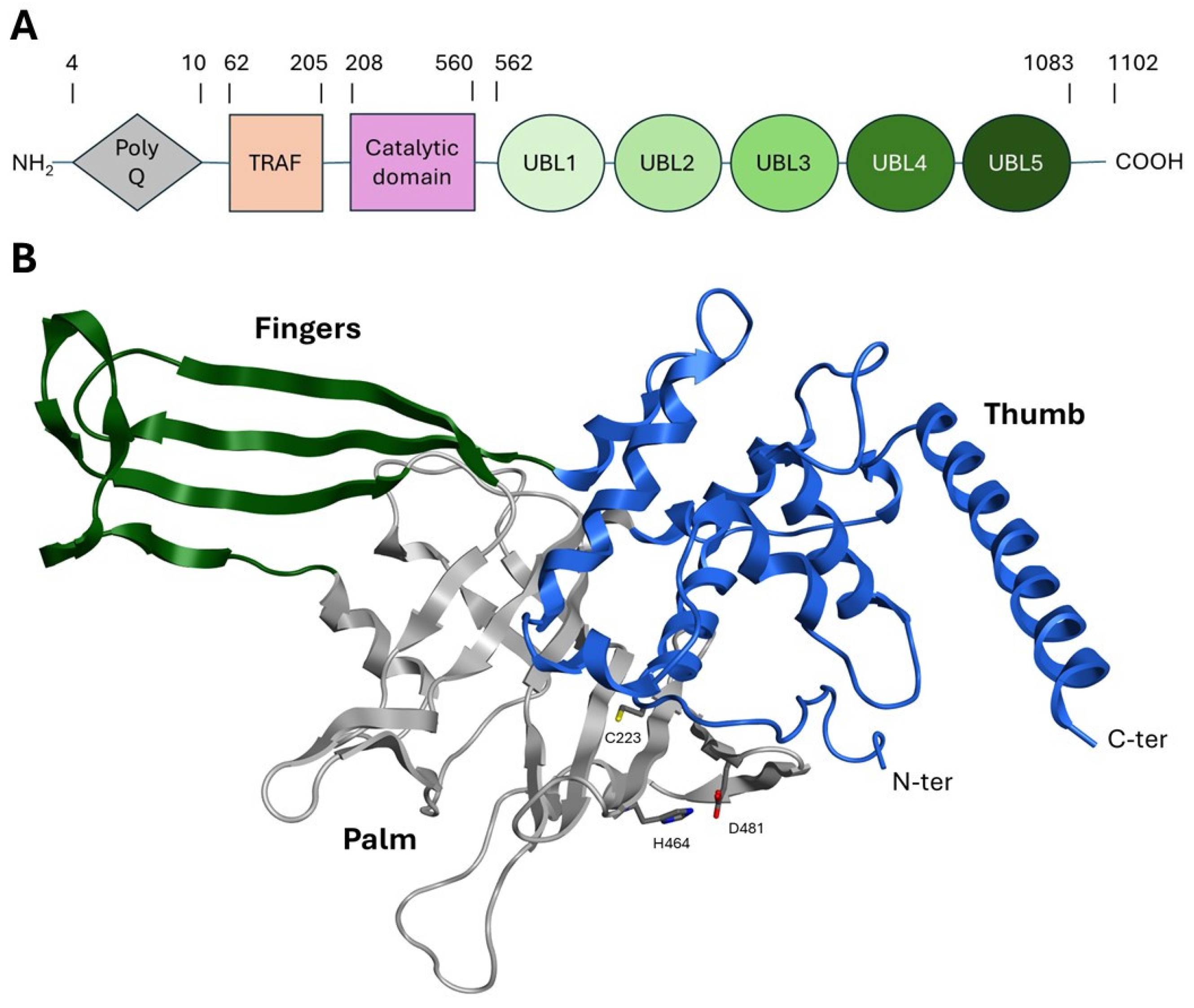
1.2. USP7 Structure
- The polyQ and TRAF-like domains are highly conserved in humans, mice, and rats and are responsible for the USP7 nuclear localization. Despite the lack of a proper localization signal in this region, it has been postulated that the binding of USP7 to its substrates (commonly nuclear proteins) could favor the translocation into the nucleus [39,40].
- The TRAF-like domain consists of eight β-sheets and one α-helix. This region contains several protein–protein interaction patterns that allow USP7 to recognize the different substrates involved in the ubiquitination pathway, such as MDM2, p53, Epstein–Barr nuclear antigen 1 (EBNA1), viral interferon regulatory factor 4 (IRF-4), latency-associated nuclear antigen (LANA), and testis-specific protein Y-encoded-like 5 (TSPYL5) [41,42].
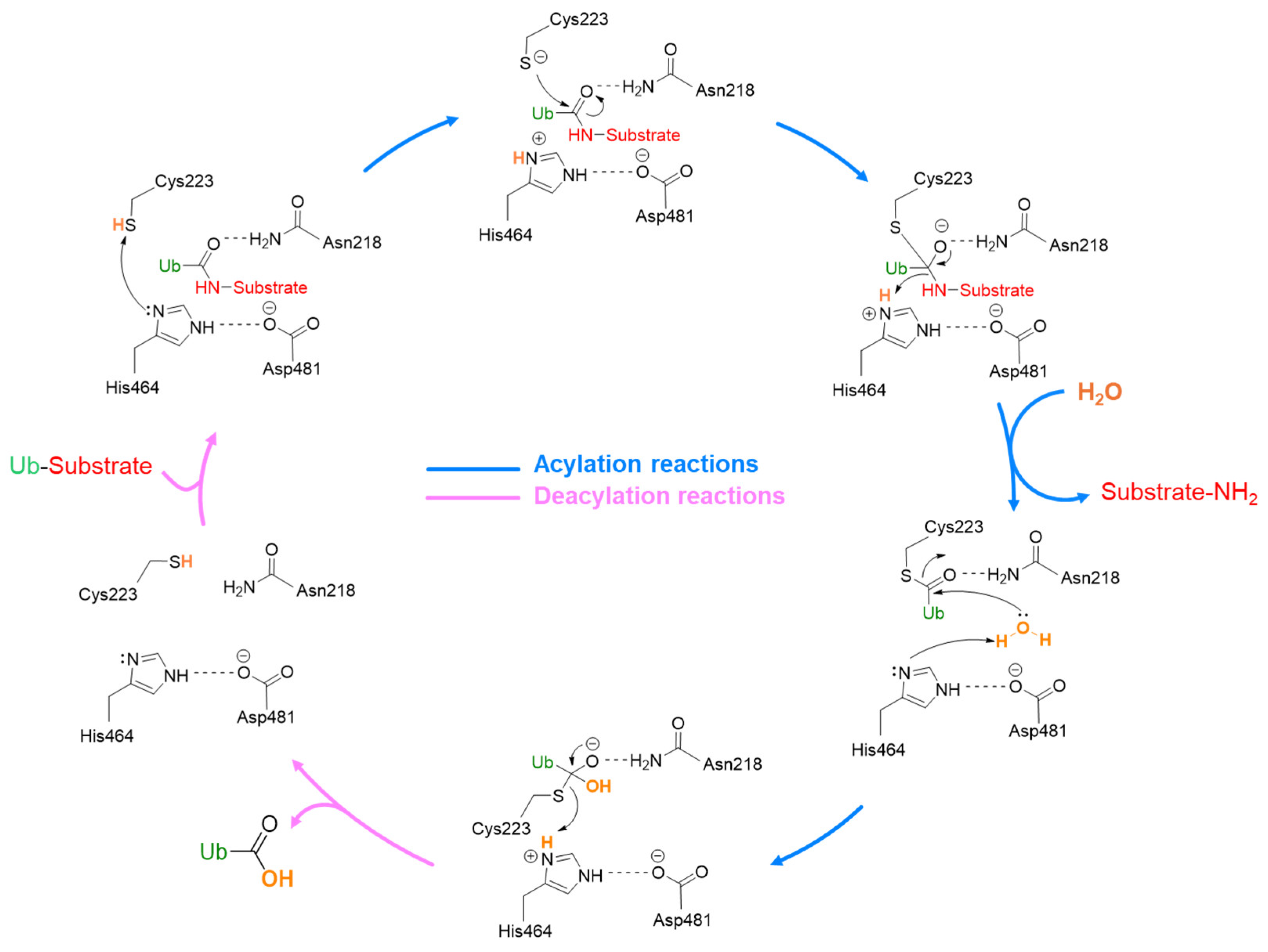
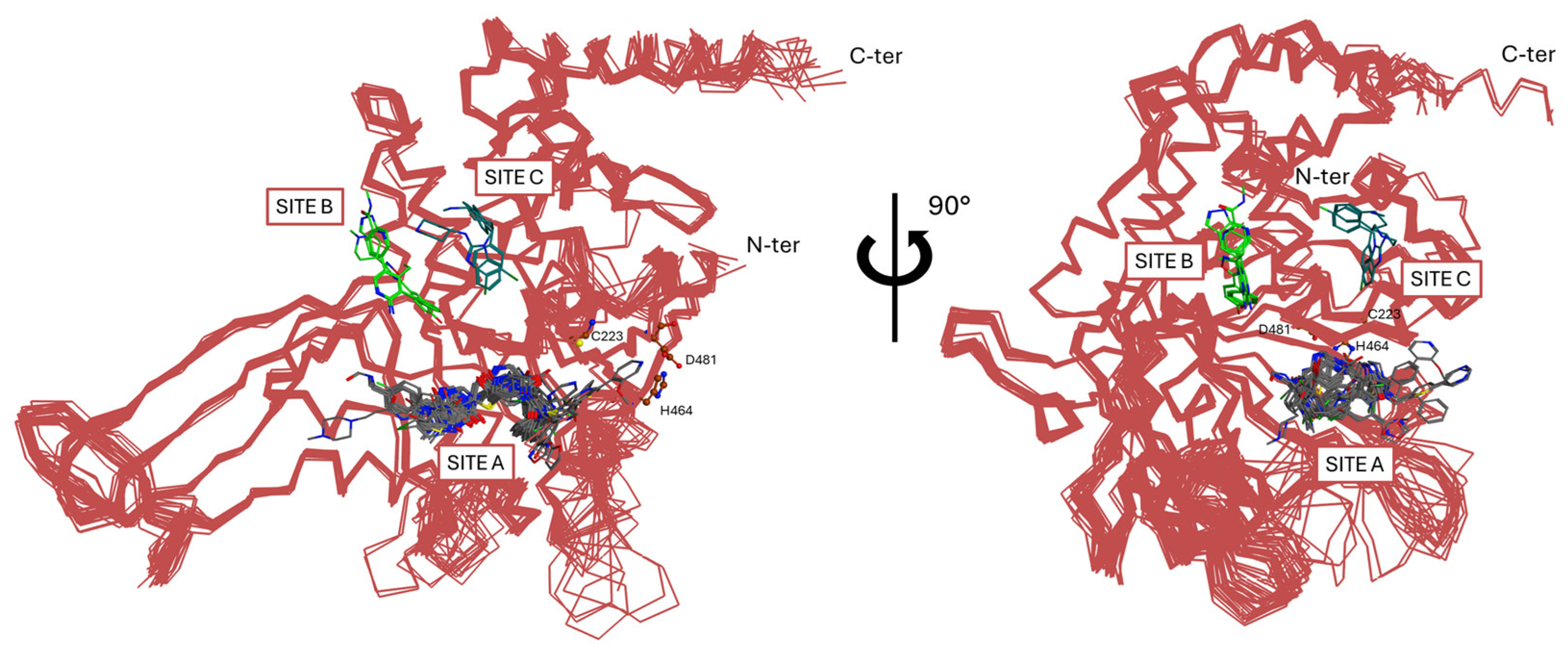
- The catalytic domain is structurally arranged as an outstretched right hand consisting of thumb, finger, and palm domains. Its structure contains ten α-helices and fourteen β-sheets and includes a conserved catalytic triad composed of Cys223, His464, and Asp481 (Figure 3B) [43]. These three residues are usually in a non-reactive conformation and switch to an active state upon ubiquitin binding. The activation mechanism is facilitated by a region known as the “switching loop,” which brings the catalytic triad residues into proximity, enabling ubiquitin interaction [41].
- The UBL domain consists of five ubiquitin-like ββαβαβ-fold domains possibly involved in substrate recognition and protein–protein interactions [44]. Specifically, UBL 1, 2, and 3 domains interact with infected cellular polypeptide 0 (ICP0), ubiquitin-like with PHD and ring finger domain 1 (UHRF1), and DNA methyltransferase-1 (DNMT1), among others [45]. UBL structural domains 4 and 5 bind to forkhead box O4 (FoxO4) [46]. Notably, the UBL4 domain interacts with the UbcH5c E2 conjugating enzyme, facilitating the transfer of ubiquitin molecules from E1 to the substrate protein, thereby enhancing its stability and mediating various cellular processes [47,48].
1.3. USP7 Roles and Functions
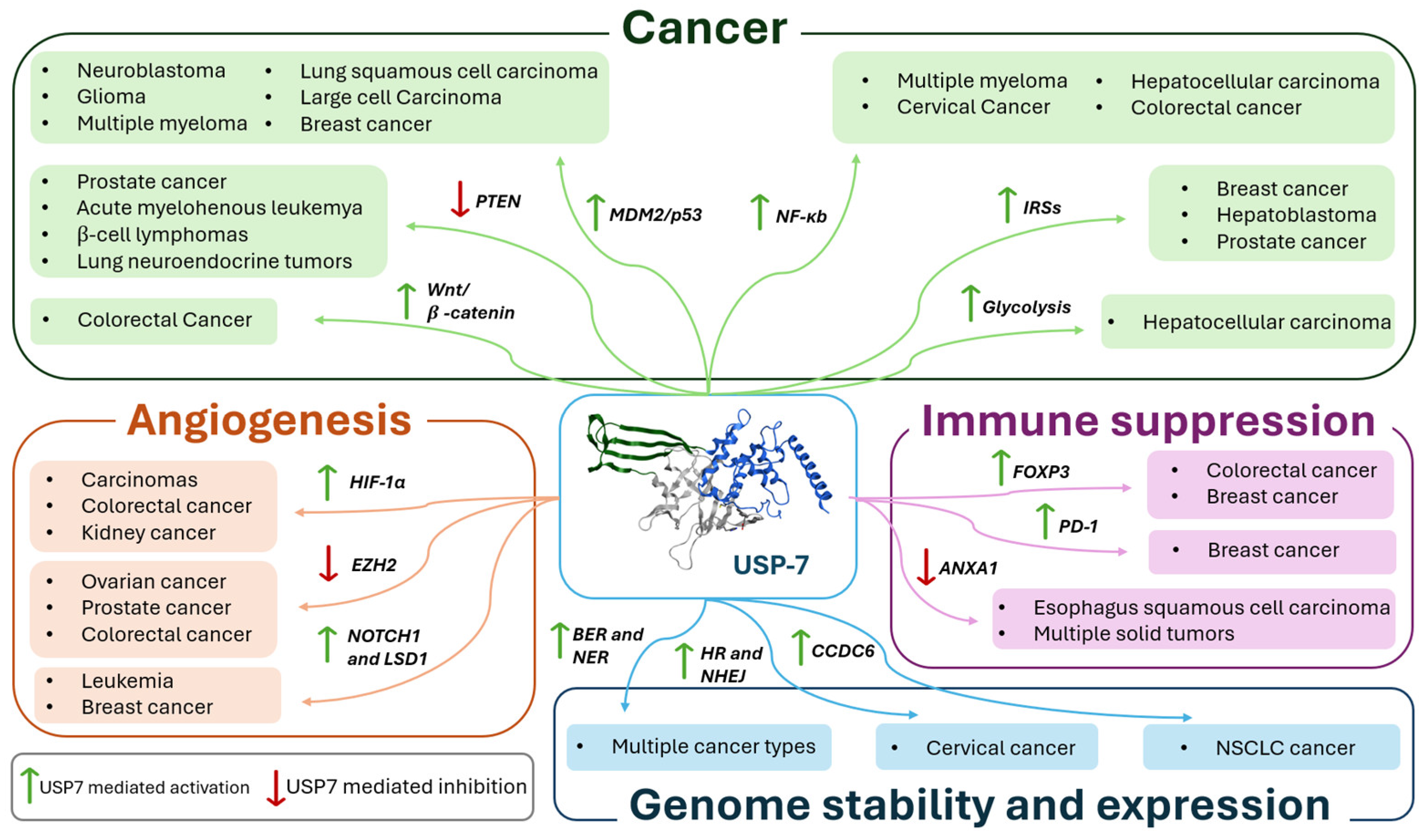
2. USP7 Implications in Cancer
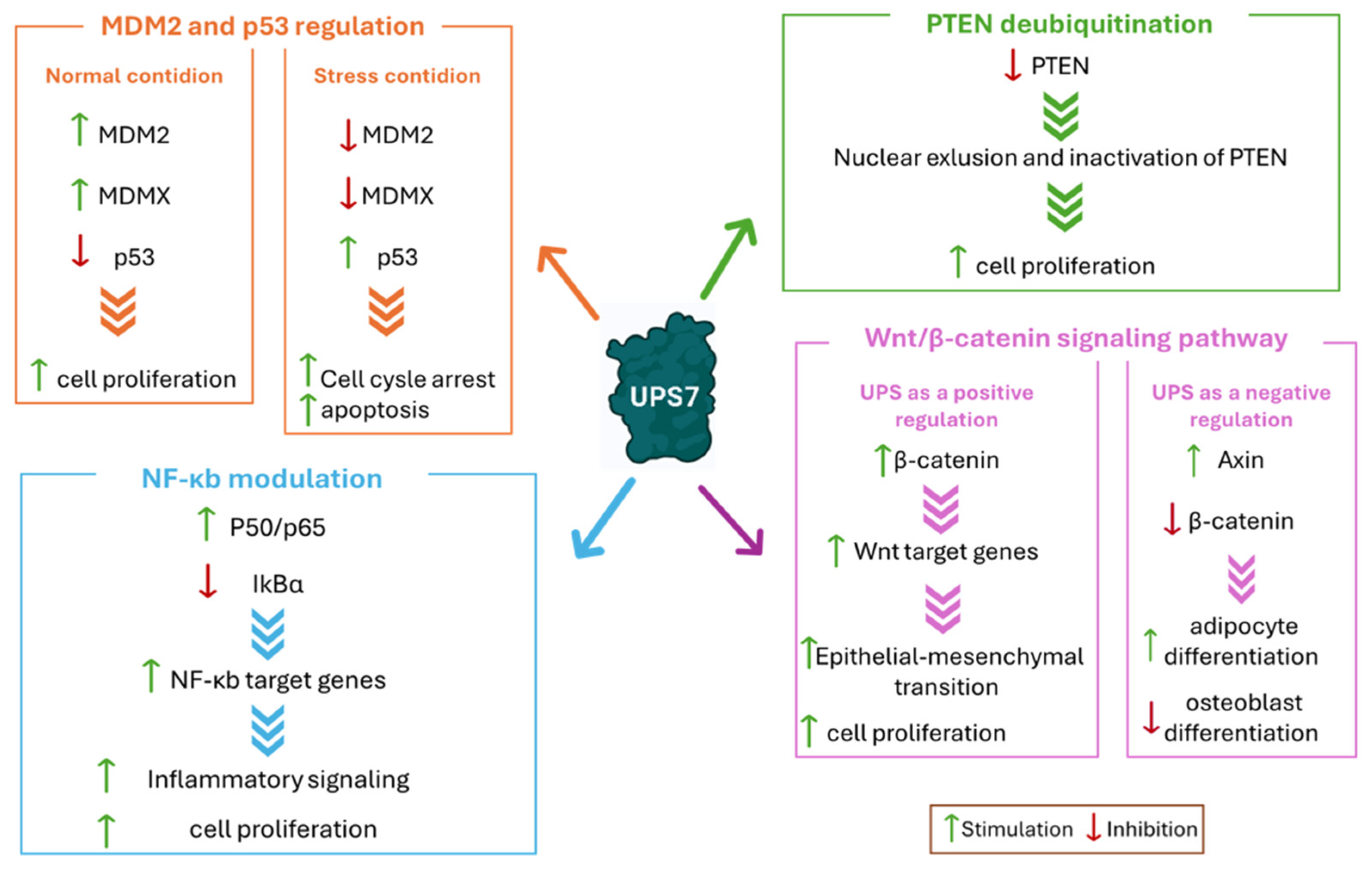
2.1. MDM2 and p53 Regulation
2.2. PTEN Deubiquitination
2.3. Wnt/β-Catenin Signaling Pathway
2.4. NF-κb Modulation
2.5. Interaction with Insulin Receptor Substrates
2.6. Cellular Energetics Deregulation
2.7. Other USP7 Implications in Tumors
3. USP7 Role in Angiogenesis and Metastasis
3.1. HIF-1α Stabilization
3.2. EZH2 and Wnt/β-Catenin Pathways Activation
3.3. NOTCH1 and LSD1 Regulation
4. USP7 Influence on Immune Suppression
4.1. Treg Cells and FOXP3 Activation
4.2. The USP7 Impact on Immune Checkpoint Inhibitors
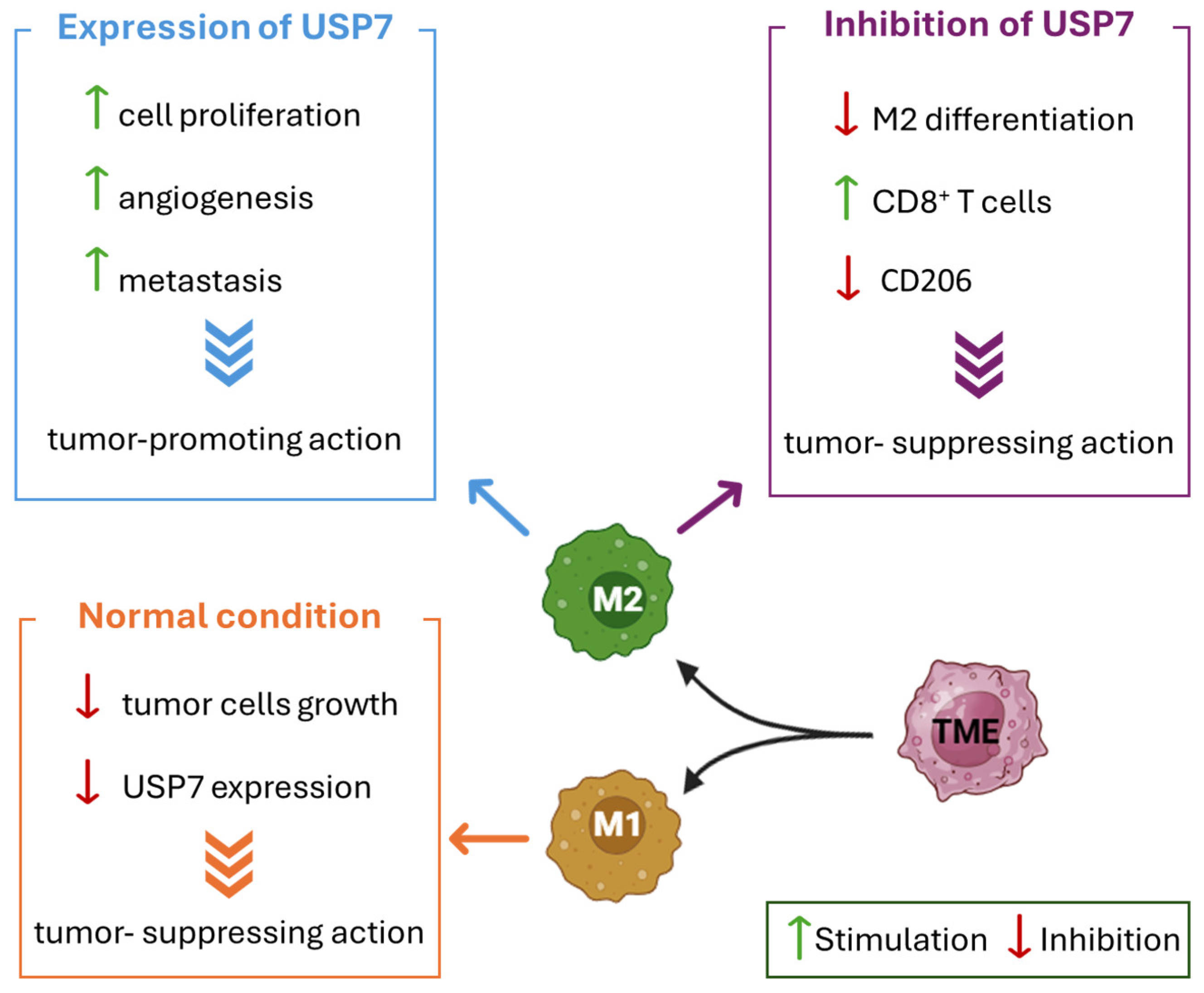
4.3. USP7 Expression in Tumor-Associated Macrophages and ANXA1
5. USP7 Regulation of Genome Stability and Expression
5.1. BER and NER
5.2. HR and NHEJ
5.3. Cell Cycle Arrest
6. USP7 Inhibitors
6.1. Covalent Inhibitors
6.2. Noncovalent Allosteric Inhibitors
6.3. Covalent Allosteric Inhibitors
6.4. USP7 PROTACs
6.5. Natural Inhibitors
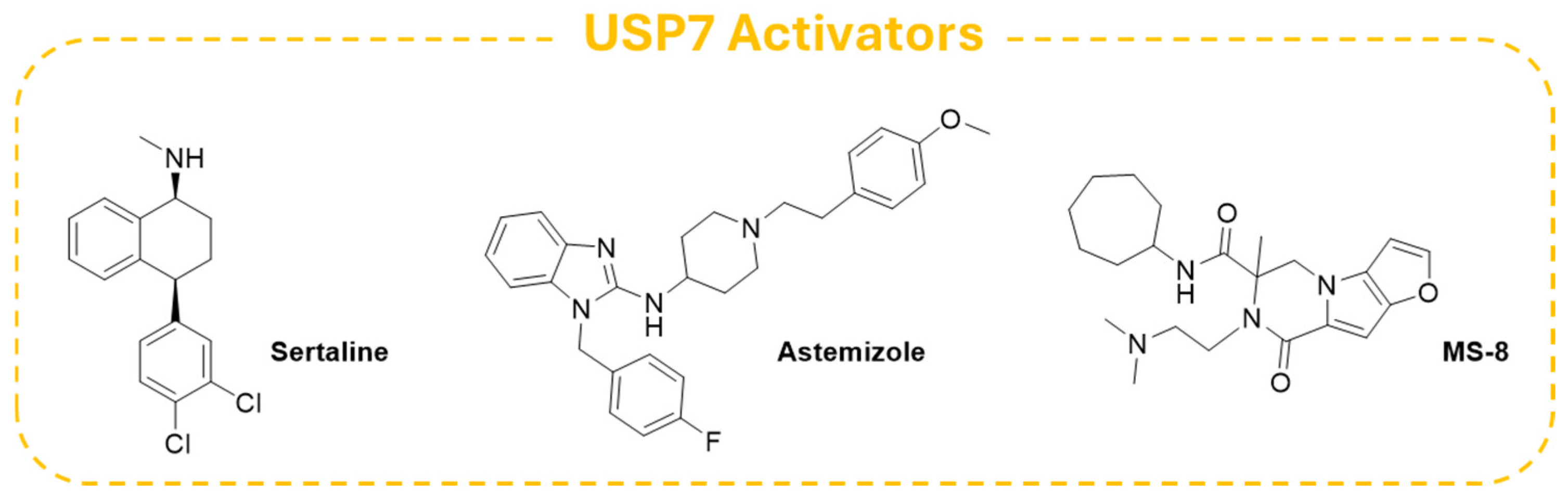
7. USP7 Activators
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 53BP1 | p53-binding protein 1 |
| ABCB1 | ATP-binding cassette sub-family B member 1 |
| Akt | Protein kinase B |
| AML | Acute myelogenous leukemia |
| ANXA1 | Annexin A1 |
| APC | Adenomatous polyposis coli |
| ARF | Alternate Reading Frame |
| ATR-Chk1 | Ataxia Telangiectasia and Rad3-related protein Checkpoint kinase-1 |
| BER | Base excision repair |
| BUB3 | BUB3 Mitotic Checkpoint Protein |
| CARM1 | Coactivator Associated Arginine Methyltransferase 1 |
| CBP | CREB-binding protein |
| CCDC6 | Coiled-coil domain-containing protein 6 |
| CCF | Cytoplasmic chromatin fragments |
| CDC25A | Cell Division Cycle 25A |
| cGAS-STING | Cyclic GMP-AMP synthase-stimulator of interferon genes |
| CID | Catenin inhibitory domain |
| CK1α | Casein kinase 1α |
| CLL | Chronic lymphoid leukemia |
| CRBN | Cereblon |
| CRC | Colorectal cancer |
| DAXX | Death domain-associated protein |
| DDX3X | DEAD-box helicase 3 X-linked |
| DNMT1 | DNA methyltransferase-1 |
| DNMT1 | DNA methyltransferase 1 |
| DSB | Double Strand Break |
| DUB | Deubiquitinate |
| DUBTACs | DUB Targeting Chimeras |
| EBNA1 | Epstein–Barr nuclear antigen 1 |
| ELK4 | ETS-like transcription factor 4 |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| ERK | Extracellular signal-regulated kinase |
| ETS | Electron transport system |
| EZH2 | Zeste homolog 2 enhancer |
| FOXO1 | Forkhead box protein O1 |
| FoxO4 | Forkhead box O4 |
| FOXO6 | Forkhead box protein O7 |
| FOXP3 | Forkhead box P3 |
| GG-NER | Global genome NER |
| GLUT1 | Glucose transporter 1 |
| Grb2 | Growth factor receptor-bound protein 2 |
| GSK3β | Glycogen synthase kinase 3β |
| H3K4me1/2 | Histone H3 at lysine 4 |
| H3K9me1/2 | Histone H3 at lysine 9 |
| HAFOUS | Hao–Fountain Syndrome |
| HAUSP | Herpesvirus-associated ubiquitin-specific protease |
| HCC | Hepatocellular carcinoma |
| HDAC1 | Histone deacetylase 1 |
| HER2 | Human epidermal growth factor receptor |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HIFs | Hypoxia-inducible factors |
| hnRNPA1 | Heterogeneous Nuclear Ribonucleoprotein A1 |
| HNSSC | Head and neck squamous cell carcinoma |
| HR | Homologous recombination |
| ICP0 | Infected cellular polypeptide 0 |
| IFNGR1 | Interferon gamma receptor 1 |
| IGF | Insulin/insulin-like growth factor |
| IL-6 | Interleukin 6 |
| IRF-4 | Viral interferon regulatory factor 4 |
| IRS | Insulin receptor substrate |
| IκBs | NF-κB inhibitors |
| JAMM | JAMM/MPN domain-associated metallopeptidase |
| JMJD3 | Jumonji domain-containing protein-3 |
| K63 | Lysine residue |
| LANA | Latency-associated nuclear antigen |
| LSD1 | Lysine-specific histone demethylase 1 |
| HLSD1 | Histone lysine demethylase 1 |
| Maged1 | Melanoma-associated antigen D1 |
| MCPIP | Monocyte chemotactic protein-induced proteins |
| MDC1 | Mediator of DNA damage checkpoint protein 1 |
| MDM2 | Murine double minute 2 |
| MDMX | Murine double minute X |
| MIG-6 | Mitogen-inducible gene-6 |
| MINDY | MIU-containing novel DUB family |
| MJD | Machado–Joseph disease protein domain proteases |
| MM1.S | Multiple myeloma |
| MMR | Mismatch repair |
| MRN | MRE11-RAD50-NBS1 |
| NB | Neuroblastoma |
| NEK2 | NIMA-related kinase 2 |
| NER | Nucleotide excision repair |
| NF-κB | Nuclear factor-kappa B |
| NHEJ | Non-homologous end joining |
| NOTCH | Notch signaling pathway |
| NPM1 | Nucleophosmin 1 |
| NSCLC | Non-small cell lung cancer |
| OTU | Otubain domain ubiquitin-binding proteins |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PLK1 | Polo-like kinase 1 |
| PPPDE | Permuted papain fold peptidase of dsDNA viruses and eukaryotes |
| PROTAC | Proteolysis-targeting chimera |
| PTEN | Phosphatase and tensin homolog |
| PVT | Paraventricular thalamus |
| RAP80 | Receptor-associated protein 80 |
| ROS | Reactive oxygen species |
| SIRT7 | Sirtuin 7 |
| SMAD | Small mother against decapentaplegic |
| TC-NER | Transcription coupled NER |
| Teff | Effector T cells |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| TPD | Targeted protein degradation |
| TRAF | Tumor necrosis receptor-associated factor |
| Treg | Regulatory T cells |
| TRIM27 | Tripartite Motif Containing 27 |
| TRIP13 | Thyroid hormone receptor interactor 13 |
| TSP-1 | Thrombospondin-1 |
| TSPYL5 | Testis-specific protein Y-encoded-like 5 |
| UBL1-5 | Ubiquitin-like domains 1-5 |
| UCH | Ubiquitin C-terminal hydrolases |
| UHRF1 | Ubiquitin-like with PHD and ring finger domain 1 |
| UPS | Ubiquitin–proteasome system |
| USP | Ubiquitin-specific proteases |
| USP7 | Ubiquitin-specific proteases 7 |
| USP7S | Ser18-containing isoform of USP7 |
| VEGF | Vascular endothelial growth factor |
| ZUFSP | Zinc finger with UFM1-specific peptidase domain protein |
| βTrCp | β-transducin repeat-containing protein |
References
- Carreira, L.D.; Oliveira, R.I.; Moreira, V.M.; Salvador, J.A.R. Ubiquitin-Specific Protease 7 (USP7): An Emerging Drug Target for Cancer Treatment. Expert Opin. Ther. Targets 2023, 27, 1043–1058. [Google Scholar] [CrossRef]
- Georges, A.; Marcon, E.; Greenblatt, J.; Frappier, L. Identification and Characterization of USP7 Targets in Cancer Cells. Sci. Rep. 2018, 8, 15833. [Google Scholar] [CrossRef]
- Selvaraju, K.; Mazurkiewicz, M.; Wang, X.; Gullbo, J.; Linder, S.; D’Arcy, P. Inhibition of Proteasome Deubiquitinase Activity: A Strategy to Overcome Resistance to Conventional Proteasome Inhibitors? Drug Resist. Updates 2015, 21–22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ouyang, T.; Li, M.; Hong, T.; Mhs, A.; Meng, W.; Zhang, N. Ubiquitin-Specific Peptidase 7: A Novel Deubiquitinase That Regulates Protein Homeostasis and Cancers. Front. Oncol. 2021, 11, 784672. [Google Scholar] [CrossRef] [PubMed]
- Ndubaku, C.; Tsui, V. Inhibiting the Deubiquitinating Enzymes (DUBs): Miniperspective. J. Med. Chem. 2015, 58, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.-J.; Wang, B.; Zhang, Y.; Kang, H.-Q.; Nie, H.-Q.; Feng, M.-K.; Zhang, X.-Y.; Zhao, L.-J.; Wang, N.; Liu, H.-M.; et al. USP7 as an Emerging Therapeutic Target: A Key Regulator of Protein Homeostasis. Int. J. Biol. Macromol. 2024, 263, 130309. [Google Scholar] [CrossRef]
- Mevissen, T.E.T.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef]
- Snyder, N.A.; Silva, G.M. Deubiquitinating Enzymes (DUBs): Regulation, Homeostasis, and Oxidative Stress Response. J. Biol. Chem. 2021, 297, 101077. [Google Scholar] [CrossRef]
- Park, H.-B.; Baek, K.-H. Current and Future Directions of USP7 Interactome in Cancer Study. Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 1878, 188992. [Google Scholar] [CrossRef]
- Yeasmin Khusbu, F.; Chen, F.; Chen, H. Targeting Ubiquitin Specific Protease 7 in Cancer: A Deubiquitinase with Great Prospects. Cell Biochem. Funct. 2018, 36, 244–254. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, T.; Dong, X.; Guo, Q.; Zheng, L.; Wang, X.; Zhang, N.; Li, D.; Ren, L.; Yi, F.; et al. De-Ubiquitination of SAMHD1 by USP7 Promotes DNA Damage Repair to Overcome Oncogenic Stress and Affect Chemotherapy Sensitivity. Oncogene 2023, 42, 1843–1856. [Google Scholar] [CrossRef]
- Pozhidaeva, A.; Bezsonova, I. USP7: Structure, Substrate Specificity, and Inhibition. DNA Repair 2019, 76, 30–39. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, F.; Zhang, G.; Zhang, Q.; Liu, Y.; Wang, Q.; Elsharkawy, M.S.; Zheng, M.; Wen, J.; Zhao, G.; et al. USP7 Promotes Deubiquitination and Stabilization of MyD88 to Enhance Immune Responses. Front. Immunol. 2022, 13, 900243. [Google Scholar] [CrossRef] [PubMed]
- Große, M.; Setz, C.; Rauch, P.; Auth, J.; Morokutti-Kurz, M.; Temchura, V.; Schubert, U. Inhibitors of Deubiquitinating Enzymes Interfere with the SARS-CoV-2 Papain-like Protease and Block Virus Replication In Vitro. Viruses 2022, 14, 1404. [Google Scholar] [CrossRef] [PubMed]
- Saha, G.; Roy, S.; Basu, M.; Ghosh, M.K. USP7—A Crucial Regulator of Cancer Hallmarks. Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 1878, 188903. [Google Scholar] [CrossRef]
- Qi, S.-M.; Cheng, G.; Cheng, X.-D.; Xu, Z.; Xu, B.; Zhang, W.-D.; Qin, J.-J. Targeting USP7-Mediated Deubiquitination of MDM2/MDMX-P53 Pathway for Cancer Therapy: Are We There Yet? Front. Cell Dev. Biol. 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Carracedo, A.; Egia, A.; Lo-Coco, F.; Teruya-Feldstein, J.; Pandolfi, P.P. The Deubiquitinylation and Localization of PTEN Are Regulated by a HAUSP–PML Network. Nature 2008, 455, 813–817. [Google Scholar] [CrossRef]
- Colleran, A.; Collins, P.E.; O’Carroll, C.; Ahmed, A.; Mao, X.; McManus, B.; Kiely, P.A.; Burstein, E.; Carmody, R.J. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 Promotes Transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 618–623. [Google Scholar] [CrossRef]
- An, T.; Gong, Y.; Li, X.; Kong, L.; Ma, P.; Gong, L.; Zhu, H.; Yu, C.; Liu, J.; Zhou, H.; et al. USP7 Inhibitor P5091 Inhibits Wnt Signaling and Colorectal Tumor Growth. Biochem. Pharmacol. 2017, 131, 29–39. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Fleskens, V.; Fu, J.; Brenkman, A.B.; Bekker, C.P.J.; Pals, C.E.G.M.; Meerding, J.; Berkers, C.R.; Barbi, J.; Gröne, A.; et al. Stabilization of the Transcription Factor Foxp3 by the Deubiquitinase USP7 Increases Treg-Cell-Suppressive Capacity. Immunity 2013, 39, 259–271. [Google Scholar] [CrossRef]
- Oliveira, R.I.; Guedes, R.A.; Salvador, J.A.R. Highlights in USP7 Inhibitors for Cancer Treatment. Front. Chem. 2022, 10, 1005727. [Google Scholar] [CrossRef] [PubMed]
- Chondrogianni, N.; Gonos, E.S. Structure and Function of the Ubiquitin–Proteasome System. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2012; Volume 109, pp. 41–74. ISBN 978-0-12-397863-9. [Google Scholar]
- Chen, S.; Liu, Y.; Zhou, H. Advances in the Development Ubiquitin-Specific Peptidase (USP) Inhibitors. Int. J. Mol. Sci. 2021, 22, 4546. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, M. Lingering Mysteries of Ubiquitin-Chain Assembly. Cell 2006, 124, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nininahazwe, L.; Liu, B.; He, C.; Zhang, H.; Chen, Z.-S. The Emerging Nature of Ubiquitin-Specific Protease 7 (USP7): A New Target in Cancer Therapy. Drug Discov. Today 2021, 26, 490–502. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The Role of Ubiquitination in Tumorigenesis and Targeted Drug Discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef]
- Ravid, T.; Hochstrasser, M. Diversity of Degradation Signals in the Ubiquitin–Proteasome System. Nat. Rev. Mol. Cell Biol. 2008, 9, 679–689. [Google Scholar] [CrossRef]
- Pickart, C.M.; Fushman, D. Polyubiquitin Chains: Polymeric Protein Signals. Curr. Opin. Chem. Biol. 2004, 8, 610–616. [Google Scholar] [CrossRef]
- Sewduth, R.N.; Baietti, M.F.; Sablina, A.A. Cracking the Monoubiquitin Code of Genetic Diseases. Int. J. Mol. Sci. 2020, 21, 3036. [Google Scholar] [CrossRef]
- Chen, Z.J.; Sun, L.J. Nonproteolytic Functions of Ubiquitin in Cell Signaling. Mol. Cell 2009, 33, 275–286. [Google Scholar] [CrossRef]
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018, 87, 697–724. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbé, S.; Komander, D. Breaking the Chains: Deubiquitylating Enzyme Specificity Begets Function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Nijman, S.M.B.; Luna-Vargas, M.P.A.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.G.; Sixma, T.K.; Bernards, R. A Genomic and Functional Inventory of Deubiquitinating Enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.J.; Liu, X.; Magin, R.S.; Doherty, L.M.; Chan, W.C.; Ficarro, S.B.; Hu, W.; Roberts, R.M.; Iacob, R.E.; Stolte, B.; et al. Selective USP7 Inhibition Elicits Cancer Cell Killing through a P53-Dependent Mechanism. Sci. Rep. 2020, 10, 5324. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.M.; Armstrong, L.A.; Kulathu, Y. Deubiquitinases: From Mechanisms to Their Inhibition by Small Molecules. Mol. Cell 2022, 82, 15–29. [Google Scholar] [CrossRef]
- Komander, D.; Clague, M.J.; Urbé, S. Breaking the Chains: Structure and Function of the Deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef]
- Tavana, O.; Gu, W. Modulation of the P53/MDM2 Interplay by HAUSP Inhibitors. J. Mol. Cell Biol. 2017, 9, 45–52. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chakraborty, D.; Basu, M.; Ghosh, M.K. Emerging Insights into HAUSP (USP7) in Physiology, Cancer and Other Diseases. Signal Transduct. Target Ther. 2018, 3, 17. [Google Scholar] [CrossRef]
- Fernández-Montalván, A.; Bouwmeester, T.; Joberty, G.; Mader, R.; Mahnke, M.; Pierrat, B.; Schlaeppi, J.; Worpenberg, S.; Gerhartz, B. Biochemical Characterization of USP7 Reveals Post-translational Modification Sites and Structural Requirements for Substrate Processing and Subcellular Localization. FEBS J. 2007, 274, 4256–4270. [Google Scholar] [CrossRef]
- Zapata, J.M.; Pawlowski, K.; Haas, E.; Ware, C.F.; Godzik, A.; Reed, J.C. A Diverse Family of Proteins Containing Tumor Necrosis Factor Receptor-Associated Factor Domains. J. Biol. Chem. 2001, 276, 24242–24252. [Google Scholar] [CrossRef]
- Valles, G.J.; Bezsonova, I.; Woodgate, R.; Ashton, N.W. USP7 Is a Master Regulator of Genome Stability. Front. Cell Dev. Biol. 2020, 8, 717. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.-M. Recent Advances in the Development of Ubiquitin-Specific-Processing Protease 7 (USP7) Inhibitors. Eur. J. Med. Chem. 2020, 191, 112107. [Google Scholar] [CrossRef]
- Hu, M.; Li, P.; Li, M.; Li, W.; Yao, T.; Wu, J.-W.; Gu, W.; Cohen, R.E.; Shi, Y. Crystal Structure of a UBP-Family Deubiquitinating Enzyme in Isolation and in Complex with Ubiquitin Aldehyde. Cell 2002, 111, 1041–1054. [Google Scholar] [CrossRef]
- Faesen, A.C.; Dirac, A.M.G.; Shanmugham, A.; Ovaa, H.; Perrakis, A.; Sixma, T.K. Mechanism of USP7/HAUSP Activation by Its C-Terminal Ubiquitin-like Domain and Allosteric Regulation by GMP-Synthetase. Mol. Cell 2011, 44, 147–159. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.; Fang, J.; Ma, L.; Gong, R.; Wang, P.; Li, Z.; Xu, Y. Molecular Mechanism for USP7-Mediated DNMT1 Stabilization by Acetylation. Nat. Commun. 2015, 6, 7023. [Google Scholar] [CrossRef] [PubMed]
- Van Der Horst, A.; De Vries-Smits, A.M.M.; Brenkman, A.B.; Van Triest, M.H.; Van Den Broek, N.; Colland, F.; Maurice, M.M.; Burgering, B.M.T. FOXO4 Transcriptional Activity Is Regulated by Monoubiquitination and USP7/HAUSP. Nat. Cell Biol. 2006, 8, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, F.; Wheaton, K.; La Delfa, A.; Mohamed, M.; Shaikh, F.; Khatun, R.; Arrowsmith, C.H.; Frappier, L.; Saridakis, V.; Sheng, Y. Ubiquitin-Specific Protease 7 Is a Regulator of Ubiquitin-Conjugating Enzyme UbE2E1. J. Biol. Chem. 2013, 288, 16975–16985. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Demichev, V.; Backman, M.; Ohmayer, U.; Ihmor, P.; Müller, S.; Ralser, M.; Daub, H. Time-Resolved in Vivo Ubiquitinome Profiling by DIA-MS Reveals USP7 Targets on a Proteome-Wide Scale. Nat. Commun. 2021, 12, 5399. [Google Scholar] [CrossRef]
- Cstorer, A.; Ménard, R. Catalytic Mechanism in Papain Family of Cysteine Peptidases. In Methods in Enzymology; Elsevier: San Diego, CA, USA, 1994; Volume 244, pp. 486–500. ISBN 978-0-12-182145-6. [Google Scholar]
- Zhou, J.; Wang, J.; Chen, C.; Yuan, H.; Wen, X.; Sun, H. USP7: Target Validation and Drug Discovery for Cancer Therapy. Med. Chem. 2018, 14, 3–18. [Google Scholar] [CrossRef]
- Khoronenkova, S.V.; Dianov, G.L. USP7S-Dependent Inactivation of Mule Regulates DNA Damage Signalling and Repair. Nucleic Acids Res. 2013, 41, 1750–1756. [Google Scholar] [CrossRef]
- Rougé, L.; Bainbridge, T.W.; Kwok, M.; Tong, R.; Di Lello, P.; Wertz, I.E.; Maurer, T.; Ernst, J.A.; Murray, J. Molecular Understanding of USP7 Substrate Recognition and C-Terminal Activation. Structure 2016, 24, 1335–1345. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin Modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, W.; You, Y.; Pang, J.; Ren, H.; Suo, Z.; Liu, H.; Zheng, Y. USP7: Novel Drug Target in Cancer Therapy. Front. Pharmacol. 2019, 10, 427. [Google Scholar] [CrossRef]
- Bojagora, A.; Saridakis, V. USP7 Manipulation by Viral Proteins. Virus Res. 2020, 286, 198076. [Google Scholar] [CrossRef]
- Lusardi, M.; Morretta, E.; Spallarossa, A.; Monti, M.C.; Rosano, C.; Iervasi, E.; Ponassi, M.; Mori, M.; Meneghetti, F.; Brullo, C. Targeting Ubiquitin-Specific Protease 7 with Novel 5-Amino-Pyrazole Inhibitors: Design, Synthesis, and Biological Evaluation. ChemMedChem 2025, 20, e202500185. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Tischler, V.; Atanassoff, A.; Wiedl, T.; Komminoth, P.; Oehlschlegel, C.; Weder, W.; Soltermann, A. Lung Neuroendocrine Tumors: Correlation of Ubiquitinylation and Sumoylation with Nucleo-Cytosolic Partitioning of PTEN. BMC Cancer 2015, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Tian, Z.; Nicholson, B.; Kumar, K.G.S.; Zhou, B.; Carrasco, R.; McDermott, J.L.; Leach, C.A.; Fulcinniti, M.; Kodrasov, M.P.; et al. A Small Molecule Inhibitor of Ubiquitin-Specific Protease-7 Induces Apoptosis in Multiple Myeloma Cells and Overcomes Bortezomib Resistance. Cancer Cell 2012, 22, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ma, M. Ubiquitin-Specific Protease 7 Expression Is a Prognostic Factor in Epithelial Ovarian Cancer and Correlates with Lymph Node Metastasis. OncoTargets Ther. 2016, 9, 1559–1569. [Google Scholar] [CrossRef]
- Das, S.; Chandrasekaran, A.P.; Jo, K.-S.; Ko, N.R.; Oh, S.J.; Kim, K.-S.; Ramakrishna, S. HAUSP Stabilizes Cdc25A and Protects Cervical Cancer Cells from DNA Damage Response. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2020, 1867, 118835. [Google Scholar] [CrossRef]
- Cheng, C.; Niu, C.; Yang, Y.; Wang, Y.; Lu, M. Expression of HAUSP in Gliomas Correlates with Disease Progression and Survival of Patients. Oncol. Rep. 2013, 29, 1730–1736. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Z.; Liu, P.; Zheng, Y.; Cai, S.; Sun, Y.; Wang, B. Upregulation of Deubiquitinase USP7 by Transcription Factor FOXO6 Promotes EC Progression via Targeting the JMJD3/CLU Axis. Mol. Ther. Oncolytics 2021, 20, 583–595. [Google Scholar] [CrossRef]
- Wang, L.; Kumar, S.; Dahiya, S.; Wang, F.; Wu, J.; Newick, K.; Han, R.; Samanta, A.; Beier, U.H.; Akimova, T.; et al. Ubiquitin-Specific Protease-7 Inhibition Impairs Tip60-Dependent Foxp3 + T-Regulatory Cell Function and Promotes Antitumor Immunity. eBioMedicine 2016, 13, 99–112. [Google Scholar] [CrossRef]
- Fu, C.; Zhu, X.; Xu, P.; Li, Y. Pharmacological Inhibition of USP7 Promotes Antitumor Immunity and Contributes to Colon Cancer Therapy. Onco Targets Ther. 2019, 12, 609–617. [Google Scholar] [CrossRef]
- Cheron, J.; Beccari, L.; Hagué, P.; Icick, R.; Despontin, C.; Carusone, T.; Defrance, M.; Bhogaraju, S.; Martin-Garcia, E.; Capellan, R.; et al. USP7/Maged1-Mediated H2A Monoubiquitination in the Paraventricular Thalamus: An Epigenetic Mechanism Involved in Cocaine Use Disorder. Nat. Commun. 2023, 14, 8481. [Google Scholar] [CrossRef]
- Fischer, M. Census and Evaluation of P53 Target Genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, M.; Dong, J.; Li, C.; Xu, W.; Zhan, Y.; Wang, X.; Yu, M.; Ge, C.; Ge, Z.; et al. ABRO1 Suppresses Tumourigenesis and Regulates the DNA Damage Response by Stabilizing P53. Nat. Commun. 2014, 5, 5059. [Google Scholar] [CrossRef]
- Hu, M.; Gu, L.; Li, M.; Jeffrey, P.D.; Gu, W.; Shi, Y. Structural Basis of Competitive Recognition of P53 and MDM2 by HAUSP/USP7: Implications for the Regulation of the P53–MDM2 Pathway. PLoS Biol. 2006, 4, e27. [Google Scholar] [CrossRef]
- Tang, J.; Qu, L.; Pang, M.; Yang, X. Daxx Is Reciprocally Regulated by Mdm2 and Hausp. Biochem. Biophys. Res. Commun. 2010, 393, 542–545. [Google Scholar] [CrossRef]
- Giovinazzi, S.; Morozov, V.M.; Summers, M.K.; Reinhold, W.C.; Ishov, A.M. USP7 and Daxx Regulate Mitosis Progression and Taxane Sensitivity by Affecting Stability of Aurora-A Kinase. Cell Death Differ. 2013, 20, 721–731. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, H.; Ji, Y.; Zhou, Q.; Du, Z.; Tian, L.; Jiang, Y.; Yao, K.; Zhou, Z. Targeting P53–MDM2 Interaction by Small-Molecule Inhibitors: Learning from MDM2 Inhibitors in Clinical Trials. J. Hematol. Oncol. 2022, 15, 91. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Cheng, J.; Vasudevan, S.A.; Dou, J.; Zhang, H.; Patel, R.H.; Ma, I.T.; Rojas, Y.; Zhao, Y.; Yu, Y.; et al. USP7 Inhibitor P22077 Inhibits Neuroblastoma Growth via Inducing P53-Mediated Apoptosis. Cell Death Dis. 2013, 4, e867. [Google Scholar] [CrossRef]
- Zhao, G.-Y.; Lin, Z.-W.; Lu, C.-L.; Gu, J.; Yuan, Y.-F.; Xu, F.-K.; Liu, R.-H.; Ge, D.; Ding, J.-Y. USP7 Overexpression Predicts a Poor Prognosis in Lung Squamous Cell Carcinoma and Large Cell Carcinoma. Tumor Biol. 2015, 36, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ghosh, M.K. HAUSP, a Novel Deubiquitinase for Rb–MDM 2 the Critical Regulator. FEBS J. 2014, 281, 3061–3078. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, Y.; Xu, Q.; Jiang, Y. Stabilization of LSD1 by Deubiquitinating Enzyme USP7 Promotes Glioblastoma Cell Tumorigenesis and Metastasis through Suppression of the P53 Signaling Pathway. Oncol. Rep. 2016, 36, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN Loss in Cancer: It’s All about Diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, M.; Pandolfi, P.P. The Functions and Regulation of the PTEN Tumour Suppressor: New Modes and Prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Fedorova, O.; Parfenyev, S.; Daks, A.; Shuvalov, O.; Barlev, N.A. The Role of PTEN in Epithelial–Mesenchymal Transition. Cancers 2022, 14, 3786. [Google Scholar] [CrossRef]
- Morotti, A.; Panuzzo, C.; Crivellaro, S.; Pergolizzi, B.; Familiari, U.; Berger, A.H.; Saglio, G.; Pandolfi, P.P. BCR-ABL Disrupts PTEN Nuclear-Cytoplasmic Shuttling through Phosphorylation-Dependent Activation of HAUSP. Leukemia 2014, 28, 1326–1333. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, C.M.; Kim, J.H. USP7 Deubiquitinates and Stabilizes EZH2 in Prostate Cancer Cells. Genet. Mol. Biol. 2020, 43, e20190338. [Google Scholar] [CrossRef]
- Noguera, N.I.; Song, M.S.; Divona, M.; Catalano, G.; Calvo, K.L.; García, F.; Ottone, T.; Florenzano, F.; Faraoni, I.; Battistini, L.; et al. Nucleophosmin/B26 Regulates PTEN through Interaction with HAUSP in Acute Myeloid Leukemia. Leukemia 2013, 27, 1037–1043. [Google Scholar] [CrossRef]
- Park, H.-B.; Hwang, S.; Baek, K.-H. USP7 Regulates the ERK1/2 Signaling Pathway through Deubiquitinating Raf-1 in Lung Adenocarcinoma. Cell Death Dis. 2022, 13, 698. [Google Scholar] [CrossRef]
- Park, H.-B.; Kim, J.-W.; Baek, K.-H. Regulation of Wnt Signaling through Ubiquitination and Deubiquitination in Cancers. Int. J. Mol. Sci. 2020, 21, 3904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lu, B.; Zamponi, R.; Charlat, O.; Aversa, R.; Yang, Z.; Sigoillot, F.; Zhu, X.; Hu, T.; Reece-Hoyes, J.S.; et al. USP7 Inhibits Wnt/β-Catenin Signaling through Promoting Stabilization of Axin. Nat. Commun. 2019, 10, 4184. [Google Scholar] [CrossRef] [PubMed]
- Novellasdemunt, L.; Foglizzo, V.; Cuadrado, L.; Antas, P.; Kucharska, A.; Encheva, V.; Snijders, A.P.; Li, V.S.W. USP7 Is a Tumor-Specific WNT Activator for APC -Mutated Colorectal Cancer by Mediating β-Catenin Deubiquitination. Cell Rep. 2017, 21, 612–627. [Google Scholar] [CrossRef]
- Basu, B.; Karmakar, S.; Basu, M.; Ghosh, M.K. USP7 Imparts Partial EMT State in Colorectal Cancer by Stabilizing the RNA Helicase DDX3X and Augmenting Wnt/β-Catenin Signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2023, 1870, 119446. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Franqui-Machin, R.; Hao, M.; Bai, H.; Gu, Z.; Zhan, X.; Habelhah, H.; Jethava, Y.; Qiu, L.; Frech, I.; Tricot, G.; et al. Destabilizing NEK2 Overcomes Resistance to Proteasome Inhibition in Multiple Myeloma. J. Clin. Investig. 2018, 128, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; He, Y.; Meng, B.; Chen, S.; Zhang, J.; Wu, X.; Zhu, Y.; Shen, Y.; Feng, X.; Guan, Y.; et al. NEK2 Induces Autophagy-mediated Bortezomib Resistance by Stabilizing Beclin-1 in Multiple Myeloma. Mol. Oncol. 2020, 14, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.; Shi, M.; Sun, Y.; Chen, C.; Niu, M.; Zhang, Q.; Zeng, L.; Yao, R.; Li, H.; et al. Blockade of Deubiquitinase USP7 Overcomes Bortezomib Resistance by Suppressing NF-κB Signaling Pathway in Multiple Myeloma. J. Leukoc. Biol. 2018, 104, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Fisher, T.L.; Thomas, J.; White, M.F. Regulation of Insulin/Insulin-like Growth Factor-1 Signaling by Proteasome-Mediated Degradation of Insulin Receptor Substrate-2. J. Biol. Chem. 2001, 276, 40362–40367. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Jones, J.I. Insulin-like Growth Factors and Their Binding Proteins: Biological Actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Yoshihara, H.; Fukushima, T.; Hakuno, F.; Saeki, Y.; Tanaka, K.; Ito, A.; Yoshida, M.; Iemura, S.; Natsume, T.; Asano, T.; et al. Insulin/Insulin-like Growth Factor (IGF) Stimulation Abrogates an Association between a Deubiquitinating Enzyme USP7 and Insulin Receptor Substrates (IRSs) Followed by Proteasomal Degradation of IRSs. Biochem. Biophys. Res. Commun. 2012, 423, 122–127. [Google Scholar] [CrossRef]
- Chen, S.-T.; Okada, M.; Nakato, R.; Izumi, K.; Bando, M.; Shirahige, K. The Deubiquitinating Enzyme USP7 Regulates Androgen Receptor Activity by Modulating Its Binding to Chromatin. J. Biol. Chem. 2015, 290, 21713–21723. [Google Scholar] [CrossRef]
- Kim, S.-H.; Baek, K.-H. Regulation of Cancer Metabolism by Deubiquitinating Enzymes: The Warburg Effect. Int. J. Mol. Sci. 2021, 22, 6173. [Google Scholar] [CrossRef]
- Hall, J.A.; Tabata, M.; Rodgers, J.T.; Puigserver, P. USP7 Attenuates Hepatic Gluconeogenesis Through Modulation of FoxO1 Gene Promoter Occupancy. Mol. Endocrinol. 2014, 28, 912–924. [Google Scholar] [CrossRef]
- Kiran, S.; Anwar, T.; Kiran, M.; Ramakrishna, G. Sirtuin 7 in Cell Proliferation, Stress and Disease: Rise of the Seventh Sirtuin! Cell. Signal. 2015, 27, 673–682. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhu, K.S.; Wang, H.; Zhu, W.-G. Advances in Cellular Characterization of the Sirtuin Isoform, SIRT7. Front. Endocrinol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xiong, J.; Zhan, J.; Yuan, F.; Tang, M.; Zhang, C.; Cao, Z.; Chen, Y.; Lu, X.; Li, Y.; et al. Ubiquitin-Specific Peptidase 7 (USP7)-Mediated Deubiquitination of the Histone Deacetylase SIRT7 Regulates Gluconeogenesis. J. Biol. Chem. 2017, 292, 13296–13311. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, C.; Lee, H.; Shin, D.; Chern, Y.; Pereira De Carvalho, B.; Chan, C. MIG-6 Is Essential for Promoting Glucose Metabolic Reprogramming and Tumor Growth in Triple-negative Breast Cancer. EMBO Rep. 2021, 22, e50781. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lin, J.; Liu, Y.-E.; Chen, Y.-C.; Liu, S.-T.; Hsu, K.-W.; Chen, D.-R.; Wu, H.-T. USP7 Induces Chemoresistance in Triple-Negative Breast Cancer via Deubiquitination and Stabilization of ABCB1. Cells 2022, 11, 3294. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Liu, Z.; Deng, Y.; Sun, Z.; Chen, B.; Wu, L.; Wang, X.; Miao, L.; Zeng, L.; et al. USP7 Promotes Temozolomide Resistance by Stabilizing MGMT in Glioblastoma. Cell Death Dis. 2025, 16, 631. [Google Scholar] [CrossRef]
- Sun, X.; Ding, Y.; Zhan, M.; Li, Y.; Gao, D.; Wang, G.; Gao, Y.; Li, Y.; Wu, S.; Lu, L.; et al. Usp7 Regulates Hippo Pathway through Deubiquitinating the Transcriptional Coactivator Yorkie. Nat. Commun. 2019, 10, 411. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kuboki, S.; Furukawa, K.; Takayashiki, T.; Takano, S.; Yoshizumi, A.; Ohtsuka, M. TRIM27-USP7 Complex Promotes Tumour Progression via STAT3 Activation in Human Hepatocellular Carcinoma. Liver Int. 2023, 43, 194–207. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Zee, Y.-K.; O’Connor, J.P.B.; Parker, G.J.M.; Jackson, A.; Clamp, A.R.; Taylor, M.B.; Clarke, N.W.; Jayson, G.C. Imaging Angiogenesis of Genitourinary Tumors. Nat. Rev. Urol. 2010, 7, 69–82. [Google Scholar] [CrossRef]
- Lu, X.; Kang, Y. Hypoxia and Hypoxia-Inducible Factors: Master Regulators of Metastasis. Clin. Cancer Res. 2010, 16, 5928–5935. [Google Scholar] [CrossRef]
- Episkopou, H.; Diman, A.; Claude, E.; Viceconte, N.; Decottignies, A. TSPYL5 Depletion Induces Specific Death of ALT Cells through USP7-Dependent Proteasomal Degradation of POT1. Mol. Cell 2019, 75, 469–482.e6. [Google Scholar] [CrossRef] [PubMed]
- Draskovic, I.; Arnoult, N.; Steiner, V.; Bacchetti, S.; Lomonte, P.; Londoño-Vallejo, A. Probing PML Body Function in ALT Cells Reveals Spatiotemporal Requirements for Telomere Recombination. Proc. Natl. Acad. Sci. USA 2009, 106, 15726–15731. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, M.; Luo, J.; Gu, W. Direct Interactions between HIF-1α and Mdm2 Modulate P53 Function. J. Biol. Chem. 2003, 278, 13595–13598. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, H.; Luo, D.; Gan, L.; Mo, S.; Dai, W.; Liang, L.; Yang, Y.; Xu, M.; Li, J.; et al. Lnc-RP11-536 K7.3/SOX2/HIF-1α Signaling Axis Regulates Oxaliplatin Resistance in Patient-Derived Colorectal Cancer Organoids. J. Exp. Clin. Cancer Res. 2021, 40, 348. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Li, C.-L.; Chen, W.-J.; Liu, J.; Wu, H.-T. Diverse Roles of FOXO Family Members in Gastric Cancer. World J. Gastrointest. Oncol. 2021, 13, 1367–1382. [Google Scholar] [CrossRef]
- Jiramongkol, Y.; Lam, E.W.-F. FOXO Transcription Factor Family in Cancer and Metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Dansen, T.B.; Burgering, B.M.T. Unravelling the Tumor-Suppressive Functions of FOXO Proteins. Trends Cell Biol. 2008, 18, 421–429. [Google Scholar] [CrossRef]
- Kim, C.G.; Lee, H.; Gupta, N.; Ramachandran, S.; Kaushik, I.; Srivastava, S.; Kim, S.-H.; Srivastava, S.K. Role of Forkhead Box Class O Proteins in Cancer Progression and Metastasis. Semin. Cancer Biol. 2018, 50, 142–151. [Google Scholar] [CrossRef]
- Brenkman, A.B.; De Keizer, P.L.J.; Van Den Broek, N.J.F.; Jochemsen, A.G.; Burgering, B.M. Mdm2 Induces Mono-Ubiquitination of FOXO4. PLoS ONE 2008, 3, e2819. [Google Scholar] [CrossRef]
- Gagarina, V.; Bojagora, A.; Lacdao, I.K.; Luthra, N.; Pfoh, R.; Mohseni, S.; Chaharlangi, D.; Tan, N.; Saridakis, V. Structural Basis of the Interaction Between Ubiquitin Specific Protease 7 and Enhancer of Zeste Homolog 2. J. Mol. Biol. 2020, 432, 897–912. [Google Scholar] [CrossRef]
- Duan, D.; Shang, M.; Han, Y.; Liu, J.; Liu, J.; Kong, S.H.; Hou, J.; Huang, B.; Lu, J.; Zhang, Y. EZH2–CCF–cGAS Axis Promotes Breast Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 1788. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the Cytoskeleton, and Cancer Cell Invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Maurice, M.M. The Various Roles of Ubiquitin in Wnt Pathway Regulation. Cell Cycle 2010, 9, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Kong, D.; Sarkar, H.F. The Role of Notch Signaling Pathway in Epithelial-Mesenchymal Transition (EMT) During Development and Tumor Aggressiveness. Curr. Drug Targets 2010, 11, 745–751. [Google Scholar] [CrossRef]
- Jin, Q.; Martinez, C.A.; Arcipowski, K.M.; Zhu, Y.; Gutierrez-Diaz, B.T.; Wang, K.K.; Johnson, M.R.; Volk, A.G.; Wang, F.; Wu, J.; et al. USP7 Cooperates with NOTCH1 to Drive the Oncogenic Transcriptional Program in T-Cell Leukemia. Clin. Cancer Res. 2019, 25, 222–239. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.; Li, L.; Lin, L.; Ji, J.; Lin, C.; Liu, L.; Zhang, N.; Duan, D.; Li, Z.; et al. Arginine Methylation-dependent LSD1 Stability Promotes Invasion and Metastasis of Breast Cancer. EMBO Rep. 2020, 21, e48597. [Google Scholar] [CrossRef]
- Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G.; et al. USP7 Targeting Modulates Anti-Tumor Immune Response by Reprogramming Tumor-Associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332–9347. [Google Scholar] [CrossRef]
- Powell, D.J.; Felipe-Silva, A.; Merino, M.J.; Ahmadzadeh, M.; Allen, T.; Levy, C.; White, D.E.; Mavroukakis, S.; Kreitman, R.J.; Rosenberg, S.A.; et al. Administration of a CD25-Directed Immunotoxin, LMB-2, to Patients with Metastatic Melanoma Induces a Selective Partial Reduction in Regulatory T Cells In Vivo. J. Immunol. 2007, 179, 4919–4928. [Google Scholar] [CrossRef]
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel Cancer Immunotherapy Agents with Survival Benefit: Recent Successes and next Steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Elkord, E. FoxP3+ T Regulatory Cells in Cancer: Prognostic Biomarkers and Therapeutic Targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, C.; Pan, F. Post-Translational Regulations of Foxp3 in Treg Cells and Their Therapeutic Applications. Front. Immunol. 2021, 12, 626172. [Google Scholar] [CrossRef] [PubMed]
- De Ceuninck, L.; Wauman, J.; Masschaele, D.; Peelman, F.; Tavernier, J. Reciprocal Cross-Regulation between RNF41 and USP8 Controls Cytokine Receptor Sorting and Processing. J. Cell Sci. 2013, 126, jcs.131250. [Google Scholar] [CrossRef]
- Colombo, M.; Vallese, S.; Peretto, I.; Jacq, X.; Rain, J.; Colland, F.; Guedat, P. Synthesis and Biological Evaluation of 9-Oxo-9 H-indeno[1,2-b]Pyrazine-2,3-dicarbonitrile Analogues as Potential Inhibitors of Deubiquitinating Enzymes. ChemMedChem 2010, 5, 552–558. [Google Scholar] [CrossRef]
- Kim, J.-E.; Shin, J.-S.; Moon, J.-H.; Hong, S.-W.; Jung, D.-J.; Kim, J.H.; Hwang, I.-Y.; Shin, Y.J.; Gong, E.-Y.; Lee, D.H.; et al. Foxp3 Is a Key Downstream Regulator of P53-Mediated Cellular Senescence. Oncogene 2017, 36, 219–230. [Google Scholar] [CrossRef]
- Khosravi, M.; Bidmeshkipour, A.; Cohen, J.L.; Moravej, A.; Hojjat-Assari, S.; Naserian, S.; Karimi, M.H. Induction of CD4+CD25+FOXP3+ Regulatory T Cells by Mesenchymal Stem Cells Is Associated with Modulation of Ubiquitination Factors and TSDR Demethylation. Stem Cell Res. Ther. 2018, 9, 273. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, X.; Fu, J.; Wang, H. Progress and Challenges in Precise Treatment of Tumors with PD-1/PD-L1 Blockade. Front. Immunol. 2020, 11, 339. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, W.; Li, O.; Qi, F.; Wang, J.; You, Y.; He, P.; Suo, Z.; Zheng, Y.; Liu, H.-M. Abrogation of USP7 Is an Alternative Strategy to Downregulate PD-L1 and Sensitize Gastric Cancer Cells to T Cells Killing. Acta Pharm. Sin. B 2021, 11, 694–707. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, Z.; Huang, Y.; Chen, Y.; Huang, M.; Liu, H.; Ye, Q.A.; Zhao, J.; Jia, B. Ferroptosis: The Silver Lining of Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 765859. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, L.; Wang, H.; Wu, P.; Gu, W.; Chen, Y.; Hao, H.; Tang, K.; Yi, P.; Liu, M.; et al. Pleiotropic Regulation of Macrophage Polarization and Tumorigenesis by Formyl Peptide Receptor-2. Oncogene 2011, 30, 3887–3899. [Google Scholar] [CrossRef] [PubMed]
- Henrique, T.; Zanon, C.D.F.; Girol, A.P.; Stefanini, A.C.B.; Contessoto, N.S.D.A.; Da Silveira, N.J.F.; Bezerra, D.P.; Silveira, E.R.; Barbosa-Filho, J.M.; Cornélio, M.L.; et al. Biological and Physical Approaches on the Role of Piplartine (Piperlongumine) in Cancer. Sci. Rep. 2020, 10, 22283. [Google Scholar] [CrossRef]
- Park, J.-J.; Lim, K.-H.; Baek, K.-H. Annexin-1 Regulated by HAUSP Is Essential for UV-Induced Damage Response. Cell Death Dis. 2015, 6, e1654. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic Regulation of Gene Expression: How the Genome Integrates Intrinsic and Environmental Signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Higa, M.; Zhang, X.; Tanaka, K.; Saijo, M. Stabilization of Ultraviolet (UV)-Stimulated Scaffold Protein A by Interaction with Ubiquitin-Specific Peptidase 7 Is Essential for Transcription-Coupled Nucleotide Excision Repair. J. Biol. Chem. 2016, 291, 13771–13779. [Google Scholar] [CrossRef]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide Excision Repair: From Molecular Defects to Neurological Abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef]
- Zhu, Q.; Ding, N.; Wei, S.; Li, P.; Wani, G.; He, J.; Wani, A.A. USP7-Mediated Deubiquitination Differentially Regulates CSB but Not UVSSA upon UV Radiation-Induced DNA Damage. Cell Cycle 2020, 19, 124–141. [Google Scholar] [CrossRef]
- Jeggo, P.A.; Pearl, L.H.; Carr, A.M. DNA Repair, Genome Stability and Cancer: A Historical Perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef]
- Löbrich, M.; Shibata, A.; Beucher, A.; Fisher, A.; Ensminger, M.; Goodarzi, A.A.; Barton, O.; Jeggo, P.A. γH2AX Foci Analysis for Monitoring DNA Double-Strand Break Repair: Strengths, Limitations and Optimization. Cell Cycle 2010, 9, 662–669. [Google Scholar] [CrossRef]
- Lane, D.P. P53, Guardian of the Genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, Q.; Wani, G.; Sharma, N.; Han, C.; Qian, J.; Pentz, K.; Wang, Q.; Wani, A.A. Ubiquitin-Specific Protease 7 Regulates Nucleotide Excision Repair through Deubiquitinating XPC Protein and Preventing XPC Protein from Undergoing Ultraviolet Light-Induced and VCP/P97 Protein-Regulated Proteolysis. J. Biol. Chem. 2014, 289, 27278–27289. [Google Scholar] [CrossRef] [PubMed]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 Promotes the Rapid Degradation of P53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sharma, N.; He, J.; Wani, G.; Wani, A.A. USP7 Deubiquitinase Promotes Ubiquitin-Dependent DNA Damage Signaling by Stabilizing RNF168*. Cell Cycle 2015, 14, 1413–1425. [Google Scholar] [CrossRef]
- Poulsen, M.; Lukas, C.; Lukas, J.; Bekker-Jensen, S.; Mailand, N. Human RNF169 Is a Negative Regulator of the Ubiquitin-Dependent Response to DNA Double-Strand Breaks. J. Cell Biol. 2012, 197, 189–199. [Google Scholar] [CrossRef]
- Bartocci, C.; Denchi, E.L. Put a RING on It: Regulation and Inhibition of RNF8 and RNF168 RING Finger E3 Ligases at DNA Damage Sites. Front. Genet. 2013, 4, 128. [Google Scholar] [CrossRef]
- Noubissi, F.K.; McBride, A.A.; Leppert, H.G.; Millet, L.J.; Wang, X.; Davern, S.M. Detection and Quantification of γ-H2AX Using a Dissociation Enhanced Lanthanide Fluorescence Immunoassay. Sci. Rep. 2021, 11, 8945. [Google Scholar] [CrossRef]
- Su, D.; Ma, S.; Shan, L.; Wang, Y.; Wang, Y.; Cao, C.; Liu, B.; Yang, C.; Wang, L.; Tian, S.; et al. Ubiquitin-Specific Protease 7 Sustains DNA Damage Response and Promotes Cervical Carcinogenesis. J. Clin. Investig. 2018, 128, 4280–4296. [Google Scholar] [CrossRef]
- Mailand, N.; Falck, J.; Lukas, C.; Syljuåsen, R.G.; Welcker, M.; Bartek, J.; Lukas, J. Rapid Destruction of Human Cdc25A in Response to DNA Damage. Science 2000, 288, 1425–1429. [Google Scholar] [CrossRef]
- Smith, J.; Mun Tho, L.; Xu, N.; Gillespie, D.A. The ATM–Chk2 and ATR–Chk1 Pathways in DNA Damage Signaling and Cancer. In Advances in Cancer Research; Academic Press: San Diego, CA, USA, 2010; Volume 108, pp. 73–112. ISBN 978-0-12-380888-2. [Google Scholar]
- Alonso-de Vega, I.; Martín, Y.; Smits, V.A. USP7 Controls Chk1 Protein Stability by Direct Deubiquitination. Cell Cycle 2014, 13, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Luise, C.; Visconti, R.; Staibano, S.; Merolla, F.; Ilardi, G.; Guggino, G.; Paladino, S.; Sarnataro, D.; Franco, R.; et al. New Therapeutic Perspectives in CCDC 6 Deficient Lung Cancer Cells. Int. J. Cancer 2015, 136, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, Y.; Gao, Y.; Yuan, B.; Qi, X.; Fu, Y.; Zhu, Q.; Cao, T.; Zhang, S.; Yin, L.; et al. USP7 Is a Novel Deubiquitinase Sustaining PLK1 Protein Stability and Regulating Chromosome Alignment in Mitosis. J. Exp. Clin. Cancer Res. 2019, 38, 468. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, H.; Yu, C.; Kang, Y.; Yang, X. Targeting Ubiquitin-Specific Protease 7 (USP7) in Cancer: A New Insight to Overcome Drug Resistance. Front. Pharmacol. 2021, 12, 648491. [Google Scholar] [CrossRef]
- Bushman, J.W.; Donovan, K.A.; Schauer, N.J.; Liu, X.; Hu, W.; Varca, A.C.; Buhrlage, S.J.; Fischer, E.S. Proteomics-Based Identification of DUB Substrates Using Selective Inhibitors. Cell Chem. Biol. 2021, 28, 78–87.e3. [Google Scholar] [CrossRef]
- Altun, M.; Kramer, H.B.; Willems, L.I.; McDermott, J.L.; Leach, C.A.; Goldenberg, S.J.; Kumar, K.G.S.; Konietzny, R.; Fischer, R.; Kogan, E.; et al. Activity-Based Chemical Proteomics Accelerates Inhibitor Development for Deubiquitylating Enzymes. Chem. Biol. 2011, 18, 1401–1412. [Google Scholar] [CrossRef]
- Kategaya, L.; Di Lello, P.; Rougé, L.; Pastor, R.; Clark, K.R.; Drummond, J.; Kleinheinz, T.; Lin, E.; Upton, J.-P.; Prakash, S.; et al. USP7 Small-Molecule Inhibitors Interfere with Ubiquitin Binding. Nature 2017, 550, 534–538. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyazaki, M.; Kodrasov, M.P.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J.; Yokosawa, H.; Nicholson, B.; Tsukamoto, S. Spongiacidin C, a Pyrrole Alkaloid from the Marine Sponge Stylissa Massa, Functions as a USP7 Inhibitor. Bioorganic Med. Chem. Lett. 2013, 23, 3884–3886. [Google Scholar] [CrossRef]
- Tanokashira, N.; Kukita, S.; Kato, H.; Nehira, T.; Angkouw, E.D.; Mangindaan, R.E.P.; De Voogd, N.J.; Tsukamoto, S. Petroquinones: Trimeric and Dimeric Xestoquinone Derivatives Isolated from the Marine Sponge Petrosia alfiani. Tetrahedron 2016, 72, 5530–5540. [Google Scholar] [CrossRef]
- Afifi, A.H.; Kagiyama, I.; El-Desoky, A.H.; Kato, H.; Mangindaan, R.E.P.; De Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Sulawesins A–C, Furanosesterterpene Tetronic Acids That Inhibit USP7, from a Psammocinia sp. Marine Sponge. J. Nat. Prod. 2017, 80, 2045–2050. [Google Scholar] [CrossRef]
- Weinstock, J.; Wu, J.; Cao, P.; Kingsbury, W.D.; McDermott, J.L.; Kodrasov, M.P.; McKelvey, D.M.; Suresh Kumar, K.G.; Goldenberg, S.J.; Mattern, M.R.; et al. Selective Dual Inhibitors of the Cancer-Related Deubiquitylating Proteases USP7 and USP47. ACS Med. Chem. Lett. 2012, 3, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Leonhardt, H.; Spada, F. Usp7 and Uhrf1 Control Ubiquitination and Stability of the Maintenance DNA Methyltransferase Dnmt1. J. Cell. Biochem. 2011, 112, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, J.; Wang, J.; Xu, C.; Chen, C.; Gu, W.; Sun, H.; Wen, X. Synthesis and Biological Evaluation of Thiazole Derivatives as Novel USP7 Inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Pozhidaeva, A.; Valles, G.; Wang, F.; Wu, J.; Sterner, D.E.; Nguyen, P.; Weinstock, J.; Kumar, K.G.S.; Kanyo, J.; Wright, D.; et al. USP7-Specific Inhibitors Target and Modify the Enzyme’s Active Site via Distinct Chemical Mechanisms. Cell Chem. Biol. 2017, 24, 1501–1512.e5. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Wu, J.; Sokirniy, I.; Nguyen, P.; Bregnard, T.; Weinstock, J.; Mattern, M.; Bezsonova, I.; Hancock, W.W.; et al. Active Site-Targeted Covalent Irreversible Inhibitors of USP7 Impair the Functions of Foxp3+ T-Regulatory Cells by Promoting Ubiquitination of Tip60. PLoS ONE 2017, 12, e0189744. [Google Scholar] [CrossRef]
- Turnbull, A.P.; Ioannidis, S.; Krajewski, W.W.; Pinto-Fernandez, A.; Heride, C.; Martin, A.C.L.; Tonkin, L.M.; Townsend, E.C.; Buker, S.M.; Lancia, D.R.; et al. Molecular Basis of USP7 Inhibition by Selective Small-Molecule Inhibitors. Nature 2017, 550, 481–486. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Chen, H.; Zhou, X.; Zhou, J.; Zhou, S.; Yuan, H.; Xu, Q.-L.; Liu, J.; Cheng, K.; et al. N-Benzylpiperidinol Derivatives as Novel USP7 Inhibitors: Structure–Activity Relationships and X-Ray Crystallographic Studies. Eur. J. Med. Chem. 2020, 199, 112279. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Zhuang, Z.; Miao, Y.-L.; Song, S.-S.; Bao, X.-B.; Yang, C.-H.; He, J.-X. Identification of YCH2823 as a Novel USP7 Inhibitor for Cancer Therapy. Biochem. Pharmacol. 2024, 222, 116071. [Google Scholar] [CrossRef]
- Lamberto, I.; Liu, X.; Seo, H.-S.; Schauer, N.J.; Iacob, R.E.; Hu, W.; Das, D.; Mikhailova, T.; Weisberg, E.L.; Engen, J.R.; et al. Structure-Guided Development of a Potent and Selective Non-Covalent Active-Site Inhibitor of USP7. Cell Chem. Biol. 2017, 24, 1490–1500.e11. [Google Scholar] [CrossRef]
- Gavory, G.; O’Dowd, C.R.; Helm, M.D.; Flasz, J.; Arkoudis, E.; Dossang, A.; Hughes, C.; Cassidy, E.; McClelland, K.; Odrzywol, E.; et al. Discovery and Characterization of Highly Potent and Selective Allosteric USP7 Inhibitors. Nat. Chem. Biol. 2018, 14, 118–125. [Google Scholar] [CrossRef]
- Ohol, Y.M.; Sun, M.T.; Cutler, G.; Leger, P.R.; Hu, D.X.; Biannic, B.; Rana, P.; Cho, C.; Jacobson, S.; Wong, S.T.; et al. Novel, Selective Inhibitors of USP7 Uncover Multiple Mechanisms of Antitumor Activity In Vitro and In Vivo. Mol. Cancer Ther. 2020, 19, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Colland, F.; Formstecher, E.; Jacq, X.; Reverdy, C.; Planquette, C.; Conrath, S.; Trouplin, V.; Bianchi, J.; Aushev, V.N.; Camonis, J.; et al. Small-Molecule Inhibitor of USP7/HAUSP Ubiquitin Protease Stabilizes and Activates P53 in Cells. Mol. Cancer Ther. 2009, 8, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Reverdy, C.; Conrath, S.; Lopez, R.; Planquette, C.; Atmanene, C.; Collura, V.; Harpon, J.; Battaglia, V.; Vivat, V.; Sippl, W.; et al. Discovery of Specific Inhibitors of Human USP7/HAUSP Deubiquitinating Enzyme. Chem. Biol. 2012, 19, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; Murray, J.M. Structurally-Defined Deubiquitinase Inhibitors Provide Opportunities to Investigate Disease Mechanisms. Drug Discov. Today: Technol. 2019, 31, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, M.; Yang, Y.; Du, C.; Zhou, H.; Liu, C.; Chen, Y.; Fan, L.; Ma, H.; Gong, Y.; et al. An Overview of PROTACs: A Promising Drug Discovery Paradigm. Mol. Biomed. 2022, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Chamberlain, P.P.; Hamann, L.G. Development of Targeted Protein Degradation Therapeutics. Nat. Chem. Biol. 2019, 15, 937–944. [Google Scholar] [CrossRef]
- Murgai, A.; Sosič, I.; Gobec, M.; Lemnitzer, P.; Proj, M.; Wittenburg, S.; Voget, R.; Gütschow, M.; Krönke, J.; Steinebach, C. Targeting the Deubiquitinase USP7 for Degradation with PROTACs. Chem. Commun. 2022, 58, 8858–8861. [Google Scholar] [CrossRef]
- Pei, Y.; Fu, J.; Shi, Y.; Zhang, M.; Luo, G.; Luo, X.; Song, N.; Mi, T.; Yang, Y.; Li, J.; et al. Discovery of a Potent and Selective Degrader for USP7. Angew. Chem. Int. Ed. 2022, 61, e202204395. [Google Scholar] [CrossRef]
- Inaba, K.; Sato, H.; Tsuda, M.; Kobayashi, J. Spongiacidins A−D, New Bromopyrrole Alkaloids from Hymeniacidon Sponge. J. Nat. Prod. 1998, 61, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Kato, H. Structural Analysis of Previously Unknown Natural Products Using Computational Methods. J. Nat. Med. 2022, 76, 719–724. [Google Scholar] [CrossRef] [PubMed]
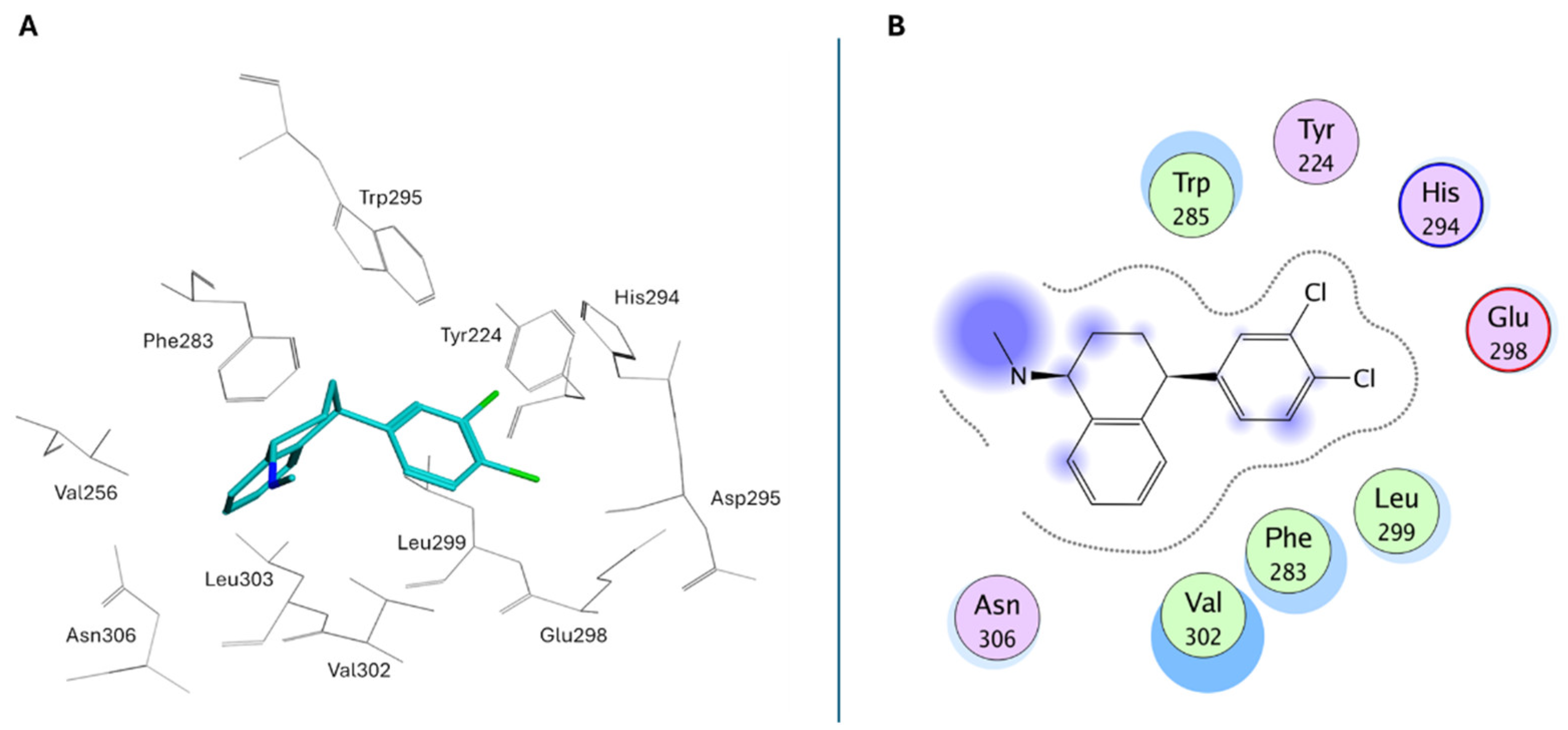
- Shi, L.; Xu, Z.; Chen, X.; Meng, Q.; Zhou, H.; Xiong, B.; Zhang, N. Sertraline and Astemizole Enhance the Deubiquitinase Activity of USP7 by Binding to Its Switching Loop Region. J. Med. Chem. 2025, 68, 5874–5890. [Google Scholar] [CrossRef] [PubMed]
- Jaen Maisonet, I.; Sharafi, M.; Korchak, E.J.; Salazar-Chaparro, A.; Bratt, A.; Parikh, T.; Varca, A.C.; Shah, B.; Darnowski, M.; Chung, M.; et al. Small-Molecule Allosteric Activator of Ubiquitin-Specific Protease 7 (USP7). bioRxiv 2025. [Google Scholar] [CrossRef]
- Korchak, E.J.; Sharafi, M.; Jaen Maisonet, I.; Salazar-Chaparro, A.; Semenova, I.V.; Khan, H.; O’Neil, A.L.; Caro, P.; Schaaf, C.P.; Buhrlage, S.J.; et al. Functional Spectrum of USP7 Pathogenic Variants in Hao–Fountain Syndrome: Insights into the Enzyme’s Activity, Stability, and Allosteric Modulation. Proc. Natl. Acad. Sci. USA 2025, 122, e2510252122. [Google Scholar] [CrossRef]
- Ojedele, O.A.; Umar, H.I.; Baammi, S.; Metouekel, A.; Mengistie, A.A.; Bin Jardan, Y.A.; Shazly, G.A.; Victor, O. Cheminformatics-Aided Discovery of Potential Allosteric Site Modulators of Ubiquitin-Specific Protease 7. Sci. Rep. 2024, 14, 24995. [Google Scholar] [CrossRef]
- Javaid, S.; Zadi, S.; Awais, M.; Wahab, A.; Zafar, H.; Maslennikov, I.; Choudhary, M.I. Identification of New Leads against Ubiquitin Specific Protease-7 (USP7): A Step towards the Potential Treatment of Cancers. RSC Adv. 2024, 14, 33080–33093. [Google Scholar] [CrossRef]
- Song, Y.; Ren, X.; Xiong, J.; Wang, W.; Zhao, Q.; Chang, J.; Yu, B. Ubiquitin-Specific Protease 7 (USP7) as a Promising Therapeutic Target for Drug Discovery: From Mechanisms to Therapies. J. Med. Chem. 2025, 68, 7914–7931. [Google Scholar] [CrossRef]
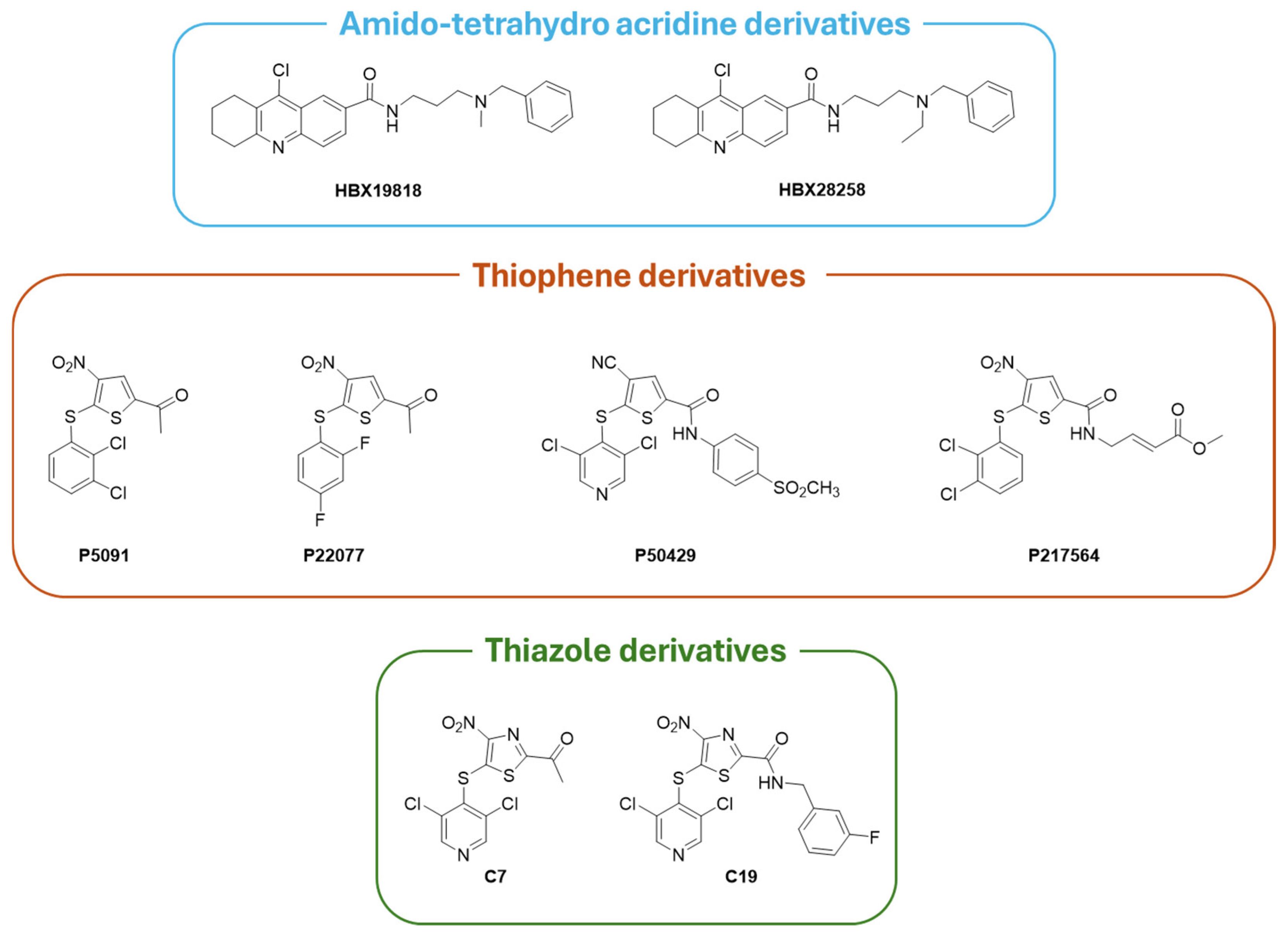


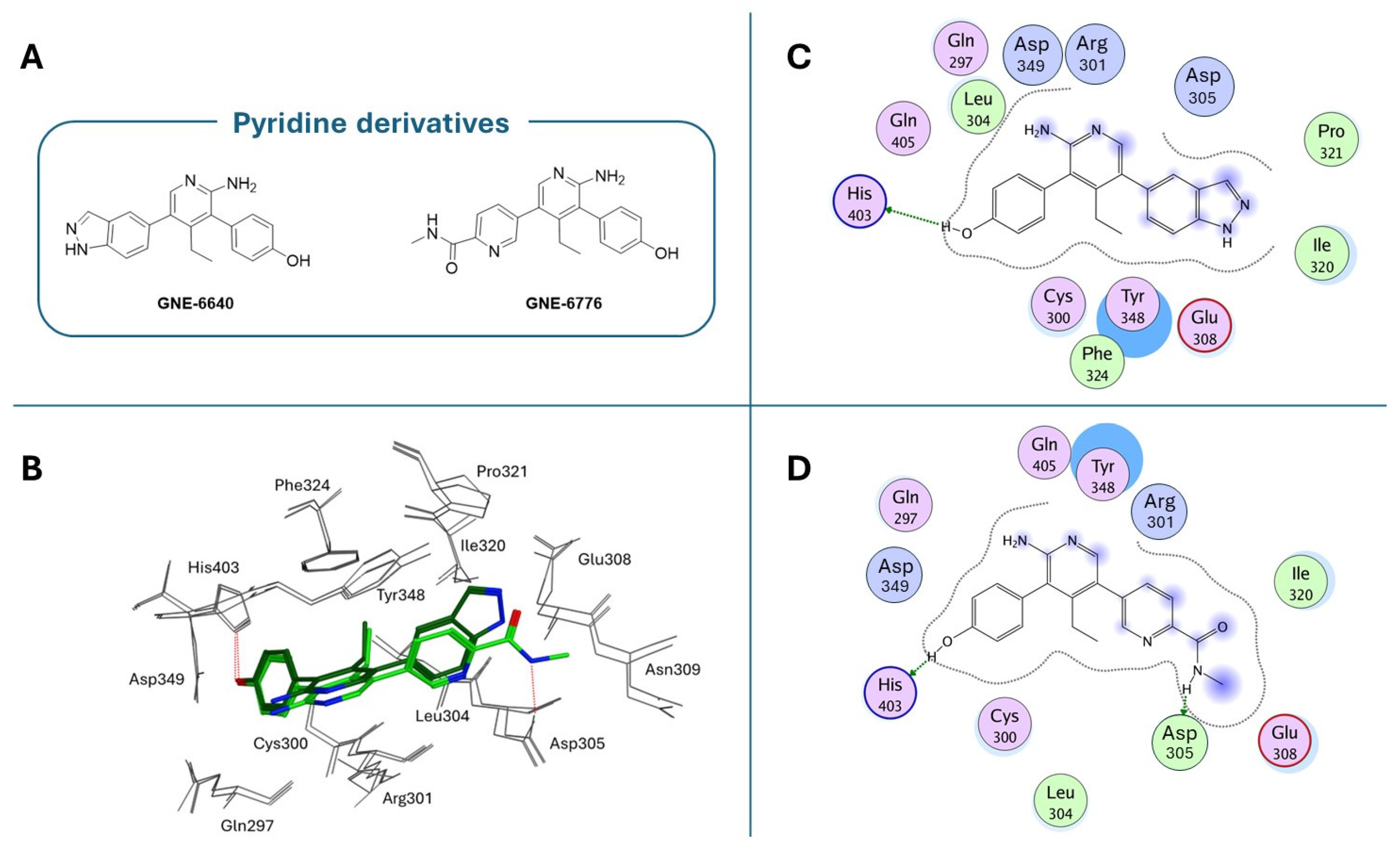
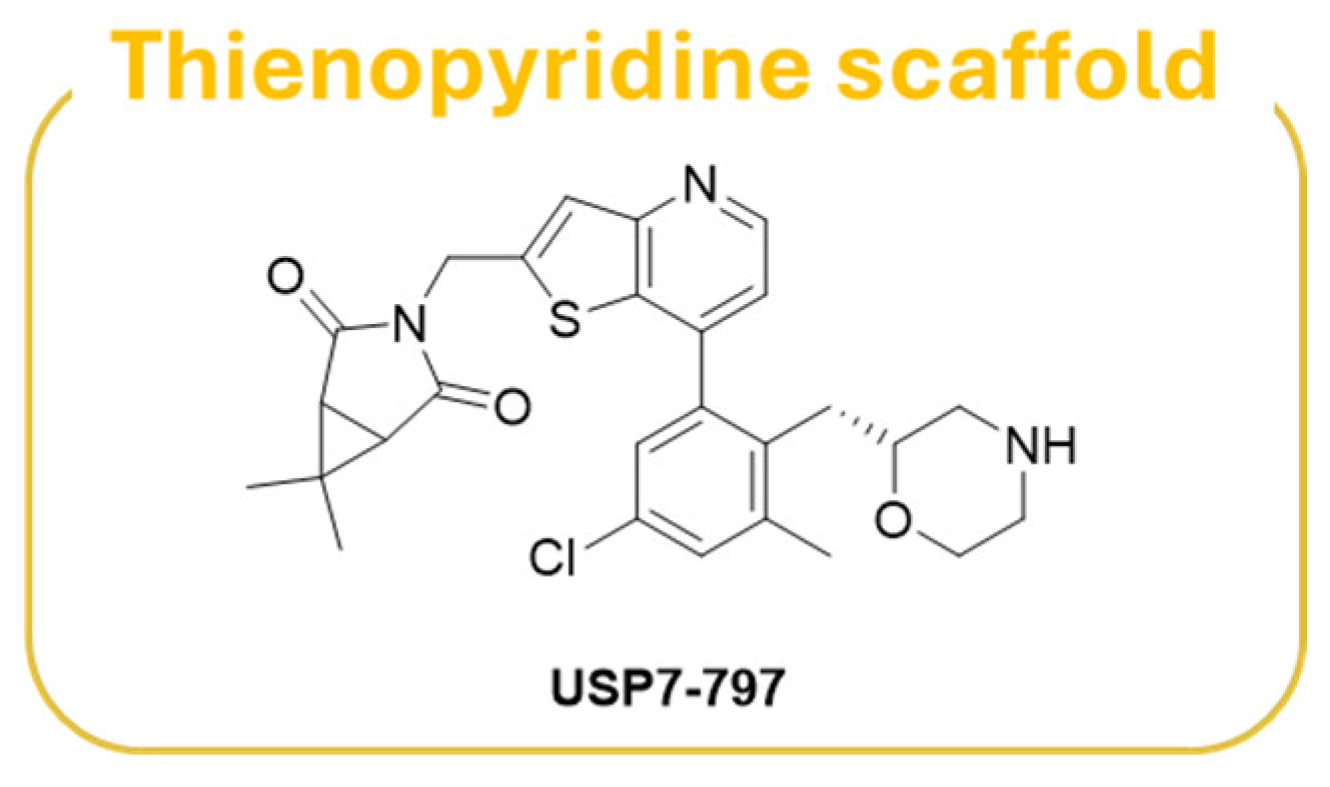
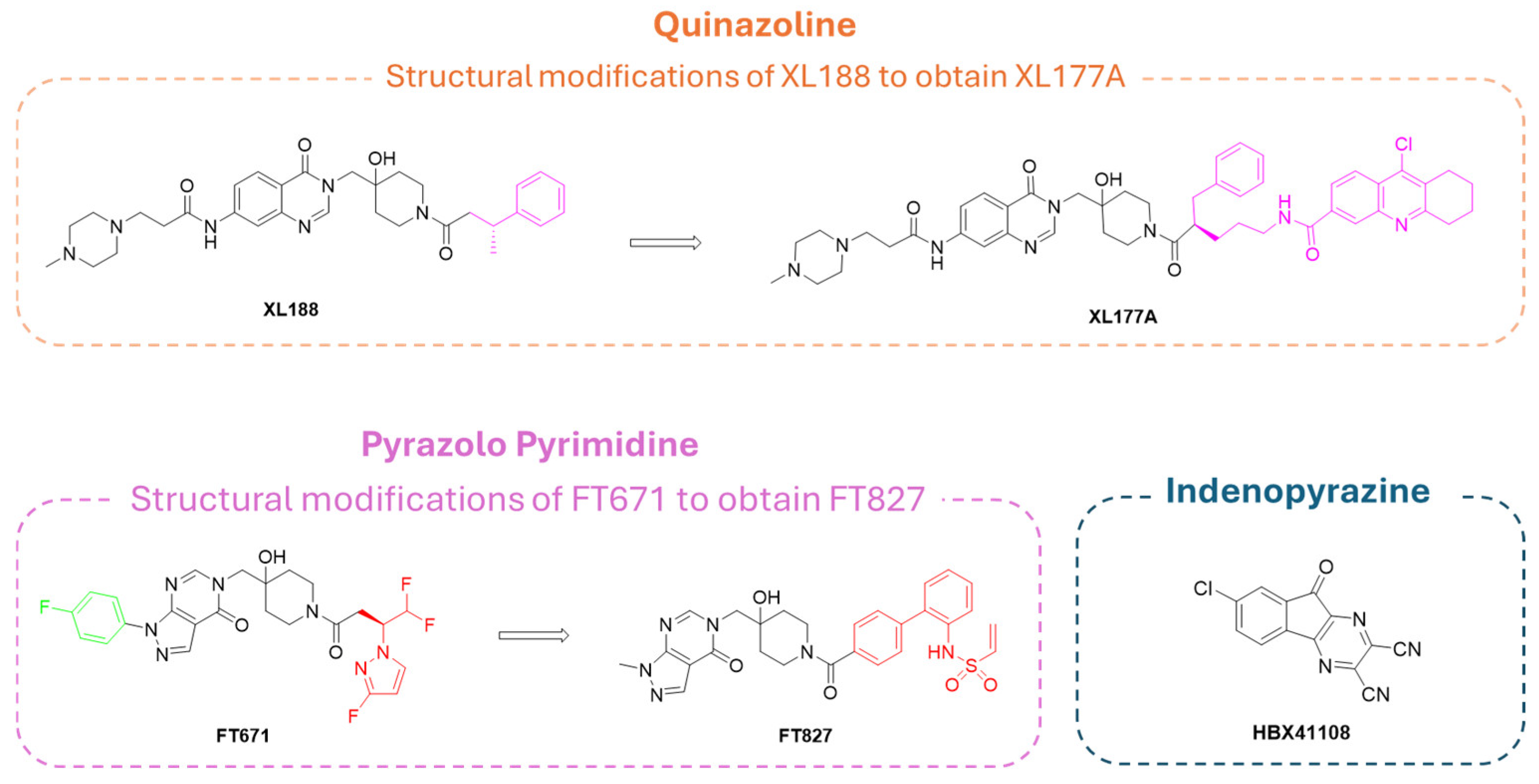

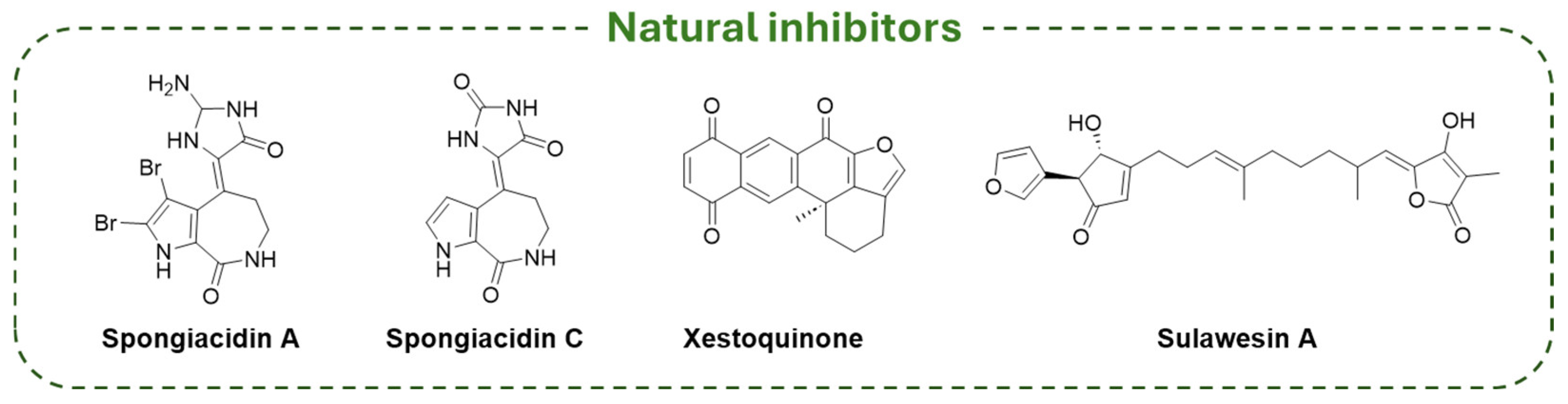
| Compound | Molecular Formula | Molecular Weigh | IC50 (USP7) | Preclinical Studies | |
|---|---|---|---|---|---|
| Covalent Inhibitors | HBX19818 | C25H28ClN3O | 421.96 | 28.1 µM | Immunology, DUB selectivity, mice tumor models, TAM targeting [188]. |
| HBX28258 | C26H30ClN3O | 435.99 | 22.6 µM | Immunology, DUB selectivity, mice tumor models, TAM targeting [188]. | |
| P5091 | C12H7Cl2NO3S2 | 348.22 | 4.2 µM | Tumor cell lines, xenograft, combination therapies [18]. | |
| P22077 | C12H7F2NO3S2 | 315.32 | 8.0 µM | DUB selectivity, preliminary in vivo evaluation, activity-based protein profiling [73]. | |
| P50429 | C18H11Cl2N3O3S3 | 484.39 | 0.42 µM | DUB selectivity, preliminary in vivo evaluation [178]. | |
| P217564 | C16H12Cl2N2O5S2 | 447.30 | 0.48 µM | DUB selectivity, preliminary in vivo evaluation [181]. | |
| C7 | C10H5Cl2N3O3S2 | 350.19 | 0.67 µM | / | |
| C19 | C16H9Cl2FN4O3S2 | 459.29 | 1.35 µM | / | |
| Noncovalent allosteric inhibitors | L55 | C28H31ClN6O4 | 551.04 | 40.8 nM | / |
| FT671 | C24H23F4N7O3 | 533.48 | 52.0 nM | Tumor cell lines, MM1.S xenograft, DUB selectivity [182]. | |
| YCH2823 | C29H32N4O4 | 500.59 | 49.6 nM | / | |
| XL188 | C32H42N6O4 | 574.71 | 90 nM | Tumor cell lines, mice tumor models, p53/MDM2 pathway regulation, DUB selectivity [184]. | |
| 1 | C22H24BrN3O3S | 490.42 | 0.30 µM | / | |
| 2a | C29H34N6O3 | 514.63 | 0.25 µM | / | |
| 2b | C29H31F3N6O3 | 568.60 | 22.00 nM | / | |
| GNE-6640 | C20H18N4O | 330.38 | 0.75 µM | Triple-negative breast cancer, DUB selectivity, p53/MDM2 pathway regulation, combination therapies [171]. | |
| GNE-6776 | C20H20N4O2 | 348.40 | 1.35 µM | Triple-negative breast cancer, DUB selectivity, in vivo tumor models, combination therapies [171]. | |
| USP7-797 | C27H28ClN3O3S | 510.05 | 0.50 nM | Tumor cell lines, DUB selectivity, V517F resistance models, in vivo tumor models [187]. | |
| Covalent allosteric inhibitors | HBX41108 | C13H3ClN4O | 266.64 | 0.42 µM | Tumor cell lines, p53 stabilization, p53 target activation, apoptosis [188]. |
| XL177A | C48H57ClN8O5 | 861.47 | 0.34 nM | Large tumor cell panel, xenograft, activity-based protein profiling [34]. | |
| FT827 | C27H28N6O5S | 548.61 | 52.00 nM | Tumor cell lines, crystallography, activity-based protein profiling [182]. | |
| PROTACs | PROTAC 17 | C50H57N9O9 | 928.06 | 1.60 µM | / |
| U7D-1 | C53H65N9O7 | 940.16 | 6.00 nM | / | |
| Natural inhibitors | Spongiacidin A | C11H11Br2N5O2 | 405.05 | 8.50 µM | / |
| Spongiacidin C | C11H10N4O3 | 246.23 | 3.80 µM | / | |
| Xestoquinone | C20H14O4 | 318.32 | 0.13 µM | / | |
| Sulawesin A | C25H30O6 | 426.20 | 2.8 µM | / | |
| USP7 activators | Asthemizole | C28H31FN4O | 458.57 | / | / |
| Sertraline | C17H17Cl2N | 306.22 | / | / | |
| MS-8 | C22H32N4O3 | 400.52 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lusardi, M.; Rapetti, F.; Spallarossa, A.; Massone, M.; Cichero, E.; Brullo, C. USP7 at the Crossroads of Ubiquitin Signaling, Cell Cycle, and Tumorigenesis. Molecules 2025, 30, 4038. https://doi.org/10.3390/molecules30204038
Lusardi M, Rapetti F, Spallarossa A, Massone M, Cichero E, Brullo C. USP7 at the Crossroads of Ubiquitin Signaling, Cell Cycle, and Tumorigenesis. Molecules. 2025; 30(20):4038. https://doi.org/10.3390/molecules30204038
Chicago/Turabian StyleLusardi, Matteo, Federica Rapetti, Andrea Spallarossa, Marta Massone, Elena Cichero, and Chiara Brullo. 2025. "USP7 at the Crossroads of Ubiquitin Signaling, Cell Cycle, and Tumorigenesis" Molecules 30, no. 20: 4038. https://doi.org/10.3390/molecules30204038
APA StyleLusardi, M., Rapetti, F., Spallarossa, A., Massone, M., Cichero, E., & Brullo, C. (2025). USP7 at the Crossroads of Ubiquitin Signaling, Cell Cycle, and Tumorigenesis. Molecules, 30(20), 4038. https://doi.org/10.3390/molecules30204038










