The Facile Solid-Phase Synthesis of Thiazolo-Pyrimidinone Derivatives
Abstract
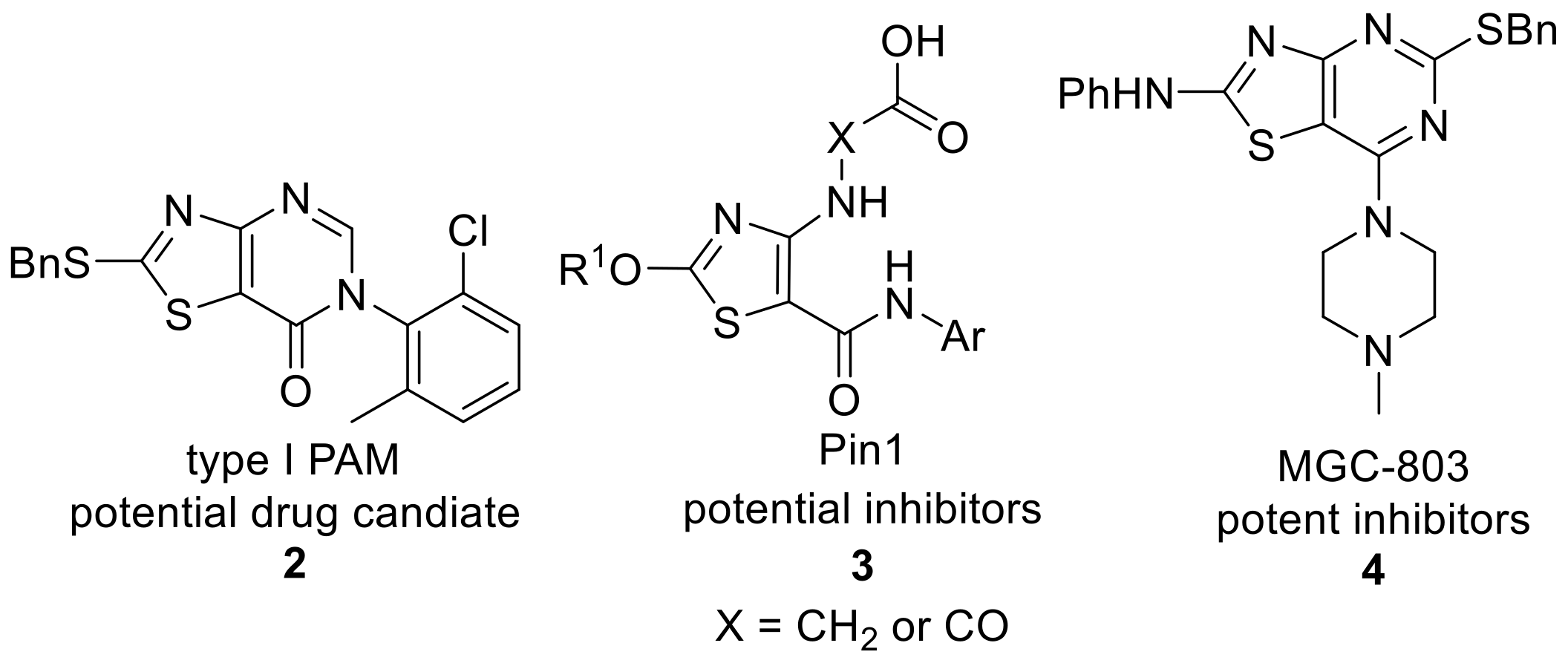
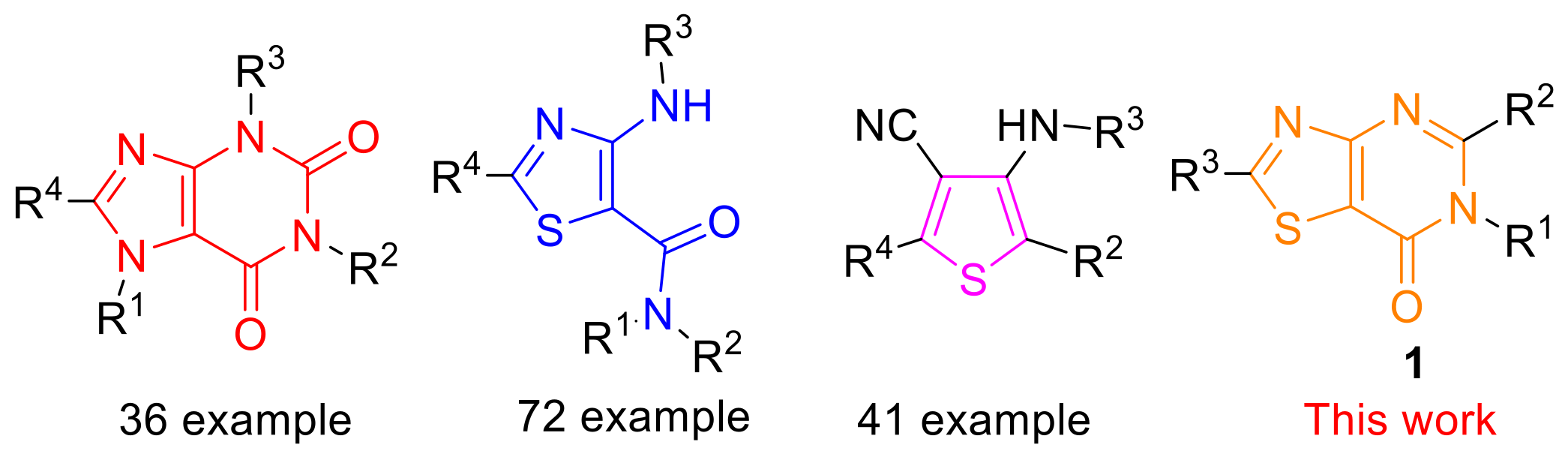
1. Introduction
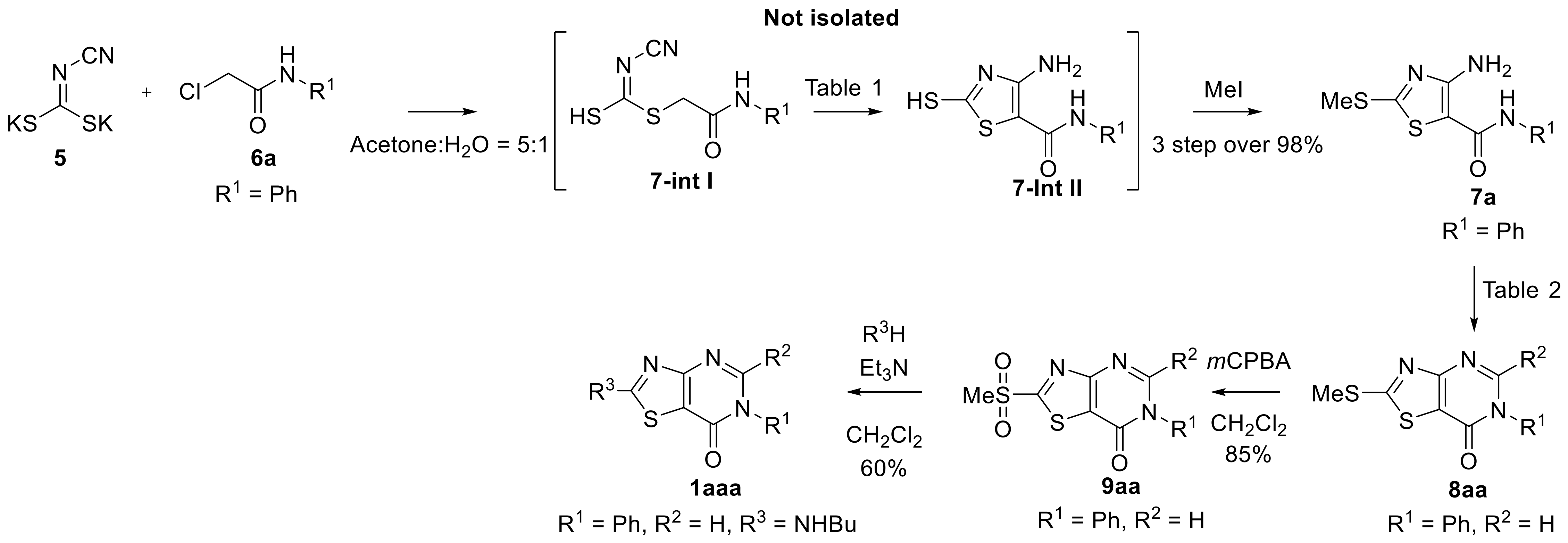
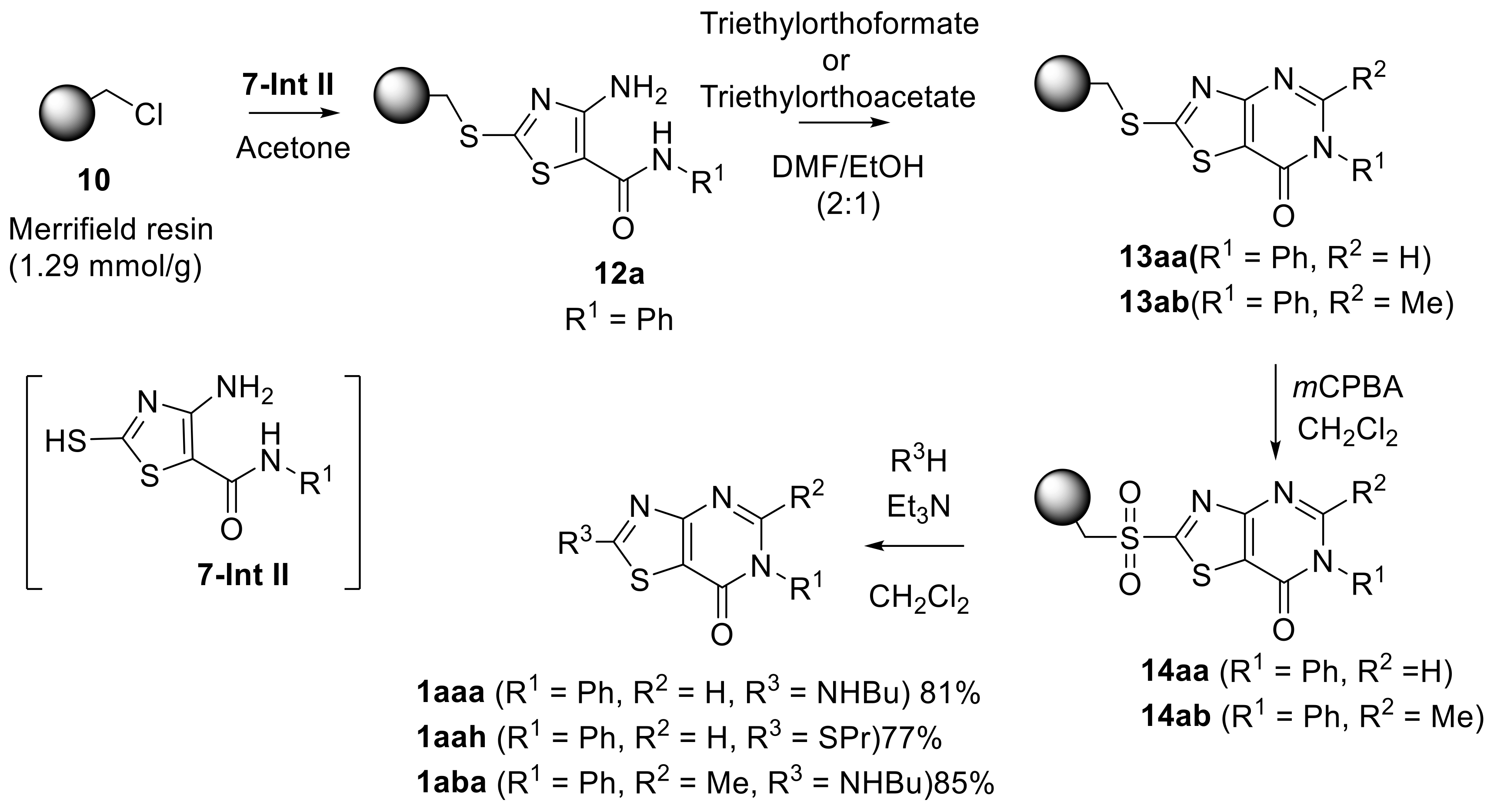
2. Results and Discussion
3. Materials and Methods
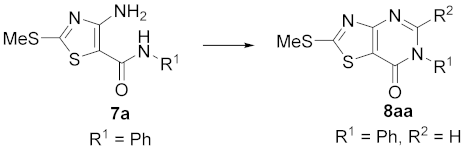
3.1. Synthesis of 4-Amino-2-(methylthio)-N-phenylthiazole-5-carboxamide (7a)
3.2. Synthesis of 2-(Methylthio)-6-phenylthiazolo [4,5-d]pyrimidin-7(6H)-one (8aa)
3.3. Synthesis of 2-(Methylsulfonyl)-6-phenylthiazolo [4,5-d]pyrimidin-7(6H)-one (9aa)
3.4. Synthesis of 2-(Butylamino)-6-phenylthiazolo [4,5-d]pyrimidin-7(6H)-One (1aaa)
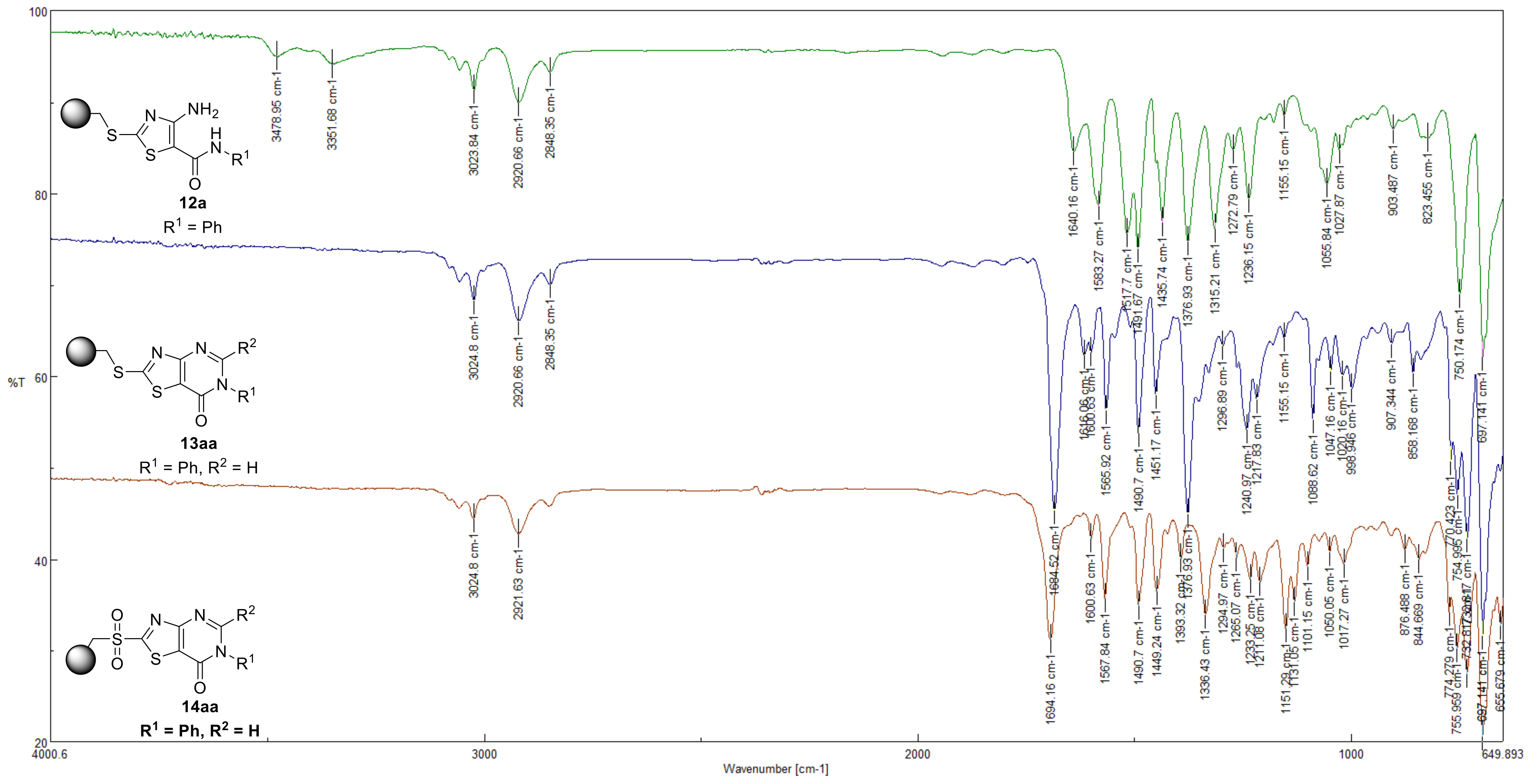
3.5. Preparation of 4-Amino-N-(substituted)thiazole-5-carboxamide resin (12a) (R1 = Ph)
3.6. Prepared of 2-(Methylthio)-6-phenylthiazolo [4,5-d]pyrimidin-7(6H)-one resin (13aa)
3.7. Prepared of 2-(Methylsulfonyl)-6-phenylthiazolo[4,5-d]pyrimidin-7(6H)-one resin (14aa)
3.8. Synthesis of 2-(Butylamino)-6-phenylthiazolo[4,5-d]pyrimidin-7(6H)-one (1aaa)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franzini, R.M.; Randolph, C. Chemical Space of DNA-Encoded Libraries. J. Med. Chem. 2016, 59, 6629–6644. [Google Scholar] [CrossRef] [PubMed]
- Goodnow, R.A., Jr.; Dumelin, C.E.; Keefe, A.D. DNA-encoded chemistry: Enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 2017, 16, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G. An Analysis of Successful Hit-to-Clinical Candidate Pairs. J. Med. Chem. 2023, 66, 7101–7139. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, Y.R.; Yu, J.Q.; Zhang, W.N.; Zhuang, C.L. DNA-encoded libraries (DELs): A review of on-DNA chemistries and their output. RSC Adv. 2021, 11, 2359–2376. [Google Scholar] [CrossRef]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in Heterocyclic Compounds, Endowed with Biological Activity, through 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Nadar, S.; Khan, T. Pyrimidine: An elite heterocyclic leitmotif in drug discovery-synthesis and biological activity. Chem. Biol. Drug Des. 2022, 100, 818–842. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Xu, H.; Zheng, Z.; Meng, Z.; Xu, Z.; Li, J.; Xue, M. Design and synthesis of five-membered heterocyclic derivatives of istradefylline with comparable pharmacological activity. Chem. Biol. Drug Des. 2022, 100, 534–552. [Google Scholar] [CrossRef]
- Nath, R.; Pathania, S.; Grover, G.; Akhtar, M.J. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020, 1222, 128900. [Google Scholar] [CrossRef]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Shahar Yar, M. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef]
- Fascio, M.L.; Errea, M.I.; D’Accorso, N.B. Imidazothiazole and related heterocyclic systems. Synthesis, chemical and biological properties. Eur. J. Med. Chem. 2015, 90, 666–683. [Google Scholar] [CrossRef]
- Abid, M.A.; Abed, A.A.; Musa, M.; Izuagie, T.; Agwamba, E.C. Anti-diabetic, anti-bacterial and anti-oxidant potential of new biologically active thiazole-derivatives: Experimental and molecular docking studies. J. Mol. Struct. 2025, 1321, 139482. [Google Scholar] [CrossRef]
- Almatari, A.S.; Saeed, A.; Abdel-Ghani, G.E.; Abdullah, M.M.S.; Al-Lohedan, H.A.; Abdel-Latif, E.; El-Demerdash, A. Synthesis of Some Novel Thiophene Analogues as Potential Anticancer Agents. Chem. Biodivers. 2024, 21, e202400313. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Anas, M.; Manhas, A.; Saha, S.; Kumar, N.; Panda, G. Synthesis, biological evaluation, Structure - Activity relationship studies of quinoline-imidazole derivatives as potent antimalarial agents. Bioorg. Chem. 2022, 121, 105671. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, K.; Liu, S.; Ren, R.; Hou, H.; Zeng, Q.; Zhang, Y.; Liu, Y. Design, synthesis and biological evaluation of novel oxazole derivatives as potential hypoglycemic agents. Bioorg. Med. Chem. 2024, 114, 117961. [Google Scholar] [CrossRef]
- Houghten, R.A.; Yu, Y. “Volatilizable” Supports for High-Throughput Organic Synthesis. J. Am. Chem. Soc. 2005, 127, 8582–8583. [Google Scholar] [CrossRef]
- Abboud, S.A.; Kodadek, T. Solid-Phase Synthesis of Diverse Macrocycles by Regiospecific 2-Pyridone Formation: Scope and Applications. JACS Au 2024, 4, 3018–3027. [Google Scholar] [CrossRef]
- Greco, A.; Tani, S.; De Marco, R.; Gentilucci, L. Synthesis and analysis of the conformational preferences of 5-aminomethyloxazolidine-2,4-dione scaffolds: First examples of beta(2)- and beta(2, 2)-homo-Freidinger lactam analogues. Chem. Eur. J. 2014, 20, 13390–13404. [Google Scholar] [CrossRef]
- De Marco, R.; Zhao, J.; Greco, A.; Ioannone, S.; Gentilucci, L. In-Peptide Synthesis of Imidazolidin-2-one Scaffolds, Equippable with Proteinogenic or Taggable/Linkable Side Chains, General Promoters of Unusual Secondary Structures. J. Org. Chem. 2019, 84, 4992–5004. [Google Scholar] [CrossRef]
- De Marco, R.; Tolomelli, A.; Campitiello, M.; Rubini, P.; Gentilucci, L. Expedient synthesis of pseudo-Pro-containing peptides: Towards constrained peptidomimetics and foldamers. Org. Biomol. Chem. 2012, 10, 2307–2317. [Google Scholar] [CrossRef]
- Aparna, E.P.; Devaky, K.S. Advances in the Solid-Phase Synthesis of Pyrimidine Derivatives. ACS Comb. Sci. 2019, 21, 35–68. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Yang, T.; Jiao, W.; Tang, J.; Huang, X.; Huang, Z.; Meng, Y.; Luo, L.; Wang, X.; et al. Design and Synthesis of Novel Positive Allosteric Modulators of alpha7 Nicotinic Acetylcholine Receptors with the Ability To Rescue Auditory Gating Deficit in Mice. J. Med. Chem. 2019, 62, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cui, G.; Jin, J.; Chen, X.; Xu, B. Synthesis and Pin1 inhibitory activity of thiazole derivatives. Bioorg. Med. Chem. 2016, 24, 5911–5920. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Liu, X.Q.; Geng, P.F.; Ma, J.L.; Zhao, T.Q.; Wei, H.M.; Yu, B.; Liu, H.M. Design, synthesis, and biological evaluation of new thiazolo[5,4-d]pyrimidine derivatives as potent antiproliferative agents. MedChemComm 2017, 8, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Liu, K.H.; Bae, J.S.; Baek, D.J.; Lee, T. Solid-Phase Synthesis of 1,3,7,8-Tetrasubstituted Xanthine Derivatives on Traceless Solid Support. ACS Comb. Sci. 2016, 18, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Baek, D.J.; Lee, D.; Liu, K.-H.; Bae, J.-S.; Gong, Y.-D.; Min, K.H.; Lee, T. Efficient solid-phase synthesis of 2,4-disubstituted 5-carbamoyl-thiazole derivatives using a traceless support. Tetrahedron 2015, 71, 3367–3377. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Song, K.-S.; Liu, K.-H.; Gong, Y.-D.; Lee, T. Parallel synthesis of 2,4,5-trisubstituted thiophene-3-carbonitrile derivatives on traceless solid support. Tetrahedron 2014, 70, 9183–9190. [Google Scholar] [CrossRef]
 | ||||
|---|---|---|---|---|
| Entry a | Base | Solvent | Temp (°C) | Yield (%) b |
| 1 | 1 M NaOH | Acetone/H2O | 60 | 57 |
| 2 | KOH | Acetone/H2O | 60 | 41 |
| 3 | LiOH | Acetone/H2O | 60 | 98 |
| 4 | NaOEt | EtOH | Reflux | 16 |
| 5 | K2CO3 | Acetone/H2O | 60 | N.R d |
| 6 | NaH | THF | R.T c | N.R d |
| 7 | Et3N | Acetone/H2O | 60 | N.R d |
| 8 | DBU | Acetone/H2O | 60 | 21 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry a | Catalyst (mol%) | R2 Reagent | Solvent | Temp (°C) | Time (h) | Yield (%) b |
| 1 | HC(OEt)3 | Ac2O | 100 | 10 | NR c | |
| 2 | p-TsOH(10) | HC(OEt)3 | Toluene | 120 | 23 | 14 |
| 3 | p-TsOH(10) | HC(OEt)3 | EtOH | Reflux | 2 | 70 |
| 4 | p-TsOH(10) | HC(OEt)3 | DMF | 120 | 10 | 64 |
| 5 | HC(OEt)3 | AcOH | Reflux | 48 | 43 | |
| 6 | p-TsOH(10) | HC(OEt)3 | THF | Reflux | 24 | NR c |
| 7 | AlCl3(10) | HC(OEt)3 | EtOH | Reflux | 10 | Trace |
| 8 | BF3OEt2(10) | HC(OEt)3 | EtOH | Reflux | 5 | 64 |
| 9 | CSA(10) | HC(OEt)3 | EtOH | 60 | 3 | 79 |
| 10 | CSA(10) | MeC(OEt)3 | EtOH | 60 | 10 | 75 |
| 11 | I2(120) | Benzaldehyde | DMSO | 100 | 0.5 | 80 |
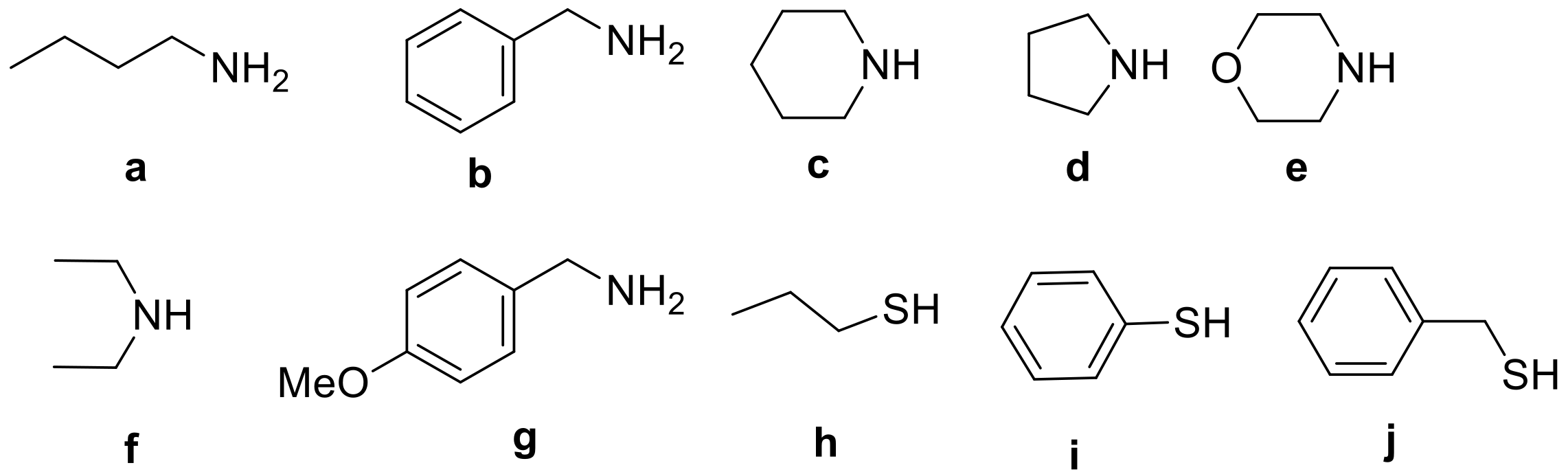
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry a | R1 | R2 | R3 | Yield b (%) | Entry a | R1 | R2 | R3 | Yield b (%) |
| 1 | Ph | H | a | 81 | 30 | 4-OMe-Ph | Me | b | 74 |
| 2 | Ph | H | b | 77 | 31 | 4-OMe-Ph | Me | c | 77 |
| 3 | Ph | H | c | 70 | 32 | 4-OMe-Ph | Me | d | 88 |
| 4 | Ph | H | e | 65 | 33 | 4-OMe-Ph | Me | e | 83 |
| 5 | Ph | H | f | 36 | 34 | 4-OMe-Ph | Me | f | 33 |
| 6 | Ph | H | g | 67 | 35 | 4-OMe-Ph | Me | g | 73 |
| 7 | Ph | H | h | 77 | 36 | 4-OMe-Ph | Me | h | 93 |
| 8 | Ph | H | i | 82 | 37 | 4-OMe-Ph | Me | i | 85 |
| 9 | Ph | H | j | 90 | 38 | 4-OMe-Ph | Me | j | 86 |
| 10 | Ph | Me | a | 85 | 39 | 4-Me-Ph | Me | a | 40 |
| 11 | Ph | Me | b | 77 | 40 | 4-Me-Ph | Me | b | 47 |
| 12 | Ph | Me | c | 82 | 41 | 4-Me-Ph | Me | c | 51 |
| 13 | Ph | Me | d | 76 | 42 | 4-Me-Ph | Me | e | 46 |
| 14 | Ph | Me | e | 79 | 43 | 4-Me-Ph | Me | g | 48 |
| 15 | Ph | Me | f | 37 | 44 | 4-Me-Ph | Me | h | 49 |
| 16 | Ph | Me | g | 75 | 45 | 4-Me-Ph | Me | j | 64 |
| 17 | Ph | Me | h | 91 | 46 | 4-NO2-Ph | H | a | 52 |
| 18 | Ph | Me | i | 88 | 47 | 4-NO2-Ph | H | c | 39 |
| 19 | Ph | Me | j | 92 | 48 | 4-NO2-Ph | H | e | 55 |
| 20 | 4-OMe-Ph | H | a | 68 | 49 | 4-NO2-Ph | H | f | 49 |
| 21 | 4-OMe-Ph | H | b | 86 | 50 | 4-NO2-Ph | H | h | 65 |
| 22 | 4-OMe-Ph | H | c | 77 | 51 | 4-NO2-Ph | H | i | 53 |
| 23 | 4-OMe-Ph | H | d | 76 | 52 | 4-NO2-Ph | H | j | 53 |
| 24 | 4-OMe-Ph | H | e | 63 | 53 | 4-NO2-Ph | Me | a | 48 |
| 25 | 4-OMe-Ph | H | g | 68 | 54 | 4-NO2-Ph | Me | b | 18 |
| 26 | 4-OMe-Ph | H | h | 80 | 55 | 4-NO2-Ph | Me | d | 47 |
| 27 | 4-OMe-Ph | H | i | 75 | 56 | 4-NO2-Ph | Me | e | 53 |
| 28 | 4-OMe-Ph | H | j | 79 | 57 | 4-NO2-Ph | Me | j | 57 |
| 29 | 4-OMe-Ph | Me | a | 72 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, S.; Moon, J.; Lee, T. The Facile Solid-Phase Synthesis of Thiazolo-Pyrimidinone Derivatives. Molecules 2025, 30, 430. https://doi.org/10.3390/molecules30020430
Hua S, Moon J, Lee T. The Facile Solid-Phase Synthesis of Thiazolo-Pyrimidinone Derivatives. Molecules. 2025; 30(2):430. https://doi.org/10.3390/molecules30020430
Chicago/Turabian StyleHua, Shuanghui, Jimin Moon, and Taeho Lee. 2025. "The Facile Solid-Phase Synthesis of Thiazolo-Pyrimidinone Derivatives" Molecules 30, no. 2: 430. https://doi.org/10.3390/molecules30020430
APA StyleHua, S., Moon, J., & Lee, T. (2025). The Facile Solid-Phase Synthesis of Thiazolo-Pyrimidinone Derivatives. Molecules, 30(2), 430. https://doi.org/10.3390/molecules30020430






