Phenolic and Iridoid Glycosides from Leonurus cardiaca L. and Their Effects on the α, δ, and γ Subtypes of the PPAR System—Including the Discovery of the Novel Phenylethanoid Cardiaphenyloside A and the Most Active 7-Chloro-6-desoxy-harpagide
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Phenylethanoids and Iridoids
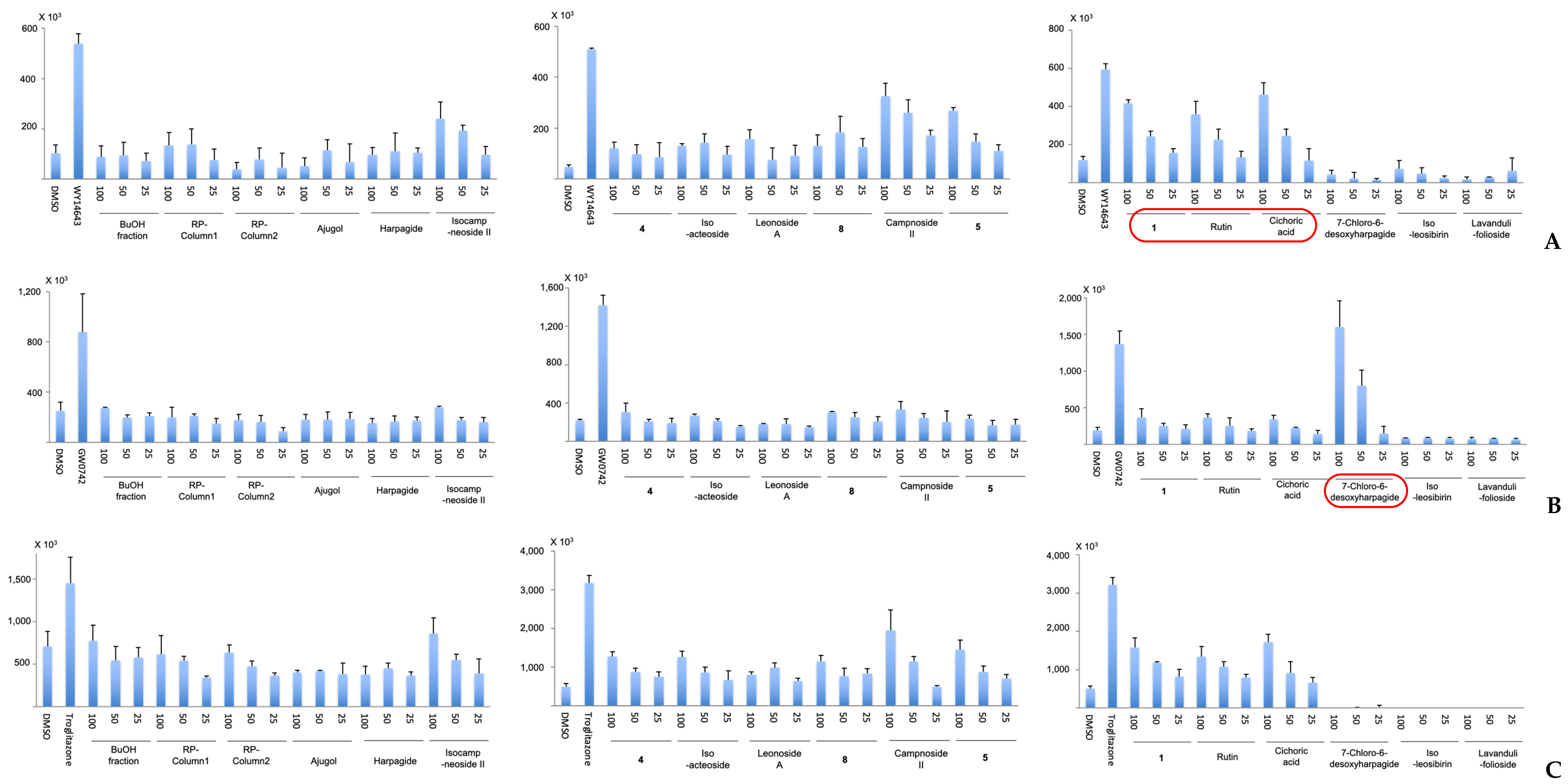
2.2. Results and Effects of the 19 Leonurus Constituents on PPAR
3. Materials and Methods
3.1. General Procedures
3.2. Chemicals
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Luciferase Reporter Assay for the 19 Leonurus Constituents
3.6. HPLC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, L. New Kreutterbuch; Michael Isingrin: Basel, Switzerland, 1543. [Google Scholar]
- Keller, K. European Medicines Agency (EMA) (2010) Committee on Herbal Medicinal Products (HMPC): Assessment Report on Leonurus cardiaca L., Herba, Final. Doc. Ref.: EMA/HMPC/127430/2010, (1995–2019). Available online: http://www.ema.europa.eu (accessed on 16 September 2010).
- Ritter, M.; Melichar, K.; Strahler, S.; Kuchta, K.; Schulte, J.; Sartiani, L.; Cerbai, E.; Mugelli, A.; Mohr, F.W.; Rauwald, H.W.; et al. Cardiac and electrophysiological effects of primary and refined extracts from Leonurus cardiaca L. (Ph.Eur.). Planta Med. 2010, 76, 572–582. [Google Scholar] [CrossRef]
- Yang, S.Z. The Divine Farmer’s Materia Medica; Blue Poppy Press: Boulder, CO, USA, 1998; p. 30. A translation of the “Shennong Bencao Jing”, first published before 200 AD. [Google Scholar]
- Rauwald, H.W.; Savtschenko, A.; Merten, A.; Rusch, C.; Appel, K.; Kuchta, K. GABAA Receptor Binding Assays of Standardized Leonurus cardiaca and Leonurus japonicus Extracts as well as their Isolated Constituents. Planta Med. 2015, 81, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef]
- Elisaf, M. Effects of fibrates on serum metabolic parameters. Curr. Med. Res. Opin. 2002, 18, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B.; Zang, H.; Rasmussen, R.K.; Flindt, E.N.; Kristiansen, K. Peroxisome proliferator-activated receptor delta (PPARdelta)-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J. Biol. Chem. 2001, 276, 3175–3182. [Google Scholar] [CrossRef]
- Joseph, E.L.; Jack, L.J.; Ira, G.D. Novel approach to treat insulin resistance, type 2 diabetes, and the metabolic syndrome: Simultaneous activation of PPARalpha, PPARgamma, and PPARdelta. Curr. Diabetes Rev. 2005, 1, 299–307. [Google Scholar]
- Coll, T.; Rodrïguez-Calvo, R.; Barroso, E.; Serrano, L.; Eyre, E.; Palomer, X.; Vázquez-Carrera, M. Peroxisome proliferator-activated receptor (PPAR) β/δ: A new potential therapeutic target for the treatment of metabolic syndrome. Curr. Mol. Pharmacol. 2009, 2, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Ortwein, J.; Çaliş, İ.; Volk, R.B.; Rauwald, H.W. Identification of Cardioactive Leonurus and Leonotis Drugs by Quantitative HPLC Determination and HPTLC Detection of Phenolic Marker Constituents. Nat. Product. Commun. 2016, 11, 1129–1133. [Google Scholar] [CrossRef]
- Wichtl, M.; Stahl-Biskup, E. Herzgespannkraut—Leonuri cardiacae herba. In Kommentar zum Europäischen Arzneibuch; Bracher, F., Heisig, P., Langguth, P., Mutschler, E., Rücker, G., Scriba, G., Stahl-Biskup, E., Troschütz, R., Eds.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2005; (19. Lfg.) 4.03/1833. [Google Scholar]
- Frohne, D. Heilpflanzenlexikon, ein Leitfaden auf Wissenschaftlicher Grundlage; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 2006; pp. 298–299. [Google Scholar]
- Rusch, C.; Hennig, L.; Rauwald, H.W. 7R-Chloro-6-desoxy-harpagide, a major iridoid glucoside from Leonurus cardiaca L. (Ph. Eur). Planta Med. 2010, 76, P235, Dissertation in preparation. [Google Scholar] [CrossRef]
- Sharma, A.R.; Harunari, E.; Oku, N.; Matsuura, N.; Trianto, A.; Igarashi, Y. Two antibacterial and PPARα/γ-agonistic unsaturated keto fatty acids from a coral-associated actinomycete of the genus Micrococcus. Beilstein J. Org. Chem. 2020, 16, 297–304. [Google Scholar] [CrossRef]
- Imakura, Y.; Kobayashi, S.; Mima, A. Bitter phenyl propanoid glycosides from Campsis chinensis. Phytochemistry 1985, 24, 139–146. [Google Scholar] [CrossRef]
- Miyase, T.; Yamamoto, R.; Ueno, A. Phenylethanoid glycosides from Stachys officinalis. Phytochemistry 1996, 43, 475–479. [Google Scholar] [CrossRef]
- Ikeda, T.; Miyase, T.; Ueno, A. Phenylethanoid glycosides from Stachys riederi. Nat. Med. 1994, 48, 32–38. [Google Scholar]
- Miyase, T.; Koizumi, A.; Ueno, A.; Noro, T.; Kuroyanagi, M.; Fukushima, S.; Akiyama, Y.; Takemoto, T. Studies on the acyl glycosides from Leucoseptrum japonicum (Miq.) Kitamura et Murata. Chem. Pharm. Bull. 1982, 30, 2732–2737. [Google Scholar] [CrossRef]
- Tasdemir, D.; Scapozza, L.; Zerbe, O.; Linden, A.; Calis, I.; Sticher, O. Iridoid glycosides of Leonurus persicus. J. Nat. Prod. 1999, 62, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Kajikawa, I.; Nishiya, K.; Watanabe, Y.; Suzuki, N.; Takeya, K.; Itokawa, H. Studies on the constituents of the European Mistletoe, Viscum album L. II. Chem. Pharm. Bull. 1988, 36, 1185–1189. [Google Scholar] [CrossRef][Green Version]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharide and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Kuang, H.X.; Xia, Y.G.; Liang, J.; Yang, B.Y.; Wang, Q.H. Lianqiaoxinoside B, a novel caffeoyl phenylethanoid glycoside from Forsythia suspensa. Molecules 2011, 16, 5674–5681. [Google Scholar] [CrossRef]
- Dressel, U.; Allen, T.L.; Pippal, J.B.; Rohde, P.R.; Lau, P.; Muscat, G.E. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol. Endocrinol. 2003, 17, 2477–2493. [Google Scholar] [CrossRef]
- Barish, G.D.; Narkar, V.A.; Evans, R.M. PPAR delta: A dagger in the heart of the metabolic syndrome. J. Clin. Investig. 2006, 116, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. New peroxisome proliferator-activated receptor agonists: Potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert. Opin. Pharmacother. 2014, 15, 493–503. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.Y. Cardarine (GW501516) Effects on Improving Metabolic Syndrome. J. Health Sports Kinesiol. 2021, 2, 22–27. [Google Scholar] [CrossRef]
- Meister, T.; Uphoff, M.A.; Heinecke, A.; Domagk, D.; Kunsch, S.; Lindhorst, A.; Ellenrieder, V.; Heinzow, H.S. Novel score for prediction of malignant bile duct obstruction based on biochemical and clinical markers. Aliment. Pharmacol. Ther. 2015, 41, 877–887. [Google Scholar] [CrossRef]
- Nass, R.; Rimpler, H. Distribution of iridoids in different populations of Physostegia virginiana and some remarks on iridoids from Avicennia officinalis and Scrophularia ningpoensis. Phytochemistry 1996, 41, 489–498. [Google Scholar] [CrossRef]

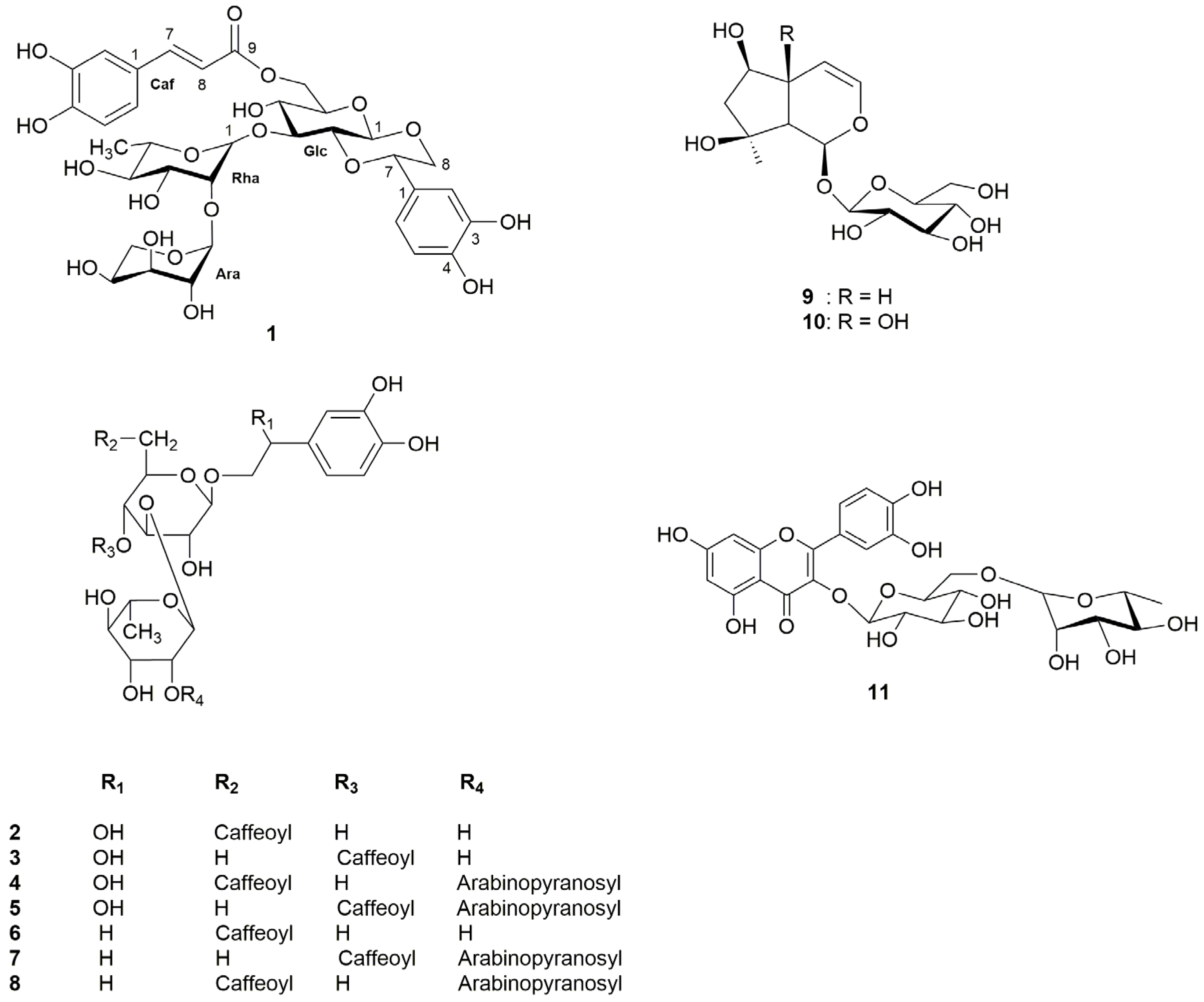
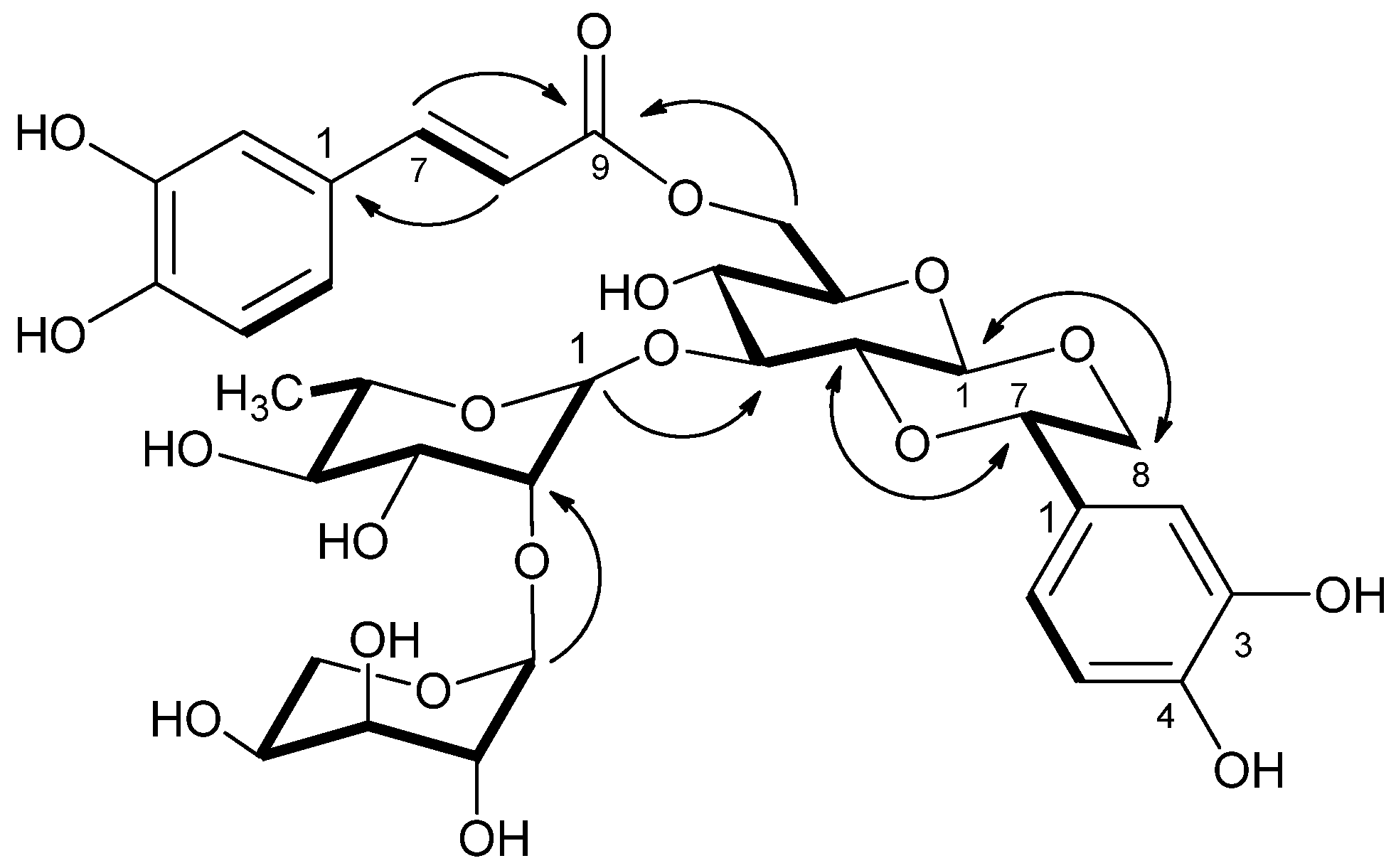
| Position | δC | δH |
|---|---|---|
| Aglycone moiety | ||
| 1 | 130.0 | |
| 2 | 115.4 | 6.80 (d, J = 1.6 Hz) |
| 3 | 146.4 | |
| 4 | 146.8 | |
| 5 | 116.4 | 6.71 (d, J = 8.0 Hz) |
| 6 | 119.8 | 6.69 (dd, J = 8.0, 1.6 Hz) |
| 7 | 78.7 | 4.50 (dd, J = 10.4, 2.0 Hz) |
| 8 | 72.5 | 3.93 #; 3.66 (br d, J = 12.0 Hz) |
| Caffeoyl moiety | ||
| 1 | 127.7 | |
| 2 | 115.1 | 7.01 (d, J = 1.6 Hz) |
| 3 | 146.8 | |
| 4 | 149.7 | |
| 5 | 116.5 | 6.74 (d, J = 8.0 Hz) |
| 6 | 123.1 | 6.91 (dd, J = 8.0, 2.0 Hz) |
| 7 | 147.3 | 7.54 (d, J = 16.0 Hz) |
| 8 | 114.7 | 6.26 (d, J = 16.0 Hz) |
| 9 | 169.0 | |
| Sugar chain | ||
| Glucose (Glc) | ||
| 1 | 99.0 | 4.44 (d, J = 8.0 Hz) |
| 2 | 82.0 | 3.35 # |
| 3 | 79.6 | 3.72 # |
| 4 | 70.2 | 3.48 # |
| 5 | 77.2 | 3.70 # |
| 6 | 64.5 | 4.50 (dd, J = 11.6, 2.0 Hz); 4.32 (dd, J = 12.0, 5.2 Hz) |
| Rhamnose (Rha) | ||
| 1 | 100.6 | 5.33 (br s) |
| 2 | 83.2 | 3.74 # |
| 3 | 71.8 | 3.66 # |
| 4 | 74.1 | 3.28 # |
| 5 | 69.7 | 3.92 # |
| 6 | 17.9 | 1.17 (d, J = 6.4 Hz) |
| Arabinopyranose (Ara) | ||
| 1 | 107.4 | 3.90 (d, J = 7.6 Hz) |
| 2 | 72.8 | 3.43 # |
| 3 | 74.3 | 3.30 # |
| 4 | 69.8 | 3.48 # |
| 5 | 66.9 | 3.25 #; 2.69 (br d, J = 12.4 Hz) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchta, K.; Matsuura, N.; Nguyen, T.H.; Rusch, C.; Iinuma, M.; Shoyama, Y.; Rauwald, H.W. Phenolic and Iridoid Glycosides from Leonurus cardiaca L. and Their Effects on the α, δ, and γ Subtypes of the PPAR System—Including the Discovery of the Novel Phenylethanoid Cardiaphenyloside A and the Most Active 7-Chloro-6-desoxy-harpagide. Molecules 2025, 30, 419. https://doi.org/10.3390/molecules30020419
Kuchta K, Matsuura N, Nguyen TH, Rusch C, Iinuma M, Shoyama Y, Rauwald HW. Phenolic and Iridoid Glycosides from Leonurus cardiaca L. and Their Effects on the α, δ, and γ Subtypes of the PPAR System—Including the Discovery of the Novel Phenylethanoid Cardiaphenyloside A and the Most Active 7-Chloro-6-desoxy-harpagide. Molecules. 2025; 30(2):419. https://doi.org/10.3390/molecules30020419
Chicago/Turabian StyleKuchta, Kenny, Nobuyasu Matsuura, Tung Huu Nguyen, Christian Rusch, Munekazu Iinuma, Yukihiro Shoyama, and Hans Wilhelm Rauwald. 2025. "Phenolic and Iridoid Glycosides from Leonurus cardiaca L. and Their Effects on the α, δ, and γ Subtypes of the PPAR System—Including the Discovery of the Novel Phenylethanoid Cardiaphenyloside A and the Most Active 7-Chloro-6-desoxy-harpagide" Molecules 30, no. 2: 419. https://doi.org/10.3390/molecules30020419
APA StyleKuchta, K., Matsuura, N., Nguyen, T. H., Rusch, C., Iinuma, M., Shoyama, Y., & Rauwald, H. W. (2025). Phenolic and Iridoid Glycosides from Leonurus cardiaca L. and Their Effects on the α, δ, and γ Subtypes of the PPAR System—Including the Discovery of the Novel Phenylethanoid Cardiaphenyloside A and the Most Active 7-Chloro-6-desoxy-harpagide. Molecules, 30(2), 419. https://doi.org/10.3390/molecules30020419







