6-Bromoindole- and 6-Bromoindazole-Based Inhibitors of Bacterial Cystathionine γ-Lyase Containing 3-Aminothiophene-2-Carboxylate Moiety
Abstract
1. Introduction
2. Results and Discussion
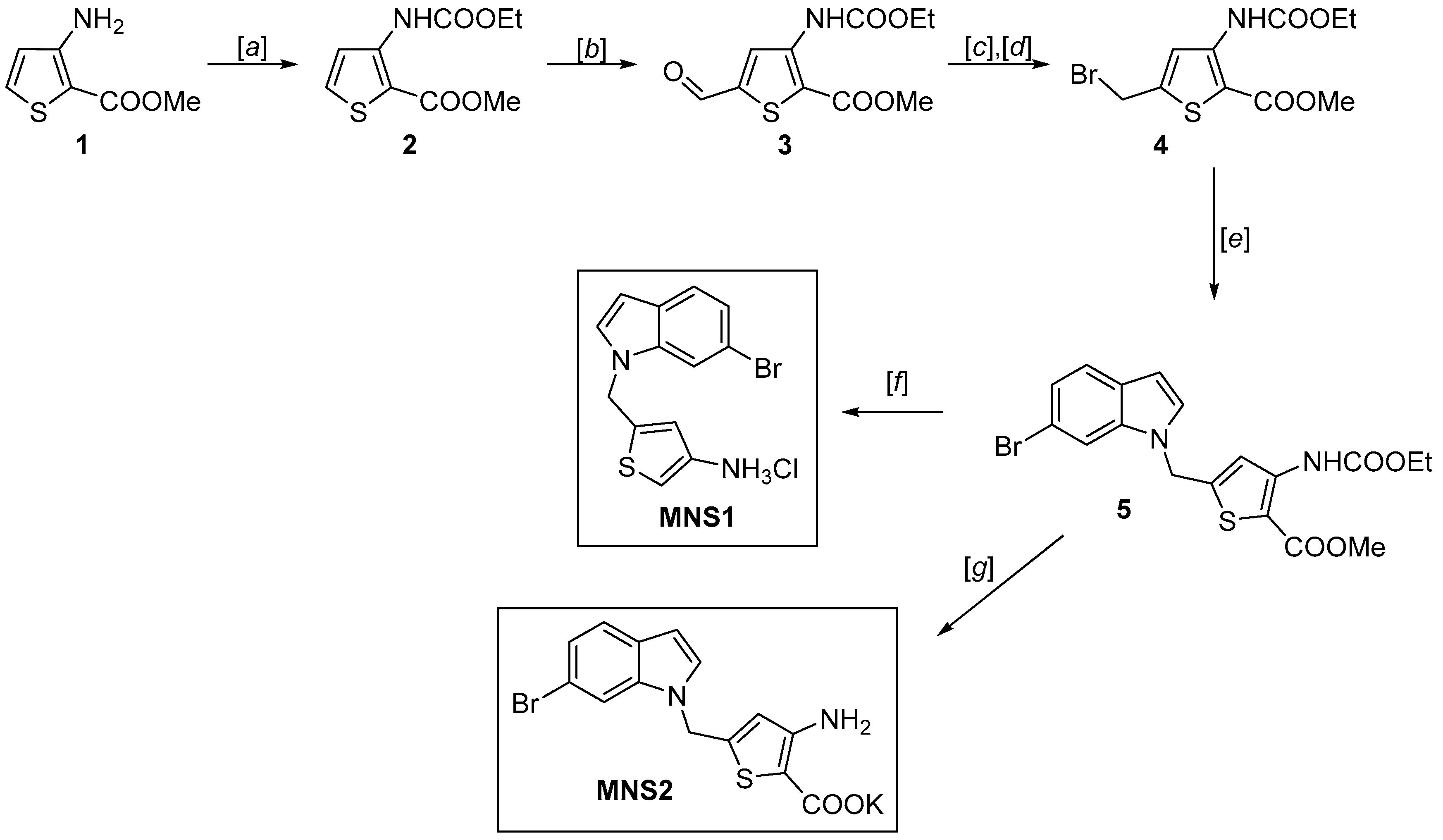
2.1. Synthesis of Thiophene Analog MNS2
2.2. Synthesis of 6-Bromoindazole Derivatives MNS3 and MNS4
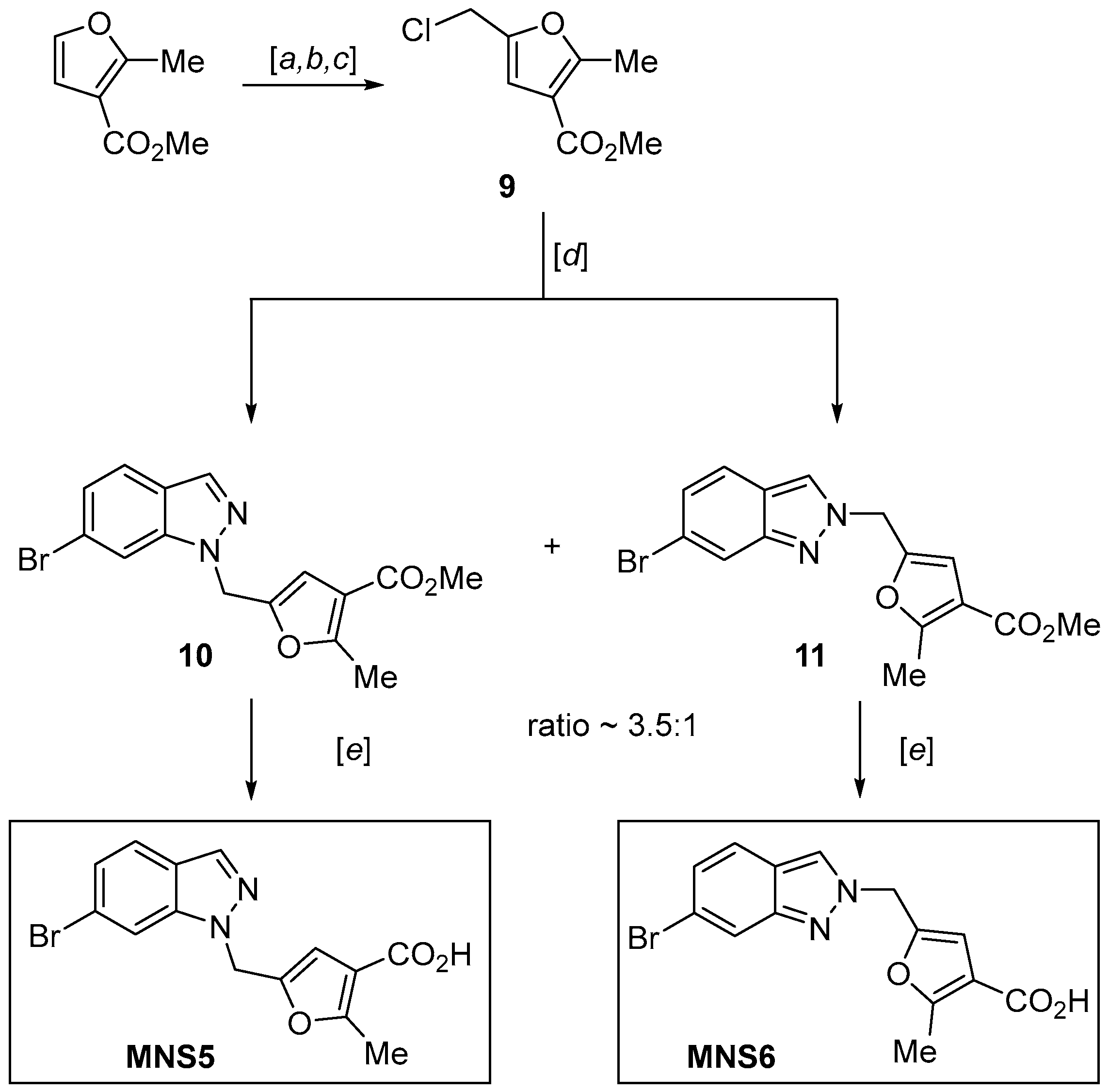
2.3. Synthesis of the 6-Bromoindazole Analogs of NL2 (MNS5 and MNS6)
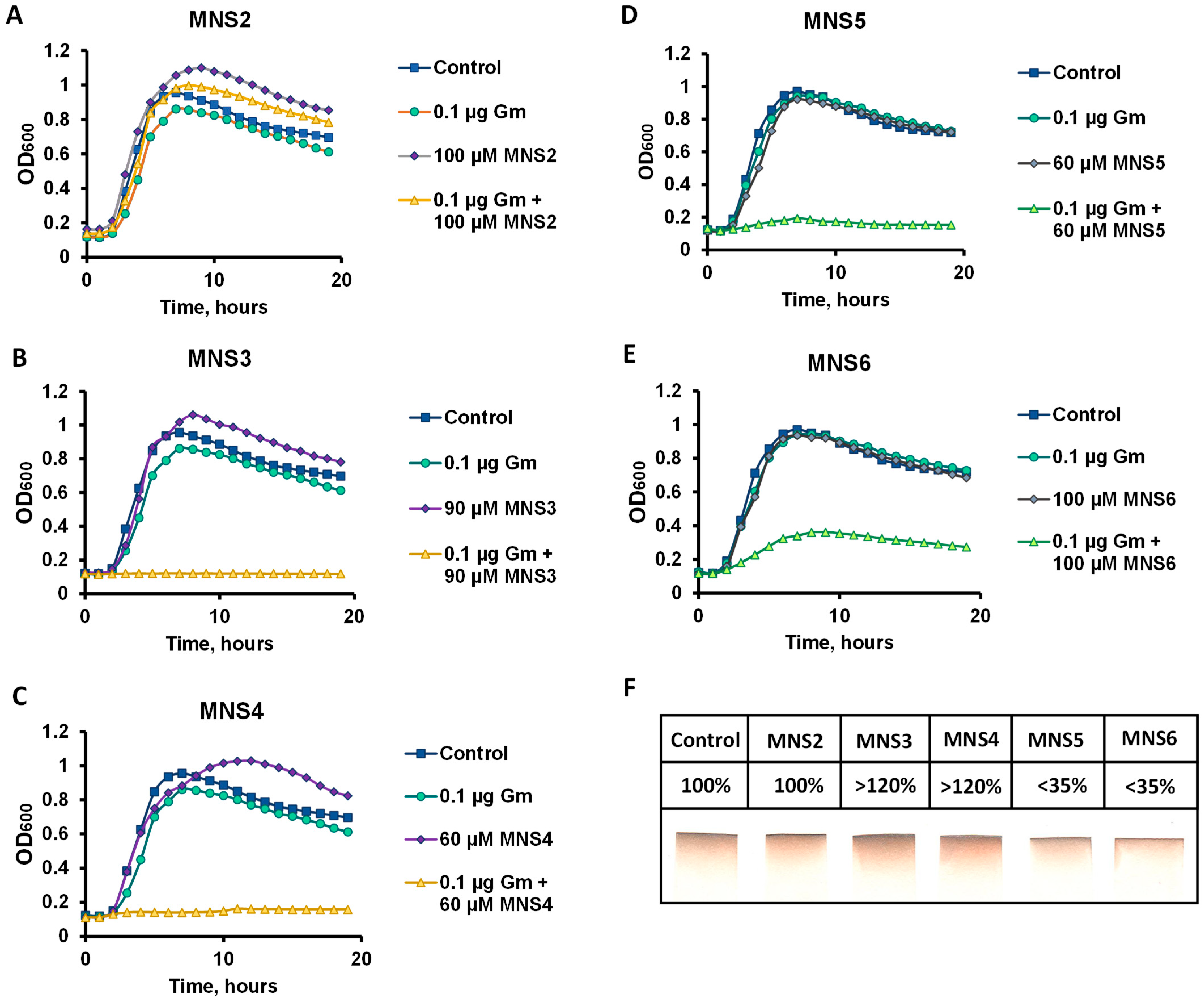
2.4. Determination of the Potentiating Activity in Bacillus subtilis Cells
3. Materials and Methods
3.1. General Experimental Details
3.2. Methyl 3-((ethoxycarbonyl)amino)thiophene-2-carboxylate (2)
3.3. Methyl 3-((ethoxycarbonyl)amino)-5-formylthiophene-2-carboxylate (3)
3.4. Methyl 5-(bromomethyl)-3-((ethoxycarbonyl)amino)thiophene-2-carboxylate (4)
3.5. Methyl 5-((6-bromo-1H-indol-1-yl)methyl)-3-((ethoxycarbonyl)amino)thiophene-2-carboxylate (5)
3.6. 6-Bromo-1H-indazol (6)
3.7. Methyl 5-((6-bromo-1H-indazol-1-yl)methyl)-3-((ethoxycarbonyl)amino)thiophene-2-carboxylate (7) and Methyl 5-((6-bromo-2H-indazol-2-yl)methyl)-3-((ethoxycarbonyl)amino)thiophene-2-carboxylate (8)
3.8. General Method for Hydrolysis of Carboxylic Esters 5, 7 and 8
3.9. Potassium 3-Amino-5-((6-bromo-1H-indol-1-yl)methyl)thiophene-2-carboxylate (MNS2)
3.10. Potassium 3-Amino-5-((6-bromo-1H-indazol-1-yl)methyl)thiophene-2-carboxylate (MNS3)
3.11. Potassium 3-Amino-5-((6-bromo-2H-indazol-2-yl)methyl)thiophene-2-carboxylate (MNS4)
3.12. Methyl 5-Formyl-2-Methylfuran-3-Carboxylate
3.13. Methyl 5-(chloromethyl)-2-methylfuran-3-carboxylate (9)
3.14. Methyl 5-((6-Bromo-1H-indazol-1-yl)methyl)-2-methylfuran-3-carboxylate (10) and Methyl 5-((6-Bromo-2H-indazol-2-yl)methyl)-2-methylfuran-3-carboxylate (11)
3.15. 5-((6-Bromo-1H-indazol-1-yl)methyl)-2-methylfuran-3-carboxylic Acid (MNS5)
3.16. 5-((6-Bromo-2H-indazol-2-yl)methyl)-2-methylfuran-3-Carboxylic Acid (MNS6)
3.17. Estimation of the Minimum Inhibitory Concentration (MIC) of Gentamycin in B. subtilis Cells
3.18. Cell Culture
3.19. MTT Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, D.; Chawla, M.; Ahrodia, T.; Narendrakumar, L.; Das, B. Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens. Antibiotics 2023, 12, 1715. [Google Scholar] [CrossRef] [PubMed]
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic Potentiators Against Multidrug-Resistant Bacteria: Discovery, Development, and Clinical Relevance. Front. Microbiol. 2022, 13, 887251. [Google Scholar] [CrossRef]
- Hyun, S.; Choi, Y.; Seolah, J.; Choo, D.; Park, T.W.; Park, S.-J.; Kim, S.; Lee, S.; Park, S.; Jin, S.M.; et al. Proline Hinged Amphipathic α-Helical Peptide Sensitizes Gram-Negative Bacteria to Various Gram-Positive Antibiotics. J. Med. Chem. 2020, 63, 14937–14950. [Google Scholar] [CrossRef] [PubMed]
- MacNair, C.R.; Brown, E.D. Outer Membrane Disruption Overcomes Intrinsic, Acquired, and Spontaneous Antibiotic Resistance. mBio 2020, 11, e01615-20. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Vermote, A.; Calenbergh, S.V. Small-Molecule Potentiators for Conventional Antibiotics against Staphylococcus aureus. ACS Infect. Dis. 2017, 3, 780–796. [Google Scholar] [CrossRef]
- Xiao, T.; Chen, S.; Yan, G.; Zheng, J.; Qiu, Q.; Lin, S.; Zong, Y.; Chang, H.; Chia, A.; Chang, Y.; et al. Cystathionine γ-lyase inhibits mitochondrial oxidative stress by releasing H2S nearby through the AKT/NRF2 signaling pathway. Front. Microbiol. 2024, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shefa, U.; Kim, M.-S.; Jeong, N.Y.; Jung, J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxid. Med. Cell. Longev. 2018, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, M.; Fernández-Rodríguez, C.; Conter, C.; Oyenarte, I.; Favretto, F.; di Matteo, A.; Dominici, P.; Petrosino, M.; Martinez-Chantar, M.L.; Majtan, T.; et al. Catalytic specificity and crystal structure of cystathionine γ-lyase from Pseudomonas aeruginosa. Sci. Rep. 2024, 14, 9364. [Google Scholar] [CrossRef]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef]
- Potapov, K.V.; Novikov, R.A.; Novikov, M.A.; Solyev, P.N.; Tomilov, Y.V.; Kochetkov, S.N.; Makarov, A.A.; Mitkevich, V.A. Synthesis of the Indole-Based Inhibitors of Bacterial Cystathionine γ-Lyase NL1-NL3. Molecules 2023, 28, 3568. [Google Scholar] [CrossRef]
- Novikov, M.A.; Potapov, K.V.; Novikov, R.A.; Solyev, P.N.; Tomilov, Y.V.; Kochetkov, S.N.; Makarov, A.A.; Mitkevich, V.A. A convenient synthesis of a chlorobenzothiophenyl-indole-based inhibitor of bacterial cystathionine γ-lyase. Mendeleev Commun. 2024, 34, 255–258. [Google Scholar] [CrossRef]
- Novikov, R.A.; Platonov, D.N.; Belyy, A.Y.; Potapov, K.V.; Novikov, M.A.; Tomilov, Y.V.; Kechko, O.I.; Seregina, T.A.; Soyev, P.N.; Mitkevich, V.A. Development of a New Inhibitor of Bacterial Cystathionine γ-Lyase Based on 6-Bromoindole and Aminothiophene. Mol. Biol. 2024, 58, 1082–1088. [Google Scholar] [CrossRef]
- Golovina, A.; Proia, E.; Fiorentino, F.; Yunin, M.; Kasatkina, M.; Zigangirova, N.; Soloveva, A.; Sysolyatina, E.; Ermolaeva, S.; Novikov, R.; et al. (Heteroarylmethyl)benzoic Acids as a New Class of Bacterial Cystathionine γ-Lyase Inhibitors: Synthesis, Biological Evaluation, and Molecular Modeling. ACS Infect. Dis. 2024, 10, 2127–2150. [Google Scholar] [CrossRef]
- Kuzovlev, A.S.; Zybalov, M.D.; Golovin, A.V.; Gureev, M.A.; Kasatkina, M.A.; Biryukov, M.V.; Belik, A.R.; Silonov, S.A.; Yunin, M.A.; Zigangirova, N.A.; et al. Naphthyl-Substituted Indole and Pyrrole Carboxylic Acids as Effective Antibiotic Potentiators—Inhibitors of Bacterial Cystathionine γ-Lyase. Int. J. Mol. Sci. 2023, 24, 16331. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Li, R.; Zhang, Y.; Bao, G.; Le, Y.; Yan, L. Design, synthesis and antitumor activity of 5-trifluoromethylpyrimidine derivatives as EGFR inhibitors. J. Enzyme Inhib. Med. Chem. 2022, 37, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, P.; Barker, J. A Convenient Synthesis of 2-Substituted 3-Hydroxy- and 3-Amino-Thiophens from Derivatives of 2-Chloroacrylic Acid. Synth. Commun. 1979, 9, 731–734. [Google Scholar] [CrossRef]
- Sigel, S.; Haendler, B.; Stresemann, C.; Fernandez, M.A.E.; Ter, L.A.; Stoeckigt, D.; Harb, H.Y.; Kosemund, D.; Eheim, A.; Moen Ning, U. 2,7-Diazaspiro[4.4]nonanes. Patent WO2018024602, 8 February 2018. [Google Scholar]
- Duan, W.; Shen, X.; Wang, W.; Wang, J.; Cao, S.; Liu, J.; Xu, X. Preparation and Application of Compound with AMPK Agonistic Activity and Prodrug of Compound. Patent CN113549010, 26 November 2021. [Google Scholar]
- Garofalo, A.W.; Schwarz, J.B.; Sabbatini, F.M.; Miglipre, M.; Bernardi, S.; Budassi, F.A. Indazoles and Azaindazoles as LRRK2 Inhibitors. Patent WO2022155419, 21 June 2022. [Google Scholar]
- Chen, Q.; Mao, Z.; Guo, F.; Liu, X. Indazolium halides as efficient ligands for Pd-catalyzed Suzuki–Miyaura cross-coupling of aryl bromides with arylboronic acids. Tetrahedron Lett. 2016, 57, 3735–3738. [Google Scholar] [CrossRef]
- Wang, P.; Farmer, M.E.; Huo, X.; Jain, P.; Shen, P.-X.; Ishoey, M.; Bradner, J.E.; Wisniewski, S.R.; Eastgate, M.D.; Yu, J.-Q. Ligand-Promoted Meta-C–H Arylation of Anilines, Phenols, and Heterocycles. J. Am. Chem. Soc. 2016, 138, 9269–9276. [Google Scholar] [CrossRef] [PubMed]
- Pattenden, G.; Palframan, M. Indirect Support for a Stepwise Carbonium Ion Pathway Operating in (4+3)-Cycloaddition Reactions between Furanoxonium Ions and 1,3-Dienes. Synlett 2013, 24, 2720–2722. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Wölfle, M.; Ata, F.; Hamzic, M.; Salathé, R.; Frey, W. Gold Catalysis: Dihydroisobenzofurans and Isochromanes by the Intramolecular Furan/Alkyne Reaction. Adv. Synth. Catal. 2006, 348, 2501–2508. [Google Scholar] [CrossRef]
- Agersø, Y.; Stuer-Lauridsen, B.; Bjerre, K.; Jensen, M.G.; Johansen, E.; Bennedsen, M.; Brockmann, E.; Nielsen, B. Antimicrobial Susceptibility Testing and Tentative Epidemiological Cutoff Values for Five Bacillus Species Relevant for Use as Animal Feed Additives or for Plant Protection. Appl. Environ. Microbiol. 2018, 84, e01108-18. [Google Scholar] [CrossRef] [PubMed]
- Fukumi, H.; Sugiyama, M.; Sakamoto, T. A Novel Heterocyclic Compound. Synthesis and Reactivities of an Oxazolo[3, 2-a]thieno[3, 2-d]pyrimidine Derivative. Chem. Pharm. Bull. 1989, 37, 1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kolesnikov, A.; Tay, S.; Chan, G.; Chao, Q.; Do, S.; Drummond, J.; Ebens, A.J.; Liu, N.; Ly, J.; et al. Discovery of 5-Azaindazole (GNE-955) as a Potent Pan-Pim Inhibitor with Optimized Bioavailability. J. Med. Chem. 2017, 60, 4458. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Method for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically; Approved Standard, 10th ed.; CLSI Document M07–A10; National Committee for Clinical and Laboratory Standards: Wayne, PA, USA, 2015. [Google Scholar]
- Forbes, B.A.; Sahm, D.F.; Weissfeld, A.S. Bailey and Scott’s Diagnostic Microbiology, 10th ed.; Mosby: St. Louis, MO, USA, 1998. [Google Scholar]



| MNS1 | MNS2 | MNS3 | MNS4 | MNS5 | MNS6 | |
|---|---|---|---|---|---|---|
| MIC with Gm (B. subtilis) a | ~50 μM | ~100 μM | ~90 μM | ~60 μM | ~60 μM | ~100 μM |
| MIC with Km (B. subtilis) b | 100 | – | 90 | 70 | 100 | – |
| CC50 of compounds (HEK273T) c | 30 μM | 25 μM | 190 μM | 87 μM | 250 μM | 260 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikov, R.A.; Platonov, D.N.; Belyy, A.Y.; Potapov, K.V.; Novikov, M.A.; Tomilov, Y.V.; Kechko, O.I.; Seregina, T.A.; Zemskaya, A.S.; Solyev, P.N.; et al. 6-Bromoindole- and 6-Bromoindazole-Based Inhibitors of Bacterial Cystathionine γ-Lyase Containing 3-Aminothiophene-2-Carboxylate Moiety. Molecules 2025, 30, 388. https://doi.org/10.3390/molecules30020388
Novikov RA, Platonov DN, Belyy AY, Potapov KV, Novikov MA, Tomilov YV, Kechko OI, Seregina TA, Zemskaya AS, Solyev PN, et al. 6-Bromoindole- and 6-Bromoindazole-Based Inhibitors of Bacterial Cystathionine γ-Lyase Containing 3-Aminothiophene-2-Carboxylate Moiety. Molecules. 2025; 30(2):388. https://doi.org/10.3390/molecules30020388
Chicago/Turabian StyleNovikov, Roman A., Dmitry N. Platonov, Alexander Yu. Belyy, Konstantin V. Potapov, Maxim A. Novikov, Yury V. Tomilov, Olga I. Kechko, Tatiana A. Seregina, Anastasia S. Zemskaya, Pavel N. Solyev, and et al. 2025. "6-Bromoindole- and 6-Bromoindazole-Based Inhibitors of Bacterial Cystathionine γ-Lyase Containing 3-Aminothiophene-2-Carboxylate Moiety" Molecules 30, no. 2: 388. https://doi.org/10.3390/molecules30020388
APA StyleNovikov, R. A., Platonov, D. N., Belyy, A. Y., Potapov, K. V., Novikov, M. A., Tomilov, Y. V., Kechko, O. I., Seregina, T. A., Zemskaya, A. S., Solyev, P. N., & Mitkevich, V. A. (2025). 6-Bromoindole- and 6-Bromoindazole-Based Inhibitors of Bacterial Cystathionine γ-Lyase Containing 3-Aminothiophene-2-Carboxylate Moiety. Molecules, 30(2), 388. https://doi.org/10.3390/molecules30020388







