Self-Assembly Strategy for Synthesis of WO3@TCN Heterojunction: Efficient for Photocatalytic Degradation and Hydrogen Production via Water Splitting
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology
2.2. Surface Chemical Composition Analysis
2.3. Structural Investigation
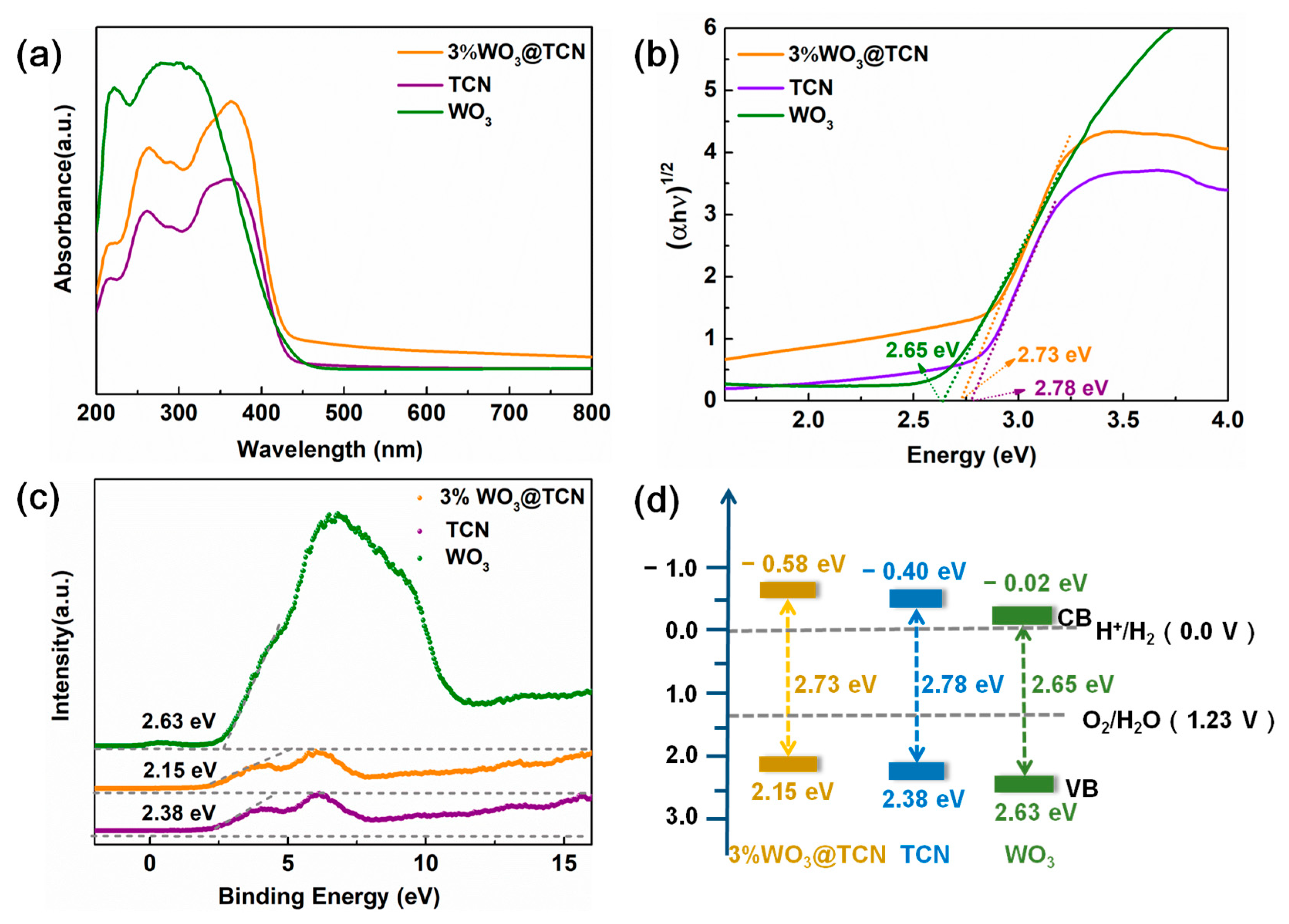
2.4. Photophysical Properties
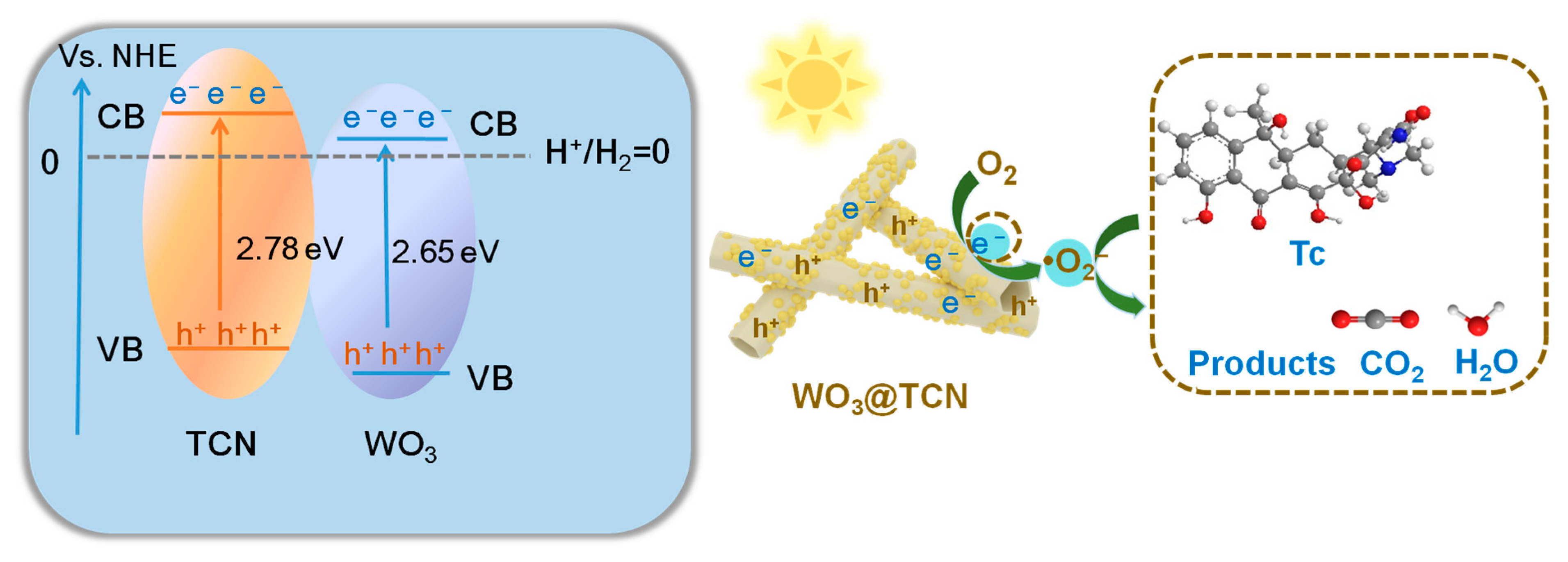
2.5. DFT Calculation and Mechanism Analysis
2.6. Photocatalytic Degradation of Tetracycline
2.7. Visible Photocatalytic Hydrogen Production Performance
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of Carbon Nitride Nanotubes (TCN)
3.3. Synthesis of WO3 Modified WO3@TCN Nanorods (WO3@TCN)
3.4. Measurements of Photocatalytic Degradation Performance
3.5. Photocatalytic Hydrogen Evolution from Water Splitting on WO3@TCN with Pt as the Cocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kumar, A.; Sharma, G.; Wang, T.; Iglesias-Juez, A.; Dhiman, P. Recent advances in g-C3N4/Metal organic frameworks heterojunctions for high-performance photocatalytic environmental remediation and energy production. J. Mol. Liq. 2023, 382, 121890. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tian, X.; Chen, Y.; Xiao, X.; Chen, T.; Wang, Y. Recent advances in carbon nitride-based S-scheme photocatalysts for solar energy conversion. Materials 2023, 16, 3745. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Song, H.; Guo, Y.; Hu, S.; Zheng, H.; Zhang, S.; Li, X.; Gao, Q.; Li, C.; et al. Photocatalytic hydrogen under visible light by nitrogen-doped rutile titania graphitic carbon nitride composites: An experimental and theoretical study. Adv. Compos. Hybrid Mater. 2023, 6, 83. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhan, X.; Hong, B.; Xia, Y.; Ding, Y.; Cai, T.; Yin, K.; Wang, X.; Yang, L.; Luo, S. Surface atom rearrangement on carbon nitride for enhanced photocatalysis degradation of antibiotics under visible light. Chem. Eng. J. 2023, 452, 139434. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K.; Vo, D.N. Removal of tetracycline from wastewater using g-C3N4 based photocatalysts: A review. Environ. Res. 2023, 216, 114660. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.K.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent advances in g-C3N4-based materials and their application in energy and environmental sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Mo, Z.; Di, J.; Yan, P.; Lv, C.; Zhu, X.; Liu, D.; Song, Y.; Liu, C.; Yu, Q.; Li, H.; et al. An all-organic D-A system for visible-light-driven overall water splitting. Small 2020, 16, e2003914. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.A.; Pham, C.T.N.; Ngoc, T.N.; Phi, H.N.; Ta, Q.T.H.; Truong, D.H.; Nguyen, V.T.; Luc, H.H.; Nguyen, L.T.; Dao, N.N.; et al. One-step synthesis of oxygen doped g-C3N4 for enhanced visible-light photodegradation of Rhodamine B. J. Phys. Chem. Solids 2021, 151, 109900. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Noh, M.F.M.; Arzaee, N.A.; Mansor, N.N.; Ibrahim, M.A.; Ludin, N.A.; Sagu, J.S.; Mat Teridi, M.A. Fabrication of exfoliated graphitic carbon nitride, (g-C3N4) thin film by methanolic dispersion. J. Alloys Compd. 2020, 818, 152916. [Google Scholar] [CrossRef]
- Gan, C.; Sheng, M.; Hu, Z.; Li, Y.; Peng, Y.; Xiang, Z.; Sun, B.; Jiang, H. Novel and efficient strategy for chlorophenols and CO2 transformation over carbon nitride nanotubes: Effect of the hydroxyl grafting and surface electron polarization. J. Catal. 2022, 413, 636–647. [Google Scholar] [CrossRef]
- Deng, Q.; Han, M.; Li, H.; Wang, G.; Hu, W.; Feng, L.; Hou, W. Interfacial affinity determined photocatalytic activity: A comparison between defective and bulk polymeric carbon nitride. ACS Appl. Mater. Interfaces 2023, 15, 31502–31513. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Sundar, J.K.; Alqarni, S.A.; Pandiaraj, S.; Hwan Oh, T. Cobalt oxide coupled with graphitic carbon nitride composite heterojunction for efficient Z-scheme photocatalytic environmental pollutants degradation performance. Environ. Res. 2023, 235, 116574. [Google Scholar] [CrossRef]
- Tang, R.; Zhou, Y.; Xiong, S.; Deng, Y.; Li, L.; Zhou, Z.; Zeng, H.; Wang, J.; Zhao, J.; Gong, D. Multiscale modification of carbon nitride-based homojunction for enhanced photocatalytic atrazine decomposition. J. Colloid Interface Sci. 2023, 630, 127–139. [Google Scholar] [CrossRef]
- Li, F.; Yue, X.; Liao, Y.; Qiao, L.; Lv, K.; Xiang, Q. Understanding the unique S-scheme charge migration in triazine/heptazine crystalline carbon nitride homojunction. Nat. Commun. 2023, 14, 3901. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, H.J.; Li, C.F.; Liu, J.; Dong, W.; Zhao, H.; Wang, C.; Liu, Y.; Hu, Z.Y.; Chen, L.; et al. Type II heterojunction in hierarchically porous zinc oxide/graphitic carbon nitride microspheres promoting photocatalytic activity. J. Colloid Interface Sci. 2019, 538, 99–107. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, S.Z.; Zhao, M.B.; Liu, L.Y.; Zhang, Z.F.; Ma, W.L. Acid-induced tubular g-C3N4 for the selective generation of singlet oxygen by energy transfer: Implications for the photocatalytic degradation of parabens in real water environments. Sci. Total Environ. 2023, 896, 165316. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, F.; Liu, W.; Chen, L.; Qi, J.; Sun, W.; Pan, F.; Duan, T.; Sun, F. Oxygen vacancy-induced spin polarization of tungsten oxide nanowires for efficient photocatalytic reduction and immobilization of uranium(VI) under simulated solar light. Appl. Catal. B Environ. 2023, 324, 122202. [Google Scholar] [CrossRef]
- Wang, T.; Quan, W.; Jiang, D.; Chen, L.; Li, D.; Meng, S.; Chen, M. Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity. Chem. Eng. J. 2016, 300, 280–290. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Li, C.; Dai, L.; Hao, Z.; Yu, J.; He, H.; Si, C.; Shen, Z.; Qiu, Z.; et al. Metal-free graphitic carbon nitride/black phosphorus quantum dots heterojunction photocatalyst for the removal of ARG contamination. Adv. Compos. Hybrid Mater. 2023, 6, 145. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.; Premkumar, S.; Yang, J.H.; Umapathy, S.; Vinu, A. Thermodynamically stable mesoporous C3N7 and C3N6 with ordered structure and their excellent performance for oxygen reduction reaction. Small 2020, 16, e1903572. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Z.; Muthu, M.; Zhang, Y. Construction of an in-situ Fenton-like system based on a g-C3N4 composite photocatalyst. J. Hazard. Mater. 2019, 373, 565–571. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, X.; Chang, B.; Zhou, Y.; Zhang, S.; He, G.; Yang, B.; Li, J. Fabrication of B doped g-C3N4/TiO2 heterojunction for efficient photoelectrochemical water oxidation. Electrochim. Acta 2018, 282, 767–774. [Google Scholar] [CrossRef]
- Hou, X.; Cui, L.; Du, H.; Gu, L.; Li, Z.; Yuan, Y. Lowering the schottky barrier of g-C3N4/carbon graphite heterostructure by N-doping for increased photocatalytic hydrogen generation. Appl. Catal. B Environ. 2020, 278, 119253. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Yu, Y.; Zhou, Y.; Zheng, Y.; Hao, S. The sulfur-bubble template-mediated synthesis of uniform porous g-C3N4 with superior photocatalytic performance. Chem. Commun. 2015, 51, 425–427. [Google Scholar] [CrossRef]
- Guan, S.; Zhou, F.; Tan, J.; Pan, M. Influence of pore size optimization in catalyst layer on the mechanism of oxygen transport resistance in PEMFCs. Prog. Nat. Sci. Mater. Int. 2020, 30, 839–845. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, H.; Cui, X.; Wang, X.; Ji, R.; Zhang, Y.; Qin, W. New strategy for enhancing the photocatalytic degradation of sulfadiazine by polymerized carbon nitride: Modulation of short-lived radicals to long-lifetime reactive species. Appl. Catal. B Environ. Energy 2024, 357, 124301. [Google Scholar] [CrossRef]
- Cai, H.; Wang, B.; Xiong, L.; Bi, J.; Yuan, L.; Yang, G.; Yang, S. Orienting the charge transfer path of type-II heterojunction for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 256, 117853. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Guo, X.; Jin, Z. Type-II CoMoO4/Graphdiyne heterojunction promotes visible-light-driven photocatalytic hydrogen production activity. Sep. Purif. Technol. 2024, 332, 125786. [Google Scholar] [CrossRef]
- Chen, J.; Tan, P.; Yang, L.; Liu, H.; Zhang, M.; Ren, R.; Zhai, H.; Liu, X.; Pan, J. Multiple chemical valences induced interface regulation in perovskite nickelate/carbon nitride for boosting photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 631, 102–111. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Niu, C.; Yuan, J.; Xu, F. Mpg-C3N4-ZIF-8 composites for the degradation of tetracycline hydrochloride using visible light. Water. Sci. Technol. 2019, 80, 2206–2217. [Google Scholar] [CrossRef]
- Xu, W.; Lai, S.; Pillai, S.C.; Chu, W.; Hu, Y.; Jiang, X.; Fu, M.; Wu, X.; Li, F.; Wang, H. Visible light photocatalytic degradation of tetracycline with porous Ag/graphite carbon nitride plasmonic composite: Degradation pathways and mechanism. J. Colloid Interface Sci. 2020, 574, 110–121. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Li, J.; Han, D.; Ma, Y.; Fan, Y.; Han, D.; Niu, L. Type II Heterojunction Formed between {010} or {012} Facets Dominated Bismuth Vanadium Oxide and Carbon Nitride to Enhance the Photocatalytic Degradation of Tetracycline. Int. J. Environ. Res. Pub. Health 2022, 19, 14770. [Google Scholar] [CrossRef]
- Xu, F.; An, N.; Lai, C.; Zhang, M.; Li, B.; Liu, S.; Li, L.; Qin, L.; Fu, Y.; Yi, H.; et al. Nitrogen-doping coupled with cerium oxide loading co-modified graphitic carbon nitride for highly enhanced photocatalytic degradation of tetracycline under visible light. Chemosphere 2022, 293, 133648. [Google Scholar] [CrossRef]
- Cao, M.; Zuo, J.; Huang, Y.; Liu, Z. Synthesis of tubular g-C3N4 via a H2SO4-assisted precursor self-assembly strategy for enhanced photocatalytic degradation of organic pollutant. J. Mater. Sci. Mater. Electron. 2020, 31, 2022–2029. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, J.; Qian, Y.; Chen, X.; Han, Y.; Zeng, X.; Qiu, B.; Zhu, Q. Order-disorder engineering of carbon nitride for photocatalytic H2O2 generation coupled with pollutant removal. ACS Appl. Mater. Interfaces 2024, 16, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Zhang, W.; Zhou, M.; Jiang, H.L. Heteroatom-doped Ag(25) nanoclusters encapsulated in metal-organic frameworks for photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2024, 63, e202401443. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Jiang, R.; Zhao, Y.; Mao, L.; Gu, X.; Cai, X.; Zhu, M. Hierarchical NiCo2S4/ZnIn2S4 heterostructured prisms: High-efficient photocatalysts for hydrogen production under visible-light. J. Colloid Interface Sci. 2022, 619, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, R.; Shang, L.; Wu, L.Z.; Tung, C.H.; Zhang, T. Template-free large-scale synthesis of g-C3N4 microtubes for enhanced visible light-driven photocatalytic H2 production. Nano Res. 2018, 11, 3462–3468. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, F.; Cao, F.; Zhao, X.; Meng, S.; Cui, Y. Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis. Appl. Catal. B Environ. 2017, 206, 417–425. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhang, W.; Huang, Z.; Hu, F.; Li, P.; Yao, X. Self-Assembly Strategy for Synthesis of WO3@TCN Heterojunction: Efficient for Photocatalytic Degradation and Hydrogen Production via Water Splitting. Molecules 2025, 30, 379. https://doi.org/10.3390/molecules30020379
Zhou L, Zhang W, Huang Z, Hu F, Li P, Yao X. Self-Assembly Strategy for Synthesis of WO3@TCN Heterojunction: Efficient for Photocatalytic Degradation and Hydrogen Production via Water Splitting. Molecules. 2025; 30(2):379. https://doi.org/10.3390/molecules30020379
Chicago/Turabian StyleZhou, Li, Wenjie Zhang, Zezhao Huang, Feng Hu, Peng Li, and Xiaoquan Yao. 2025. "Self-Assembly Strategy for Synthesis of WO3@TCN Heterojunction: Efficient for Photocatalytic Degradation and Hydrogen Production via Water Splitting" Molecules 30, no. 2: 379. https://doi.org/10.3390/molecules30020379
APA StyleZhou, L., Zhang, W., Huang, Z., Hu, F., Li, P., & Yao, X. (2025). Self-Assembly Strategy for Synthesis of WO3@TCN Heterojunction: Efficient for Photocatalytic Degradation and Hydrogen Production via Water Splitting. Molecules, 30(2), 379. https://doi.org/10.3390/molecules30020379





