Acid-Catalyzed, Metal- and Oxidant-Free C=C Bond Cleavage of Enaminones: One-Pot Synthesis of 3,4-Dihydroquinazolines
Abstract
1. Introduction
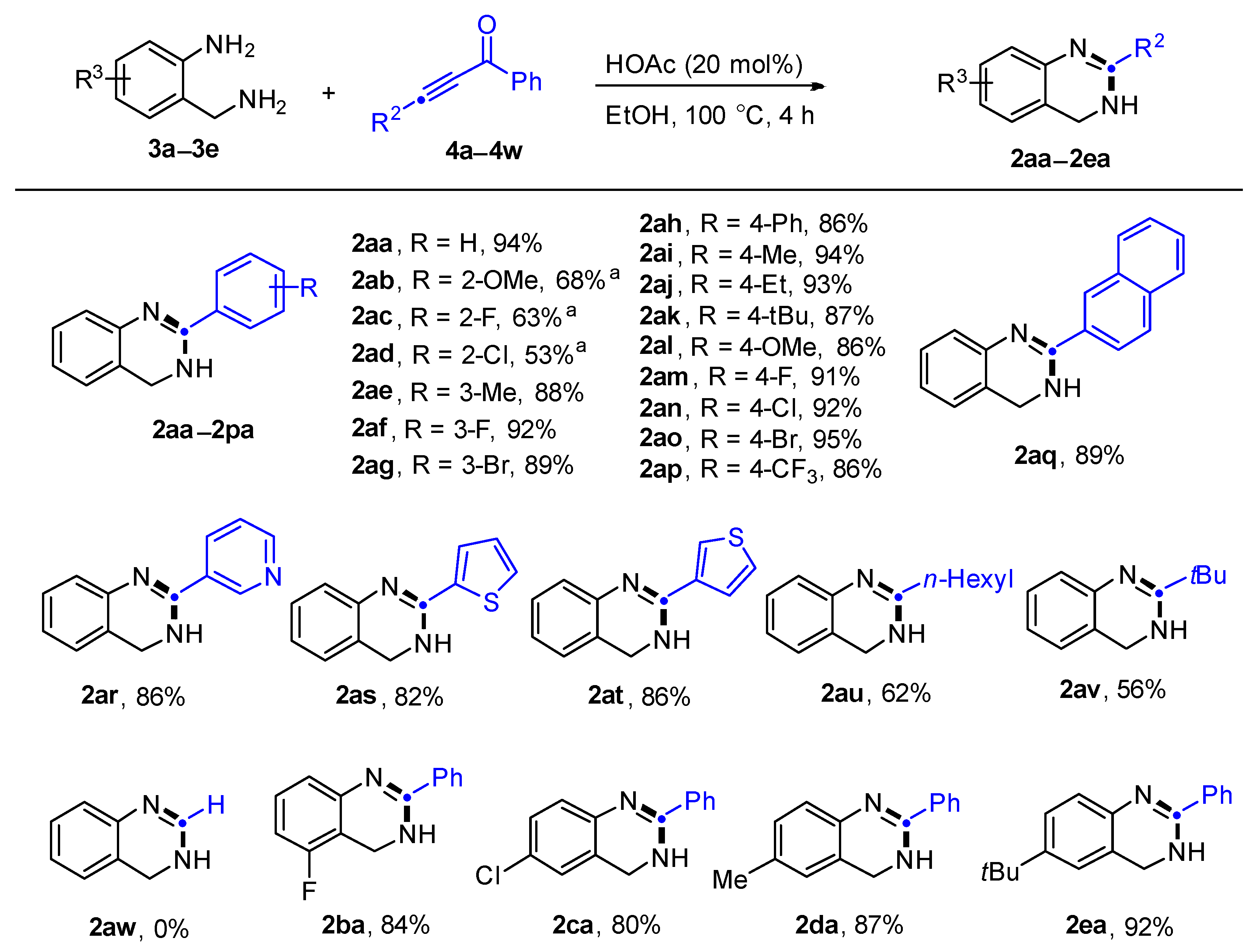
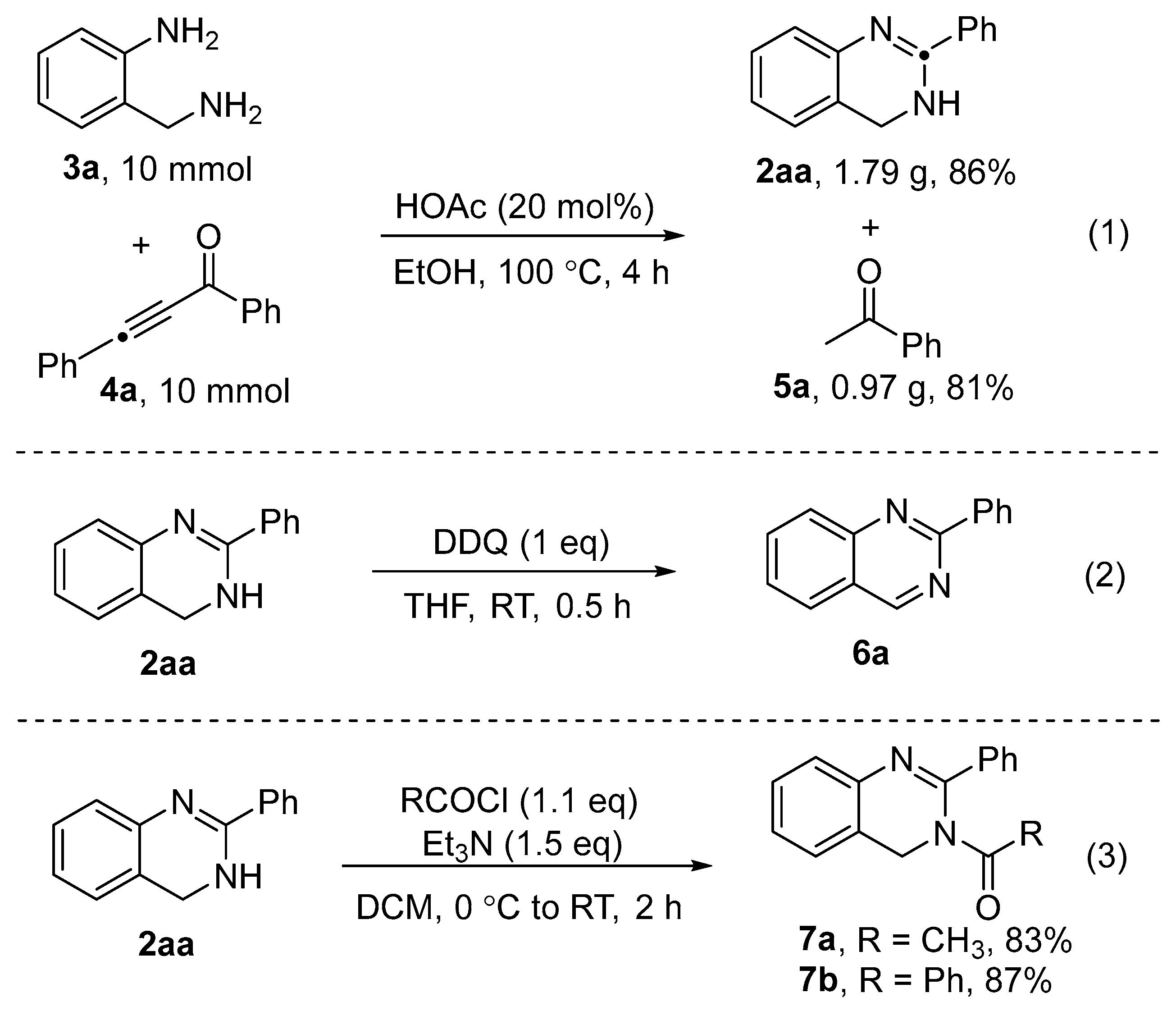
2. Results and Discussion
3. Experimental Sections
3.1. General Information
3.2. General Procedure for the Synthesis of 3,4-Dihydroquinazolines 2
3.3. Procedure for Synthesis of 6a
3.4. Procedure for Synthesis of 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claeson, U.P.; Malmfors, T.; Wikman, G.; Bruhn, J.G. Traditional herbal medicine research and applications in Europe. J. Ethnopharmacol. 2000, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Ojha, R.; Dhar, K.L. Antioxidant and free radical scavenging activities of some medicinal plants from the Nepalese Himalayas. Med. Chem. Res. 2013, 22, 1–15. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; He, D.-D.; Li, S.-P.; Zhu, Y.-D.; Jiang, B.; Cheng, X.-M.; Wang, Z.-T.; Wang, C.-H. Anti-inflammatory and antioxidant activities of rhizoma Dioscoreae extract. Phytomedicine 2015, 22, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Alphey, M.S.; Jones, D.C.; Shanks, E.J.; Street, I.P.; Frearson, J.A.; Wyatt, P.G.; Gilbert, I.H.; Fairlamb, A.H. Exploring the mechanism of action of antitrypanosomal compounds. J. Med. Chem. 2011, 54, 6514–6530. [Google Scholar] [CrossRef]
- Goldner, T.; Hewlett, G.; Ettischer, N.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. Identification of novel inhibitors of the cytomegalovirus DNA polymerase. J. Virol. 2011, 85, 10884–10893. [Google Scholar] [CrossRef]
- Marschall, M.; Stamminger, T.; Urban, A.; Wildum, S.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. Identification of novel antiviral agents targeting herpes simplex virus DNA polymerase. Antimicrob. Agents Chemother. 2012, 56, 1135–1137. [Google Scholar] [CrossRef]
- Li, W.-J.; Li, Q.; Liu, D.-L.; Ding, M.-W. Chemical composition and antimicrobial activity of essential oil from Mentha longifolia L. J. Agric. Food Chem. 2013, 61, 1419–1426. [Google Scholar] [CrossRef]
- Rim, H.-K.; Lee, H.-W.; Choi, I.S.; Park, J.Y.; Choi, H.W.; Choi, J.-H.; Cho, Y.-W.; Lee, J.Y.; Lee, K.-T. Synthesis and biological evaluation of new antibacterial agents. Bioorg. Med. Chem. Lett. 2012, 22, 7123–7126. [Google Scholar] [CrossRef]
- Jang, S.J.; Choi, H.W.; Choi, D.L.; Cho, S.; Rim, H.-K.; Choi, H.-E.; Kim, K.-S.; Huang, M.; Rhim, H.; Lee, K.-T.; et al. Synthesis and evaluation of new antitumor agents. Bioorg. Med. Chem. Lett. 2013, 23, 6656–6662. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, S.H.; Kang, H.B.; Park, H.A.; Chang, S.K.; Kim, J.; Choo, D.J.; Oh, C.R.; Kim, Y.D.; Seo, J.H. Antitumor activity of 3, 4-dihydroquinazoline dihydrochloride in A549 xenograft nude mice. Bioorg. Med. Chem. Lett. 2010, 20, 6633–6636. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X.-F. Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065–12080. [Google Scholar] [CrossRef]
- Campbell, M.V.; Iretskii, A.V.; Mosey, R.A. One-Pot Tandem Assembly of Amides, Amines, and Ketones: Synthesis of C4-Quaternary 3,4- and 1,4-Dihydroquinazolines. J. Org. Chem. 2020, 85, 11211–11225. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Saidulu, G.; Sridhar, B.; Liu, S.T.; Reddy, K.R. Highly Efficient Nickel-Catalyzed Synthesis of α-Ketoamides via Cross-Dehydrogenative Coupling of Secondary Amines with 1,3-Dicarbonyl Compounds. J. Org. Chem. 2013, 78, 10240–10250. [Google Scholar] [CrossRef]
- Kobayashi, K.; Matsumoto, N.; Nagashima, M.; Inouchi, H. Synthesis of Substituted Benzoxazinones Using Oxidative Coupling Reactions. Helv. Chim. Acta 2015, 98, 184–189. [Google Scholar] [CrossRef]
- Ren, J.; Pi, C.; Wu, Y.; Cui, X. Copper-Catalyzed Oxidative Cross-Dehydrogenative Cyclization: Synthesis of 3,4-Dihydroquinazolines. Org. Lett. 2019, 21, 4067–4071. [Google Scholar] [CrossRef]
- Carlson, H.M.; Smith, S.R.; Mosey, R.A. Direct Formation of C–C, C–N, and C–O Bonds in Dihydroquinazolines via Hypervalent Iodine (III)-Mediated sp3 C–H Functionalization. J. Org. Chem. 2024, 89, 1160–1174. [Google Scholar] [CrossRef]
- Chen, S.; Ji, Y.S.; Choi, Y.; Youn, S.W. One-Pot Three-Component Reaction for the Synthesis of 3,4-Dihydroquinazolines and Quinazolin-4(3H)-ones. J. Org. Chem. 2024, 89, 6428–6443. [Google Scholar] [CrossRef]
- Gruber, N.; Díaz, J.E.; Orelli, L.R. Synthesis of Dihydroquinazolines from 2-Aminobenzylamine: N3-Aryl Derivatives with Electron-Withdrawing Groups. Beilstein J. Org. Chem. 2018, 14, 2510–2519. [Google Scholar] [CrossRef]
- Li, C.; An, S.; Zhu, Y.; Zhang, J.; Kang, Y.; Liu, P.; Wang, Y.; Li, J. Copper-catalyzed intermolecular cyclization of nitriles and 2-aminobenzylamine for 3,4-dihydroquinazolines and quinazolines synthesis via cascade coupling and aerobic oxidation. RSC Adv. 2014, 4, 49888–49891. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Grishin, I.Y.; Aksenov, N.A.; Malyuga, V.V.; Aksenov, D.A.; Nobi, M.A.; Rubin, M. Electrophilically Activated Nitroalkanes in Synthesis of 3,4-Dihydroquinozalines. Molecules 2021, 26, 4274. [Google Scholar] [CrossRef]
- Xie, Z.K.; Ding, J.J.; Ou, Y.M.; Shi, J.X.; Shen, M.L.; Yao, C.Z.; Jiang, H.J.; Yu, J. De novo Synthesis of Chiral 3,4-DihydroquinazolinesviaOne-Pot Enantioselective Ugi-Azide/Cyclization Sequences. Chin. J. Chem. 2024, 42, 2140–2146. [Google Scholar] [CrossRef]
- Xiong, J.; He, H.-T.; Yang, H.-Y.; Zeng, Z.-G.; Zhong, C.-R.; Shi, H.; Ouyang, M.-L.; Tao, Y.-Y.; Pang, Y.-L.; Zhang, Y.-H.; et al. Synthesis of 4-Tetrazolyl-Substituted 3,4-Dihydroquinazoline Derivatives with Anticancer Activity via a One-Pot Sequential Ugi-Azide/Palladium-Catalyzed Azide-Isocyanide Cross-Coupling/Cyclization Reaction. J. Org. Chem. 2022, 87, 9488–9496. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Zhang, X.; Wan, J.-P. Recent advances in transition metal-free annulation toward heterocycle diversity based on the C–N bond cleavage of enaminone platform. Org. Biomol. Chem. 2022, 20, 2356–2369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kumar, M.; Kumar, S.; Bhattacherjee, D.; Shil, A.K.; Mehta, M.; Das, P. β-Enaminones from cyclohexane-1,3-diones: Versatile precursors for nitrogen and oxygen-containing heterocycles synthesis. Synth. Commun. 2023, 53, 953–993. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, B.; Liu, Y.; Wan, J.P. Recent advances in reactions using enaminone in water or aqueous medium. Adv. Synth. Catal. 2022, 364, 1508–1521. [Google Scholar] [CrossRef]
- Yu, F.; Huang, J. Recent advances in organic synthesis based on N,N-dimethyl enaminones. Synthesis 2020, 53, 587–610. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J.-P. Recent advances in visible light-mediated chemical transformations of enaminones. Chin. Chem. Lett. 2024, 35, 108977. [Google Scholar] [CrossRef]
- Wan, J.-P.; Lin, Y.; Cao, X.; Liu, Y.; Wei, L. Copper-catalyzed, hypervalent iodine mediated CC bond activation of enaminones for the synthesis of α-keto amides. Chem. Commun. 2016, 52, 1270–1273. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.; Wan, J.P. Ambient and aerobic carbon–carbon bond cleavage toward α-ketoester synthesis by transition-metal-free photocatalysis. Green Chem. 2019, 21, 3436–3441. [Google Scholar] [CrossRef]
- Cao, S.; Zhong, S.; Xin, L.; Wan, J.-P.; Wen, C. Visible-light-induced C–C bond cleavage of enaminones for the synthesis of 1,2-diketones and quinoxalines in sustainable medium. ChemCatChem 2015, 7, 1478–1482. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Liu, H.; Guo, M. Metal-free TBAI-catalyzed Oxidative Csp3-S Bond Formation through Csp2-Csp2 Bond and S-N Bond Cleavage: A New Route to β-Keto-Sulfones. Tetrahedron Lett. 2018, 59, 3703–3705. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, B.; Li, L.; Rao, K.; Yang, J.; Yu, F. Mn(OAc)3-Promoted Oxidative Csp3-P Bond Formation through Csp2-Csp2 and P-H Bond Cleavage: Access to β-Ketophosphonates. J. Org. Chem. 2017, 82, 13268–13276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, T.; Wan, J.-P. Ultrasound-Promoted Synthesis of α-Thiocyanoketones via Enaminone C=C Bond Cleavage and Tunable One-Pot Access to 4-Aryl-2-aminothiazoles. J. Org. Chem. 2022, 87, 8248–8255. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Gao, Y.; Wei, L.; Wan, J.-P. Synthesis of a-keto thioamides by metal-free C=C bond cleavage in enaminones using elemental sulfur. J. Org. Chem. 2019, 84, 1064–1069. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, J.; Wei, L.; Wan, J.-P. Switchable synthesis of α,α-dihalomethyl and α,α,α-trihalomethyl ketones by metal-free decomposition of enaminone C=C double bond. Adv. Synth. Catal. 2020, 362, 877–883. [Google Scholar] [CrossRef]
- Gan, L.; Yu, Q.; Liu, Y.; Wan, J.P. Scissoring Enaminone C=C Double Bond by Free Radical Process for the Synthesis of α-Trifluoromethyl Ketones with CF3SO2Na. J. Org. Chem. 2021, 86, 1231–1237. [Google Scholar] [CrossRef]
- Tian, L.; Guo, Y.; Wei, L.; Wan, J.-P.; Sheng, S. Thermo-induced free-radical cleavage of enaminone C=C double bond for α-acyloxyl ketone synthesis. Asian J. Org. Chem. 2019, 8, 1484–1489. [Google Scholar] [CrossRef]
- Gan, L.; Wei, L.; Wan, J.-P. Catalyst-Free Synthesis of α-Diazoketones in Water by Microwave Promoted Enaminone C=C Double Bond Cleavage. ChemistrySelect 2020, 5, 7822–7825. [Google Scholar] [CrossRef]
- Ge, B.; Peng, Y.; Liu, J.; Wen, S.; Peng, C.; Cheng, G. Acid-Promoted cleavage of the C−C double bond of N-(2-hydroxylphenyl)enaminones for the synthesis of benzoxazoles. Tetrahedron 2020, 76, 130818–130825. [Google Scholar] [CrossRef]
- Wan, J.P.; Zhou, Y.; Liu, Y.; Sheng, S. Metal-free Oxidative Carbonylation on Enaminone C = C Bond for the Cascade Synthesis of Benzothiazole-containing Vicinal Diketones. Green Chem. 2016, 18, 402–405. [Google Scholar] [CrossRef]
- Xie, C.; Feng, L.; Li, W.; Ma, X.; Ma, X.; Liu, Y.; Ma, C. Efficient synthesis of pyrrolo [1,2-a]quinoxalines catalyzed by a Brønsted acid through cleavage of C–C bonds. Org. Biomol. Chem. 2016, 14, 8529–8535. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhou, H.; Du, P.; Liu, S.; Zou, K.; Uozumi, Y. Brønsted acid-catalyzed selective C–C bond cleavage of 1,3-diketones: A facile synthesis of 4 (3 H)-quinazolinones in aqueous ethyl lactate. RSC Adv. 2015, 5, 85646–85651. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, G.; Shen, J.; Kuai, C.; Cui, X. Cleavage of the C–C triple bond of ketoalkynes: Synthesis of 4(3 H)-quinazolinones. Org. Chem. Front. 2015, 2, 366–368. [Google Scholar] [CrossRef]
- Xu, L.; Wu, L.; Chen, T.; Xu, S.; Huang, C.; Wang, Y.; You, Q.; Shen, J. Superbase-promoted N-α-sp3C-H functionalization of enaminones: Synthesis of polysubstituted pyrroles. ChemistrySelect 2020, 5, 655–659. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, X.; Wang, W.; Feng, Y.; Wang, Y.; Shen, J. C–C Bond Cleavage Initiated Cascade Reaction of β-Enaminones: One-Pot Synthesis of 5-Hydroxy-1H-pyrrol-2(5H)-ones. J. Org. Chem. 2021, 86, 2917–2928. [Google Scholar] [CrossRef]
- Cui, X.; Chen, Y.; Wang, W.; Zeng, T.; Li, Y.; Wang, X. Chemoselective synthesis of β-enaminones from ynones and aminoalkyl-, phenol- and thioanilines under metal-free conditions. Chem. Pap. 2021, 75, 3625–3634. [Google Scholar] [CrossRef]
- Cox, R.J.; Ritson, D.J.; Dane, T.A.; Berge, J.; Charmant, J.P.H.; Kantacha, A. Room temperature palladium catalysed coupling of acyl chlorides with terminal alkynes. Chem. Commun. 2005, 1037–1039. [Google Scholar] [CrossRef]
- Chatterjee, T.; Kim, D.I.; Cho, E.J. Base-Promoted Synthesis of 2-Aryl Quinazolines from 2-Aminobenzylamines in Water. J. Org. Chem. 2018, 83, 7423–7430. [Google Scholar] [CrossRef]
- Diaz, J.E.; Ranieri, S.; Gruber, N.; Orelli, L.R. Syntheses of 3,4- and 1,4-dihydroquinazolines from 2-aminobenzylamine. Beilstein J. Org. Chem. 2017, 13, 1470–1477. [Google Scholar] [CrossRef]
- Wiedemann, S.H.; Ellman, J.A.; Bergman, R.G. Rhodium-Catalyzed Direct C–H Addition of 3,4-Dihydroquinazolines to Alkenes and Their Use in the Total Synthesis of Vasicoline. J. Org. Chem. 2006, 71, 1969–1976. [Google Scholar] [CrossRef]




 | |||||
|---|---|---|---|---|---|
| Entry | Additive | Solvent | T (°C) | R1 | Yield(%) b |
| 1 | no | EtOH | 100 | Ph | 43 |
| 2 | NaOH | EtOH | 100 | Ph | 52 |
| 3 | KOH | EtOH | 100 | Ph | 46 |
| 4 | Et3N | EtOH | 100 | Ph | 53 |
| 5 | HOAc | EtOH | 100 | Ph | 96 |
| 6 | TFA | EtOH | 100 | Ph | 88 |
| 7 | TsOH·H2O | EtOH | 100 | Ph | 91 |
| 8 | NH4Cl | EtOH | 100 | Ph | 94 |
| 9 | (NH4)2S2O4 | EtOH | 100 | Ph | 93 |
| 10 | HOAc | MeOH | 100 | Ph | 88 |
| 11 | HOAc | iPrOH | 100 | Ph | 91 |
| 12 | HOAc | DMSO | 100 | Ph | 38 |
| 13 | HOAc | DMF | 100 | Ph | 88 |
| 14 | HOAc | CH3CN | 100 | Ph | 55 |
| 15 | HOAc | PhMe | 100 | Ph | 37 |
| 16 | HOAc | dioxane | 100 | Ph | 70 |
| 17 | HOAc | EtOH | 90 | Ph | 92 |
| 18 | HOAc | EtOH | 110 | Ph | 96 |
| 19 | HOAc | EtOH | 100 | 4–OMeC6H4– | 89 |
| 20 | HOAc | EtOH | 100 | 4–FC6H4– | 94 |
| 21 | HOAc | EtOH | 100 | Me | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Huang, T.; Ye, M.; Shen, J. Acid-Catalyzed, Metal- and Oxidant-Free C=C Bond Cleavage of Enaminones: One-Pot Synthesis of 3,4-Dihydroquinazolines. Molecules 2025, 30, 350. https://doi.org/10.3390/molecules30020350
Chen T, Huang T, Ye M, Shen J. Acid-Catalyzed, Metal- and Oxidant-Free C=C Bond Cleavage of Enaminones: One-Pot Synthesis of 3,4-Dihydroquinazolines. Molecules. 2025; 30(2):350. https://doi.org/10.3390/molecules30020350
Chicago/Turabian StyleChen, Ting, Ting Huang, Moudan Ye, and Jinhai Shen. 2025. "Acid-Catalyzed, Metal- and Oxidant-Free C=C Bond Cleavage of Enaminones: One-Pot Synthesis of 3,4-Dihydroquinazolines" Molecules 30, no. 2: 350. https://doi.org/10.3390/molecules30020350
APA StyleChen, T., Huang, T., Ye, M., & Shen, J. (2025). Acid-Catalyzed, Metal- and Oxidant-Free C=C Bond Cleavage of Enaminones: One-Pot Synthesis of 3,4-Dihydroquinazolines. Molecules, 30(2), 350. https://doi.org/10.3390/molecules30020350






