Multivariate Statistical Analyses of the Temporal Variation in the Chemical Composition of the Essential Oil of Eucalyptus torquata in Cyprus
Abstract
1. Introduction
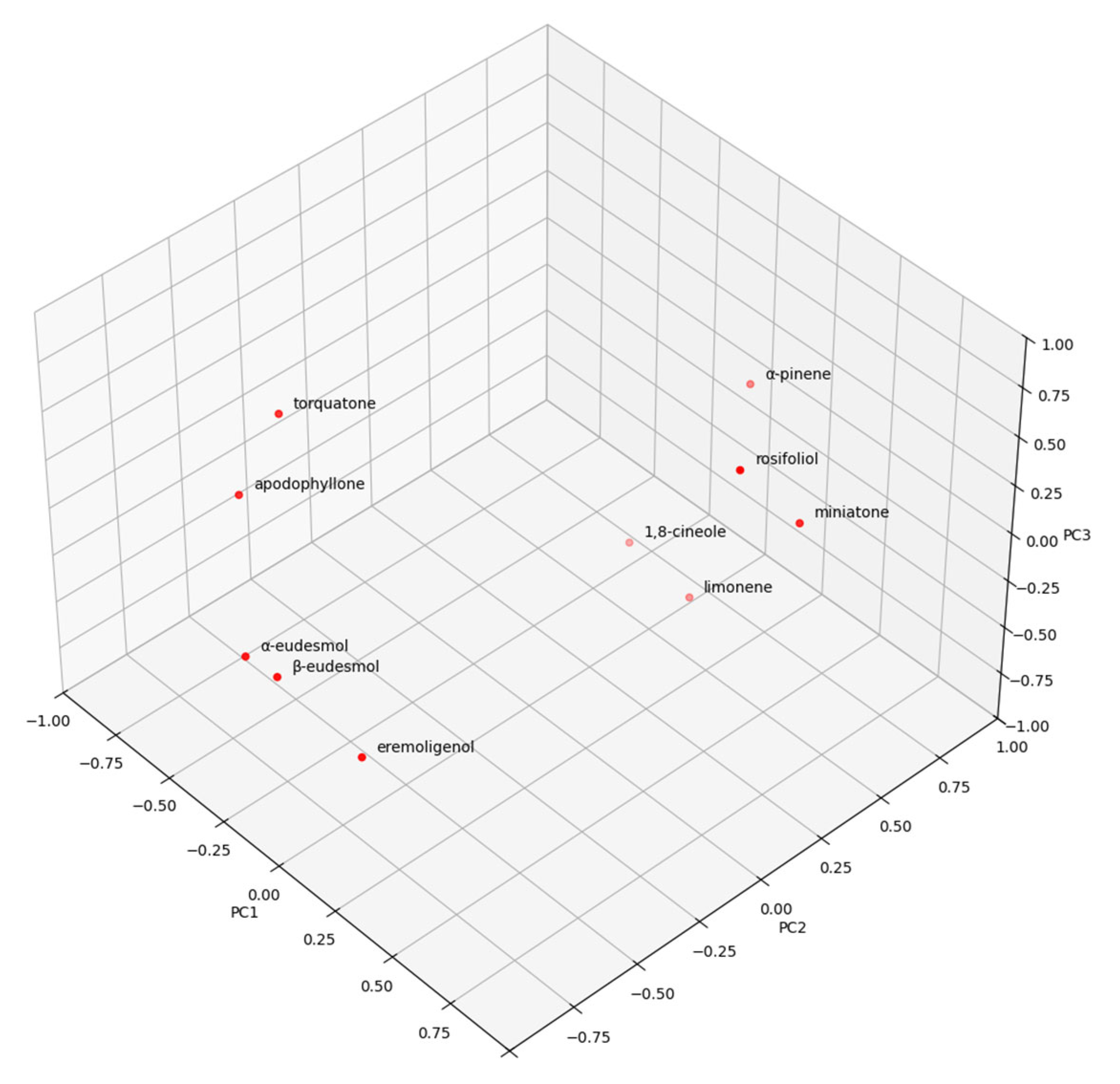
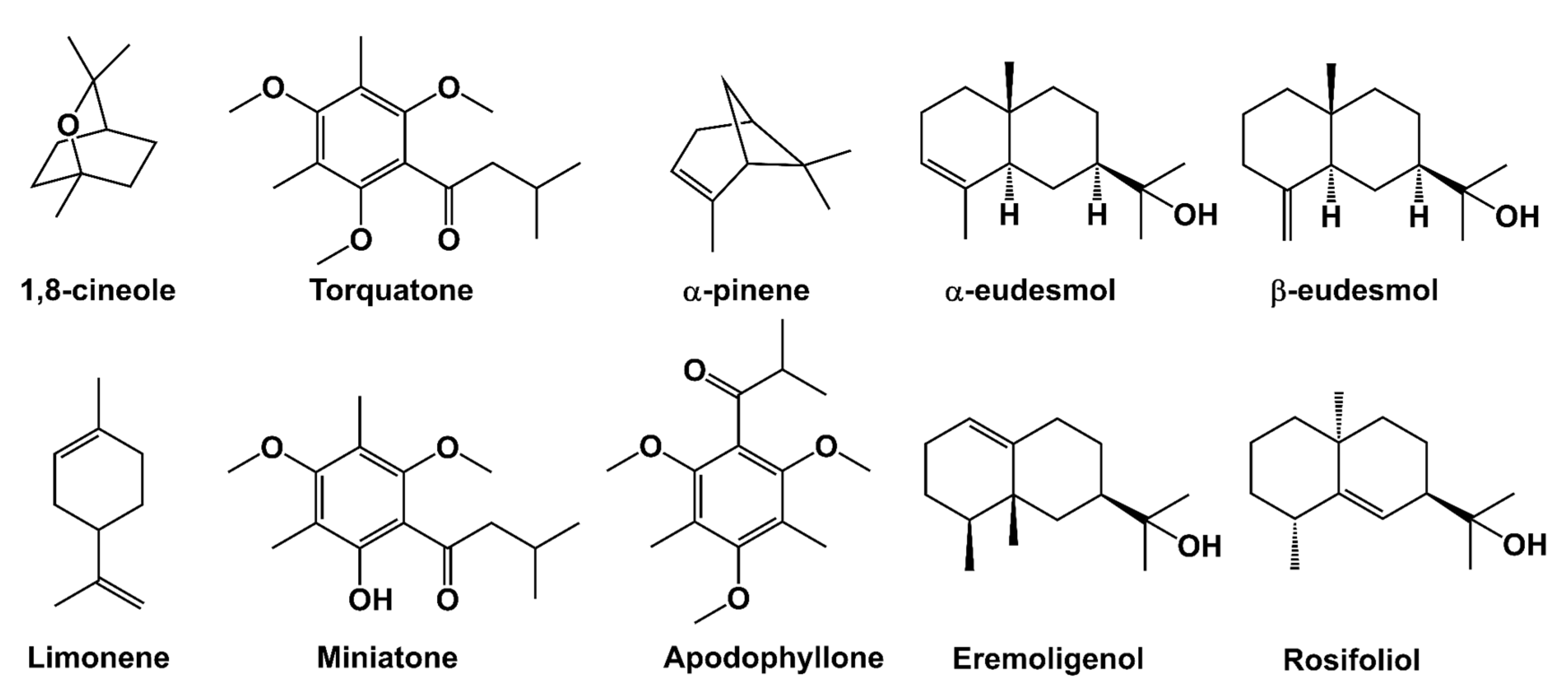
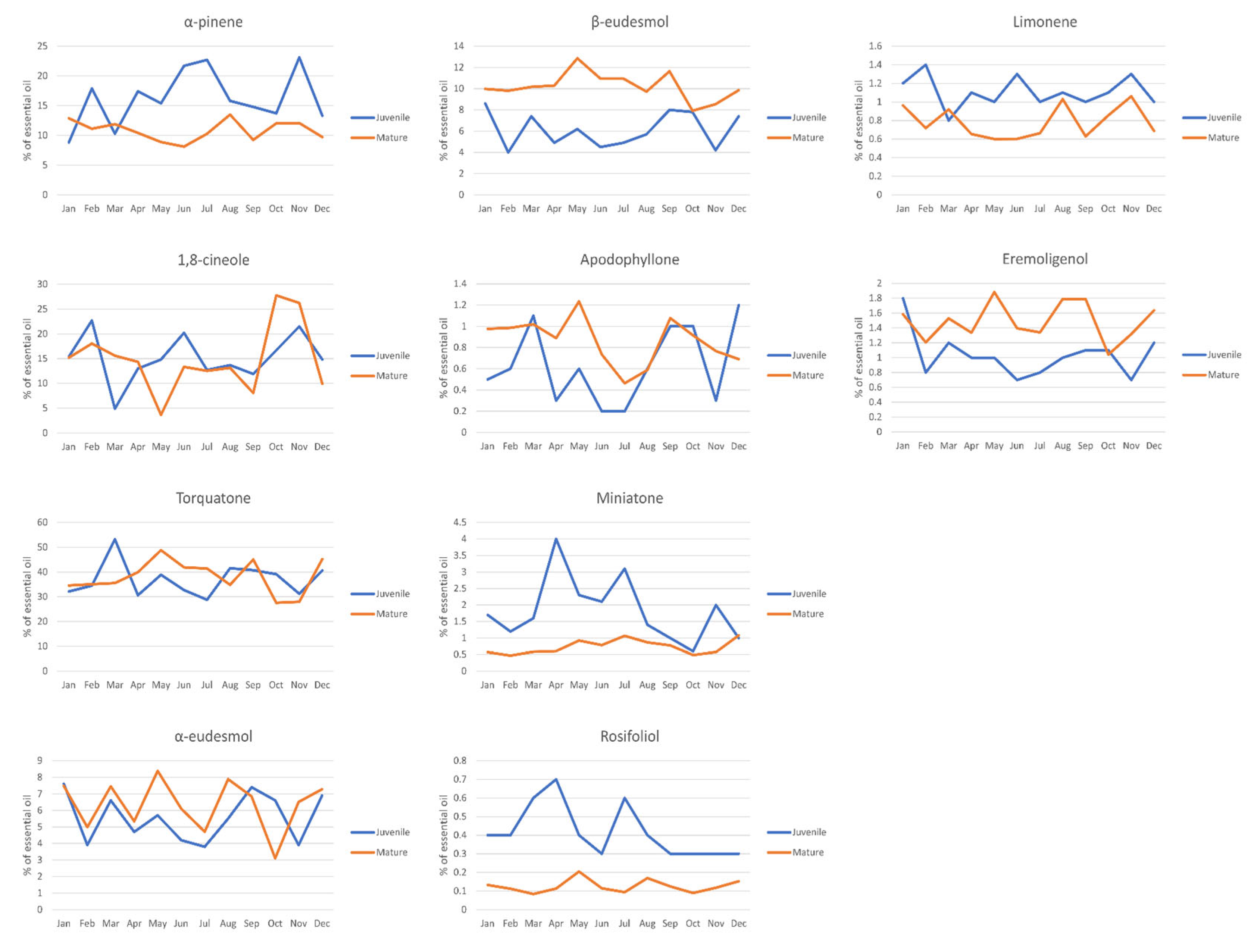
2. Results and Discussion
| Code | Collection Date | Essential Oil Yields (%, v/w) | |
|---|---|---|---|
| Juvenile Leaves | Mature Leaves | ||
| Jan | 13 Janaury 2023 | 1.56 | 1.50 |
| Feb | 12 February 2023 | 0.96 | 2.00 |
| Mar | 15 March 2023 | 0.53 | 2.00 |
| Apr | 10 April 2023 | 0.91 | 1.00 |
| May | 11 May 2023 | 2.59 | 1.00 |
| Jun | 13 June 2023 | 1.71 | 1.60 |
| Jul | 11 July 2023 | 1.08 | 2.00 |
| Aug | 10 August 2023 | 2.36 | 1.00 |
| Sep | 10 September 2023 | 3.12 | 2.00 |
| Oct | 10 October 2023 | 2.77 | 1.60 |
| Nov | 10 November 2023 | 3.24 | 1.80 |
| Dec | 10 December 2023 | 1.81 | 3.18 |
| LRI Lit. | LRI PS | Compound Name | Relative Percentage Amounts in Juvenile Leaves (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | Jun | Jul | Agu | Sep | Oct | Nov | Dec | |||
| 1008–1039 b | 1015 | α-Pinene | 8.8 | 17.9 | 10.3 | 17.4 | 15.4 | 21.7 | 22.7 | 15.8 | 14.8 | 13.7 | 23.1 | 13.3 |
| 1043–1086 b | 1035 | Camphene | - | - | - | 0.1 | tr | - | 0.1 | tr | 0.1 | tr | - | tr |
| 1085–1130 b | 1082 | β-Pinene | 1.0 | 0.6 | 0.3 | 0.4 | 0.4 | 0.6 | 0.5 | 0.5 | 0.4 | 0.4 | 0.7 | 0.4 |
| 1140–1175 b | 1136 | Myrcene | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.1 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 |
| 1148–1186 b | 1140 | α-Phellandrene | 0.8 | 0.6 | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 | 0.6 | 0.6 | 0.7 | 0.4 | 0.3 |
| 1154–1195 b | 1155 | α-Terpinene * | - | 0.1 | - | - | - | - | - | 0.1 | 0.1 | tr | - | tr |
| 1178–1219 b | 1173 | Limonene | 1.2 | 1.4 | 0.8 | 1.1 | 1.0 | 1.3 | 1.0 | 1.1 | 1.0 | 1.1 | 1.3 | 1.0 |
| 1188–1233 b | 1184 | β-Phellandrene | - | - | - | - | 0.1 | - | - | 0.1 | 0.1 | 0.1 | - | tr |
| 1186–1231 b | 1186 | 1,8-Cineole | 15.4 | 22.7 | 4.9 | 13.0 | 14.8 | 20.2 | 12.7 | 13.7 | 11.9 | 16.7 | 21.5 | 14.8 |
| 1222–1266 b | 1218 | γ-Terpinene | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1246–1291 b | 1246 | p-Cymene | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 | 0.4 | 0.6 | 0.5 | 0.4 | 0.6 | 0.2 | 0.3 |
| 1261–1300 b | 1256 | Terpinolene | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1511–1545 b | 1497 | α-Gurjunene * | 0.4 | 0.2 | 0.1 | 0.5 | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 |
| 1545–1590 b | 1551 | Pinocarvone | 0.3 | 0.1 | tr | 0.3 | 0.2 | 0.2 | 0.5 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
| 1564–1618 b | 1562 | β-Gurjunene * | - | - | - | 0.1 | tr | - | 0.1 | - | tr | tr | - | tr |
| 1570–1685 b | 1568 | β-Caryophyllene | - | - | - | - | 0.1 | 0.1 | tr | - | 0.1 | tr | - | tr |
| 1564–1630 b | 1573 | Terpinen-4-ol | - | 0.2 | 0.1 | 0.4 | 0.2 | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| 1583–1668 b | 1577 | Aromadendrene | 3.2 | 1.1 | 0.8 | 3.8 | 1.2 | 1.6 | 3.5 | 0.9 | 0.6 | 0.4 | 1.4 | 0.6 |
| 1585 | Selina-5,11-diene * | - | - | - | 0.1 | 0.1 | 0.1 | tr | tr | tr | 0.2 | tr | ||
| 1624–1668 b | 1616 | Alloaromadendrene | 0.3 | 0.1 | 0.1 | 0.4 | 0.2 | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 | - | 0.1 |
| 1643–1671 b | 1629 | trans-Pinocarveol | 0.9 | 0.2 | 0.1 | 0.6 | 0.5 | 0.6 | 1.7 | 0.1 | 0.2 | 0.4 | 0.7 | 0.2 |
| 1637–1689 b | 1641 | α-Humulene * | - | - | - | - | tr | - | tr | tr | tr | tr | - | tr |
| 1620–1679 a | 1658 | E-Methyl geranate * | - | - | - | - | 0.1 | - | tr | tr | - | tr | - | 0.1 |
| 1656–1707 a | 1663 | Ledene * | 0.4 | 0.3 | 0.2 | 0.5 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 |
| 1629–1724 b | 1665 | α-Terpineol | 0.9 | 0.7 | 0.4 | 1.1 | 0.5 | 0.6 | 0.9 | 0.5 | 0.4 | 0.3 | 0.5 | 0.4 |
| 1653–1728 b | 1672 | Borneol | 0.3 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1688–1761 b | 1691 | Valencene * | - | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 |
| 1664–1688 a | 1694 | Selina-4,11-diene * | - | - | - | 0.1 | tr | 0.1 | tr | 0.1 | 0.1 | tr | - | tr |
| 1704–1737 a | 1697 | β-Dihydro agarofuran * | - | 0.1 | - | tr | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | - | 0.1 |
| 1840–1949 b | 1711 | Piperitone | - | 0.1 | - | - | tr | - | tr | tr | tr | tr | - | tr |
| 1722–1774 b | 1720 | δ-Cadinene * | - | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | - | tr |
| 1743–1808 b | 1762 | Myrtenol | - | 0.1 | - | - | 0.1 | tr | 0.1 | tr | - | tr | - | 0.1 |
| 1790–1886 a | 1770 | Calamenene * | - | - | - | 0.1 | tr | 0.1 | 0.1 | tr | tr | tr | - | tr |
| 1893 | 5,11-Epoxy-1(10)-cadinene * | - | - | - | 0.1 | tr | - | 0.1 | tr | - | - | - | - | |
| 1911–1938 b | 1904 | Palustrol | - | - | - | 0.1 | tr | tr | 0.1 | tr | tr | tr | - | tr |
| 1969 | Amyl phenyl acetate * | - | - | - | 0.1 | tr | 0.1 | 0.1 | tr | - | tr | - | tr | |
| 1973 a | 1974 | Maaliol | 0.3 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2025–2033 a | 1982 | epi-globulol | 1.1 | 0.5 | 0.5 | 1.1 | 0.4 | 0.4 | 0.9 | 0.5 | 0.3 | 0.3 | 0.4 | 0.4 |
| 2014–2062 b | 2006 | Ledol | - | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2061–2074 a | 2025 | Cubeban-11-ol | 0.3 | 0.1 | 0.2 | 0.3 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2065 a | 2043 | 10-epi-elemol * | - | - | - | - | tr | - | tr | - | 0.1 | 0.1 | - | 0.1 |
| 2049–2104 b | 2049 | Globulol | 4.4 | 2.3 | 2.6 | 4.7 | 1.8 | 1.6 | 4.0 | 2.2 | 1.4 | 1.5 | 1.7 | 1.9 |
| 2041–2110 b | 2057 | Viridiflorol | 0.6 | 0.3 | 0.4 | 0.7 | 0.3 | 0.3 | 0.5 | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 |
| 2133–2144 a | 2085 | Rosifoliol | 0.4 | 0.4 | 0.6 | 0.7 | 0.4 | 0.3 | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| 2117 | Muurola-4,10(14)-diene-1-ol * | - | - | - | 0.1 | 0.1 | - | tr | - | - | tr | 0.1 | tr | |
| 2147–2199 b | 2135 | γ-Eudesmol | 3.6 | 2.0 | 3.0 | 2.5 | 2.9 | 2.2 | 2.2 | 3.0 | 3.9 | 3.0 | 1.9 | 3.2 |
| 2178–2204 a | 2147 | Eremoligenol | 1.8 | 0.8 | 1.2 | 1.0 | 1.0 | 0.7 | 0.8 | 1.0 | 1.1 | 1.1 | 0.7 | 1.2 |
| 2081–2108 a | 2157 | α-Guaiol | 1.1 | 0.5 | 0.8 | 0.6 | 0.7 | 0.5 | 0.5 | 0.6 | 0.8 | 0.8 | 0.4 | 0.8 |
| 2140–2246 b | 2170 | Carvacrol | - | - | - | - | 0.1 | - | tr | - | - | tr | 0.2 | 0.1 |
| 2176 | Agarospirol * | - | - | - | - | tr | - | tr | - | - | tr | - | - | |
| 2215–2231 a | 2184 | Valerianol | - | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - |
| 2186–2250 b | 2194 | α-Eudesmol | 7.6 | 3.9 | 6.6 | 4.7 | 5.7 | 4.2 | 3.8 | 5.5 | 7.4 | 6.6 | 3.9 | 6.9 |
| 2180–2255 b | 2199 | α-Cadinol * | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| 2196–2272 b | 2205 | β-Eudesmol | 8.6 | 4.0 | 7.4 | 4.9 | 6.2 | 4.5 | 4.9 | 5.7 | 8.0 | 7.8 | 4.2 | 7.4 |
| 2207–2274 b | 2226 | Selin-11-en-4-a-ol | - | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2286 c | 2244 | Apodophyllone | 0.5 | 0.6 | 1.1 | 0.3 | 0.6 | 0.2 | 0.2 | 0.6 | 1.0 | 1.0 | 0.3 | 1.2 |
| 2278–2387 b | 2305 | (E,E)-Farnesol * | - | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 |
| 2379 c | 2334 | Isotorquatone | - | 0.4 | 0.6 | 0.4 | 0.4 | 0.3 | 0.3 | - | 0.5 | 0.4 | 0.3 | 0.5 |
| 2424 a | 2376 | Torquatone | 32.1 | 34.5 | 53.2 | 30.6 | 38.9 | 32.7 | 28.8 | 41.5 | 40.7 | 39.1 | 31.2 | 40.6 |
| 2518 c | 2482 | Miniatone | 1.7 | 1.2 | 1.6 | 4.0 | 2.3 | 2.1 | 3.1 | 1.4 | 1.0 | 0.6 | 2.0 | 1.0 |
| Total | 99.0 | 99.6 | 99.6 | 98.9 | 99.1 | 99.6 | 99.0 | 99.2 | 99.6 | 99.6 | 99.6 | 99.6 | ||
| LRI Lit. | LRI PS | Compound Name | Relative Percentage Amounts in Mature Leaves (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | Jun | Jul | Agu | Sep | Oct | Nov | Dec | |||
| 1008–1039 b | 1015 | α-Pinene | 12.9 | 11.1 | 11.9 | 10.4 | 8.9 | 8.1 | 10.3 | 13.5 | 9.2 | 12.0 | 12.1 | 9.7 |
| 1043–1086 b | 1035 | Camphene | tr | - | tr | tr | - | tr | tr | tr | tr | 0.1 | tr | tr |
| 1085–1130 b | 1082 | β-Pinene | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.4 | 0.3 |
| 1101–1136 a | 1101 | Isoamyl acetate * | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | tr | 0.1 | 0.1 | 0.1 | 0.2 | tr |
| 1140–1175 b | 1136 | Myrcene | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | tr | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 |
| 1148–1186 b | 1140 | α-Phellandrene | 0.8 | 0.2 | 0.9 | 0.1 | 0.2 | 0.2 | 0.1 | 1.2 | 0.3 | 0.1 | 0.6 | 0.8 |
| 1154–1195 b | 1155 | α-Terpinene * | 0.1 | - | 0.1 | - | - | tr | - | 0.1 | tr | tr | tr | 0.1 |
| 1178–1219 b | 1173 | Limonene | 1.0 | 0.7 | 0.9 | 0.7 | 0.6 | 0.6 | 0.7 | 1.0 | 0.6 | 0.9 | 1.1 | 0.7 |
| 1188–1233 b | 1184 | β-Phellandrene | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 |
| 1186–1231 b | 1186 | 1,8-Cineole | 15.2 | 18.0 | 15.6 | 14.3 | 3.6 | 13.3 | 12.5 | 13.1 | 8.1 | 27.8 | 26.2 | 9.9 |
| 1222–1266 b | 1218 | γ-Terpinene | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 |
| 1246–1291 b | 1246 | p-Cymene | 1.6 | 1.8 | 1.4 | 1.4 | 0.6 | 1.7 | 2.5 | 0.8 | 1.3 | 2.3 | 1.1 | 0.5 |
| 1261–1300 b | 1256 | Terpinolene | tr | - | tr | tr | - | tr | tr | 0.1 | tr | tr | 0.1 | 0.1 |
| 1277–1304 a | 1268 | Isoamyl isovalerate * | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1380–421 a | 1343 | 2-Octanol * | tr | - | tr | tr | - | tr | 0.1 | tr | tr | 0.1 | 0.1 | tr |
| 1519 | 1-isopropyl-3-methyl butyl acetate * | 0.1 | - | 0.1 | 0.1 | - | 0.1 | 1.3 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | |
| 1545–1590 b | 1553 | Pinocarvone | 0.6 | 1.6 | 0.7 | 1.1 | 0.2 | 1.0 | - | 0.3 | 0.4 | 1.7 | 0.5 | 0.2 |
| 1570–1685 b | 1570 | β-Caryophyllene | 0.1 | - | 0.1 | tr | - | tr | - | 0.1 | tr | 0.1 | 0.1 | 0.1 |
| 1564–1630 b | 1575 | Terpinen-4-ol | 0.1 | - | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 |
| 1583–1668 b | 1579 | Aromadendrene | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.7 | 0.7 | 0.5 | 0.7 | 1.0 |
| 1624–1668 b | 1617 | Alloaromadendrene | 0.1 | - | 0.1 | tr | 0.1 | tr | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1643–1671 b | 1632 | trans-Pinocarveol | 2.0 | 4.8 | 2.3 | 3.5 | 0.6 | 3.1 | 4.2 | 0.8 | 1.5 | 5.4 | 1.5 | 0.7 |
| 1662–1717 b | 1658 | Limonene-4-ol * | tr | - | tr | 0.1 | - | tr | 0.1 | tr | 0.1 | 0.1 | 0.1 | - |
| 1686–1697 a | 1664 | Carvotan acetone * | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | |
| 1629–1724 b | 1667 | α-Terpineol | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.5 | 0.3 | 0.4 | 0.5 | 0.3 | 0.2 |
| 1653–1728 b | 1675 | Borneol | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 |
| 1699 | 2-Acetoxy-1,8-cineole * | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| 1840–1949 b | 1713 | Piperitone | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1743–1808 b | 1764 | Myrtenol | 0.1 | 0.2 | 0.1 | 0.1 | - | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | tr |
| 1810–1821 a | 1767 | trans-p-mentha-1,(7),8-diene-2-ol * | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | tr | tr | 0.2 | 0.1 | tr |
| 1779 | p-mentha-1,(7),5-diene-2-ol * | 0.1 | - | 0.1 | tr | - | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | tr | |
| 1801–1879 a | 1803 | trans-Carveol * | 0.1 | 0.2 | 0.1 | 0.1 | - | 0.1 | 0.2 | tr | 0.1 | 0.2 | 0.1 | tr |
| 1820–1881 a | 1818 | p-Cymene-8-ol * | tr | 0.1 | tr | 0.1 | - | tr | 0.1 | tr | tr | 0.1 | tr | tr |
| 1885–1903 a | 1857 | cis-p-mentha-1,(7),8-diene-2-ol * | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.1 | tr | 0.1 | 0.2 | 0.1 | tr |
| 1874 | α-Phellandrene epoxide * | 0.1 | - | 0.1 | tr | - | 0.1 | 0.1 | tr | tr | 0.1 | tr | - | |
| 1911–1938 b | 1907 | Palustrol | tr | - | tr | tr | - | tr | tr | 0.1 | 0.1 | tr | tr | 0.1 |
| 1973 a | 1977 | Maaliol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2025–2033 a | 1984 | epi-globulol | 0.3 | 0.3 | 0.3 | 0.4 | 0.5 | 0.4 | 0.2 | 0.4 | 0.4 | 0.3 | 0.3 | 0.4 |
| 2014–2062 b | 2008 | Ledol | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2061–2074 a | 2027 | Cubeban-11-ol | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2043–2013 b | 2046 | Elemol * | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2049–2104 b | 2052 | Globulol | 1.5 | 1.9 | 1.5 | 2.1 | 2.5 | 2.0 | 1.3 | 2.0 | 2.0 | 1.4 | 1.6 | 1.9 |
| 2041–2110 b | 2059 | Viridiflorol | 0.2 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 |
| 2133–2144 a | 2079 | Rosifoliol | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 |
| 2088 | α-Thujaplicin * | 0.4 | 0.2 | 0.2 | 0.2 | - | 0.2 | 0.2 | 0.4 | - | - | - | - | |
| 2074–2150 b | 2096 | Spathulenol * | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.4 | 0.1 | 0.2 | 0.2 |
| 2147–2199 b | 2138 | γ-Eudesmol | 2.8 | 1.2 | 2.7 | 1.6 | 2.8 | 1.8 | 1.4 | 3.5 | 2.0 | 0.7 | 2.4 | 2.8 |
| 2178–2204 a | 2150 | Eremoligenol | 1.6 | 1.2 | 1.5 | 1.3 | 1.9 | 1.4 | 1.3 | 1.8 | 1.8 | 1.0 | 1.3 | 1.6 |
| 2081–2108 a | 2160 | α-Guaiol | 1.0 | 0.7 | 1.0 | 0.8 | 1.2 | 0.9 | 0.7 | 1.1 | 1.1 | 0.5 | 0.9 | 1.0 |
| 2140–2246 b | 2173 | Carvacrol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| 2187 | Valerianol | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | |
| 2186–2250 b | 2197 | α-Eudesmol | 7.5 | 5.0 | 7.5 | 5.3 | 8.4 | 6.1 | 4.7 | 7.9 | 6.8 | 3.1 | 6.5 | 7.3 |
| 2196–2272 | 2207 | β-Eudesmol | 10.0 | 9.8 | 10.2 | 10.3 | 12.9 | 10.9 | 10.9 | 9.7 | 11.6 | 7.9 | 8.5 | 9.9 |
| 2207–2274 b | 2229 | Selin-11-en-4-α-ol | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2286 c | 2256 | Apodophyllone | 1.0 | 1.0 | 1.0 | 0.9 | 1.2 | 0.7 | 0.5 | 0.6 | 1.1 | 0.9 | 0.8 | 0.7 |
| 2379 c | 2337 | Isotorquatone | 0.4 | 0.5 | 0.4 | 0.5 | 0.7 | 0.4 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| 2424 a | 2378 | Torquatone | 34.6 | 35.1 | 35.5 | 39.9 | 48.8 | 41.9 | 41.4 | 34.8 | 45.1 | 27.6 | 28.0 | 45.2 |
| 2518 c | 2484 | Miniatone | 0.6 | 0.5 | 0.6 | 0.6 | 0.9 | 0.8 | 1.1 | 0.9 | 0.8 | 0.5 | 0.6 | 1.1 |
| Total | 99.7 | 99.7 | 99.8 | 99.7 | 99.5 | 99.7 | 99.9 | 99.8 | 99.3 | 99.5 | 99.5 | 99.4 | ||
| Country | Major Compounds (%) | Reference |
|---|---|---|
| Cyprus | α-pinene (18.6%), 1,8-cineole (18.8%), β-eudesmol (10.3%), torquatone (29.2%) | [2] |
| Tunisia | α-pinene (10.5%), 1,8-cineole (12.0%), β-eudesmol (10.1%), torquatone (42.0%) | [5] |
| Iran | 1,8-cineole (28.6%), α-pinene (15.7%), globulol (13.1%) | [15] |
| Iran | 1,8-cineole (69.6%), α-pinene (9.5%), aromadendrene (4.5%), alloaromadendrene (7.8%) | [16] |
| Iran | 1,8-cineole (66.9%), α-pinene (13.9%), trans-pinocarveol (6.3%) | [17] |
| Australia | α-pinene (18.79%), torquatone (40.91%) | [18] |
| Iran | α-pinene (20.0%), 1,8-cineole (24.2%), globulol (8.4%), aromadendrene (7.8%) | [20] |
| Morocco | α-pinene (16.7 and 20.0%), 1,8-cineole (46.9 and 28.9%) borneol (10.8 and 22.6%) | [21] |
| Australia | Torquatone (42.0%), 1,8-cineole (11.2%), α-pinene (10.2%), α-eudesmol (10.2%), β-eudesmol (11.1%), γ-eudesmol (4.8%) | [22] |
3. Materials and Method
3.1. Collection of Plant Samples
3.2. Isolation of the Essential Oil
3.3. Gas Chromatography
3.4. Gas Chromatography/Mass Spectrometry (GC/MS)
3.5. Identification of the Constituents
3.6. Statistical Analysis
Exclusions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J. Volatile Leaf Oils of Some South-Western and Southern Australian Species of the Genus Eucalyptus (Series 1). Part XIX. Flavour Fragr. J. 1998, 13, 131–139. [Google Scholar] [CrossRef]
- Yiğit Hanoğlu, D.; Hanoğlu, A.; Adediran, S.B.; Baser, K.H.C.; Özkum Yavuz, D. The Essential Oil Compositions of Two Eucalyptus sp. (E. camaldulensis Dehnh. and E. torquata Luehm.) Naturalized to Cyprus. J. Essent. Oil Res. 2023, 35, 136–142. [Google Scholar] [CrossRef]
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A Systematic and Comprehensive Review on Current Understanding of the Pharmacological Actions, Molecular Mechanisms, and Clinical Implications of the Genus Eucalyptus. Phytomedicine Plus 2021, 1, 100089. [Google Scholar] [CrossRef]
- Ashour, H.M. Antibacterial, Antifungal, and Anticancer Activities of Volatile Oils and Extracts from Stems, Leaves, and Flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2008, 7, 399–403. [Google Scholar] [CrossRef]
- Elaissi, A.; Medini, H.; Marzouki, H.; Larbi Khouja, M.; Lynene, F.; Chemli, R.; Harzallah-Skhiri, F. Variation in Volatile Leaf Oils of Twelve Eucalyptus Species Harvested from Hajeb Layoun Arboreta (Tunisia). Chem. Biodivers. 2010, 7, 705–716. [Google Scholar] [CrossRef]
- Coppen, J.J.W. (Ed.) Eucalyptus: The Genus Eucalyptus; CRC Press: London, UK, 2002; ISBN 978-0-429-21889-7. [Google Scholar]
- Zhang, J.; An, M.; Wu, H.; Stanton, R.; Lemerle, D. Chemistry and Bioactivity of Eucalyptus Essential Oils. Allelopathy J. 2010, 25, 313–330. [Google Scholar]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical Variability and Biological Activities of Eucalyptus spp. Essential Oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus Genus Chemical Constituents, Biological Activities and Health-Promoting Effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Chalmers, A.C.; Jyoti Bhuyan, D.; Bowyer, M.C.; Scarlett, C.J. Botanical, Phytochemical, and Anticancer Properties of the Eucalyptus Species. Chem. Biodivers. 2015, 12, 907–924. [Google Scholar] [CrossRef]
- Yıkıcı, Z. Okaliptüs Dünü ve Geleceği (The Past and Future of Eucalyptus); Orman Mühendisliği Odası (Chamber of Forest Engineers): Nicosia, Cyprus, 2015. [Google Scholar]
- İlseven, S.; Baştaş, M. The Place of Eucalyptus Within the Vegetation of Mesaoria Plain (Cyprus) and the Views of Vegetation Geography Lecturers. Eurasia J. Math. Sci. Technol. Educ. 2018, 14, 3381–3388. [Google Scholar] [CrossRef]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal Plants in the Mediterranean Area: Synthesis of the Results of the Project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Yöney, A.; Prieto, J.M.; Lardos, A.; Heinrich, M. Ethnopharmacy of Turkish-Speaking Cypriots in Greater London. Phytother. Res. PTR 2010, 24, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, A.; Sendi, J.J.; Maroufpoor, M.; Rahimi-Nasrabadi, M. Acaricidal Potentials of the Terpene-Rich Essential Oils of Two Iranian Eucalyptus Species against Tetranychus urticae Koch. J. Oleo Sci. 2017, 66, 307–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nikbakht, M.R.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Gandomi, H.; Abbaszadeh, S.; Batooli, H. The Chemical Composition and in vitro Antifungal Activities of Essential Oils of Five Eucalyptus Species. J. Essent. Oil Bear. Plants 2015, 18, 666–677. [Google Scholar] [CrossRef]
- Sefidkon, F.; Assareh, M.H.; Abravesh, Z.; Barazandeh, M.M. Chemical Composition of the Essential Oils of Four Cultivated Eucalyptus Species in Iran as Medicinal Plants (E. microtheca, E. spathulata, E. largiflorens and E. torquata). Iran. J. Pharm. Res. 2022, 6, 135–140. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J.; Jackson, J.F. Volatile Leaf Oils of Some South-Western and Southern Australian Species of the Genus Eucalyptus. Part II—Subgenus Symphyomyrtus, Section Dumaria, Series Torquatae. Flavour Fragr. J. 1994, 9, 167–171. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Skelton, B.W.; White, A.H. Structural Study of Torquatone, an Acylphloroglucinol Derivative From Eucalyptus Species. Aust. J. Chem. 1995, 48, 1771–1774. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Naseri, B.; Abedi, Z.; Setzer, W.N.; Changbunjong, T. Promising Insecticidal Efficiency of Essential Oils Isolated from Four Cultivated Eucalyptus Species in Iran against the Lesser Grain Borer, Rhyzopertha Dominica (F.). Insects 2022, 13, 517. [Google Scholar] [CrossRef]
- Zrira, S.; Khiranr, F.; Benjllalr, B. Huiles essentielles de six espèces xérophyles d’Eucalyptus: Effet du milieu sur les rendements et la èomposition-chimique [Essential oils of six xerophilic Eucalyptus species: Effect of location on the yield and the chemical composition]. Rev. Marocaine Sci. Agron. Vétérinaires 1994, 14, 5–9. [Google Scholar]
- Baranska, M.; Schulz, H.; Reitzenstein, S.; Uhlemann, U.; Strehle, M.A.; Krüger, H.; Quilitzsch, R.; Foley, W.; Popp, J. Vibrational Spectroscopic Studies to Acquire a Quality Control Method of Eucalyptus Essential Oils. Biopolymers 2005, 78, 237–248. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook NIST Standard Reference Database Last Update to Data: 2018; The National Institute of Standards and Technology: Gaithersburg, MD, USA, 2001. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Ireland, B.F.; Goldsack, R.J.; Brophy, J.J.; Fookes, C.J.; Clarkson, J.R. The leaf essential oils of Eucalyptus miniata and its allies. J. Essent. Oil Res. 2004, 16, 89–94. [Google Scholar] [CrossRef]
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; John Wiley & Sons: Hoboken, NJ, USA, 1989. [Google Scholar]
- Joulain, D.; Koenig, W.A. The Atlas of Spectra Data of Sesquiterpene Hydrocarbons; EB-Verlag: Hamburg, Germany, 1998. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulama Modu, M.; Yiğit Hanoğlu, D.; Hanoğlu, A.; Alkaş, F.B.; Başer, K.H.C.; Özkum Yavuz, D. Multivariate Statistical Analyses of the Temporal Variation in the Chemical Composition of the Essential Oil of Eucalyptus torquata in Cyprus. Molecules 2025, 30, 332. https://doi.org/10.3390/molecules30020332
Bulama Modu M, Yiğit Hanoğlu D, Hanoğlu A, Alkaş FB, Başer KHC, Özkum Yavuz D. Multivariate Statistical Analyses of the Temporal Variation in the Chemical Composition of the Essential Oil of Eucalyptus torquata in Cyprus. Molecules. 2025; 30(2):332. https://doi.org/10.3390/molecules30020332
Chicago/Turabian StyleBulama Modu, Mustapha, Duygu Yiğit Hanoğlu, Azmi Hanoğlu, Fehmi Burak Alkaş, K. Hüsnü Can Başer, and Dudu Özkum Yavuz. 2025. "Multivariate Statistical Analyses of the Temporal Variation in the Chemical Composition of the Essential Oil of Eucalyptus torquata in Cyprus" Molecules 30, no. 2: 332. https://doi.org/10.3390/molecules30020332
APA StyleBulama Modu, M., Yiğit Hanoğlu, D., Hanoğlu, A., Alkaş, F. B., Başer, K. H. C., & Özkum Yavuz, D. (2025). Multivariate Statistical Analyses of the Temporal Variation in the Chemical Composition of the Essential Oil of Eucalyptus torquata in Cyprus. Molecules, 30(2), 332. https://doi.org/10.3390/molecules30020332







