Porous Single-Crystalline Rare Earth Phosphates Monolith to Enhance Catalytic Activity and Durability
Abstract
1. Introduction
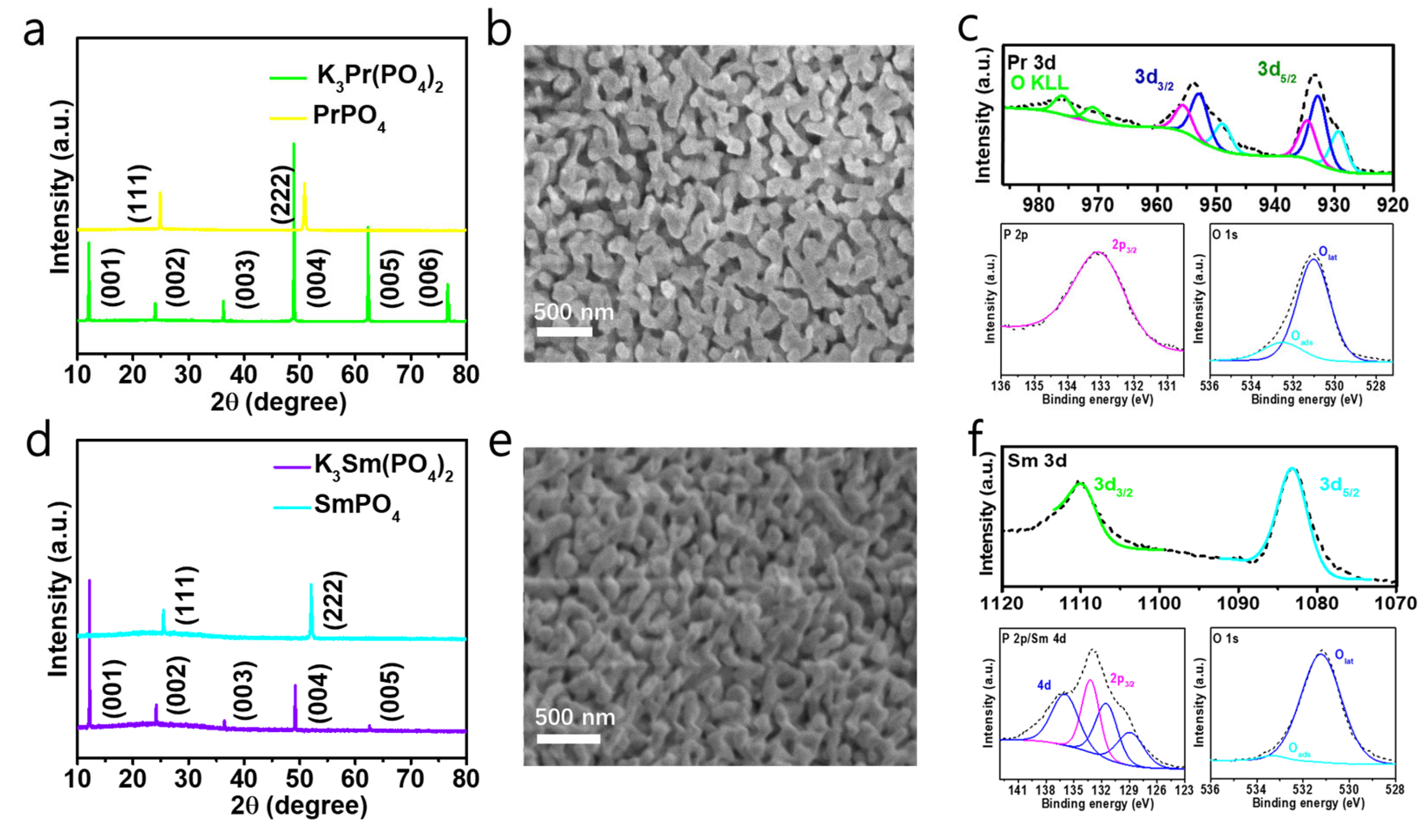
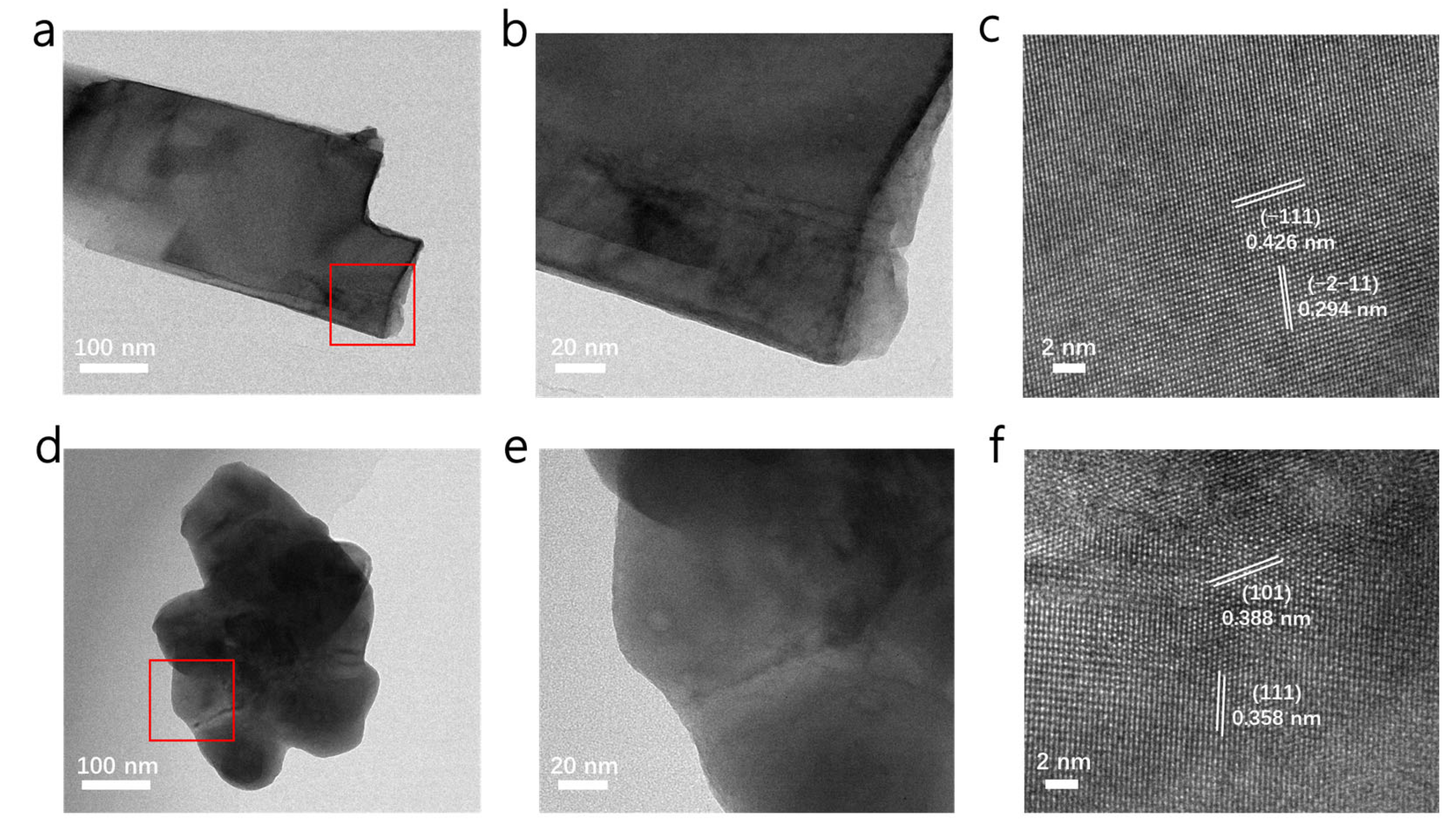
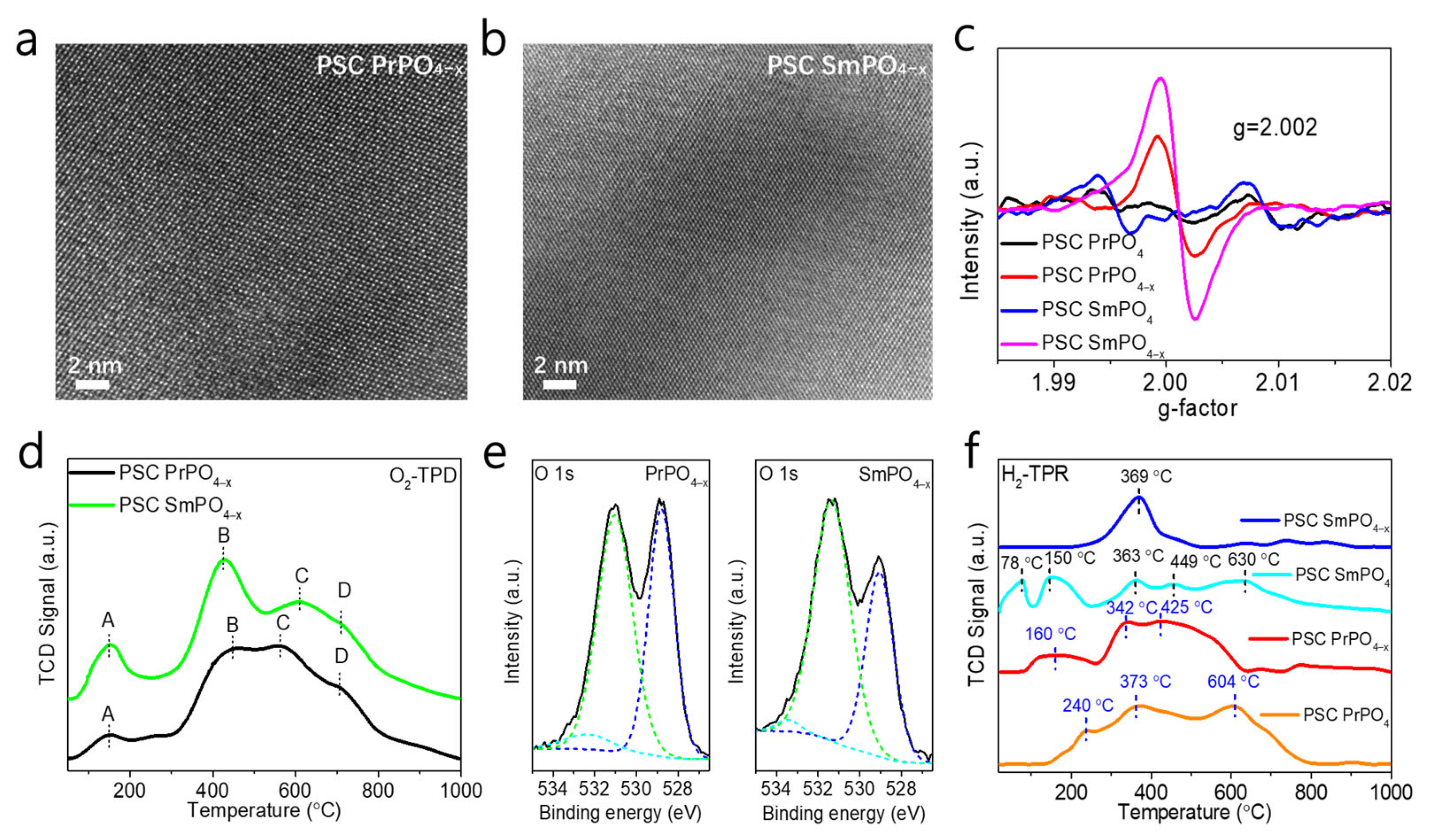
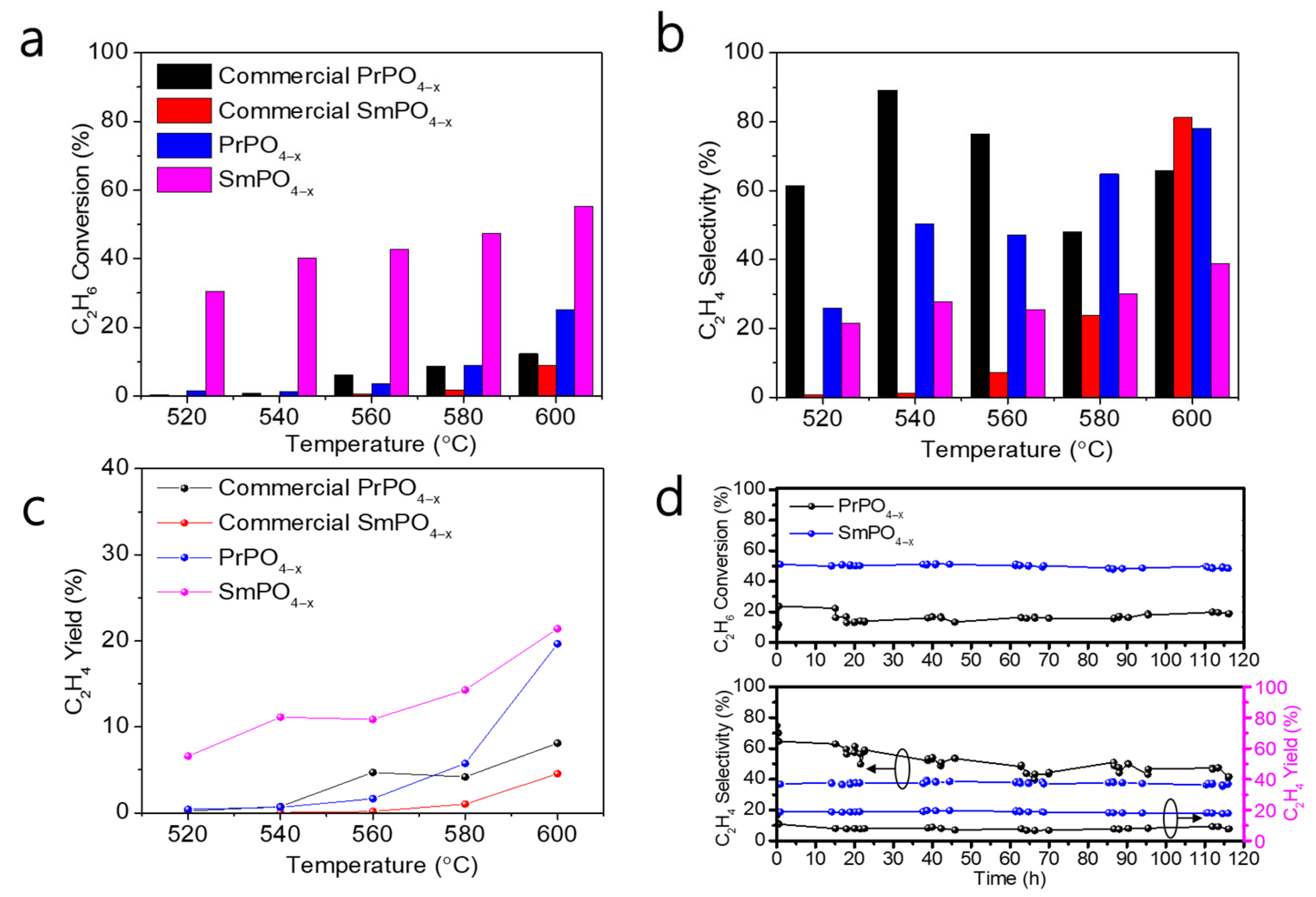
2. Results and Discussion
3. Experiment
3.1. Material Preparation
3.2. Material Characterization
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achary, S.N.; Bevara, S.; Tyagi, A.K. Recent progress on synthesis and structural aspects of rare-earth phosphates. Coord. Chem. Rev. 2017, 340, 266–297. [Google Scholar] [CrossRef]
- Nunez, N.O.; Liviano, S.R.; Ocana, M. Citrate mediated synthesis of uniform monazite LnPO4 (Ln = La, Ce) and Ln:LaPO4 (Ln = Eu, Ce, Ce plus Tb) spheres and their photoluminescence. J. Colloid Interface Sci. 2010, 349, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Teng, Y.C.; Wu, L.; Huang, Y.; Ma, J.Y.; Wang, G.L. Chemical durability and leaching mechanism of Ce0.5Eu0.5PO4 ceramics: Effects of temperature and pH values. J. Nucl. Mater. 2015, 466, 187–193. [Google Scholar] [CrossRef]
- Lin, G.M.; Li, H.; Xie, K. Twisted surfaces in porous single crystals to deliver enhanced catalytic activity and stability. Angew. Chem. Int. Ed. 2020, 59, 16440–16444. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Shale Gas Revolution: An Opportunity for the Production of Biobased Chemicals? Angew. Chem. Int. Ed. 2013, 52, 11980–11987. [Google Scholar] [CrossRef]
- Gaertner, C.A.; van Veen, A.C.; Lercher, J.A. Oxidative Dehydrogenation of Ethane: Common Principles and Mechanistic Aspects. ChemCatChem 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Xie, T.Y.; Mcauley, K.B.; Hsu, J.C.C.; Bacon, D.W. Gas Phase ethylene polymerization: Production processes, polymer properties, and reactor modeling. Ind. Eng. Chem. Res. 1994, 33, 449–479. [Google Scholar] [CrossRef]
- Li, W.T.; Xie, K. Porous single crystals at the macroscale: From growth to application. Acc. Chem. Res. 2023, 56, 374–384. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Zhang, J.; Xie, K. In situ Observation of Porosity Formation in Porous Single-crystalline TiO2 Monolith for Enhanced and Stable Catalytic CO Oxidation. Angew. Chem. Int. Ed. 2023, 62, e202300480. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Lin, G.M.; Hu, X.L.; Xi, S.B.; Xie, K. Porous single-crystalline titanium dioxide at 2 cm scale delivering enhanced photoelectrochemical performance. Nat. Commun. 2019, 10, 3618. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.M.; Boatner, L.A.; Chakoumakos, B.C.; Rawn, C.J.; Richardson, J. Structural and crystal chemical properties of alkali rare-earth double phosphates. J. Alloys Compd. 2015, 655, 253–265. [Google Scholar] [CrossRef]
- Toumi, M.; Smiri-Dogguy, L.; Bulou, A. Crystal structure, infrared, and polarized Raman spectra of K3Sm(PO4)2. Eur. J. Inorg. Chem. 1999, 9, 1545–1550. [Google Scholar] [CrossRef]
- Ni, Y.X.; Hughes, J.M.; Mariano, A.N. Crystal chemistry of the monazite and xenotime structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-ray photoelectron-spectroscopy of rare-earth compounds. J. Electron Spectrosc. Relat. Phenom. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Z.J.; Qiu, L.M.; Zhao, L.Z. Tables of peak positions for XPS photoelectron and auger electron peaks. Anal. Test. Technol. Instrum. 2009, 15, 1–17. [Google Scholar]
- Glorieux, B.; Berjoan, R.; Matecki, M.; Kammouni, A.; Perarnau, D. XPS analyses of lanthanides phosphates. Appl. Surf. Sci. 2007, 253, 3349–3359. [Google Scholar] [CrossRef]
- Pemba-Mabiala, J.M.; Lenzi, M.; Lenzi, J.; Lebugle, A. XPS study of mixed cerium-terbium orthophosphate catalysts. Surf. Interface Anal. 1990, 15, 663–667. [Google Scholar] [CrossRef]
- Winer, K.; Colmenares, C.A.; Smith, R.L.; Wooten, F. Interaction of water vapor with clean and oxygen-covered uranium surfaces. Surf. Sci. 1987, 183, 67–99. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.S.; Lin, Z.J.; Wang, D.X. Study of the sorption of elements on the surface of feldspars, muscovite and hornblende by means of XPS. J. Nucl. Radiochem. 1992, 14, 71–78. [Google Scholar]
- Mullica, D.F.; Lok, C.K.C.; Perkins, H.O.; Benesh, G.A.; Young, V. The X-ray photoemission spectra of Nd(OH)3, Sm(OH)3, Eu(OH)3 and Gd(OH)3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 1–20. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.Y.; Ye, H.C.; Chen, S.M.; Ju, H.X.; Liu, D.B.; Lin, Y.; Ye, W.; Wang, C.M.; Xu, Q.; et al. Oxide defect engineering enables to couple solar energy into oxygen activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.K.; Yao, Y.Y.; Wei, L.Q.; Shi, Z.F.; Han, L.Y.; Yuan, H.X.; Li, B.; Dong, L.H.; Wang, F.; Sun, C.Z. Preparation and evaluation of copper manganese oxide as a high-efficiency catalyst for CO oxidation and NO reduction by CO. J. Phys. Chem. C 2017, 121, 12757–12770. [Google Scholar] [CrossRef]
- Cai, W.; Zhong, Q.; Zhao, W.; Bu, Y.F. Focus on the modified CexZr1-xO2 with the rigid benzene-muti-carboxylate ligands and its catalysis in oxidation of NO. Appl. Catal. B Environ. Energy 2014, 158, 258–268. [Google Scholar] [CrossRef]
- Concepcion, P.; Hernandez, S.; Lopez Nieto, J.M. On the nature of active sites in MoVTeO and MoVTeNbO catalysts: The influence of catalyst activation temperature. Appl. Catal. A-Gen. 2011, 391, 92–101. [Google Scholar] [CrossRef]
- Yisup, N.; Cao, Y.; Feng, W.L.; Dai, W.L.; Fan, K.N. Catalytic oxidation of methane over novel Ce-Ni-O mixed oxide catalysts prepared by oxalate gel-coprecipitation. Catal. Lett. 2005, 99, 207–213. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wang, L.C.; Chen, M.; Xu, J.; Cao, Y.; He, H.Y.; Fan, K.N. Highly selective Ce-Ni-O catalysts for efficient low temperature oxidative dehydrogenation of propane. Catal. Lett. 2009, 130, 350–354. [Google Scholar] [CrossRef]
- Li, X.; Li, W.T.; Zhang, J.; Yin, W.; Xia, Y.G.; Xie, K. Porous single-crystalline centimeter-sized α-Al2O3 monoliths for selective and durable non-oxidative dehydrogenation of ethane. Angew. Chem. Int. Ed. 2024, 63, e202315274. [Google Scholar] [CrossRef]
- Wood, J.; Alldrick, M.J.; Winterbottom, J.M.; Stitt, E.H.; Bailey, S. Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) study of ethyne hydrogenation on Pd/Al2O3. Catal. Today 2007, 128, 52–62. [Google Scholar] [CrossRef]
- Karamullaoglu, G.; Onen, S.; Dogu, T. Oxidative dehydrogenation of ethane and isobutane with chromium-vanadium-niobium mixed oxide catalysts. Chem. Eng. Process. Process Intensif. 2002, 41, 337–347. [Google Scholar] [CrossRef]
- Lin, X.F.; Hoel, C.A.; Sachtler, W.M.H.; Poeppelmeier, K.R.; Weitz, E. Oxidative dehydrogenation (ODH) of ethane with O2 as oxidant on selected transition metal-loaded zeolites. J. Catal. 2009, 265, 54–62. [Google Scholar] [CrossRef]
- Shee, D.; Deo, G. In situ DRIFT studies of alkane adsorption on vanadia supported titania-doped catalysts. Catal. Today 2019, 325, 25–32. [Google Scholar] [CrossRef]
- Karamullaoglu, G.; Dogu, T. Oxidative dehydrogenation of ethane over a monolith coated by molybdenum-vanadium-niobium mixed-oxide catalyst. Chem. Eng. Commun. 2003, 190, 1427–1438. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Trevisanut, C.; Millet, J.M.M.; Busca, G.; Cavani, F. In situ DRIFTS-MS study of the anaerobic oxidation of ethanol over spinel mixed oxides. J. Phys. Chem. C 2013, 117, 23908–23918. [Google Scholar] [CrossRef]
- Coronado, J.M.; Kataoka, S.; Tejedor-Tejedor, I.; Anderson, M.A. Dynamic phenomena during the photocatalytic oxidation of ethanol and acetone over nanocrystalline TiO2: Simultaneous FTIR analysis of gas and surface species. J. Catal. 2003, 219, 219–230. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhang, Y.M.; Krishna, R.; Yao, K.X.; Han, Y.; Wu, Z.L.; Ma, D.X.; Shi, Z.; Pham, T.; Space, B.; et al. Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane. J. Am. Chem. Soc. 2014, 136, 8654–8660. [Google Scholar] [CrossRef]
- Busca, G.; Finocchio, E.; Lorenzelli, V.; Ramis, G.; Baldi, M. IR studies on the activation of C-H hydrocarbon bonds on oxidation catalysts. Catal. Today 1999, 49, 453–465. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Subbotina, I.R.; Rane, N.; van Santen, R.A.; Hensen, E.J.M. On two alternative mechanisms of ethane activation over ZSM-5 zeolite modified by Zn2+ and Ga1+ cations. Phys. Chem. Chem. Phys. 2005, 7, 3088–3092. [Google Scholar] [CrossRef]
- He, Y.; Song, Y.J.; Laursen, S. The Origin of the Special Surface and Catalytic Chemistry of Ga-Rich Ni3Ga in the Direct Dehydrogenation of Ethane. ACS Catal. 2019, 9, 10464–10468. [Google Scholar] [CrossRef]
- Barman, S.; Maity, N.; Bhatte, K.; Ould-Chikh, S.; Dachwald, O.; Haessner, C.; Saih, Y.; Abou-Hamad, E.; Llorens, I.; Hazemann, J.L.; et al. Single-Site VOx Moieties Generated on Silica by Surface Organometallic Chemistry: A Way To Enhance the Catalytic Activity in the Oxidative Dehydrogenation of Propane. ACS Catal. 2016, 6, 5908–5921. [Google Scholar] [CrossRef]
- Dawson, R.D.; Elwell, D.; Brice, J.C. Top seeded solution growth of sodium niobate. J. Cryst. Growth 1974, 23, 65–70. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Chai, Y.C.; Li, X.Y.; Wu, Z.H.; Han, Y.J.; Li, L.; Qi, H.F.; Gu, Y.M.; Kang, L.L.; Wang, X.D. Defect-rich TiO2 in situ evolved from MXene for the enhanced oxidative dehydrogenation of ethane to ethylene. ACS Catal. 2021, 11, 15223–15233. [Google Scholar] [CrossRef]
- Lu, J.L.; Fu, B.S.; Kung, M.C.; Xiao, G.M.; Elam, J.M.; Kung, H.H.; Stair, P.C. Coking- and sintering-resistant palladium catalysts achieved through atomic layer deposition. Science 2012, 335, 1205–1208. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Ye, L.; Tu, C.; Xie, K. Porous Single-Crystalline Rare Earth Phosphates Monolith to Enhance Catalytic Activity and Durability. Molecules 2025, 30, 331. https://doi.org/10.3390/molecules30020331
Li W, Ye L, Tu C, Xie K. Porous Single-Crystalline Rare Earth Phosphates Monolith to Enhance Catalytic Activity and Durability. Molecules. 2025; 30(2):331. https://doi.org/10.3390/molecules30020331
Chicago/Turabian StyleLi, Wenting, Lingting Ye, Chaoyang Tu, and Kui Xie. 2025. "Porous Single-Crystalline Rare Earth Phosphates Monolith to Enhance Catalytic Activity and Durability" Molecules 30, no. 2: 331. https://doi.org/10.3390/molecules30020331
APA StyleLi, W., Ye, L., Tu, C., & Xie, K. (2025). Porous Single-Crystalline Rare Earth Phosphates Monolith to Enhance Catalytic Activity and Durability. Molecules, 30(2), 331. https://doi.org/10.3390/molecules30020331





