Abstract
Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) have emerged as extraordinary bioactive lipids, exhibiting diverse bioactivities, from the enhancement of insulin secretion and the optimization of blood glucose absorption to anti-inflammatory effects. The intricate nature of FAHFAs’ structure reflects a synthetic challenge that requires the strategic introduction of ester bonds along the hydroxy fatty acid chain. Our research seeks to create an effective methodology for generating varied FAHFA derivatives. Our primary approach centers on a photochemical hydroacylation reaction, merging terminal alkenes, either ω-alkenoic acids or ω-alkenyl alcohols, with commercially available aldehydes. This transformative, environmentally friendly process, orchestrated by phenylglyoxylic acid as the photoinitiator, serves as the linchpin in establishing a practical and relatively simple method for constructing a library of racemic FAHFAs. The ketones produced by the photochemical reactions are easily converted to hydroxy derivatives, which are coupled with caproic, palmitic, or oleic acid, providing a large set of FAHFAs, which broaden our ability for future structure–activity relationship studies.
1. Introduction
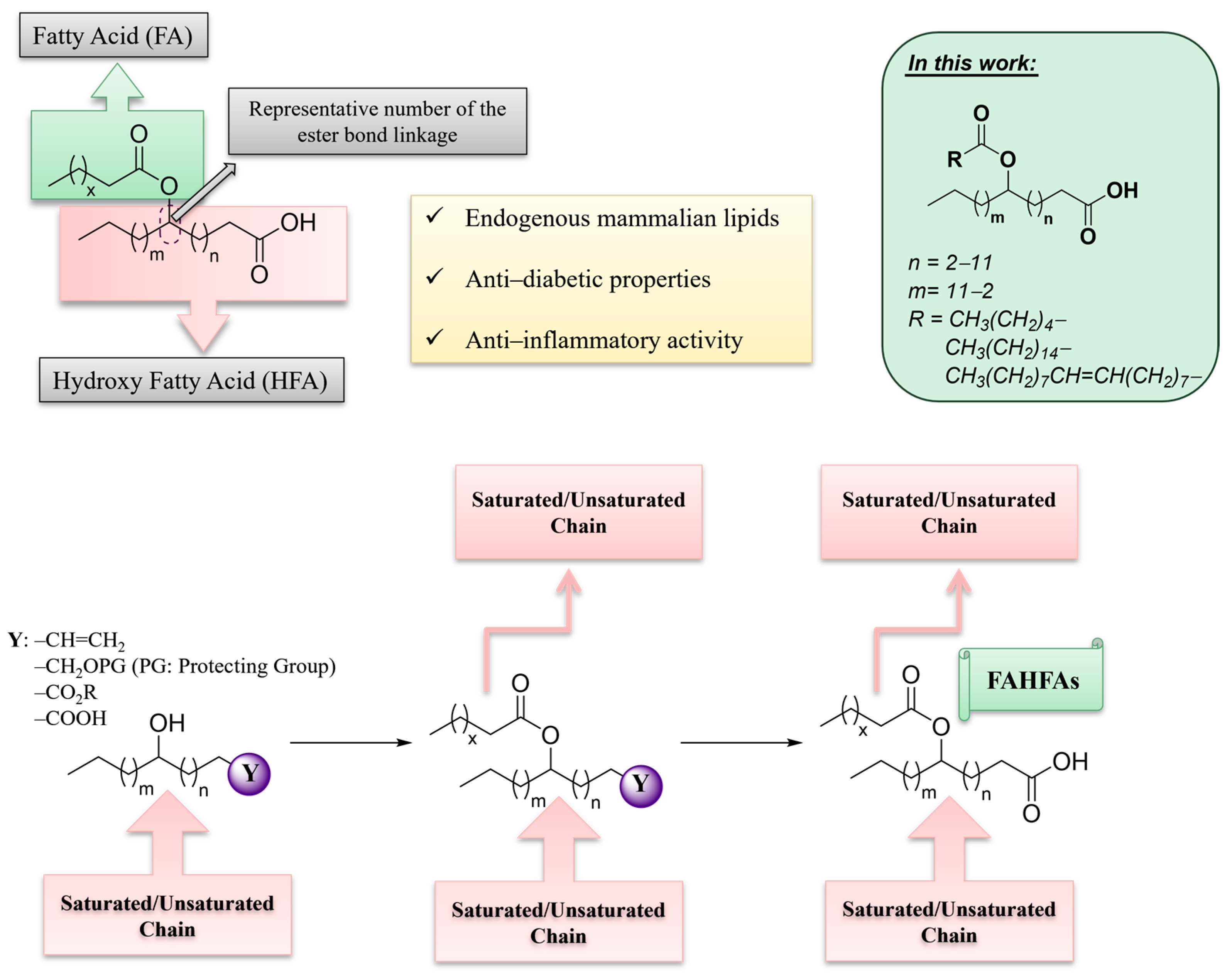
Among the diverse classes of bioactive lipids, Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) constitute a unique class of endogenous lipids, exhibiting fascinating biological properties [1,2,3]. In their seminal work in 2014, Kahn and her colleagues, performing lipidomics analysis of adipose tissue from mice engineered to overexpress the glucose transporter GLUT4 (AG4OX mice), uncovered a novel category of mammalian lipids distinguished by a branched ester connection linking a fatty acid (FA) with a hydroxy fatty acid (HFA), referred to as FAHFAs (Figure 1) [4]. For convenience, abbreviations will be used throughout this work (e.g., CA for caproic acid, PA for palmitic acid, SA for stearic acid and OA for oleic acid, prefixed with “H” for hydroxy fatty acids [HFAs] and a unique number representing the location of the branch). FAHFAs belong to a family of estolides, which are fatty acid oligomers [1]. They are endogenously produced in insects [5] and mammals, such as mice [4,6] and humans [6,7]. The most studied family of FAHFAs is PAHSAs, which are synthesized in vivo. FAHFAs are naturally derived compounds identified in microalgae [8], breast milk [9], and various food sources, including grains, fruits and vegetables, oils, eggs, and meat [3,4,10,11], suggesting that FAHFAs may occur in other types of food and that mammals could potentially obtain FAHFAs through dietary intake.
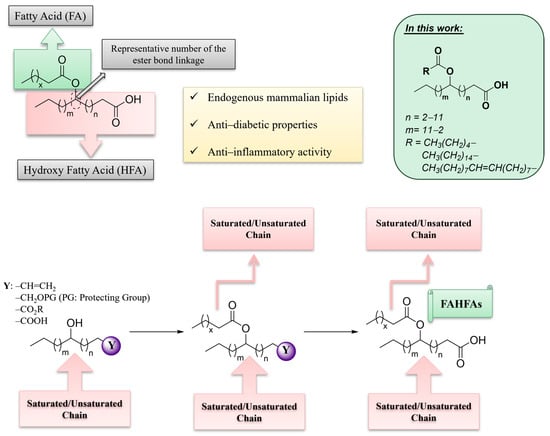
Figure 1.
General structure of Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) and schematic overview of the basic strategies for the synthesis of FAHFAs.
FAHFAs can improve blood glucose uptake, enhance insulin secretion, and alleviate obesity-related inflammation in mammals, as well as reduce various pro-inflammatory responses [1]. More specifically, a correlation of FAHFA levels with insulin sensitivity was observed, and their presence was found to be diminished in the adipose tissue and serum of individuals with insulin resistance. Consequently, it is anticipated that these natural lipids could be potentially employed in the treatment of diabetes [4]. Kahn claimed that the administration of PAHSAs to mice lowers the body’s glycemia, improves glucose tolerance, and stimulates glucagon-like peptide-1 (GLP-1) and insulin secretion [4]. Additionally, within the scope of studying the bioactivities of FAHFAs, several groups aimed at exploring the potential anti-inflammatory effects of PAHSAs, during which they observed successful anti-inflammatory activity primarily attributed to the activation of G protein-coupled receptors and the downregulation of macrophage activation, as well as the reduction of pro-inflammatory cytokines [4,12,13,14,15]. Simultaneously, research on FAHFAs has exhibited promising outcomes in combatting ulcerative colitis [16], and these compounds are noted for their potential to fortify the immune system of infants, being transmitted through breast milk from mothers to their newborns during breastfeeding [9]. Moreover, FAHFAs are purported to function as activators of Nrf2, a transcription factor responsible for regulating the gene expression of antioxidants and enzymes. This action reinforces the idea that FAHFAs may also act as antioxidants, diminishing oxidative stress and amplifying the expression of cytoprotective antioxidant enzymes [17]. It is conceivable that various FAHFAs’ regio-isomers might not demonstrate identical properties or exhibit the same level of efficacy [4,18].
The significance of conducting a structure–activity relationship study, along with the absence of synthetic techniques at that time for generating chiral FAHFAs, prompted the creation of a synthetic method for producing the two distinct enantiomeric forms of 9-PAHSA, employing commercially available enantiopure epichlorohydrin as the starting material via a chiral pool approach [18]. A five-step process starting from commercially available (R)- or (S)-epichlorohydrin led to the synthesis of (S)- or (R)-9-PAHSA. Back in 2014, Kahn’s research team conducted the first synthesis of FAHFAs using an aldehyde as the starting material [4]. The same group published yet another synthetic methodology for the preparation of racemic FAHFAs, starting from the introduction of a tetrahydropyranyl group into commercially available α,ω-diols, mainly proposing 9-PAHSA synthesis in five steps [19]. In 2016, Balas and co-workers presented a study on the effective synthesis of FAHFAs, using commercially accessible terminal alkenes and alkynes, via a seven-step synthesis, which mainly led to 5-, 7-, 9- and 10-regio-isomers [20], while in 2018, Bielohuby and Tennagel’s group pursued an enantioselective approach for the synthesis of 5- and 9-PAHSAs using cross metathesis, in an attempt to investigate the characteristics of FAHFAs and explore the interactions of these compounds with different receptors [21]. In 2019, a different route for the asymmetric synthesis of FAHFAs, employing an enantioselective organocatalytic step resulting in the formation of chiral terminal epoxides, starting from monoprotected α,ω-diols and their subsequent ring opening by a Grignard reagent as the key-steps, was developed by our group [22]. Lastly, during 2019, Siegel conducted research for the synthesis of 9-PAHSA analogs, featuring varying numbers of carbons in the fatty acid chain [14]. These synthesized analogs underwent analysis for their anti-inflammatory properties, particularly in inhibiting the production of IL-6. The results indicated that among them, 9-(hexanoyloxy)octadecanoic acid (9-CAHSA) exhibited the most promising outcome, even surpassing the known anti-inflammatory capabilities of 9-PAHSA [14]. With an overview of all the strategies developed for the synthesis of FAHFAs (Figure 1) [23], it is observed that most of them target the synthesis of specific regio-isomers, mainly 9- and 5-, while a more general method leading to a wide variety of regio-isomers would be of practical value for studying the bioactivities of such lipids.
The diverse chemical composition within the FAHFAs superfamily (regarding the position of the ester group, the fatty- or hydroxy fatty-acid chain length, and the presence or absence of unsaturation) renders their synthesis particularly challenging. The objective of this current study was the development of a practical, high-yield, and few-synthetic-step method to generate a library of racemic FAHFAs with the ability to select the position of the ester bond along the HFA chain. Meanwhile, the previously reported study concerning the anti-inflammatory activity of FAHFA derivatives, notably the superior activity observed in 9-CAHSA [14], inspired us to expand our research. We envisioned that alteration in the FA chain, like the linkage of different fatty acids to the respective HFA, may affect the biological impact of FAHFAs. Consequently, we utilized three different FAs, caproic, palmitic, and oleic acid, contributing to the production of diverse regio-isomers of CAHSAs, PAHSAs, and OAHSAs. With these considerations in mind, we successfully developed a versatile and efficient method for synthesizing FAHFAs that accommodates the diverse chemical structures of the superfamily, while providing flexibility in the choice of starting materials to generate structurally diverse libraries. This modular approach, based on a photochemical hydroacylation, is both cost-effective and well suited for structure–activity relationship studies, paving the way for advanced research into the biological functions and therapeutic potential of FAHFAs.
2. Results and Discussion
Photochemistry utilizes light to enable complex chemical transformations, facilitating reactions previously considered challenging. Most examples employ metal-based catalysts, primarily Ru or Ir, having the advantage of adjusting their electronic characteristics through ligand coordination. However, the cost of metal-based catalysts along with their limited availability prompt scientists to explore alternatives, like small organic molecules. Known as photoorganocatalysis, this approach stands out for its environmentally friendly and cost-effective nature, thanks to moderate energy input and reaction conditions, offering a promising solution [24,25,26,27,28,29,30,31,32,33,34]. In 2020, Kokotos and co-workers developed a green, mild, metal-free photochemical protocol that is easy to operate, employing cheap household lamps as the irradiation source and phenylglyoxylic acid, a cheap, organic, and commercially available compound, as the photoinitiator for the hydroacylation of olefins without the need for any directing groups or additives [35].
Drawn from this particular light-driven approach, our group developed a photochemical method in 2021 for synthesizing a class of lipids with mostly unexplored biological properties. We employed the hydroacylation reaction to produce saturated oxo fatty acids (SOFAs), bearing the oxo functionality at various positions of the long aliphatic chain [36]. In this context, we chose to broaden our research scope by utilizing the previously established photochemical hydroacylation reaction to produce oxo fatty acids and various oxo derivatives. This process served as the fundamental step, enabling the generation of different families of FAHFAs.
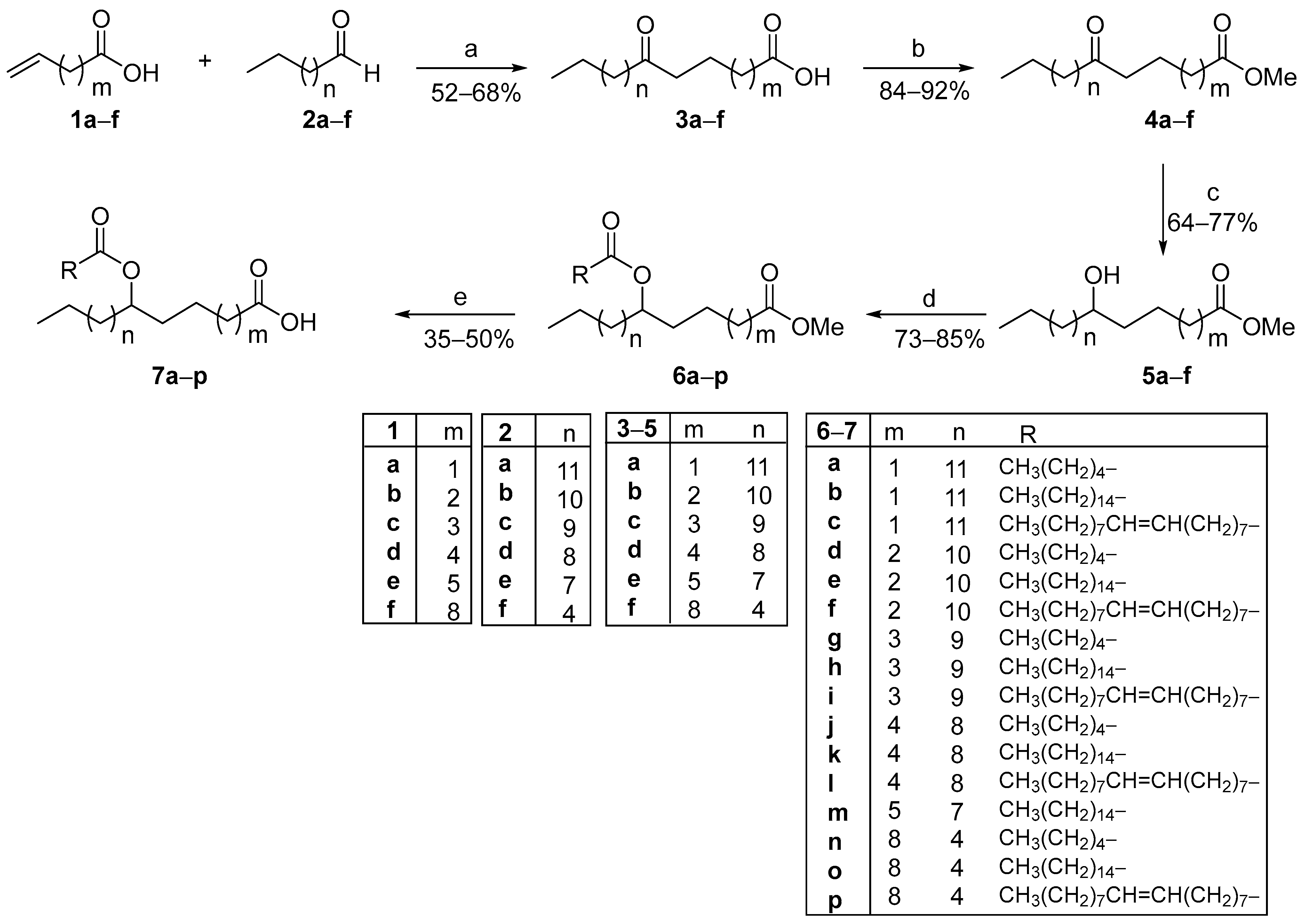
As depicted in Scheme 1, firstly, the desired OFAs were produced in a 52–68% yield from the photochemical hydroacylation reaction between commercially available terminal alkenoic acids 1a–f and aliphatic aldehydes 2a–f, utilizing phenylglyoxylic acid as the photoinitiator and water as the solvent under household lamp irradiation.
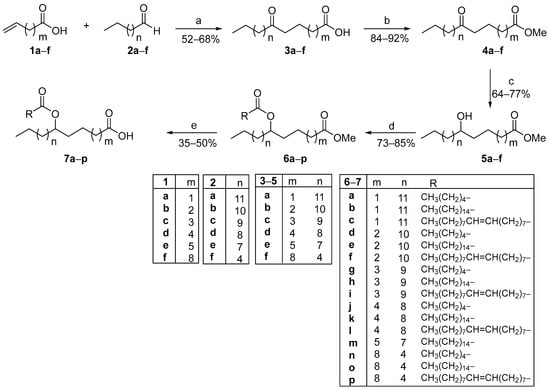
Scheme 1.
General synthetic route for the construction of FAHFAs from ω-alkenoic acids and aldehydes, using a photocatalytic hydroacylation protocol as the key step. (a) Phenylglyoxylic acid (20 mol%), H2O, hv (2 × 85 W CFL household bulbs); (b) CH3OH, conc. H2SO4; (c) NaBH4, CH3OH; (d) CH3(CH2)4COOH or CH3(CH2)14COOH or CH3(CH2)7CH=CH(CH2)7COOH, EDCI·HCl, Et3N, 4-dimethylaminopyridine, dry CH2Cl2; (e) LiOH·H2O, THF:H2O.
The synthesized OFAs 3a–f were subsequently subjected to a Fischer esterification reaction in methanol and conc. H2SO4 to afford the corresponding methyl esters 4a–f in excellent yields. In the next step, the reduction in the oxo functionality of 4a–f by the employment of NaBH4 as the reducing agent resulted in secondary alcohols 5a–f. Coupling reaction between alcohols 5a–f and three different FAs (CA, PA and OA) was accomplished using EDCI·HCl as the coupling reagent, in the presence of Et3N and 4-dimethylaminopyridine (4-DMAP), leading to the formation of methyl esters 6a–p in high yields. Finally, the desired FAHFAs 7a–p were obtained after the basic hydrolysis of the methyl-ester group using LiOH·H2O. The partial hydrolysis of the second ester moiety occurred under the reaction conditions, though to a lesser extent, resulting in a slight reduction in the yields of the hydrolysis step.
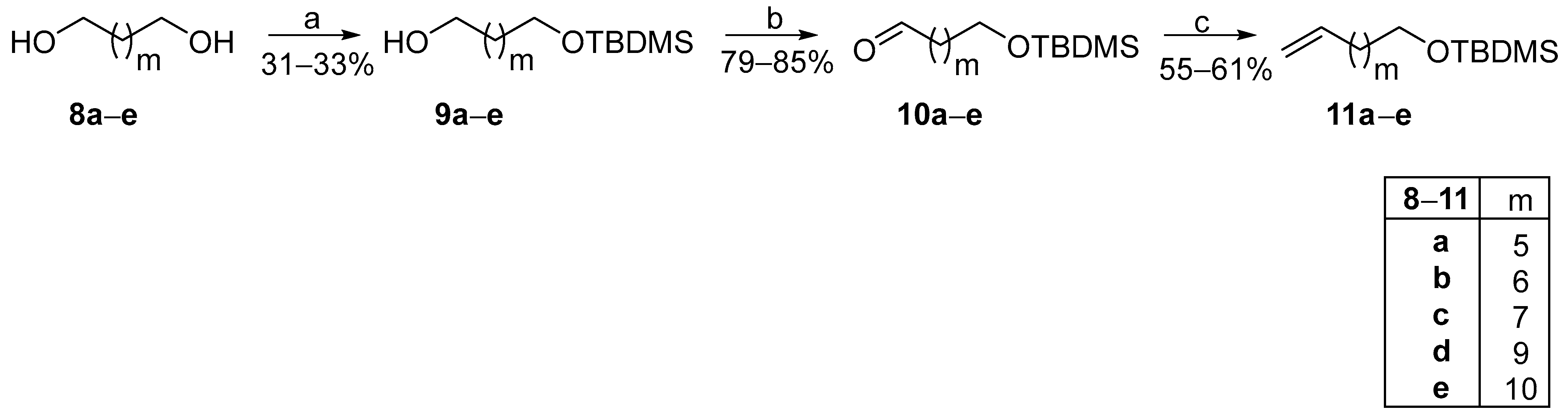
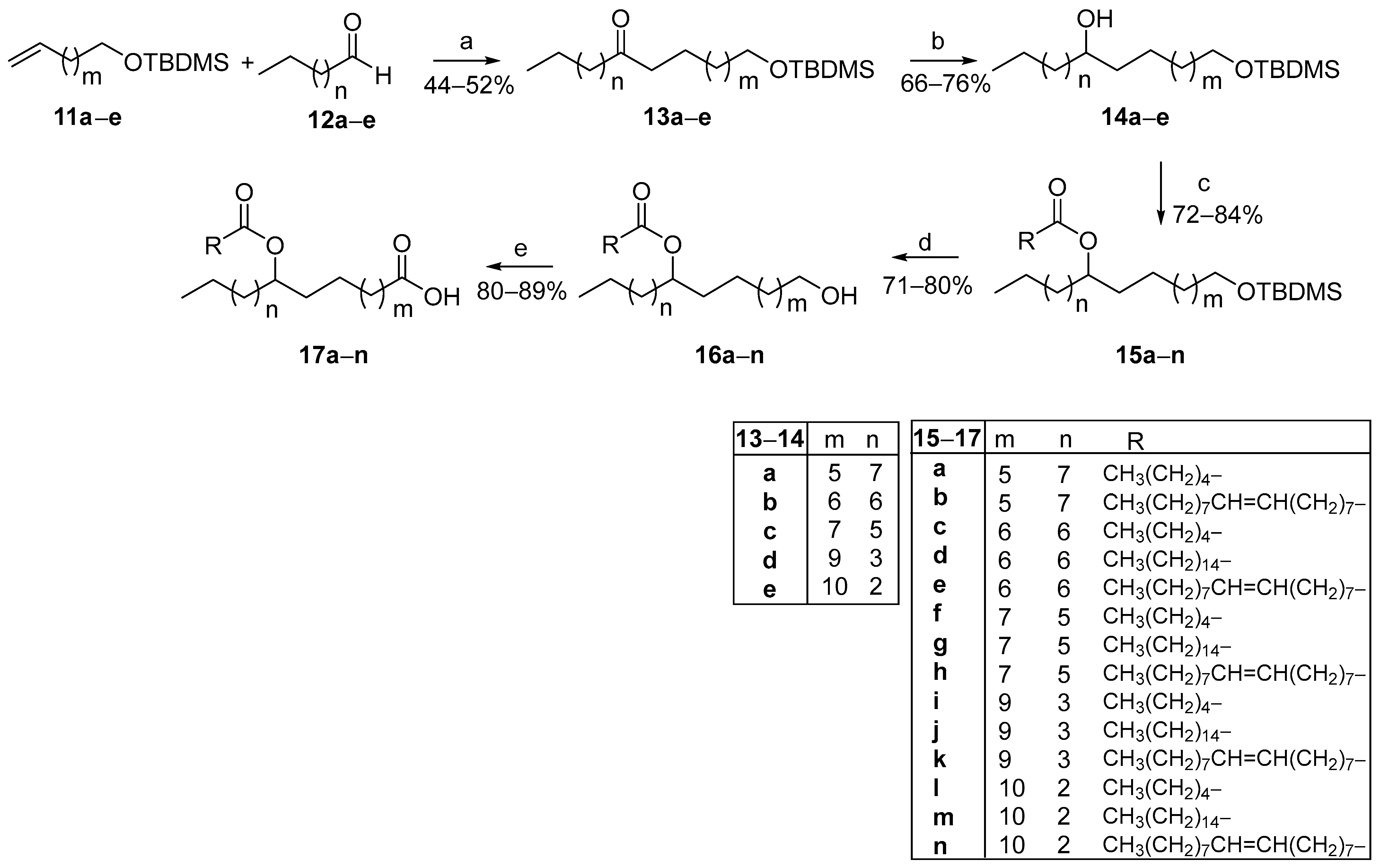
The cost of ω-terminal alkenoic acids is exponentially becoming more expensive as their chain lengthens. Thus, in such cases, we envisaged the use of cheap commercially available α,ω-diols as starting materials, proposing a modified FAHFA synthetic route. As presented in Scheme 2, α,ω-diols 8a–e were mono-protected with a tert-butyldimethylsilyl (TBDMS) protecting group and then, alcohols 9a–e were oxidized to the corresponding aldehydes 10a–e, using pyridinium chlorochromate (PCC) as the oxidant. The TBDMS protecting group was selected for its compatibility with our methodology and the specific requirements of the target molecules. Its stability ensured that other functional groups such as the oleic acid’s double bond remained unaffected. This would not have been feasible with the use of a benzyl protecting group, as its deprotection would also compromise the double bond. Although alternative protecting groups, including ester-based options, were evaluated, their deprotection via hydrolysis often led to partial hydrolysis, thereby reducing efficiency. Wittig olefination reaction between aldehydes 10a–e and phosphonium ylide MePPH3Br produced TBDMS-protected alkenes 11a–e, in good yields, which were employed in the photochemical process, along with commercially available aliphatic aldehydes 12a–e to afford TBDMS-protected ketones 13a–e (Scheme 3).
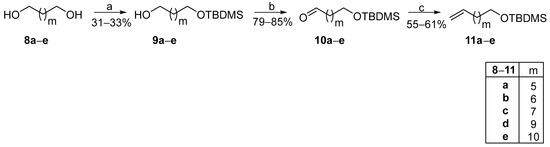
Scheme 2.
Synthetic pathway for the conversion of α,ω-commercially available diols to ω-alkenyl alcohols. (a) tert-Butyldimethylsilyl chloride, imidazole, 4-dimethylaminopyridine, dry N,N-dimethylformamide; (b) Pyridinium chlorochromate, dry CH2Cl2; (c) MePPh3Br, n-BuLi (1.6 M in hexanes), dry THF.
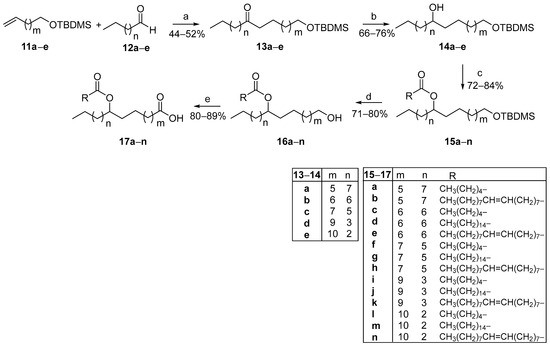
Scheme 3.
General synthetic route for the construction of FAHFAs from ω-alkenyl alcohols and aldehydes through photocatalytic hydroacylation. (a) Phenylglyoxylic acid (20 mol%), H2O, hv (2 × 85 W CFL household bulbs); (b) NaBH4, CH3OH; (c) CH3(CH2)4COOH or CH3(CH2)14COOH or CH3(CH2)7CH=CH(CH2)7COOH, EDCI·HCl, Et3N, 4-dimethylaminopyridine, dry CH2Cl2; (d) TBAF, dry THF; (e) Jones reagent, acetone.
In a manner similar to Scheme 1, the reduction of the oxo group by NaBH4 led to the corresponding alcohols 14a–e. Similarly, the coupling reaction between alcohols 14a–e and caproic, palmitic, or oleic acid afforded 15a–n in high yields. Deprotection of the TBDMS group by treatment with tetra-n-butylammonium fluoride (TBAF), followed by Jones oxidation led to the desired FAHFAs 17a–n.
3. Materials and Methods
3.1. General Remarks
All commercially available products and solvents were purchased from Fluorochem (Fluorochem Ltd., Hadfield, Glossop, UK), Sigma-Aldrich (Sigma-Aldrich, Saint Louis, MO, USA), Fluka (Fluka Chemicals Ltd., Gillingham, Dorset, UK), Merck (Merck, Darmstadt, Germany), and Alfa Aesar (Alfa Aesar, Ward Hill, MA, USA). The chromatographic purification of products was accomplished using forced-flow chromatography on Merck® (Merck, Darmstadt, Germany) Kieselgel 60 F254 230–400 mesh. Thin-layer chromatography (TLC) was performed on aluminum-backed silica plates (0.2 mm, 60 F254). The visualization of the developed chromatogram was performed by fluorescence quenching using phosphomolybdic acid. Melting points were measured on a Buchi 530 apparatus (Buchi, Flawil, Switzerland) and were uncorrected. High-resolution mass spectra were obtained on a Bruker Maxis Impact QTOF spectrometer (Bruker Daltonics, Bremen, Germany). 1H-NMR and 13C-NMR spectra were recorded on an Avance III HD Bruker 400 MHz (Bruker, Fällanden, Switzerland) (400 MHz and 100 MHz, respectively) or a Varian Mercury (Varian, Palo Alto, CA, USA) (200 MHz and 50 MHz, respectively) and are internally referenced to residual solvent signals. Data for 1H-NMR are reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, t = triplet, quin = quintet, m = multiplet, br s = broad signal), coupling constant, integration, and assignment. Data for 13C-NMR are reported in terms of a chemical shift (δ ppm). The synthesis of 3a–f and 13b was carried out as described by Batsika et al. [36], and their spectroscopic data were in accordance with the literature. Additionally, compounds 4a–f [37,38,39,40,41,42], 5a [39], 5b [43], 5c,d [40], 5e [42], 5f [44], 9a–d [45,46,47,48], 9e [36], 10a–d [49,50,51,52], 10e [36], 11a,b [53,54], 11c [51], 11d [55], and 11e [36] were prepared by known procedures, and their spectroscopic data were identical with those in the literature.
3.2. General Procedure for the Photochemical Reaction of Aldehydes with Alkenes
In a glass vial equipped with a PTFE-coated stirring bar, the appropriate alkene (0.50 mmol), the aldehyde (1.50 mmol), and the phenylglyoxylic acid (15 mg, 0.10 mmol) were added using water (1 mL) as the reaction solvent. The vial was sealed with a screw cap and left stirring under household bulb irradiation (2 × 85 W household lamps) for 48 h. The reaction mixture was extracted with CH2Cl2 (3 × 3 mL). The combined organic layers were dried over Na2SO4, and the solvent was removed in vacuo. The desired oxo fatty acids 3a–f were isolated by precipitation with hexane (3–5 mL) (cooled with an external ice bath). Ketones 13a–e were purified by flash chromatography on silica gel eluting with a petroleum ether (bp 40–60 °C)/ethyl acetate 95/5 system.
1-((tert-Βutyldimethylsilyl)oxy)octadecan-9-one (13a). Colorless oil; Yield: 44%; 1H-NMR (400 MHz, CDCl3): δ 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.37 (t, J = 7.4 Hz, 4H, 2 × COCH2), 1.59–1.44 (m, 6H, 3 × CH2), 1.31–1.23 (m, 20H, 10 × CH2), 0.91–0.84 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 211.6, 63.3, 42.8, 42.8, 32.8, 31.9, 29.4, 29.4, 29.4, 29.3, 29.3, 29.2, 26.0, 25.7, 23.9, 23.9, 22.6, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3477.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-one (13c). Colorless oil; Yield: 49%; 1H-NMR (200 MHz, CDCl3): δ 3.54 (t, J = 6.5 Hz, 2H, CH2OTBDMS), 2.33 (t, J = 7.4 Hz, 4H, 2 × COCH2), 1.57–1.41 (m, 6H, 3 × CH2), 1.28–1.16 (m, 20H, 10 × CH2), 0.86–0.78 (m, 12H, 4 × CH3), -0.01 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 211.3, 63.2, 42.7, 32.8, 31.6, 29.5, 29.3, 29.1, 29.0, 25.9, 25.7, 23.8, 22.5, 18.2, 14.0, −5.4; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3472.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-one (13d). Colorless oil; Yield: 52%; 1H-NMR (400 MHz, CDCl3): δ 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.37 (t, J = 7.4 Hz, 4H, 2 × COCH2), 1.57–1.47 (m, 6H, 3 × CH2), 1.32–1.24 (m, 20H, 10 × CH2), 0.91–0.86 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 211.7, 63.3, 42.8, 42.8, 32.9, 31.4, 29.6, 29.6, 29.5, 29.4, 29.4, 29.3, 26.0, 25.8, 23.9, 23.6, 22.4, 18.4, 13.9, −5.3; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3478.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-one (13e). Colorless oil; Yield: 46%; 1H-NMR (200 MHz, CDCl3): δ 3.54 (t, J = 6.5 Hz, 2H, CH2OTBDMS), 2.33 (t, J = 7.2 Hz, 4H, 2 × COCH2), 1.60–1.40 (m, 6H, 3 × CH2), 1.32–1.16 (m, 20H, 10 × CH2), 0.93–0.77 (m, 12H, 4 × CH3), −0.01 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 211.2, 63.2, 42.7, 42.4, 32.8, 29.5, 29.5, 29.4, 29.4, 29.2, 25.9, 25.7, 23.8, 22.3, 18.2, 13.8, −5.4; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3478.
3.3. General Procedure for the Synthesis of Alcohols via Oxo Functionality Reduction
TBDMS-protected ketone 13a–e (1.00 mmol) in MeOH (5 mL) was added to a round-bottomed flask at 0 °C. Next, NaBH4 (189 mg, 5.00 mmol) was added under vigorous stirring. The solution was stirred for 1 h, and then methanol was removed under reduced pressure. The reaction mixture was washed with a saturated aqueous solution of NH4Cl (3 × 10 mL) and the combined organic layers were collected and dried with Na2SO4, and the solvent was removed under reduced pressure. The crude mixture was purified by flash chromatography (petroleum ether (bp 40–60 °C)/ethyl acetate, 70/30 for methyl esters, or 90/10 for TBDMS-protected compounds) to afford the desired alcohol.
1-((tert-Βutyldimethylsilyl)oxy)octadecan-9-ol (14a) [22]. Colorless oil; Yield: 74%; 1H-NMR (200 MHz, CDCl3): δ 3.64–3.53 (m, 3H, CH2OTBDMS and CHOH), 1.56–1.22 (m, 31H, 15 × CH2 and OH), 0.96–0.77 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 72.0, 63.3, 37.5, 32.9, 31.9, 29.7, 29.6, 29.6, 29.6 29.4, 29.3, 26.0, 25.8, 25.6, 22.7, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3633.
18-((tert-Butyldimethylsilyl)oxy)octadecan-9-ol (14b) [20]. Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 3.68–3.47 (m, 3H, CH2OTBDMS and CHOH), 1.53–1.39 (m, 7H, 3 × CH2 and OH), 1.33–1.23 (m, 24H, 12 × CH2), 0.90–0.86 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 72.0, 63.3, 37.5, 32.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.4, 29.3, 26.0, 25.8, 25.6, 22.7, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+, 423.3629; [M + Na]+ found, 423.3630.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-ol (14c). Colorless oil; Yield: 71%; 1H-NMR (200 MHz, CDCl3): δ 3.62–3.52 (m, 3H, CH2OTBDMS and CHOH), 1.56–1.39 (m, 7H, 3 × CH2 and OH), 1.35–1.21 (m, 24H, 12 × CH2), 0.90–0.82 (m, 12H, 4 × CH3), 0.03 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 72.0, 63.3, 37.5, 32.8, 31.8, 29.7, 29.6, 29.4, 29.3, 25.9, 25.8, 25.6, 22.6, 18.3, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3632.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-ol (14d). Colorless oil; Yield: 66%; 1H-NMR (400 MHz, CDCl3): δ 3.62–3.55 (m, 3H, CH2OTBDMS and CHOH), 1.52–1.40 (m, 7H, 3 × CH2 and OH), 1.36–1.21 (m, 24H, 12 × CH2), 0.91–0.86 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 72.0, 63.3, 37.5, 37.5, 32.9, 31.9, 29.7, 29.6, 29.6, 29.4, 26.0, 25.8, 25.7, 25.3, 22.6, 18.4, 14.0, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3646.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-ol (14e). Colorless oil; Yield: 70%; 1H-NMR (400 MHz, CDCl3): δ 3.63–3.50 (m, 3H, CH2OTBDMS and CHOH), 1.58–1.16 (m, 31H, 15 × CH2 and OH), 0.98–0.76 (m, 12H, 4 × CH3), 0.03 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 71.9, 63.3, 37.5, 37.2, 32.9, 29.7, 29.6, 29.4, 27.8, 26.0, 25.8, 25.7, 22.8, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3624.
3.4. General Procedure for the Coupling Reaction Between Alcohols and FAs
To a solution of the appropriate fatty acid (caproic or palmitic or oleic acid) (4.00 mmol) in dry CH2Cl2 (10 mL) at 0 °C, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (767 mg, 4.00 mmol), triethylamine (0.56 mL, 4.00 mmol), and 4-dimethylaminopyridine (24 mg, 0.20 mmol) were added consecutively. The reaction mixture was left stirring at 0 °C for 10 min, and then a solution of the secondary alcohol (1.00 mmol) in dry CH2Cl2 (10 mL) was added. After 16 h, the reaction mixture was extracted with a saturated aqueous solution of NH4Cl (3 × 10 mL), and the combined organic layers were collected and dried with Na2SO4. The solvent was then removed under reduced pressure. The crude mixture was purified by flash silica chromatography (petroleum ether (bp 40–60 °C)/ethyl acetate, 90/10–80/20) to afford the desired ester as a colorless oil.
Methyl 5-(hexanoyloxy)octadecanoate (6a). Colorless oil; Yield: 77%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.81 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.40–2.21 (m, 4H, 2 × COCH2), 1.69–1.50 (m, 8H, 4 × CH2), 1.39–1.21 (m, 26H, 13 × CH2), 1.01–0.80 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.8, 173.6, 73.4, 51.5, 34.6, 34.0, 33.8, 33.5, 31.9, 31.3, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 25.3, 24.8, 22.7, 22.3, 20.7, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M+Na]+ found: 435.3445.
Methyl 5-(palmitoyloxy)octadecanoate (6b). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.83 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.36–2.22 (m, 4H, 2 × COCH2), 1.70–1.48 (m, 8H, 4 × CH2), 1.34–1.19 (m, 46H, 23 × CH2), 0.87 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.8, 173.6, 73.4, 51.5, 34.7, 34.0, 33.8, 33.5, 31.9, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 25.3, 25.1, 22.7, 20.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5008.
1-Methoxy-1-oxooctadecan-5-yl oleate (6c). Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 5.44–5.31 (m, 2H, 2 × =CH), 4.94–4.83 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.35–2.24 (m, 4H, 2 × COCH2), 2.07–1.94 (m, 4H, 2 × =CHCH2), 1.64–1.51 (m, 8H, 4 × CH2), 1.38–1.19 (m, 42H, 21 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.8, 173.6, 130.0, 129.7, 73.4, 51.5, 34.6, 34.0, 33.8, 33.5, 31.9, 31.9, 29.8, 29.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 22.7, 20.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5161.
Methyl 6-(hexanoyloxy)octadecanoate (6d). Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.82 (m, 1H, COOCH), 3.68 (s, 3H, OCH3), 2.42–2.22 (m, 4H, 2 × COCH2), 1.68–1.61 (m, 4H, 2 × CH2), 1.59–1.48 (m, 4H, 2 × CH2), 1.42–1.24 (m, 26H, 13 × CH2), 0.96–0.86 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.0, 173.7, 73.8, 51.5, 34.7, 34.2, 34.0, 33.8, 31.9, 31.4, 29.7, 29.7, 29.6, 29. 6, 29.5, 29.4, 25.3, 24.9, 24.9, 22.7, 22.4, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3444.
Methyl 6-(palmitoyloxy)octadecanoate (6e). Low melting point solid; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.80 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.33–2.23 (m, 4H, 2 × COCH2), 1.65–1.57 (m, 4H, 2 × CH2), 1.57–1.46 (m, 4H, 2 × CH2), 1.35–1.21 (m, 46H, 23 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.9, 173.6, 73.7, 51.4, 34.7, 34.1, 33.9, 33.8, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 25.3, 25.1, 24.9, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5011.
1-Methoxy-1-oxooctadecan-6-yl oleate (6f). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 5.46–5.27 (m, 2H, 2 × =CH), 4.96–4.77 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.37–2.18 (m, 4H, 2 × COCH2), 2.14–1.88 (m, 4H, 2 × =CHCH2), 1.65–1.58 (m, 4H, 2 × CH2), 1.55–1.46 (m, 4H, 2 × CH2), 1.36–1.23 (m, 42H, 21 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.0, 173.6, 130.0, 129.7, 73.7, 51.4, 34.7, 34.1, 33.9, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 24.9, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5161.
Methyl 7-(hexanoyloxy)octadecanoate (6g). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.80 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.33–2.23 (m, 4H, 2 × COCH2), 1.65–1.57 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.35–1.20 (m, 26H, 13 × CH2), 0.94–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.1, 173.7, 73.8, 51.4, 34.6, 34.1, 33.9, 31.9, 31.3, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.0, 25.3, 25.0, 24.8, 24.8, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3445.
Methyl 7-(palmitoyloxy)octadecanoate (6h). Off white low melting point solid; Yield: 77%; 1H-NMR (200 MHz, CDCl3): δ 4.91–4.79 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.33–2.21 (m, 4H, 2 × COCH2), 1.69–1.46 (m, 8H, 4 × CH2), 1.46–1.05 (m, 46H, 23 × CH2), 0.86 (t, J = 6.4 Hz, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 174.1, 173.7, 73.8, 51.4, 34.7, 34.1, 33.9, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.0, 25.3, 25.2, 25.0, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5020.
1-Methoxy-1-oxooctadecan-7-yl oleate (6i). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 5.43–5.26 (m, 2H, 2 × =CH), 4.89–4.81 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.31–2.24 (m, 4H, 2 × COCH2), 2.11–1.88 (m, 4H, 2 × =CHCH2), 1.64–1.56 (m, 4H, 2 × CH2), 1.54–1.45 (m, 4H, 2 × CH2), 1.36–1.20 (m, 42H, 21 × CH2), 0.87 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.1, 173.6, 129.9, 129.7, 73.8, 51.4, 34.7, 34.1, 33.9, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.1, 29.1, 29.0, 27.2, 27.1, 25.3, 25.1, 25.0, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5165.
Methyl 8-(hexanoyloxy)octadecanoate (6j). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.79 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.35–2.21 (m, 4H, 2 × COCH2), 1.65–1.58 (m, 4H, 2 × CH2), 1.54–1.46 (m, 4H, 2 × CH2), 1.36–1.21 (m, 26H, 13 × CH2), 0.96–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 73.9, 51.4, 34.7, 34.1, 34.1, 34.0, 31.9, 31.3, 29.6, 29.5, 29.5, 29.3, 29.1, 29.0, 25.3, 25.1, 24.8, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3457.
Methyl 8-(palmitoyloxy)octadecanoate (6k). Colorless oil; Yield: 85%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.79 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.32–2.23 (m, 4H, 2 × COCH2), 1.65–1.57 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.35–1.21 (m, 46H, 23 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.7, 73.9, 51.4, 34.7, 34.2, 34.1, 34.0, 31.9, 31.9, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.1, 29.0, 25.3, 25.2, 25.1, 24.9, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5009.
1-Methoxy-1-oxooctadecan-8-yl oleate (6l). Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 5.45–5.26 (m, 2H, 2 × =CH), 4.93–4.79 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.34–2.22 (m, 4H, 2 × COCH2), 2.10–1.88 (m, 4H, 2 × =CHCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.54–1.43 (m, 4H, 2 × CH2), 1.39–1.11 (m, 42H, 21 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 130.0, 129.7, 73.9, 51.4, 34.7, 34.1, 34.07, 34.0, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.2, 29.1, 29.1, 29.0, 27.2, 27.2, 25.3, 25.1, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5162.
Methyl 9-(palmitoyloxy)octadecanoate (6m) [42]. Colorless oil; Yield: 73%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.75 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.34–2.22 (m, 4H, 2 × COCH2), 1.64–1.57 (m, 4H, 2 × CH2), 1.57–1.45 (m, 4H, 2 × CH2), 1.35–1.22 (m, 46H, 23 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.7, 74.0, 51.4, 34.7, 34.2, 34.1, 34.1, 31.9, 31.9, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.1, 29.0, 25.3, 25.2, 25.2, 24.9, 22.7, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5010.
Methyl 12-(hexanoyloxy)octadecanoate (6n). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.80 (m, 1H, COOCH), 3.64 (s, 3H, OCH3), 2.33–2.22 (m, 4H, 2 × COCH2), 1.67–1.54 (m, 4H, 2 × CH2), 1.54–1.42 (m, 4H, 2 × CH2), 1.40–1.15 (m, 26H, 13 × CH2), 0.91–0.82 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 74.0, 51.3, 34.6, 34.1, 34.0, 31.7, 31.3, 29.5, 29.5, 29.4, 29.4, 29.2, 29.1, 29.1, 25.3, 25.2, 24.9, 24.8, 22.5, 22.3, 14.0, 13.8; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3446.
Methyl 12-(palmitoyloxy)octadecanoate (6o) [56]. Colorless oil; Yield: 77%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.35–2.23 (m, 4H, 2 × COCH2), 1.64–1.56 (m, 4H, 2 × CH2), 1.53–1.45 (m, 4H, 2 × CH2), 1.38–1.21 (m, 46H, 23 × CH2), 0.86 (t, J = 6.2 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 74.0, 51.3, 34.7, 34.1, 34.1, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 25.3, 25.3, 25.2, 24.9, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5024.
18-Methoxy-18-oxooctadecan-7-yl oleate (6p). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 5.40–5.27 (m, 2H, 2 × =CH), 4.90–4.81 (m, 1H, COOCH), 3.64 (s, 3H, OCH3), 2.31–2.22 (m, 4H, 2 × COCH2), 2.06–1.90 (m, 4H, 2 × =CHCH2), 1.64–1.56 (m, 4H, 2 × CH2), 1.54–1.43 (m, 4H, 2 × CH2), 1.37–1.17 (m, 42H, 21 × CH2), 0.86 (t, J = 6.1 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 129.9, 129.7, 74.0, 51.3, 34.7, 34.1, 34.1, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.1, 29.1, 27.2, 27.1, 25.3, 25.2, 25.1, 24.9, 22.6, 22.5, 14.0, 14.0; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5164.
1-((tert-Βutyldimethylsilyl)oxy)octadecan-9-yl hexanoate (15a). Colorless oil; Yield: 77%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO),1.64–159 (m, 2H, CH2), 1.53–1.46 (m, 6H, 3 × CH2), 1.32–1.23 (m, 28H, 14 × CH2), 0.91–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.4, 29.5, 29.5, 29.5, 29.4, 29.3, 26.0, 25.8, 25.3, 24.9, 22.7, 22.3, 18.4, 14.1, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4364.
1-((tert-Butyldimethylsilyl)oxy)octadecan-9-yl oleate (15b) [22]. Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 5.39–5.30 (m, 2H, 2 × =CH), 4.90–4.82 (m, 1H, COOCH), 3.59 (t, J = 7.5 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.04–1.93 (m, 4H, 2 × =CHCH2), 1.66–1.55 (m, 4H, 2 × CH2), 1.53–1.47 (m, 6H, 3 × CH2), 1.34–1.25 (m, 42H, 21 × CH2), 0.90–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.6, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6095.
18-((tert-Butyldimethylsilyl)oxy)octadecan-9-yl hexanoate (15c). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.76 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.47 (m, 8H, 4 × CH2), 1.33–1.23 (m, 28H, 14 × CH2), 0.94–0.82 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.3, 29.7, 29.5, 29.5, 29.5, 29.4, 29.2, 26.0, 25.8, 25.3, 24.9, 22.6, 22.3, 18.4, 14.1, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4371.
18-((tert-Butyldimethylsilyl)oxy)octadecan-9-yl palmitate (15d). Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.77 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 1.65–1.59 (m, 2H, CH2), 1.54–1.44 (m, 6H, 3 × CH2), 1.35–1.21 (m, 48H, 24 × CH2), 0.97–0.79 (m, 15H, 5 × CH3), 0.05 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.8, 34.2, 32.9, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.7, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5921.
18-((tert-Butyldimethylsilyl)oxy)octadecan-9-yl oleate (15e) [20]. Colorless oil; Yield: 83%; 1H-NMR (400 MHz, CDCl3): δ 5.49–5.23 (m, 2H, 2 × =CH), 4.93–4.79 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.12–1.88 (m, 4H, 2 × =CHCH2), 1.68–1.55 (m, 4H, 2 × CH2), 1.53–1.45 (m, 6H, 3 × CH2), 1.35–1.23 (m, 42H, 21 × CH2), 0.97–0.80 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.7, 18.4, 14.1, 14.1, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6080.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-yl hexanoate (15f). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.78 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.28 (t, J = 7.5 Hz, 2H, CH2COO), 1.67–1.60 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.34–1.23 (m, 28H, 14 × CH2), 0.93–0.83 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 34.1, 32.9, 31.7, 31.4, 29.6, 29.6, 29.6, 29.6, 29.5, 29.4, 26.0, 25.8, 25.3, 25.0, 24.9, 22.5, 22.3, 18.4, 14.0, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4374.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-yl palmitate (15g). Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.79 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.65–1.59 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.32–1.22 (m, 48H, 24 × CH2), 0.92–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.8, 34.2, 32.9, 31.9, 31.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5958.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-yl oleate (15h). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 5.39–5.28 (m, 2H, 2 × =CH), 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.5 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 2.06–1.94 (m, 4H, 2 × =CHCH2), 1.67–1.56 (m, 4H, 2 × CH2), 1.53–1.47 (m, 6H, 3 × CH2), 1.33–1.24 (m, 42H, 21 × CH2), 0.90–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.8, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.8, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.50, 29.4, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 18.4, 14.1, 14.1, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6109.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-yl hexanoate (15i). Colorless oil; Yield: 72%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.58 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.34–1.22 (m, 28H, 14 × CH2), 0.92–0.84 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 34.1, 32.9, 31.7, 31.4, 29.6, 29.6, 29.6, 29.6, 29.5, 29.4, 26.0, 25.8, 25.3, 25.0, 24.9, 22.5, 22.3, 18.4, 14.0, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4374.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-yl palmitate (15j). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.58 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.34–1.19 (m, 48H, 24 × CH2), 0.92–0.84 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.3, 34.7, 34.2, 34.1, 32.9, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 26.0, 25.8, 25.3, 25.2, 25.0, 22.7, 22.5, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5949.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-yl oleate (15k). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.25 (m, 2H, 2 × =CH), 4.92–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.11–1.89 (m, 4H, 2 × =CHCH2), 1.65–1.59 (m, 2H, CH2), 1.55–1.46 (m, 6H, 3 × CH2), 1.37–1.22 (m, 44H, 22 × CH2), 0.92–0.83 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 34.1, 32.9, 31.9, 31.7, 29.8, 29.7, 29.6, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 25.0, 22.7, 22.6, 22.5, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6099.
18-((tert-Βutyldimethylsilyl)oxy)octadecan-5-yl hexanoate (15l). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.59 (m, 2H, CH2), 1.56–1.45 (m, 6H, 3 × CH2), 1.36–1.23 (m, 28H, 14 × CH2), 0.94–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 33.9, 32.9, 31.4, 29.6, 29.6, 29.6, 29.6, 29.5, 27.5, 26.0, 25.8, 25.3, 24.9, 22.6, 22.3, 18.4, 14.0, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4363.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-yl palmitate (15m). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.81 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.64–1.59 (m, 2H, CH2), 1.55–1.46 (m, 6H, 3 × CH2), 1.37–1.21 (m, 48H, 24 × CH2), 0.98–0.77 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.3, 34.7, 34.2, 33.9, 32.9, 31.9, 29.7, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 27.5, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5939.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-yl oleate (15n). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 5.40–5.29 (m, 2H, 2 × =CH), 4.91–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 2.08–1.92 (m, 4H, 2 × =CHCH2), 1.64–1.48 (m, 8H, 4 × CH2), 1.35–1.22 (m, 44H, 22 × CH2), 0.92–0.84 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.6, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 33.9, 32.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 27.5, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6099.
3.5. General Procedure for the Synthesis of FAHFAs Through the Saponification of Esters
To a round-bottomed flask containing methyl esters 6a–p (1.00 mmol) diluted in a mixture of THF and H2O (3:1, 10 mL), LiOH·H2O (210 mg, 5.00 mmol) was added, and the reaction mixture was left stirring at room temperature for 16 h. The reaction was then quenched with H2O, followed by the addition of HCl 1 N (10 mL) to pH 1. Then, the mixture was extracted with EtOAc (3 × 10 mL), washed with brine (1 × 30 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure. Flash chromatography on silica gel of the crude mixture (petroleum ether (bp 40–60 °C)/ethyl acetate, 60/40) resulted in the desired FAHFA.
5-(Hexanoyloxy)octadecanoic acid (7a). White solid; Yield: 35%; m. p.: 39–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.97–4.82 (m, 1H, COOCH), 2.36 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.68–1.49 (m, 8H, 4 × CH2), 1.39–1.23 (m, 26H, 13 × CH2), 1.01–0.81 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.2, 173.7, 73.4, 34.6, 34.1, 33.5, 33.4, 31.9, 31.3, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 25.3, 24.8, 22.7, 22.3, 20.5, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3287.
5-(Palmitoyloxy)octadecanoic acid (7b) [21]. White solid; Yield: 42%; m. p.: 39–40 °C, (lit. m.p.: 39–40 °C); 1H-NMR (400 MHz, CDCl3): δ 4.97–4.81 (m, 1H, COOCH), 2.36 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.72–1.45 (m, 8H, 4 × CH2), 1.36–1.19 (m, 46H, 23 × CH2), 0.88 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.7, 73.4, 34.7, 34.1, 33.7, 33.4, 31.9, 29.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 29.1, 25.3, 25.1, 24.7, 22.7, 20.4, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4853.
5-(Oleoyloxy)octadecanoic acid (7c). Colorless oil; Yield: 41%; 1H-NMR (400 MHz, CDCl3): δ 5.46–5.23 (m, 2H, 2 × =CH), 4.97–4.81 (m, 1H, COOCH), 2.36 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 2.12–1.89 (m, 4H, 2 × =CHCH2), 1.70–1.48 (m, 8H, 4 × CH2), 1.40–1.09 (m, 42H, 21 × CH2), 0.88 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.4, 173.7, 130.0, 129.8, 73.4, 34.7, 34.1, 33.4, 31.9, 31.9, 29.8, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 22.7, 20.5, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5008.
6-(Hexanoyloxy)octadecanoic acid (7d). Colorless oil; Yield: 39%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.83 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.72–1.58 (m, 4H, 2 × CH2), 1.58–1.45 (m, 4H, 2 × CH2), 1.40–1.21 (m, 26H, 13 × CH2), 0.94–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.8, 173.7, 73.7, 34.7, 34.1, 33.8, 33.7, 31.9, 31.3, 29.7, 29.6, 29.6, 29.5, 29.5, 29.3, 25.3, 24.8, 24.5, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3286.
6-(Palmitoyloxy)octadecanoic acid (7e). White solid; Yield: 46%; m. p.: 38–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.72–1.58 (m, 4H, 2 × CH2), 1.58–1.43 (m, 4H, 2 × CH2), 1.39–1.20 (m, 46H, 23 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.4, 173.7, 73.7, 34.7, 34.1, 33.8, 33.8, 31.9, 29.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 25.3, 25.2, 24.8, 24.5, 22.7, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4851.
6-(Oleoyloxy)octadecanoic acid (7f). Colorless oil; Yield: 50%; 1H-NMR (400 MHz, CDCl3): δ 5.53–5.20 (m, 2H, 2 × =CH), 4.94–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.12–1.89 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.57–1.48 (m, 4H, 2 × CH2), 1.37–1.22 (m, 42H, 21 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.7, 130.0, 129.7, 73.7, 34.7, 34.1, 33.8, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 24.8, 24.5, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
7-(Hexanoyloxy)octadecanoic acid (7g). Off white low-melting point solid; Yield: 38%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.56 (m, 4H, 2 × CH2), 1.56–1.45 (m, 4H, 2 × CH2), 1.39–1.18 (m, 26H, 13 × CH2), 0.92–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.4, 173.8, 73.9, 34.7, 34.2, 33.9, 33.9, 31.9, 31.3, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 28.9, 25.3, 25.0, 24.8, 24.5, 22.7, 22.3, 14.1, 13.9; HRMS (ESI−): m/z calcd for C24H45O4−: 397.3323; [M − H]− found: 397.3324.
7-(Palmitoyloxy)octadecanoic acid (7h) [20]. White solid; Yield: 48%; m. p.: 39–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.40–1.17 (m, 46H, 23 × CH2), 0.87 (t, J = 6.9 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.8, 173.8, 73.9, 34.7, 34.2, 33.9, 33.9, 31.9, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 28.9, 25.3, 25.2, 25.0, 24.5, 22.7, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4870.
7-(Oleoyloxy)octadecanoic acid (7i) [20]. Colorless oil; Yield: 49%; 1H-NMR (400 MHz, CDCl3): δ 5.48–5.19 (m, 2H, 2 × =CH), 4.95–4.77 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.12–1.89 (m, 4H, 2 × =CHCH2), 1.68–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.41–1.13 (m, 42H, 21 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.7, 173.7, 130.0, 129.7, 73.9, 34.7, 34.1, 33.9, 33.9, 31.9, 29.8, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 28.9, 27.2, 27.2, 25.3, 25.1, 25.0, 24.5, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5011.
8-(Hexanoyloxy)octadecanoic acid (7j). White solid; Yield: 42%; m. p.: 35–37 °C; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.41–1.16 (m, 26H, 13 × CH2), 0.98–0.79 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.8, 173.8, 74.0, 34.7, 34.1, 34.1, 34.0, 31.9, 31.3, 29.6, 29.5, 29.5, 29.5, 29.3, 29.1, 28.9, 25.3, 25.1, 24.8, 24.6, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3289.
8-(Palmitoyloxy)octadecanoic acid (7k). White solid; Yield: 39%; m. p.: 39–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.77 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.37–1.19 (m, 46H, 23 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.7, 173.7, 74.0, 34.7, 34.2, 34.1, 34.0, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.1, 28.9, 25.3, 25.2, 25.1, 24.6, 22.7, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4854.
8-(Oleoyloxy)octadecanoic acid (7l). Colorless oil; Yield: 43%; 1H-NMR (400 MHz, CDCl3): δ 5.44–5.30 (m, 2H, 2 × =CH), 4.91–4.81 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.11–1.88 (m, 4H, 2 × =CHCH2), 1.68–1.56 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.42–1.18 (m, 42H, 21 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.7, 130.0, 129.7, 74.0, 34.7, 34.2, 34.1, 33.9, 31.9, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 29.1, 28.9, 27.2, 27.2, 25.3, 25.2, 25.1, 24.6, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5016.
9-(Palmitoyloxy)octadecanoic acid (7m) [42]. Colorless oil; Yield: 45%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.58–1.45 (m, 4H, 2 × CH2), 1.34–1.23 (m, 46H, 23 × CH2), 0.88 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.4, 173.8, 74.0, 34.8, 34.2, 34.1, 33.9, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 29.1, 29.0, 25.3, 25.2, 25.2, 24.6, 22.7, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4852.
12-(Hexanoyloxy)octadecanoic acid (7n). Colorless oil; Yield: 37%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.58 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.34–1.22 (m, 26H, 13 × CH2), 0.91–0.85 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.9, 173.8, 74.1, 34.7, 34.1, 34.1, 31.7, 31.3, 29.5, 29.5, 29.4, 29.4, 29.2, 29.2, 29.0, 25.3, 25.3, 24.8, 24.7, 22.5, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3289.
12-(Palmitoyloxy)octadecanoic acid (7o) [56]. White solid; Yield: 40%; m. p.: 39–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.57 (m, 4H, 2 × CH2), 1.55–1.46 (m, 4H, 2 × CH2), 1.38–1.18 (m, 46H, 23 × CH2), 0.87 (t, J = 6.2 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.7, 173.8, 74.1, 34.8, 34.2, 34.0, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.0, 25.3, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4846.
12-(Oleoyloxy)octadecanoic acid (7p). Colorless oil; Yield: 36%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.25 (m, 2H, 2 × =CH), 4.92–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.13–1.88 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.56–1.45 (m, 4H, 2 × CH2), 1.37–1.21 (m, 42H, 21 × CH2), 0.88 (t, J = 6.1 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.7, 130.0, 129.8, 74.1, 34.7, 34.2, 34.0, 31.9, 31.8, 29.8, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 27.2, 27.2, 25.3, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
3.6. General Procedure for the Deprotection of Tert-Butyldimethylsilyl (TBDMS) Group
The appropriate protected alcohol 15a–n (1.00 mmol) was added to a flame-dried flask in a dry THF (3 mL) and a solution of tetra-n-butylammonium fluoride (1 mL, 1.00 mmol, 1 M in THF) was added dropwise. The reaction mixture was left stirring for 1 h at room temperature, and then the solvent was removed under reduced pressure. The desired product was isolated by flash chromatography (petroleum ether (bp 40–60 °C)/ethyl acetate, 80/20–70/30) on silica gel.
1-Hydroxyoctadecan-9-yl hexanoate (16a). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.67–1.42 (m, 9H, 4 × CH2 and OH), 1.37–1.22 (m, 28H, 14 × CH2), 0.93–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.0, 34.7, 34.2, 34.1, 32.8, 31.9, 31.3, 29.5, 29.4, 29.4, 29.3, 25.7, 25.3, 25.3, 24.8, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3480.
1-Hydroxyoctadecan-9-yl oleate (16b) [22]. Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 5.43–5.26 (m, 2H, 2 × =CH), 4.93–4.79 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.10–1.88 (m, 4H, 2 × =CHCH2), 1.64–1.47 (m, 8H, 4 × CH2), 1.43–1.20 (m, 45H, 22 × CH2 and OH), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.0, 63.0, 34.7, 34.2, 34.1, 32.8, 31.9, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.7, 25.3, 25.3, 25.2, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5202.
18-Hydroxyoctadecan-9-yl hexanoate (16c). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.76 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 1.66–1.45 (m, 9H, 4 × CH2 and OH), 1.39–1.19 (m, 28H, 14 × CH2), 0.98–0.78 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.2, 34.1, 32.8, 31.9, 31.4, 29.7, 29.5, 29.5, 29.5, 29.4, 29.4, 29.2, 25.7, 25.3, 25.3, 24.9, 22.6, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3509.
18-Hydroxyoctadecan-9-yl palmitate (16d). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.79 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.65–1.46 (m, 11H, 5 × CH2 and OH), 1.35–1.22 (m, 46H, 23 × CH2), 0.87 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.0, 34.7, 34.2, 34.1, 32.8, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 25.7, 25.3, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5070.
18-Hydroxyoctadecan-9-yl oleate (16e) [20]. Colorless oil; Yield: 73%; 1H-NMR (400 MHz, CDCl3): δ 5.51–5.21 (m, 2H, 2 × =CH), 4.94–4.79 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.11–1.89 (m, 4H, 2 × =CHCH2), 1.66–1.45 (m, 10H, 5 × CH2), 1.40–1.17 (m, 43H, 21 × CH2 and OH), 0.88 (t, J = 6.3 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.0, 34.7, 34.1, 32.8, 31.9, 31.8, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 27.2, 27.2, 25.7, 25.3, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5229.
18-Hydroxyoctadecan-8-yl hexanoate (16f). Colorless oil; Yield: 78%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.47 (m, 9H, 4 × CH2 and OH), 1.35–1.24 (m, 28H, 14 × CH2), 0.93–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.2, 32.8, 31.8, 31.3, 29.5, 29.5, 29.4, 29.4, 29.2, 25.7, 25.3, 25.3, 24.8, 22.6, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3497.
18-Hydroxyoctadecan-8-yl palmitate (16g). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, OCH2), 2.27 (t, J = 7.4 Hz, 2H, COCH2), 1.68–1.44 (m, 10H, 5 × CH2), 1.36–1.23 (m, 47H, 23 × CH2 and OH), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.8, 34.2, 32.8, 31.9, 31.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5065.
18-Hydroxyoctadecan-8-yl oleate (16h). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 5.45–5.25 (m, 2H, 2 × =CH), 4.92–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.5 Hz, 2H, OCH2), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 2.05–1.95 (m, 4H, 2 × =CHCH2), 1.65–1.46 (m, 11H, 5 × CH2 and OH), 1.36–1.24 (m, 42H, 21 × CH2), 0.88 (t, J = 6.2 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.0, 34.7, 34.2, 32.8, 31.9, 31.8, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5220.
18-Hydroxyoctadecan-6-yl hexanoate (16i). Colorless oil; Yield: 71%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.71–1.40 (m, 9H, 4 × CH2 and OH), 1.37–1.22 (m, 28H, 14 × CH2), 0.94–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.1, 34.1, 32.8, 31.7, 31.3, 29.6, 29.5, 29.5, 29.5, 29.4, 25.7, 25.3, 25.0, 24.8, 22.5, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3500.
18-Hydroxyoctadecan-6-yl palmitate (16j). Colorless oil; Yield: 71%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 3.62 (t, J = 6.6 Hz, 2H, OCH2), 2.26 (t, J = 7.4 Hz, 2H, COCH2), 1.62–1.46 (m, 11H, 5 × CH2 and OH), 1.34–1.23 (m, 46H, 23 × CH2), 0.87 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.0, 34.7, 34.1, 34.1, 32.8, 31.9, 31.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 25.7, 25.3, 25.2, 25.0, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5065.
18-Hydroxyoctadecan-6-yl oleate (16k). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 5.45–5.23 (m, 2H, 2 × =CH), 4.91–4.81 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, OCH2), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.10–1.89 (m, 4H, 2 × =CHCH2), 1.71–1.42 (m, 11H, 5 × CH2 and OH), 1.35–1.23 (m, 42H, 21 × CH2), 0.87 (t, J = 6.3 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.0, 34.7, 34.1, 34.1, 32.8, 31.9, 31.7, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.1, 29.1, 27.2, 27.2, 25.7, 25.3, 25.2, 25.0, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5221.
18-Hydroxyoctadecan-5-yl hexanoate (16l). Colorless oil; Yield: 78%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.79 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.48 (m, 9H, 4 × CH2 and OH), 1.43–1.17 (m, 28H, 14 × CH2), 0.99–0.80 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.2, 33.8, 32.8, 31.3, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 27.5, 25.7, 25.3, 24.8, 22.6, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3496.
18-Hydroxyoctadecan-5-yl palmitate (16m). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.82 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 1.65–1.43 (m, 9H, 4 × CH2 and OH), 1.38–1.16 (m, 48H, 24 × CH2), 0.87 (t, J = 5.9 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.1, 34.7, 34.2, 33.9, 32.8, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 27.5, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5064.
18-Hydroxyoctadecan-5-yl oleate (16n). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.25 (m, 2H, 2 × =CH), 4.94–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.11–1.90 (m, 4H, 2 × =CHCH2), 1.65–1.48 (m, 9H, 4 × CH2 and OH), 1.38–1.21 (m, 44H, 22 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.8, 74.1, 63.1, 34.7, 34.2, 33.9, 32.8, 31.9, 29.8, 29.7, 29.6, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.1, 27.5, 27.2, 27.2, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5215.
3.7. General Procedure for the Oxidation of Alcohols 16a–n to FAHFAs
To a round-bottomed flask containing the appropriate alcohol 16a–n (1.00 mmol), acetone (10 mL) was added, and the mixture was left stirring at 0 °C until full dissolution. Jones reagent (2 M, 1.5 mL, 3.00 mmol) was added dropwise at 0 °C and the reaction mixture was left stirring for 1 h at this temperature. Then, the reaction mixture was quenched with a saturated aqueous solution of NaHSO3 (10 mL) and extracted with Et2O (3 × 20 mL). The combined organic layers were washed with brine (1 × 50 mL), dried over Na2SO4, and,] then evaporated to give a crude mixture, which was purified by flash column chromatography on silica gel (petroleum ether (bp 40–60 °C)/ethyl acetate, 60/40) to afford the desired FAHFAs.
9-(Hexanoyloxy)octadecanoic acid (17a) [14]. Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.59 (m, 4H, 2 × CH2), 1.54–1.46 (m, 4H, 2 × CH2), 1.35–1.23 (m, 26H, 13 × CH2), 0.93–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.3, 173.8, 74.0, 34.7, 34.2, 34.1, 33.9, 31.9, 31.3, 29.5, 29.5, 29.3, 29.1, 29.0, 25.3, 25.2, 24.8, 24.6, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3286.
9-(Oleoyloxy)octadecanoic acid (17b) [57]. Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 5.50–5.22 (m, 2H, 2 × =CH), 4.94–4.78 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.12–1.89 (m, 4H, 2 × =CHCH2), 1.66–1.59 (m, 4H, 2 × CH2), 1.55–1.46 (m, 4H, 2 × CH2), 1.36–1.22 (m, 42H, 21 × CH2), 0.88 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.6, 173.7, 130.0, 129.8, 74.0, 34.7, 34.2, 34.1, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.1, 29.1, 29.1, 29.0, 27.2, 27.2, 25.3, 25.3, 25.2, 24.6, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
10-(Hexanoyloxy)octadecanoic acid (17c). Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.77 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.58–1.39 (m, 4H, 2 × CH2), 1.33–1.23 (m, 26H, 13 × CH2), 0.93–0.82 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.0, 173.8, 74.1, 34.7, 34.1, 33.9, 31.9, 31.9, 31.3, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.1, 29.1, 29.0, 25.3, 25.3, 24.8, 24.7, 24.6, 22.7, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3286.
10-(Palmitoyloxy)octadecanoic acid (17d) [58]. White solid; Yield: 86%; m.p.: 36–37 °C; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.78 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.56–1.42 (m, 4H, 2 × CH2), 1.37–1.22 (m, 46H, 23 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.0, 173.8, 74.1, 34.8, 34.2, 33.9, 31.9, 31.9, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 25.3, 25.3, 25.2, 24.7, 22.7, 22.7, 14.1, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4858.
10-(Oleoyloxy)octadecanoic acid (17e) [20]. Colorless oil; Yield: 83%;1H-NMR (400 MHz, CDCl3): δ 5.49–5.25 (m, 2H, 2 × =CH), 4.93–4.78 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.17–1.82 (m, 4H, 2 × =CHCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.36–1.22 (m, 42H, 21 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.8, 173.7, 130.0, 129.8, 74.1, 34.7, 34.2, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 29.0, 27.2, 27.2, 25.3, 25.3, 25.2, 24.7, 22.7, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
11-(Hexanoyloxy)octadecanoic acid (17f). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.93–480 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.59 (m, 4H, 2 × CH2), 1.54–1.46 (m, 4H, 2 × CH2), 1.36–1.24 (m, 26H, 13 × CH2), 0.93–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.8, 74.1, 34.7, 34.2, 33.9, 31.8, 31.3, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.0, 25.3, 25.3, 24.9, 24.7, 22.6, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3288.
11-(Palmitoyloxy)octadecanoic acid (17g). Colorless oil; Yield: 85%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.65–1.59 (m, 4H, 2 × CH2), 1.53–1.47 (m, 4H, 2 × CH2), 1.34–1.23 (m, 46H, 23 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.8, 74.1, 34.8, 34.2, 33.9, 31.9, 31.8, 29.7, 29.7, 29.6, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.0, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4863.
11-(Oleoyloxy)octadecanoic acid (17h). Colorless oil; Yield: 86%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.23 (m, 2H, 2 × =CH), 4.92–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.14–1.87 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.56–1.46 (m, 4H, 2 × CH2), 1.37–1.22 (m, 42H, 21 × CH2), 0.88 (t, J = 6.4 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.0, 173.7, 130.0, 129.8, 74.1, 34.7, 34.2, 33.9, 31.9, 31.8, 29.8, 29.7, 29.7, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 29.0, 27.2, 27.2, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5009.
13-(Hexanoyloxy)octadecanoic acid (17i). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.1 Hz, 2H, COCH2), 2.27 (t, J = 7.1 Hz, 2H, COCH2), 1.68–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.38–1.19 (m, 26H, 13 × CH2), 0.94–0.81 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.7, 173.8, 74.1, 34.7, 34.1, 34.1, 34.0, 31.7, 31.3, 29.5, 29.4, 29.2, 29.0, 25.3, 25.0, 24.8, 24.7, 22.5, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3287.
13-(Palmitoyloxy)octadecanoic acid (17j). White solid; Yield: 89%; m.p.: 39–41 °C; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.82 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.59 (m, 4H, 2 × CH2), 1.54–1.47 (m, 4H, 2 × CH2), 1.35–1.23 (m, 46H, 23 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.3, 173.8, 74.1, 34.8, 34.2, 34.1, 33.9, 31.9, 31.7, 29.7, 29.7, 29.6, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 29.0, 25.3, 25.2, 25.0, 24.7, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4879.
13-(Oleoyloxy)octadecanoic acid (17k). Colorless oil; Yield: 87%; 1H-NMR (400 MHz, CDCl3): δ 5.42–5.26 (m, 2H, 2 × =CH), 4.90–4.83 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.10–1.87 (m, 4H, 2 × =CHCH2), 1.66–1.59 (m, 4H, 2 × CH2), 1.53–1.46 (m, 4H, 2 × CH2), 1.34–1.24 (m, 42H, 21 × CH2), 0.88 (t, J = 6.3 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.7, 130.0, 129.7, 74.1, 34.7, 34.2, 34.1, 34.0, 31.9, 31.7, 29.8, 29.7, 29.6, 29.6, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 29.0, 29.0, 27.2, 27.2, 25.3, 25.2, 25.0, 24.7, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
14-(Hexanoyloxy)octadecanoic acid (17l). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.55–1.46 (m, 4H, 2 × CH2), 1.38–1.21 (m, 26H, 13 × CH2), 0.94–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.8, 74.1, 34.7, 34.2, 34.0, 33.8, 31.3, 29.6, 29.5, 29.5, 29.4, 29.2, 29.0, 27.5, 25.3, 24.8, 24.7, 22.6, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3288.
14-(Palmitoyloxy)octadecanoic acid (17m). White solid; Yield: 80%; m. p.: 41–42 °C; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.54–1.47 (m, 4H, 2 × CH2), 1.39–1.20 (m, 46H, 23 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.1, 173.8, 74.1, 34.8, 34.2, 33.9, 33.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 29.1, 27.5, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M+Na]+ found: 561.4853.
14-(Oleoyloxy)octadecanoic acid (17n). Colorless oil; Yield: 85%; 1H-NMR (400 MHz, CDCl3): δ 5.49–5.23 (m, 2H, 2 × =CH), 4.95–4.79 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.28 (t, J = 7.5 Hz, 2H, CH2COO), 2.17–1.81 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.56–1.46 (m, 4H, 2 × CH2), 1.37–1.16 (m, 42H, 21 × CH2), 0.88 (t, J = 5.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.8, 130.0, 129.7, 74.1, 34.7, 34.2, 33.9, 33.8, 31.9, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.2, 29.1, 29.1, 27.5, 27.2, 27.2, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5007.
4. Conclusions
In summary, we have developed a compact and convenient strategy for synthesizing endogenous lipids, FAHFAs, which are notable for their potent antidiabetic and anti-inflammatory effects. Our innovative approach harnesses the power of a highly efficient photo-induced hydroacylation reaction between terminal alkenes, either ω-alkenoic acids or ω-alkenyl alcohols, and commercially available aldehydes, utilizing phenylglyoxylic acid as the photoinitiator under household lamp irradiation as the key process. This environmentally friendly reaction not only enables the production of a rich array of oxo fatty acids and other oxo-functionalized compounds but also lays the foundation for the facile synthesis of FAHFAs. By thoughtfully selecting suitable olefins and aldehydes, we successfully introduced the ester linkage at various positions along the aliphatic chain, leading to the formation of a spectrum of regio-isomers and adding an extra layer of versatility to our methodology. Building upon this foundation, subsequent coupling reactions with diverse fatty acids (FAs) allowed us to synthesize a broad array of FAHFA families. This not onlypaves the way for an in-depth exploration of FAHFA structural variations, but also positions our work at the forefront of understanding their diverse biological activities.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30020286/s1, Scheme S1: Graphical representation of the structures of all final FAHFA compounds; Scheme S2: The photochemical hydroacylation reaction of aldehydes with alkenes to oxo fatty acids (products with yields); Scheme S3: The synthesis of oxo methyl esters from oxo fatty acids (products with yields); Scheme S4: The synthesis of alcohols 5a–f via oxo functionality reduction (products with yields); Scheme S5: The coupling reaction between alcohols and FAs in the first synthetic route (products with yields); Scheme S6: The synthesis of FAHFAs through saponification of esters (products with yields); Scheme S7: Synthetic pathway for the conversion of α,ω-commercially available diols to ω-alkenyl alcohols (products with yields); Scheme S8: The photochemical hydroacylation reaction of ω-alkenyl alcohols and aldehydes (products with yields); Scheme S9: The synthesis of alcohols 14a–e via oxo functionality reduction (products with yields); Scheme S10: The coupling reaction between alcohols and FAs in the second synthetic route (products with yields); Scheme S11: The deprotection of tert-butyldimethylsilyl (TBDMS) group (products with yields); Scheme S12: The oxidation of alcohols 16a–n to FAHFAs (products with yields); 1H NMR and 13C NMR spectra of the compounds synthesized.
Author Contributions
Conceptualization, G.K.; methodology, O.G.M. and C.S.B.; investigation, O.G.M., C.S.B. and C.M.; writing—original draft preparation, O.G.M., C.S.B. and G.K.; writing—review and editing, G.K., O.G.M., C.S.B. and C.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented was carried out within the framework of a Stavros Niarchos Foundation grant to the National and Kapodistrian University of Athens (G.K.). The authors gratefully acknowledge the Hellenic Foundation for Research and Innovation (H.F.R.I.), since this research project was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “1st Call for H.F.R.I. Research Projects to support Faculty Members & Researchers and the Procurement of High-cost research equipment grant” (grant number 655) (C.G.K.). The research work was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the H.F.R.I. PhD Fellowship grant (Fellowship Number: 1338) (O.G.M.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting this study are included in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Riecan, M.; Paluchova, V.; Lopes, M.; Brejchova, K.; Kuda, O. Branched and linear fatty acid esters of hydroxy fatty acids (FAHFA) relevant to human health. Pharmacol. Ther. 2022, 231, 107972. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G. Analytical methods for the determination of fatty acid esters of hydroxy fatty acids (FAHFAs) in biological samples, plants and foods. Biomolecules 2021, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Olajide, T.M.; Cao, W. Exploring foods as natural sources of FAHFAs—A review of occurrence, extraction, analytical techniques and emerging bioactive potential. Trends Food Sci. Technol. 2022, 129, 591–607. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Smedley, S.R.; Schroeder, F.C.; Weibel, D.B.; Meinwald, J.; Lafleur, K.A.; Renwick, J.A.; Rutowski, R.; Eisner, T. Mayolenes: Labile defensive lipids from the glandular hairs of a caterpillar (Pieris rapae). Proc. Natl. Acad. Sci. USA 2002, 99, 6822–6827. [Google Scholar] [CrossRef]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Syed, I.; Vijayakumar, A.; Eliasson, B.; Gogg, S.; Kahn, B.B.; Smith, U. Adipose tissue dysfunction is associated with low levels of the novel palmitic acid hydroxystearic acids. Sci. Rep. 2018, 8, 15757. [Google Scholar] [CrossRef] [PubMed]
- Fais, G.; Malavasi, V.; Scano, P.; Soru, S.; Caboni, P.; Cao, G. Metabolomics and lipid profile analysis of Coccomyxa melkonianii SCCA 048. Extremophiles 2021, 25, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Brezinova, M.; Kuda, O.; Hansikova, J.; Rombaldova, M.; Balas, L.; Bardova, K.; Durand, T.; Rossmeisl, M.; Cerna, M.; Stranak, Z.; et al. Levels of palmitic acid ester of hydroxystearic acid (PAHSA) are reduced in the breast milk of obese mothers. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Liberati-Čizmek, A.-M.; Biluš, M.; Brkić, A.L.; Barić, I.C.; Bakula, M.; Hozić, A.; Cindrić, M. Analysis of fatty acid esters of hydroxyl fatty acid in selected plant food. Plant Foods Hum. Nutr. 2019, 74, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-F.; Yan, J.-W.; Zhang, T.-Y.; Xiao, H.-M.; Feng, Y.-Q. Comprehensive screening and identification of fatty acid esters of hydroxy fatty acids in plant tissues by chemical isotope labeling-assisted liquid chromatography-mass spectrometry. Anal. Chem 2018, 90, 10056–10063. [Google Scholar] [CrossRef]
- Syed, I.; Lee, J.; Moraes-Vieira, P.M.; Donaldson, C.J.; Sontheimer, A.; Aryal, P.; Wellenstein, K.; Kolar, M.J.; Nelson, A.T.; Siegel, D.; et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 2018, 27, 419–427. [Google Scholar] [CrossRef]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J., Jr.; Kudova, E.; et al. Docosahexaenoic acid-derived fatty acid esters of hydroxy fatty acids (FAHFAs) with anti-inflammatory properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chang, T.; Konduri, S.; Huang, J.; Saghatelian, A.; Siegel, D. Synthesis of chemically edited derivatives of the endogenous regulator of inflammation 9-PAHSA. J. Antibiot. 2019, 72, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Dongoran, R.A.; Lin, T.-J.; Byekyet, A.; Tang, S.-C.; Yang, J.-H.; Liu, C.-H. Determination of major endogenous FAHFAs in healthy human circulation: The correlations with several circulating cardiovascular-related biomarkers and anti-inflammatory effects on RAW 264.7 cells. Biomolecules 2020, 10, 1689. [Google Scholar] [CrossRef]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched fatty acid esters of hydroxy fatty acids (FAHFAs) protect against colitis by regulating gut innate and adaptive immune responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Fuda, H.; Tsukui, T.; Chiba, H.; Hui, S.-P. Discovery of eicosapentaenoic acid esters of hydroxy fatty acids as potent Nrf2 activators. Antioxidants 2020, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.T.; Kolar, M.J.; Chu, Q.; Syed, I.; Kahn, B.B.; Saghatelian, A.; Siegel, D. Stereochemistry of endogenous palmitic acid ester of 9-hydroxystearic acid and relevance of absolute configuration to regulation. J. Am. Chem. Soc. 2017, 139, 4943–4947. [Google Scholar] [CrossRef]
- Kahn, B.B.; Herman, M.A.; Saghatelian, A.; Homan, E. Lipids That Increase Insulin Sensitivity and Methods of Using the Same. U.S. Patent US10604473B2, 31 March 2020. [Google Scholar]
- Balas, L.; Bertrand-Michel, J.; Viars, F.; Faugere, J.; Lefort, C.; Caspar-Bauguil, S.; Langin, D.; Durand, T. Regiocontrolled syntheses of FAHFAs and LC-MS/MS differentiation of regioisomers. Org. Biomol. Chem. 2016, 14, 9012–9020. [Google Scholar] [CrossRef] [PubMed]
- Pflimlin, E.; Bielohuby, M.; Korn, M.; Breitschopf, K.; Löhn, M.; Wohlfart, P.; Konkar, A.; Podeschwa, M.; Bärenz, F.; Pfenninger, A.; et al. Acute and repeated treatment with 5-PAHSA or 9-PAHSA isomers does not improve glucose control in mice. Cell Metab. 2018, 28, 217–227. [Google Scholar] [CrossRef]
- Mountanea, O.G.; Limnios, D.; Kokotou, M.G.; Bourboula, A.; Kokotos, G. Asymmetric synthesis of saturated hydroxy fatty acids and fatty acid esters of hydroxy fatty acids. Eur. J. Org. Chem. 2019, 2019, 2010–2019. [Google Scholar] [CrossRef]
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef] [PubMed]
- Fagnoni, M.; Dondi, D.; Ravelli, D.; Albini, A. Photocatalysis for the formation of the C-C bond. Chem. Rev. 2007, 107, 2725–2756. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon-carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Silvi, M.; Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 2018, 554, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sideri, I.K.; Voutyritsa, E.; Kokotos, C.G. Photoorganocatalysis, small organic molecules and light in the service of organic synthesis: The awakening of a sleeping giant. Org. Biomol. Chem. 2018, 16, 4596–4614. [Google Scholar] [CrossRef] [PubMed]
- Nikitas, N.F.; Gkizis, P.L.; Kokotos, C.G. Thioxanthone: A powerful photocatalyst for organic reactions. Org. Biomol. Chem. 2021, 19, 5237–5253. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 2021, 1, 523–598. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C-H bonds elaboration. Chem. Rev. 2022, 122, 1875–1924. [Google Scholar] [CrossRef] [PubMed]
- Gkizis, P.L. Photochemical cross-coupling enables Csp3-C bond formation by C-H homolytic cleavage: New developments and perspectives. Eur. J. Org. Chem. 2022, 2022, e202201139. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, D.; Fagnoni, M. Designing radical chemistry by visible light-promoted homolysis. Trends Chem. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Voutyritsa, E.; Kokotos, C.G. Green metal-free photochemical hydroacylation of unactivated olefins. Angew. Chem. Int. Ed. 2020, 59, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Batsika, C.S.; Mantzourani, C.; Gkikas, D.; Kokotou, M.G.; Mountanea, O.G.; Kokotos, C.G.; Politis, P.K.; Kokotos, G. Saturated oxo fatty acids (SOFAs): A previously unrecognized class of endogenous bioactive lipids exhibiting a cell growth inhibitory activity. J. Med. Chem. 2021, 64, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A.; Vu, H.D.; Maher, T.J. Macamides and their synthetic analogs: Evaluation of in vitro FAAH inhibition. Bioorg. Med. Chem. 2013, 21, 5188–5197. [Google Scholar] [CrossRef]
- Afri, M.; Alexenberg, C.; Aped, P.; Bodner, E.; Cohen, S.; Ejgenburg, M.; Eliyahu, S.; Glinsky-Sharon, P.; Harel, Y.; Naggash, M.E.; et al. NMR-based molecular ruler for determining the depth of intercalants within the lipid bilayer: Part III: Studies on keto esters and acids. Chem. Phys. Lipids 2014, 184, 105–118. [Google Scholar] [CrossRef]
- Bergström, S.; Erdtman, G.A.; Rolander, B.; Stenhagen, E.; Östling, S. The monoketo-and monohydroxyoctadecanoic acids. Acta Chem. Scand. 1952, 6, 1157–1174. [Google Scholar] [CrossRef]
- Ebert, C.; Felluga, F.; Forzato, C.; Foscato, M.; Gardossi, L.; Nitti, P.; Pitacco, G.; Boga, C.; Caruana, P.; Micheletti, G.; et al. Enzymatic kinetic resolution of hydroxystearic acids: A combined experimental and molecular modelling investigation. J. Mol. Catal. B Enzym. 2012, 83, 38–45. [Google Scholar] [CrossRef]
- Daulatabad, C.D.; Bhat, G.G.; Jamkhandi, A.M. A keto fatty acid from Smilax macrophylla seed oil. Phytochemistry 1996, 42, 889–890. [Google Scholar] [CrossRef]
- Paul, S.; Guin, J. A general approach to intermolecular olefin hydroacylation through light-induced HAT initiation: An efficient synthesis of long-chain aliphatic ketones and functionalized fatty acids. Chem. Eur. J. 2021, 27, 4412–4419. [Google Scholar] [CrossRef] [PubMed]
- Alexenberg, C.; Afri, M.; Eliyahu, S.; Porat, H.; Ranz, A.; Frimer, A.A. Locating intercalants within lipid bilayers using fluorescence quenching by bromophospholipids and iodophospholipids. Chem. Phys. Lipids 2019, 221, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Fukuyama, T.; Ryu, I. Efficient flow Fischer esterification of carboxylic acids with alcohols using sulfonic acid-functionalized silica as supported catalyst. Bull. Chem. Soc. Jpn. 2017, 90, 607–612. [Google Scholar] [CrossRef]
- Ogawa, N.; Arai, K.; Kobayashi, Y. Total synthesis of Resolvin T4. Synlett 2022, 33, 76–79. [Google Scholar] [CrossRef]
- Zhu, H.; Wickenden, J.G.; Campbell, N.E.; Leung, J.C.T.; Johnson, K.M.; Sammis, G.M. Construction of carbo- and heterocycles using radical relay cyclizations initiated by alkoxy radicals. Org. Lett. 2009, 11, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.J.; Rowe, M.T.; Weir, A.N.M.; Akter, N.; Mbatha, S.Z.; Walker, P.D.; Williams, C.; Song, A.; Race, P.R.; Willis, C.L.; et al. Programmed iteration controls the assembly of the nonanoic acid side chain of the antibiotic mupirocin. Angew. Chem. Int. Ed. 2022, 61, e202212393. [Google Scholar] [CrossRef] [PubMed]
- Petermichl, M.; Steinert, C.; Schobert, R. A synthetic route to the MT1-MMP inhibitor Ancorinoside D. Synthesis 2019, 51, 730–738. [Google Scholar]
- Jana, S.; Sarpe, V.A.; Kulkarni, S.S. Total synthesis and structure revision of a fungal glycolipid fusaroside. Org. Lett. 2021, 23, 1664–1668. [Google Scholar] [CrossRef]
- Yadav, J.S.; Ganganna, B.; Dutta, P.; Singarapu, K.K. Synthesis and determination of absolute configuration of α-pyrones isolated from Penicillium corylophilum. J. Org. Chem. 2014, 79, 10762–10771. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, M.E.; Nash, R.J.; Pyne, S.G. Studies towards the synthesis of polyhydroxylated pyrrolidine alkaloids isolated from Broussonetia kazinoki (moraceae). Tetrahedron 2022, 128, 133104. [Google Scholar] [CrossRef]
- Makado, G.; Morimoto, T.; Sugimoto, Y.; Tsutsumi, K.; Kagawa, N.; Kakiuchi, K. Highly linear-selective hydroformylation of 1-alkenes using formaldehyde as a syngas substitute. ACS Catal. 2010, 352, 299–304. [Google Scholar] [CrossRef]
- Brimble, M.A.; Flowers, C.L.; Hutchinson, J.K.; Robinson, J.E.; Sidford, M. Synthesis of the phthalide-containing anti-Helicobacter pylori agents CJ-13,015, CJ-13,102, CJ-13,103, CJ-13,104 and CJ-13,108. Tetrahedron 2005, 61, 10036–10047. [Google Scholar] [CrossRef]
- Bissember, A.C.; Levina, A.; Fu, G.C. A mild, palladium-catalyzed method for the dehydrohalogenation of alkyl bromides: Synthetic and mechanistic studies. J. Am. Chem. Soc. 2012, 134, 14232–14237. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Sadaiah, K.; Raghu, N. An organocatalytic enantioselective synthesis of (+)-duryne. Tetrahedron Asymmetry 2012, 23, 587–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Engin Eser, B.; Guo, Z. A bi-enzymatic cascade pathway towards optically pure FAHFAs. ChemBioChem 2021, 22, 2146–2153. [Google Scholar] [CrossRef]
- Harry-O kuru, R.E.; Isbell, T.A.; Weisleder, D. Synthesis of estolide esters from cis-9-octadecenoic acid estolides. J. Am. Oil Chem. Soc. 2001, 78, 219–223. [Google Scholar] [CrossRef]
- Bogojevic, O.; Zhang, Y.; Wolff, C.D.; Johnsen, N.K.; Arevång, C.; Guo, Z. A chemo-enzymatic cascade strategy for the synthesis of phosphatidylcholine incorporated with structurally diverse FAHFAs. J. Org. Chem. 2024, 89, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).