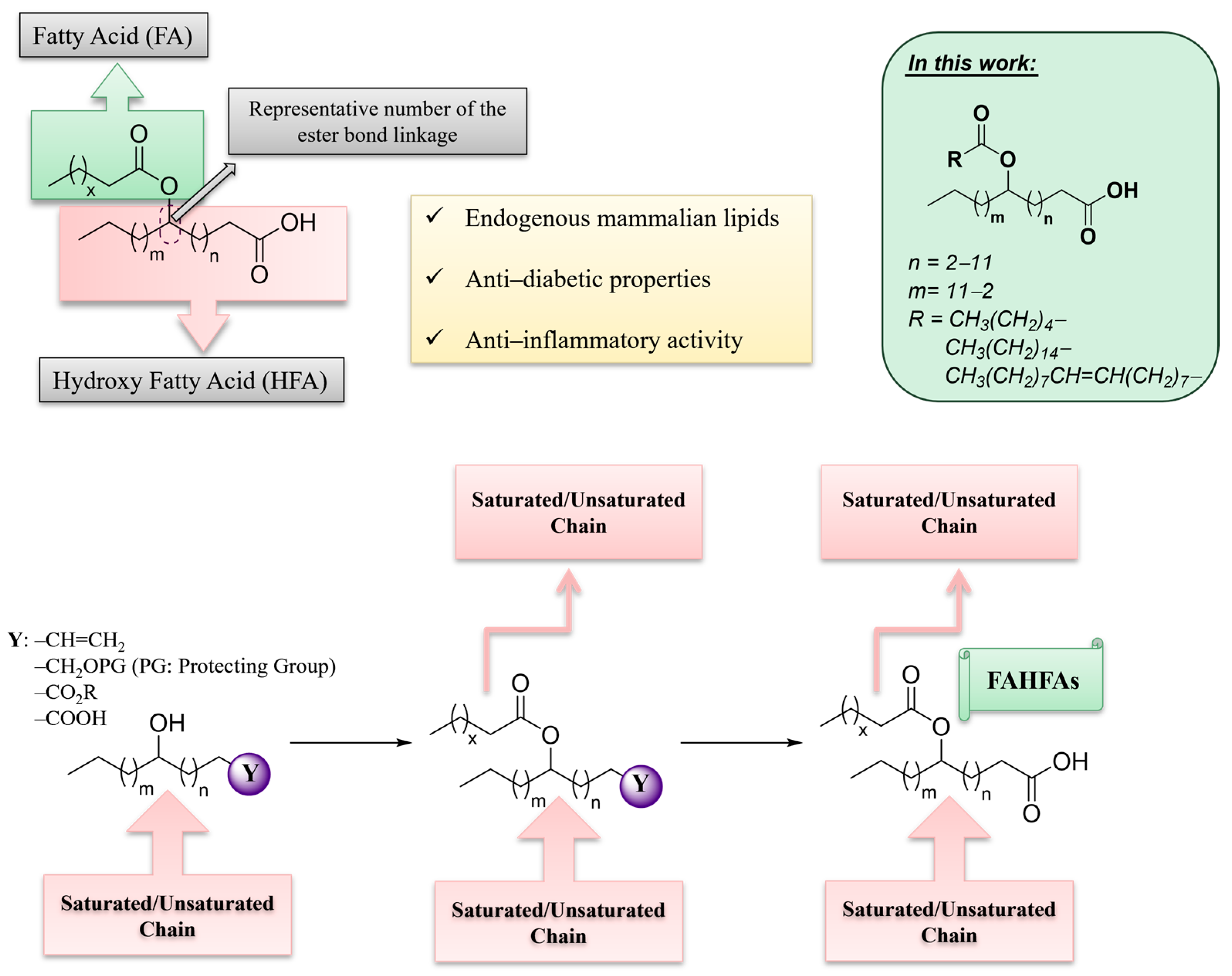
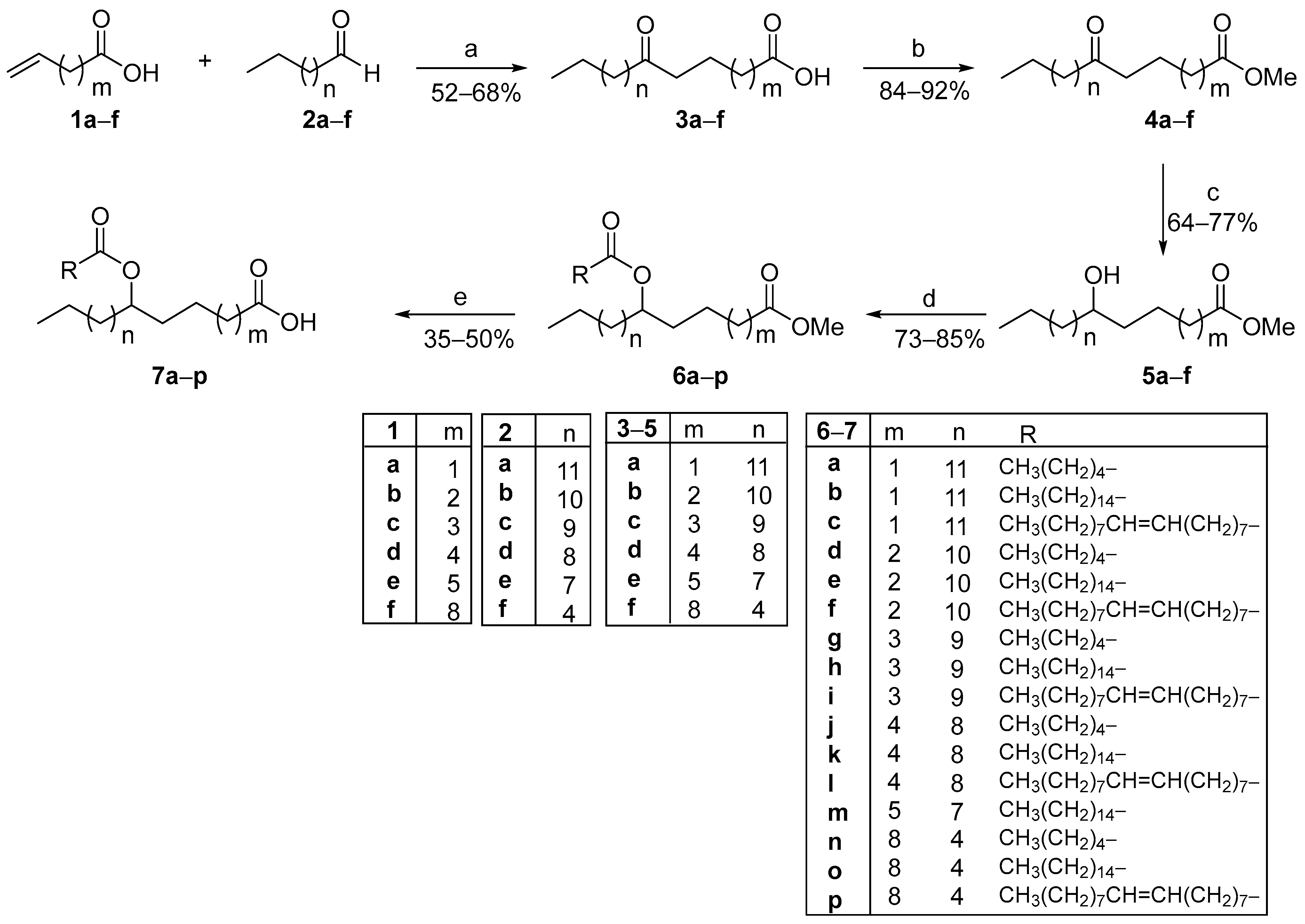
3. Materials and Methods
3.1. General Remarks
All commercially available products and solvents were purchased from Fluorochem (Fluorochem Ltd., Hadfield, Glossop, UK), Sigma-Aldrich (Sigma-Aldrich, Saint Louis, MO, USA), Fluka (Fluka Chemicals Ltd., Gillingham, Dorset, UK), Merck (Merck, Darmstadt, Germany), and Alfa Aesar (Alfa Aesar, Ward Hill, MA, USA). The chromatographic purification of products was accomplished using forced-flow chromatography on Merck
® (Merck, Darmstadt, Germany) Kieselgel 60 F254 230–400 mesh. Thin-layer chromatography (TLC) was performed on aluminum-backed silica plates (0.2 mm, 60 F254). The visualization of the developed chromatogram was performed by fluorescence quenching using phosphomolybdic acid. Melting points were measured on a Buchi 530 apparatus (Buchi, Flawil, Switzerland) and were uncorrected. High-resolution mass spectra were obtained on a Bruker Maxis Impact QTOF spectrometer (Bruker Daltonics, Bremen, Germany).
1H-NMR and
13C-NMR spectra were recorded on an Avance III HD Bruker 400 MHz (Bruker, Fällanden, Switzerland) (400 MHz and 100 MHz, respectively) or a Varian Mercury (Varian, Palo Alto, CA, USA) (200 MHz and 50 MHz, respectively) and are internally referenced to residual solvent signals. Data for
1H-NMR are reported as follows: chemical shift (
δ ppm), multiplicity (s = singlet, t = triplet, quin = quintet, m = multiplet, br s = broad signal), coupling constant, integration, and assignment. Data for
13C-NMR are reported in terms of a chemical shift (
δ ppm). The synthesis of
3a–
f and
13b was carried out as described by Batsika et al. [
36], and their spectroscopic data were in accordance with the literature. Additionally, compounds
4a–
f [
37,
38,
39,
40,
41,
42],
5a [
39],
5b [
43],
5c,d [
40],
5e [
42],
5f [
44],
9a–
d [
45,
46,
47,
48],
9e [
36],
10a–
d [
49,
50,
51,
52],
10e [
36],
11a,b [
53,
54],
11c [
51],
11d [
55], and
11e [
36] were prepared by known procedures, and their spectroscopic data were identical with those in the literature.
3.2. General Procedure for the Photochemical Reaction of Aldehydes with Alkenes
In a glass vial equipped with a PTFE-coated stirring bar, the appropriate alkene (0.50 mmol), the aldehyde (1.50 mmol), and the phenylglyoxylic acid (15 mg, 0.10 mmol) were added using water (1 mL) as the reaction solvent. The vial was sealed with a screw cap and left stirring under household bulb irradiation (2 × 85 W household lamps) for 48 h. The reaction mixture was extracted with CH2Cl2 (3 × 3 mL). The combined organic layers were dried over Na2SO4, and the solvent was removed in vacuo. The desired oxo fatty acids 3a–f were isolated by precipitation with hexane (3–5 mL) (cooled with an external ice bath). Ketones 13a–e were purified by flash chromatography on silica gel eluting with a petroleum ether (bp 40–60 °C)/ethyl acetate 95/5 system.
1-((tert-Βutyldimethylsilyl)oxy)octadecan-9-one (13a). Colorless oil; Yield: 44%; 1H-NMR (400 MHz, CDCl3): δ 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.37 (t, J = 7.4 Hz, 4H, 2 × COCH2), 1.59–1.44 (m, 6H, 3 × CH2), 1.31–1.23 (m, 20H, 10 × CH2), 0.91–0.84 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 211.6, 63.3, 42.8, 42.8, 32.8, 31.9, 29.4, 29.4, 29.4, 29.3, 29.3, 29.2, 26.0, 25.7, 23.9, 23.9, 22.6, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3477.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-one (13c). Colorless oil; Yield: 49%; 1H-NMR (200 MHz, CDCl3): δ 3.54 (t, J = 6.5 Hz, 2H, CH2OTBDMS), 2.33 (t, J = 7.4 Hz, 4H, 2 × COCH2), 1.57–1.41 (m, 6H, 3 × CH2), 1.28–1.16 (m, 20H, 10 × CH2), 0.86–0.78 (m, 12H, 4 × CH3), -0.01 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 211.3, 63.2, 42.7, 32.8, 31.6, 29.5, 29.3, 29.1, 29.0, 25.9, 25.7, 23.8, 22.5, 18.2, 14.0, −5.4; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3472.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-one (13d). Colorless oil; Yield: 52%; 1H-NMR (400 MHz, CDCl3): δ 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.37 (t, J = 7.4 Hz, 4H, 2 × COCH2), 1.57–1.47 (m, 6H, 3 × CH2), 1.32–1.24 (m, 20H, 10 × CH2), 0.91–0.86 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 211.7, 63.3, 42.8, 42.8, 32.9, 31.4, 29.6, 29.6, 29.5, 29.4, 29.4, 29.3, 26.0, 25.8, 23.9, 23.6, 22.4, 18.4, 13.9, −5.3; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3478.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-one (13e). Colorless oil; Yield: 46%; 1H-NMR (200 MHz, CDCl3): δ 3.54 (t, J = 6.5 Hz, 2H, CH2OTBDMS), 2.33 (t, J = 7.2 Hz, 4H, 2 × COCH2), 1.60–1.40 (m, 6H, 3 × CH2), 1.32–1.16 (m, 20H, 10 × CH2), 0.93–0.77 (m, 12H, 4 × CH3), −0.01 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 211.2, 63.2, 42.7, 42.4, 32.8, 29.5, 29.5, 29.4, 29.4, 29.2, 25.9, 25.7, 23.8, 22.3, 18.2, 13.8, −5.4; HRMS (ESI+): m/z calcd for C24H50NaO2Si+: 421.3472; [M + Na]+ found: 421.3478.
3.3. General Procedure for the Synthesis of Alcohols via Oxo Functionality Reduction
TBDMS-protected ketone 13a–e (1.00 mmol) in MeOH (5 mL) was added to a round-bottomed flask at 0 °C. Next, NaBH4 (189 mg, 5.00 mmol) was added under vigorous stirring. The solution was stirred for 1 h, and then methanol was removed under reduced pressure. The reaction mixture was washed with a saturated aqueous solution of NH4Cl (3 × 10 mL) and the combined organic layers were collected and dried with Na2SO4, and the solvent was removed under reduced pressure. The crude mixture was purified by flash chromatography (petroleum ether (bp 40–60 °C)/ethyl acetate, 70/30 for methyl esters, or 90/10 for TBDMS-protected compounds) to afford the desired alcohol.
1-((
tert-Βutyldimethylsilyl)oxy)octadecan-9-ol (
14a) [
22]. Colorless oil; Yield: 74%;
1H-NMR (200 MHz, CDCl
3):
δ 3.64–3.53 (m, 3H, CH
2OTBDMS and C
HOH), 1.56–1.22 (m, 31H, 15 × CH
2 and OH), 0.96–0.77 (m, 12H, 4 × CH
3), 0.04 (s, 6H, 2 × CH
3);
13C-NMR (50 MHz, CDCl
3):
δ 72.0, 63.3, 37.5, 32.9, 31.9, 29.7, 29.6, 29.6, 29.6 29.4, 29.3, 26.0, 25.8, 25.6, 22.7, 14.1, −5.3; HRMS (ESI
+):
m/
z calcd for C
24H
52NaO
2Si
+: 423.3629; [M + Na]
+ found: 423.3633.
18-((
tert-Butyldimethylsilyl)oxy)octadecan-9-ol (
14b) [
20]. Colorless oil; Yield: 76%;
1H-NMR (400 MHz, CDCl
3):
δ 3.68–3.47 (m, 3H, CH
2OTBDMS and C
HOH), 1.53–1.39 (m, 7H, 3 × CH
2 and OH), 1.33–1.23 (m, 24H, 12 × CH
2), 0.90–0.86 (m, 12H, 4 × CH
3), 0.04 (s, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 72.0, 63.3, 37.5, 32.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.4, 29.3, 26.0, 25.8, 25.6, 22.7, 18.4, 14.1, −5.3; HRMS (ESI
+):
m/
z calcd for C
24H
52NaO
2Si
+, 423.3629; [M + Na]
+ found, 423.3630.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-ol (14c). Colorless oil; Yield: 71%; 1H-NMR (200 MHz, CDCl3): δ 3.62–3.52 (m, 3H, CH2OTBDMS and CHOH), 1.56–1.39 (m, 7H, 3 × CH2 and OH), 1.35–1.21 (m, 24H, 12 × CH2), 0.90–0.82 (m, 12H, 4 × CH3), 0.03 (s, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 72.0, 63.3, 37.5, 32.8, 31.8, 29.7, 29.6, 29.4, 29.3, 25.9, 25.8, 25.6, 22.6, 18.3, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3632.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-ol (14d). Colorless oil; Yield: 66%; 1H-NMR (400 MHz, CDCl3): δ 3.62–3.55 (m, 3H, CH2OTBDMS and CHOH), 1.52–1.40 (m, 7H, 3 × CH2 and OH), 1.36–1.21 (m, 24H, 12 × CH2), 0.91–0.86 (m, 12H, 4 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 72.0, 63.3, 37.5, 37.5, 32.9, 31.9, 29.7, 29.6, 29.6, 29.4, 26.0, 25.8, 25.7, 25.3, 22.6, 18.4, 14.0, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3646.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-ol (14e). Colorless oil; Yield: 70%; 1H-NMR (400 MHz, CDCl3): δ 3.63–3.50 (m, 3H, CH2OTBDMS and CHOH), 1.58–1.16 (m, 31H, 15 × CH2 and OH), 0.98–0.76 (m, 12H, 4 × CH3), 0.03 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 71.9, 63.3, 37.5, 37.2, 32.9, 29.7, 29.6, 29.4, 27.8, 26.0, 25.8, 25.7, 22.8, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C24H52NaO2Si+: 423.3629; [M + Na]+ found: 423.3624.
3.4. General Procedure for the Coupling Reaction Between Alcohols and FAs
To a solution of the appropriate fatty acid (caproic or palmitic or oleic acid) (4.00 mmol) in dry CH2Cl2 (10 mL) at 0 °C, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (767 mg, 4.00 mmol), triethylamine (0.56 mL, 4.00 mmol), and 4-dimethylaminopyridine (24 mg, 0.20 mmol) were added consecutively. The reaction mixture was left stirring at 0 °C for 10 min, and then a solution of the secondary alcohol (1.00 mmol) in dry CH2Cl2 (10 mL) was added. After 16 h, the reaction mixture was extracted with a saturated aqueous solution of NH4Cl (3 × 10 mL), and the combined organic layers were collected and dried with Na2SO4. The solvent was then removed under reduced pressure. The crude mixture was purified by flash silica chromatography (petroleum ether (bp 40–60 °C)/ethyl acetate, 90/10–80/20) to afford the desired ester as a colorless oil.
Methyl 5-(hexanoyloxy)octadecanoate (6a). Colorless oil; Yield: 77%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.81 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.40–2.21 (m, 4H, 2 × COCH2), 1.69–1.50 (m, 8H, 4 × CH2), 1.39–1.21 (m, 26H, 13 × CH2), 1.01–0.80 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.8, 173.6, 73.4, 51.5, 34.6, 34.0, 33.8, 33.5, 31.9, 31.3, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 25.3, 24.8, 22.7, 22.3, 20.7, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M+Na]+ found: 435.3445.
Methyl 5-(palmitoyloxy)octadecanoate (6b). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.83 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.36–2.22 (m, 4H, 2 × COCH2), 1.70–1.48 (m, 8H, 4 × CH2), 1.34–1.19 (m, 46H, 23 × CH2), 0.87 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.8, 173.6, 73.4, 51.5, 34.7, 34.0, 33.8, 33.5, 31.9, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 25.3, 25.1, 22.7, 20.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5008.
1-Methoxy-1-oxooctadecan-5-yl oleate (6c). Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 5.44–5.31 (m, 2H, 2 × =CH), 4.94–4.83 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.35–2.24 (m, 4H, 2 × COCH2), 2.07–1.94 (m, 4H, 2 × =CHCH2), 1.64–1.51 (m, 8H, 4 × CH2), 1.38–1.19 (m, 42H, 21 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.8, 173.6, 130.0, 129.7, 73.4, 51.5, 34.6, 34.0, 33.8, 33.5, 31.9, 31.9, 29.8, 29.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 22.7, 20.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5161.
Methyl 6-(hexanoyloxy)octadecanoate (6d). Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.82 (m, 1H, COOCH), 3.68 (s, 3H, OCH3), 2.42–2.22 (m, 4H, 2 × COCH2), 1.68–1.61 (m, 4H, 2 × CH2), 1.59–1.48 (m, 4H, 2 × CH2), 1.42–1.24 (m, 26H, 13 × CH2), 0.96–0.86 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.0, 173.7, 73.8, 51.5, 34.7, 34.2, 34.0, 33.8, 31.9, 31.4, 29.7, 29.7, 29.6, 29. 6, 29.5, 29.4, 25.3, 24.9, 24.9, 22.7, 22.4, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3444.
Methyl 6-(palmitoyloxy)octadecanoate (6e). Low melting point solid; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.80 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.33–2.23 (m, 4H, 2 × COCH2), 1.65–1.57 (m, 4H, 2 × CH2), 1.57–1.46 (m, 4H, 2 × CH2), 1.35–1.21 (m, 46H, 23 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.9, 173.6, 73.7, 51.4, 34.7, 34.1, 33.9, 33.8, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 25.3, 25.1, 24.9, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5011.
1-Methoxy-1-oxooctadecan-6-yl oleate (6f). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 5.46–5.27 (m, 2H, 2 × =CH), 4.96–4.77 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.37–2.18 (m, 4H, 2 × COCH2), 2.14–1.88 (m, 4H, 2 × =CHCH2), 1.65–1.58 (m, 4H, 2 × CH2), 1.55–1.46 (m, 4H, 2 × CH2), 1.36–1.23 (m, 42H, 21 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.0, 173.6, 130.0, 129.7, 73.7, 51.4, 34.7, 34.1, 33.9, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 24.9, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5161.
Methyl 7-(hexanoyloxy)octadecanoate (6g). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.80 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.33–2.23 (m, 4H, 2 × COCH2), 1.65–1.57 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.35–1.20 (m, 26H, 13 × CH2), 0.94–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.1, 173.7, 73.8, 51.4, 34.6, 34.1, 33.9, 31.9, 31.3, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.0, 25.3, 25.0, 24.8, 24.8, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3445.
Methyl 7-(palmitoyloxy)octadecanoate (6h). Off white low melting point solid; Yield: 77%; 1H-NMR (200 MHz, CDCl3): δ 4.91–4.79 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.33–2.21 (m, 4H, 2 × COCH2), 1.69–1.46 (m, 8H, 4 × CH2), 1.46–1.05 (m, 46H, 23 × CH2), 0.86 (t, J = 6.4 Hz, 6H, 2 × CH3); 13C-NMR (50 MHz, CDCl3): δ 174.1, 173.7, 73.8, 51.4, 34.7, 34.1, 33.9, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.0, 25.3, 25.2, 25.0, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5020.
1-Methoxy-1-oxooctadecan-7-yl oleate (6i). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 5.43–5.26 (m, 2H, 2 × =CH), 4.89–4.81 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.31–2.24 (m, 4H, 2 × COCH2), 2.11–1.88 (m, 4H, 2 × =CHCH2), 1.64–1.56 (m, 4H, 2 × CH2), 1.54–1.45 (m, 4H, 2 × CH2), 1.36–1.20 (m, 42H, 21 × CH2), 0.87 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.1, 173.6, 129.9, 129.7, 73.8, 51.4, 34.7, 34.1, 33.9, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.1, 29.1, 29.0, 27.2, 27.1, 25.3, 25.1, 25.0, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5165.
Methyl 8-(hexanoyloxy)octadecanoate (6j). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.79 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.35–2.21 (m, 4H, 2 × COCH2), 1.65–1.58 (m, 4H, 2 × CH2), 1.54–1.46 (m, 4H, 2 × CH2), 1.36–1.21 (m, 26H, 13 × CH2), 0.96–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 73.9, 51.4, 34.7, 34.1, 34.1, 34.0, 31.9, 31.3, 29.6, 29.5, 29.5, 29.3, 29.1, 29.0, 25.3, 25.1, 24.8, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3457.
Methyl 8-(palmitoyloxy)octadecanoate (6k). Colorless oil; Yield: 85%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.79 (m, 1H, COOCH), 3.66 (s, 3H, OCH3), 2.32–2.23 (m, 4H, 2 × COCH2), 1.65–1.57 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.35–1.21 (m, 46H, 23 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.7, 73.9, 51.4, 34.7, 34.2, 34.1, 34.0, 31.9, 31.9, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.1, 29.0, 25.3, 25.2, 25.1, 24.9, 22.7, 14.1; HRMS (ESI+): m/z calcd for C35H68NaO4+: 575.5010; [M + Na]+ found: 575.5009.
1-Methoxy-1-oxooctadecan-8-yl oleate (6l). Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 5.45–5.26 (m, 2H, 2 × =CH), 4.93–4.79 (m, 1H, COOCH), 3.65 (s, 3H, OCH3), 2.34–2.22 (m, 4H, 2 × COCH2), 2.10–1.88 (m, 4H, 2 × =CHCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.54–1.43 (m, 4H, 2 × CH2), 1.39–1.11 (m, 42H, 21 × CH2), 0.87 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 130.0, 129.7, 73.9, 51.4, 34.7, 34.1, 34.07, 34.0, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.2, 29.1, 29.1, 29.0, 27.2, 27.2, 25.3, 25.1, 24.8, 22.7, 14.1; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5162.
Methyl 9-(palmitoyloxy)octadecanoate (
6m) [
42]. Colorless oil; Yield: 73%;
1H-NMR (400 MHz, CDCl
3):
δ 4.96–4.75 (m, 1H, COOCH), 3.65 (s, 3H, OCH
3), 2.34–2.22 (m, 4H, 2 × COCH
2), 1.64–1.57 (m, 4H, 2 × CH
2), 1.57–1.45 (m, 4H, 2 × CH
2), 1.35–1.22 (m, 46H, 23 × CH
2), 0.87 (t,
J = 6.7 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 174.2, 173.7, 74.0, 51.4, 34.7, 34.2, 34.1, 34.1, 31.9, 31.9, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.1, 29.0, 25.3, 25.2, 25.2, 24.9, 22.7, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
35H
68NaO
4+: 575.5010; [M + Na]
+ found: 575.5010.
Methyl 12-(hexanoyloxy)octadecanoate (6n). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.80 (m, 1H, COOCH), 3.64 (s, 3H, OCH3), 2.33–2.22 (m, 4H, 2 × COCH2), 1.67–1.54 (m, 4H, 2 × CH2), 1.54–1.42 (m, 4H, 2 × CH2), 1.40–1.15 (m, 26H, 13 × CH2), 0.91–0.82 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 74.0, 51.3, 34.6, 34.1, 34.0, 31.7, 31.3, 29.5, 29.5, 29.4, 29.4, 29.2, 29.1, 29.1, 25.3, 25.2, 24.9, 24.8, 22.5, 22.3, 14.0, 13.8; HRMS (ESI+): m/z calcd for C25H48NaO4+: 435.3445; [M + Na]+ found: 435.3446.
Methyl 12-(palmitoyloxy)octadecanoate (
6o) [
56]. Colorless oil; Yield: 77%;
1H-NMR (400 MHz, CDCl
3):
δ 4.91–4.81 (m, 1H, COOCH), 3.65 (s, 3H, OCH
3), 2.35–2.23 (m, 4H, 2 × COCH
2), 1.64–1.56 (m, 4H, 2 × CH
2), 1.53–1.45 (m, 4H, 2 × CH
2), 1.38–1.21 (m, 46H, 23 × CH
2), 0.86 (t,
J = 6.2 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 174.2, 173.6, 74.0, 51.3, 34.7, 34.1, 34.1, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 25.3, 25.3, 25.2, 24.9, 22.7, 22.5, 14.1, 14.0; HRMS (ESI
+):
m/
z calcd for C
35H
68NaO
4+: 575.5010; [M + Na]
+ found: 575.5024.
18-Methoxy-18-oxooctadecan-7-yl oleate (6p). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 5.40–5.27 (m, 2H, 2 × =CH), 4.90–4.81 (m, 1H, COOCH), 3.64 (s, 3H, OCH3), 2.31–2.22 (m, 4H, 2 × COCH2), 2.06–1.90 (m, 4H, 2 × =CHCH2), 1.64–1.56 (m, 4H, 2 × CH2), 1.54–1.43 (m, 4H, 2 × CH2), 1.37–1.17 (m, 42H, 21 × CH2), 0.86 (t, J = 6.1 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 174.2, 173.6, 129.9, 129.7, 74.0, 51.3, 34.7, 34.1, 34.1, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.1, 29.1, 27.2, 27.1, 25.3, 25.2, 25.1, 24.9, 22.6, 22.5, 14.0, 14.0; HRMS (ESI+): m/z calcd for C37H70NaO4+: 601.5166; [M + Na]+ found: 601.5164.
1-((tert-Βutyldimethylsilyl)oxy)octadecan-9-yl hexanoate (15a). Colorless oil; Yield: 77%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO),1.64–159 (m, 2H, CH2), 1.53–1.46 (m, 6H, 3 × CH2), 1.32–1.23 (m, 28H, 14 × CH2), 0.91–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.4, 29.5, 29.5, 29.5, 29.4, 29.3, 26.0, 25.8, 25.3, 24.9, 22.7, 22.3, 18.4, 14.1, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4364.
1-((
tert-Butyldimethylsilyl)oxy)octadecan-9-yl oleate (
15b) [
22]. Colorless oil; Yield: 84%;
1H-NMR (400 MHz, CDCl
3):
δ 5.39–5.30 (m, 2H, 2 × =CH), 4.90–4.82 (m, 1H, COOCH), 3.59 (t,
J = 7.5 Hz, 2H, CH
2OTBDMS), 2.27 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.04–1.93 (m, 4H, 2 × =CHC
H2), 1.66–1.55 (m, 4H, 2 × CH
2), 1.53–1.47 (m, 6H, 3 × CH
2), 1.34–1.25 (m, 42H, 21 × CH
2), 0.90–0.85 (m, 15H, 5 × CH
3), 0.04 (s, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 173.6, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 18.4, 14.1, −5.3; HRMS (ESI
+):
m/
z calcd for C
42H
84NaO
3Si
+: 687.6082; [M + Na]
+ found: 687.6095.
18-((tert-Butyldimethylsilyl)oxy)octadecan-9-yl hexanoate (15c). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.76 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.47 (m, 8H, 4 × CH2), 1.33–1.23 (m, 28H, 14 × CH2), 0.94–0.82 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.3, 29.7, 29.5, 29.5, 29.5, 29.4, 29.2, 26.0, 25.8, 25.3, 24.9, 22.6, 22.3, 18.4, 14.1, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4371.
18-((tert-Butyldimethylsilyl)oxy)octadecan-9-yl palmitate (15d). Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.77 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 1.65–1.59 (m, 2H, CH2), 1.54–1.44 (m, 6H, 3 × CH2), 1.35–1.21 (m, 48H, 24 × CH2), 0.97–0.79 (m, 15H, 5 × CH3), 0.05 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.8, 34.2, 32.9, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.7, 18.4, 14.1, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5921.
18-((
tert-Butyldimethylsilyl)oxy)octadecan-9-yl oleate (
15e) [
20]. Colorless oil; Yield: 83%;
1H-NMR (400 MHz, CDCl
3):
δ 5.49–5.23 (m, 2H, 2 × =CH), 4.93–4.79 (m, 1H, COOCH), 3.59 (t,
J = 6.6 Hz, 2H, CH
2OTBDMS), 2.27 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.12–1.88 (m, 4H, 2 × =CHC
H2), 1.68–1.55 (m, 4H, 2 × CH
2), 1.53–1.45 (m, 6H, 3 × CH
2), 1.35–1.23 (m, 42H, 21 × CH
2), 0.97–0.80 (m, 15H, 5 × CH
3), 0.04 (s, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 173.7, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.7, 18.4, 14.1, 14.1, −5.3; HRMS (ESI
+):
m/
z calcd for C
42H
84NaO
3Si
+: 687.6082; [M + Na]
+ found: 687.6080.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-yl hexanoate (15f). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.78 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.28 (t, J = 7.5 Hz, 2H, CH2COO), 1.67–1.60 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.34–1.23 (m, 28H, 14 × CH2), 0.93–0.83 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 34.1, 32.9, 31.7, 31.4, 29.6, 29.6, 29.6, 29.6, 29.5, 29.4, 26.0, 25.8, 25.3, 25.0, 24.9, 22.5, 22.3, 18.4, 14.0, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4374.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-yl palmitate (15g). Colorless oil; Yield: 74%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.79 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.65–1.59 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.32–1.22 (m, 48H, 24 × CH2), 0.92–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.8, 34.2, 32.9, 31.9, 31.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5958.
18-((tert-Butyldimethylsilyl)oxy)octadecan-8-yl oleate (15h). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 5.39–5.28 (m, 2H, 2 × =CH), 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.5 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 2.06–1.94 (m, 4H, 2 × =CHCH2), 1.67–1.56 (m, 4H, 2 × CH2), 1.53–1.47 (m, 6H, 3 × CH2), 1.33–1.24 (m, 42H, 21 × CH2), 0.90–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.8, 74.1, 63.3, 34.7, 34.2, 32.9, 31.9, 31.8, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.50, 29.4, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 18.4, 14.1, 14.1, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6109.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-yl hexanoate (15i). Colorless oil; Yield: 72%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.58 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.34–1.22 (m, 28H, 14 × CH2), 0.92–0.84 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 34.1, 32.9, 31.7, 31.4, 29.6, 29.6, 29.6, 29.6, 29.5, 29.4, 26.0, 25.8, 25.3, 25.0, 24.9, 22.5, 22.3, 18.4, 14.0, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4374.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-yl palmitate (15j). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.58 (m, 2H, CH2), 1.55–1.45 (m, 6H, 3 × CH2), 1.34–1.19 (m, 48H, 24 × CH2), 0.92–0.84 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.3, 34.7, 34.2, 34.1, 32.9, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 26.0, 25.8, 25.3, 25.2, 25.0, 22.7, 22.5, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5949.
18-((tert-Butyldimethylsilyl)oxy)octadecan-6-yl oleate (15k). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.25 (m, 2H, 2 × =CH), 4.92–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.11–1.89 (m, 4H, 2 × =CHCH2), 1.65–1.59 (m, 2H, CH2), 1.55–1.46 (m, 6H, 3 × CH2), 1.37–1.22 (m, 44H, 22 × CH2), 0.92–0.83 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 34.1, 32.9, 31.9, 31.7, 29.8, 29.7, 29.6, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 25.0, 22.7, 22.6, 22.5, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6099.
18-((tert-Βutyldimethylsilyl)oxy)octadecan-5-yl hexanoate (15l). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.90–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.59 (m, 2H, CH2), 1.56–1.45 (m, 6H, 3 × CH2), 1.36–1.23 (m, 28H, 14 × CH2), 0.94–0.85 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.3, 34.7, 34.2, 33.9, 32.9, 31.4, 29.6, 29.6, 29.6, 29.6, 29.5, 27.5, 26.0, 25.8, 25.3, 24.9, 22.6, 22.3, 18.4, 14.0, 13.9, −5.3; HRMS (ESI+): m/z calcd for C30H62NaO3Si+: 521.4360; [M + Na]+ found: 521.4363.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-yl palmitate (15m). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.81 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.64–1.59 (m, 2H, CH2), 1.55–1.46 (m, 6H, 3 × CH2), 1.37–1.21 (m, 48H, 24 × CH2), 0.98–0.77 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.3, 34.7, 34.2, 33.9, 32.9, 31.9, 29.7, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 27.5, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C40H82NaO3Si+: 661.5925; [M + Na]+ found: 661.5939.
18-((tert-Butyldimethylsilyl)oxy)octadecan-5-yl oleate (15n). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 5.40–5.29 (m, 2H, 2 × =CH), 4.91–4.83 (m, 1H, COOCH), 3.59 (t, J = 6.6 Hz, 2H, CH2OTBDMS), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 2.08–1.92 (m, 4H, 2 × =CHCH2), 1.64–1.48 (m, 8H, 4 × CH2), 1.35–1.22 (m, 44H, 22 × CH2), 0.92–0.84 (m, 15H, 5 × CH3), 0.04 (s, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.6, 130.0, 129.7, 74.1, 63.3, 34.7, 34.2, 33.9, 32.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 27.5, 27.2, 27.2, 26.0, 25.8, 25.3, 25.2, 22.7, 22.6, 18.4, 14.1, 14.0, −5.3; HRMS (ESI+): m/z calcd for C42H84NaO3Si+: 687.6082; [M + Na]+ found: 687.6099.
3.5. General Procedure for the Synthesis of FAHFAs Through the Saponification of Esters
To a round-bottomed flask containing methyl esters 6a–p (1.00 mmol) diluted in a mixture of THF and H2O (3:1, 10 mL), LiOH·H2O (210 mg, 5.00 mmol) was added, and the reaction mixture was left stirring at room temperature for 16 h. The reaction was then quenched with H2O, followed by the addition of HCl 1 N (10 mL) to pH 1. Then, the mixture was extracted with EtOAc (3 × 10 mL), washed with brine (1 × 30 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure. Flash chromatography on silica gel of the crude mixture (petroleum ether (bp 40–60 °C)/ethyl acetate, 60/40) resulted in the desired FAHFA.
5-(Hexanoyloxy)octadecanoic acid (7a). White solid; Yield: 35%; m. p.: 39–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.97–4.82 (m, 1H, COOCH), 2.36 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.68–1.49 (m, 8H, 4 × CH2), 1.39–1.23 (m, 26H, 13 × CH2), 1.01–0.81 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.2, 173.7, 73.4, 34.6, 34.1, 33.5, 33.4, 31.9, 31.3, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 25.3, 24.8, 22.7, 22.3, 20.5, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3287.
5-(Palmitoyloxy)octadecanoic acid (
7b) [
21]. White solid; Yield: 42%; m. p.: 39–40 °C, (lit. m.p.: 39–40 °C);
1H-NMR (400 MHz, CDCl
3):
δ 4.97–4.81 (m, 1H, COOCH), 2.36 (t,
J = 7.5 Hz, 2H, COCH
2), 2.28 (t,
J = 7.5 Hz, 2H, COCH
2), 1.72–1.45 (m, 8H, 4 × CH
2), 1.36–1.19 (m, 46H, 23 × CH
2), 0.88 (t,
J = 6.7 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.2, 173.7, 73.4, 34.7, 34.1, 33.7, 33.4, 31.9, 29.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 29.1, 25.3, 25.1, 24.7, 22.7, 20.4, 14.1; HRMS (ESI
+):
m/
z calcd for C
34H
66NaO
4+: 561.4853; [M + Na]
+ found: 561.4853.
5-(Oleoyloxy)octadecanoic acid (7c). Colorless oil; Yield: 41%; 1H-NMR (400 MHz, CDCl3): δ 5.46–5.23 (m, 2H, 2 × =CH), 4.97–4.81 (m, 1H, COOCH), 2.36 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 2.12–1.89 (m, 4H, 2 × =CHCH2), 1.70–1.48 (m, 8H, 4 × CH2), 1.40–1.09 (m, 42H, 21 × CH2), 0.88 (t, J = 6.7 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.4, 173.7, 130.0, 129.8, 73.4, 34.7, 34.1, 33.4, 31.9, 31.9, 29.8, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 22.7, 20.5, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5008.
6-(Hexanoyloxy)octadecanoic acid (7d). Colorless oil; Yield: 39%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.83 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.72–1.58 (m, 4H, 2 × CH2), 1.58–1.45 (m, 4H, 2 × CH2), 1.40–1.21 (m, 26H, 13 × CH2), 0.94–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 178.8, 173.7, 73.7, 34.7, 34.1, 33.8, 33.7, 31.9, 31.3, 29.7, 29.6, 29.6, 29.5, 29.5, 29.3, 25.3, 24.8, 24.5, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3286.
6-(Palmitoyloxy)octadecanoic acid (7e). White solid; Yield: 46%; m. p.: 38–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.72–1.58 (m, 4H, 2 × CH2), 1.58–1.43 (m, 4H, 2 × CH2), 1.39–1.20 (m, 46H, 23 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.4, 173.7, 73.7, 34.7, 34.1, 33.8, 33.8, 31.9, 29.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 25.3, 25.2, 24.8, 24.5, 22.7, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4851.
6-(Oleoyloxy)octadecanoic acid (7f). Colorless oil; Yield: 50%; 1H-NMR (400 MHz, CDCl3): δ 5.53–5.20 (m, 2H, 2 × =CH), 4.94–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.12–1.89 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.57–1.48 (m, 4H, 2 × CH2), 1.37–1.22 (m, 42H, 21 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.7, 130.0, 129.7, 73.7, 34.7, 34.1, 33.8, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.3, 25.1, 24.8, 24.5, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
7-(Hexanoyloxy)octadecanoic acid (7g). Off white low-melting point solid; Yield: 38%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.56 (m, 4H, 2 × CH2), 1.56–1.45 (m, 4H, 2 × CH2), 1.39–1.18 (m, 26H, 13 × CH2), 0.92–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.4, 173.8, 73.9, 34.7, 34.2, 33.9, 33.9, 31.9, 31.3, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 28.9, 25.3, 25.0, 24.8, 24.5, 22.7, 22.3, 14.1, 13.9; HRMS (ESI−): m/z calcd for C24H45O4−: 397.3323; [M − H]− found: 397.3324.
7-(Palmitoyloxy)octadecanoic acid (
7h) [
20]. White solid; Yield: 48%; m. p.: 39–40 °C;
1H-NMR (400 MHz, CDCl
3):
δ 4.92–4.80 (m, 1H, COOCH), 2.33 (t,
J = 7.5 Hz, 2H, COCH
2), 2.27 (t,
J = 7.5 Hz, 2H, COCH
2), 1.66–1.57 (m, 4H, 2 × CH
2), 1.55–1.44 (m, 4H, 2 × CH
2), 1.40–1.17 (m, 46H, 23 × CH
2), 0.87 (t,
J = 6.9 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.8, 173.8, 73.9, 34.7, 34.2, 33.9, 33.9, 31.9, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 28.9, 25.3, 25.2, 25.0, 24.5, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
34H
66NaO
4+: 561.4853; [M + Na]
+ found: 561.4870.
7-(Oleoyloxy)octadecanoic acid (
7i) [
20]. Colorless oil; Yield: 49%;
1H-NMR (400 MHz, CDCl
3):
δ 5.48–5.19 (m, 2H, 2 × =CH), 4.95–4.77 (m, 1H, COOCH), 2.33 (t,
J = 7.5 Hz, 2H, COCH
2), 2.27 (t,
J = 7.5 Hz, 2H, COCH
2), 2.12–1.89 (m, 4H, 2 × =CHC
H2), 1.68–1.57 (m, 4H, 2 × CH
2), 1.55–1.44 (m, 4H, 2 × CH
2), 1.41–1.13 (m, 42H, 21 × CH
2), 0.87 (t,
J = 6.7 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.7, 173.7, 130.0, 129.7, 73.9, 34.7, 34.1, 33.9, 33.9, 31.9, 29.8, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 28.9, 27.2, 27.2, 25.3, 25.1, 25.0, 24.5, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
36H
68NaO
4+: 587.5010; [M + Na]
+ found: 587.5011.
8-(Hexanoyloxy)octadecanoic acid (7j). White solid; Yield: 42%; m. p.: 35–37 °C; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.41–1.16 (m, 26H, 13 × CH2), 0.98–0.79 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.8, 173.8, 74.0, 34.7, 34.1, 34.1, 34.0, 31.9, 31.3, 29.6, 29.5, 29.5, 29.5, 29.3, 29.1, 28.9, 25.3, 25.1, 24.8, 24.6, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3289.
8-(Palmitoyloxy)octadecanoic acid (7k). White solid; Yield: 39%; m. p.: 39–40 °C; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.77 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.37–1.19 (m, 46H, 23 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.7, 173.7, 74.0, 34.7, 34.2, 34.1, 34.0, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.3, 29.3, 29.3, 29.2, 29.1, 28.9, 25.3, 25.2, 25.1, 24.6, 22.7, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4854.
8-(Oleoyloxy)octadecanoic acid (7l). Colorless oil; Yield: 43%; 1H-NMR (400 MHz, CDCl3): δ 5.44–5.30 (m, 2H, 2 × =CH), 4.91–4.81 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.11–1.88 (m, 4H, 2 × =CHCH2), 1.68–1.56 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.42–1.18 (m, 42H, 21 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.7, 130.0, 129.7, 74.0, 34.7, 34.2, 34.1, 33.9, 31.9, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 29.1, 29.1, 28.9, 27.2, 27.2, 25.3, 25.2, 25.1, 24.6, 22.7, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5016.
9-(Palmitoyloxy)octadecanoic acid (
7m) [
42]. Colorless oil; Yield: 45%;
1H-NMR (400 MHz, CDCl
3):
δ 4.93–4.80 (m, 1H, COOCH), 2.34 (t,
J = 7.5 Hz, 2H, COCH
2), 2.27 (t,
J = 7.5 Hz, 2H, COCH
2), 1.67–1.58 (m, 4H, 2 × CH
2), 1.58–1.45 (m, 4H, 2 × CH
2), 1.34–1.23 (m, 46H, 23 × CH
2), 0.88 (t,
J = 6.7 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.4, 173.8, 74.0, 34.8, 34.2, 34.1, 33.9, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 29.1, 29.0, 25.3, 25.2, 25.2, 24.6, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
34H
66NaO
4+: 561.4853; [M + Na]
+ found: 561.4852.
12-(Hexanoyloxy)octadecanoic acid (7n). Colorless oil; Yield: 37%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 2.33 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.58 (m, 4H, 2 × CH2), 1.55–1.45 (m, 4H, 2 × CH2), 1.34–1.22 (m, 26H, 13 × CH2), 0.91–0.85 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.9, 173.8, 74.1, 34.7, 34.1, 34.1, 31.7, 31.3, 29.5, 29.5, 29.4, 29.4, 29.2, 29.2, 29.0, 25.3, 25.3, 24.8, 24.7, 22.5, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3289.
12-(Palmitoyloxy)octadecanoic acid (
7o) [
56]. White solid; Yield: 40%; m. p.: 39–40 °C;
1H-NMR (400 MHz, CDCl
3):
δ 4.94–4.80 (m, 1H, COOCH), 2.34 (t,
J = 7.5 Hz, 2H, COCH
2), 2.27 (t,
J = 7.5 Hz, 2H, COCH
2), 1.66–1.57 (m, 4H, 2 × CH
2), 1.55–1.46 (m, 4H, 2 × CH
2), 1.38–1.18 (m, 46H, 23 × CH
2), 0.87 (t,
J = 6.2 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.7, 173.8, 74.1, 34.8, 34.2, 34.0, 31.9, 31.7, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.0, 25.3, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI
+):
m/
z calcd for C
34H
66NaO
4+: 561.4853; [M + Na]
+ found: 561.4846.
12-(Oleoyloxy)octadecanoic acid (7p). Colorless oil; Yield: 36%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.25 (m, 2H, 2 × =CH), 4.92–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 2.13–1.88 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.56–1.45 (m, 4H, 2 × CH2), 1.37–1.21 (m, 42H, 21 × CH2), 0.88 (t, J = 6.1 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.7, 130.0, 129.8, 74.1, 34.7, 34.2, 34.0, 31.9, 31.8, 29.8, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 27.2, 27.2, 25.3, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
3.6. General Procedure for the Deprotection of Tert-Butyldimethylsilyl (TBDMS) Group
The appropriate protected alcohol 15a–n (1.00 mmol) was added to a flame-dried flask in a dry THF (3 mL) and a solution of tetra-n-butylammonium fluoride (1 mL, 1.00 mmol, 1 M in THF) was added dropwise. The reaction mixture was left stirring for 1 h at room temperature, and then the solvent was removed under reduced pressure. The desired product was isolated by flash chromatography (petroleum ether (bp 40–60 °C)/ethyl acetate, 80/20–70/30) on silica gel.
1-Hydroxyoctadecan-9-yl hexanoate (16a). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.67–1.42 (m, 9H, 4 × CH2 and OH), 1.37–1.22 (m, 28H, 14 × CH2), 0.93–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.0, 34.7, 34.2, 34.1, 32.8, 31.9, 31.3, 29.5, 29.4, 29.4, 29.3, 25.7, 25.3, 25.3, 24.8, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3480.
1-Hydroxyoctadecan-9-yl oleate (
16b) [
22]. Colorless oil; Yield: 74%;
1H-NMR (400 MHz, CDCl
3):
δ 5.43–5.26 (m, 2H, 2 × =CH), 4.93–4.79 (m, 1H, COOCH), 3.63 (t,
J = 6.6 Hz, 2H, CH
2OH), 2.27 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.10–1.88 (m, 4H, 2 × =CHC
H2), 1.64–1.47 (m, 8H, 4 × CH
2), 1.43–1.20 (m, 45H, 22 × CH
2 and OH), 0.88 (t,
J = 6.6 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 173.7, 130.0, 129.7, 74.0, 63.0, 34.7, 34.2, 34.1, 32.8, 31.9, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.7, 25.3, 25.3, 25.2, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
36H
70NaO
3+: 573.5217; [M + Na]
+ found: 573.5202.
18-Hydroxyoctadecan-9-yl hexanoate (16c). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 4.96–4.76 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 1.66–1.45 (m, 9H, 4 × CH2 and OH), 1.39–1.19 (m, 28H, 14 × CH2), 0.98–0.78 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.2, 34.1, 32.8, 31.9, 31.4, 29.7, 29.5, 29.5, 29.5, 29.4, 29.4, 29.2, 25.7, 25.3, 25.3, 24.9, 22.6, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3509.
18-Hydroxyoctadecan-9-yl palmitate (16d). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.79 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.65–1.46 (m, 11H, 5 × CH2 and OH), 1.35–1.22 (m, 46H, 23 × CH2), 0.87 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.0, 34.7, 34.2, 34.1, 32.8, 31.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 25.7, 25.3, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5070.
18-Hydroxyoctadecan-9-yl oleate (
16e) [
20]. Colorless oil; Yield: 73%;
1H-NMR (400 MHz, CDCl
3):
δ 5.51–5.21 (m, 2H, 2 × =CH), 4.94–4.79 (m, 1H, COOCH), 3.63 (t,
J = 6.6 Hz, 2H, CH
2OH), 2.27 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.11–1.89 (m, 4H, 2 × =CHC
H2), 1.66–1.45 (m, 10H, 5 × CH
2), 1.40–1.17 (m, 43H, 21 × CH
2 and OH), 0.88 (t,
J = 6.3 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 173.7, 130.0, 129.7, 74.1, 63.0, 34.7, 34.1, 32.8, 31.9, 31.8, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 27.2, 27.2, 25.7, 25.3, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1; HRMS (ESI
+):
m/
z calcd for C
36H
70NaO
3+: 573.5217; [M + Na]
+ found: 573.5229.
18-Hydroxyoctadecan-8-yl hexanoate (16f). Colorless oil; Yield: 78%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.47 (m, 9H, 4 × CH2 and OH), 1.35–1.24 (m, 28H, 14 × CH2), 0.93–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.2, 32.8, 31.8, 31.3, 29.5, 29.5, 29.4, 29.4, 29.2, 25.7, 25.3, 25.3, 24.8, 22.6, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3497.
18-Hydroxyoctadecan-8-yl palmitate (16g). Colorless oil; Yield: 75%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, OCH2), 2.27 (t, J = 7.4 Hz, 2H, COCH2), 1.68–1.44 (m, 10H, 5 × CH2), 1.36–1.23 (m, 47H, 23 × CH2 and OH), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.8, 34.2, 32.8, 31.9, 31.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5065.
18-Hydroxyoctadecan-8-yl oleate (16h). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 5.45–5.25 (m, 2H, 2 × =CH), 4.92–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.5 Hz, 2H, OCH2), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 2.05–1.95 (m, 4H, 2 × =CHCH2), 1.65–1.46 (m, 11H, 5 × CH2 and OH), 1.36–1.24 (m, 42H, 21 × CH2), 0.88 (t, J = 6.2 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.0, 34.7, 34.2, 32.8, 31.9, 31.8, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 27.2, 27.2, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5220.
18-Hydroxyoctadecan-6-yl hexanoate (16i). Colorless oil; Yield: 71%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.81 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.71–1.40 (m, 9H, 4 × CH2 and OH), 1.37–1.22 (m, 28H, 14 × CH2), 0.94–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.1, 34.1, 32.8, 31.7, 31.3, 29.6, 29.5, 29.5, 29.5, 29.4, 25.7, 25.3, 25.0, 24.8, 22.5, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3500.
18-Hydroxyoctadecan-6-yl palmitate (16j). Colorless oil; Yield: 71%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.80 (m, 1H, COOCH), 3.62 (t, J = 6.6 Hz, 2H, OCH2), 2.26 (t, J = 7.4 Hz, 2H, COCH2), 1.62–1.46 (m, 11H, 5 × CH2 and OH), 1.34–1.23 (m, 46H, 23 × CH2), 0.87 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.0, 34.7, 34.1, 34.1, 32.8, 31.9, 31.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 25.7, 25.3, 25.2, 25.0, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5065.
18-Hydroxyoctadecan-6-yl oleate (16k). Colorless oil; Yield: 76%; 1H-NMR (400 MHz, CDCl3): δ 5.45–5.23 (m, 2H, 2 × =CH), 4.91–4.81 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, OCH2), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.10–1.89 (m, 4H, 2 × =CHCH2), 1.71–1.42 (m, 11H, 5 × CH2 and OH), 1.35–1.23 (m, 42H, 21 × CH2), 0.87 (t, J = 6.3 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.7, 74.1, 63.0, 34.7, 34.1, 34.1, 32.8, 31.9, 31.7, 29.8, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.1, 29.1, 27.2, 27.2, 25.7, 25.3, 25.2, 25.0, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5221.
18-Hydroxyoctadecan-5-yl hexanoate (16l). Colorless oil; Yield: 78%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.79 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 1.66–1.48 (m, 9H, 4 × CH2 and OH), 1.43–1.17 (m, 28H, 14 × CH2), 0.99–0.80 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.1, 63.1, 34.7, 34.2, 33.8, 32.8, 31.3, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 27.5, 25.7, 25.3, 24.8, 22.6, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H48NaO3+: 407.3496; [M + Na]+ found: 407.3496.
18-Hydroxyoctadecan-5-yl palmitate (16m). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.82 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.4 Hz, 2H, CH2COO), 1.65–1.43 (m, 9H, 4 × CH2 and OH), 1.38–1.16 (m, 48H, 24 × CH2), 0.87 (t, J = 5.9 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 74.0, 63.1, 34.7, 34.2, 33.9, 32.8, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 27.5, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H68NaO3+: 547.5061; [M + Na]+ found: 547.5064.
18-Hydroxyoctadecan-5-yl oleate (16n). Colorless oil; Yield: 79%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.25 (m, 2H, 2 × =CH), 4.94–4.80 (m, 1H, COOCH), 3.63 (t, J = 6.6 Hz, 2H, CH2OH), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.11–1.90 (m, 4H, 2 × =CHCH2), 1.65–1.48 (m, 9H, 4 × CH2 and OH), 1.38–1.21 (m, 44H, 22 × CH2), 0.88 (t, J = 6.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 173.7, 130.0, 129.8, 74.1, 63.1, 34.7, 34.2, 33.9, 32.8, 31.9, 29.8, 29.7, 29.6, 29.6, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.1, 27.5, 27.2, 27.2, 25.7, 25.3, 25.2, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H70NaO3+: 573.5217; [M + Na]+ found: 573.5215.
3.7. General Procedure for the Oxidation of Alcohols 16a–n to FAHFAs
To a round-bottomed flask containing the appropriate alcohol 16a–n (1.00 mmol), acetone (10 mL) was added, and the mixture was left stirring at 0 °C until full dissolution. Jones reagent (2 M, 1.5 mL, 3.00 mmol) was added dropwise at 0 °C and the reaction mixture was left stirring for 1 h at this temperature. Then, the reaction mixture was quenched with a saturated aqueous solution of NaHSO3 (10 mL) and extracted with Et2O (3 × 20 mL). The combined organic layers were washed with brine (1 × 50 mL), dried over Na2SO4, and,] then evaporated to give a crude mixture, which was purified by flash column chromatography on silica gel (petroleum ether (bp 40–60 °C)/ethyl acetate, 60/40) to afford the desired FAHFAs.
9-(Hexanoyloxy)octadecanoic acid (
17a) [
14]. Colorless oil; Yield: 84%;
1H-NMR (400 MHz, CDCl
3):
δ 4.90–4.83 (m, 1H, COOCH), 2.34 (t,
J = 7.5 Hz, 2H, COCH
2), 2.28 (t,
J = 7.5 Hz, 2H, COCH
2), 1.66–1.59 (m, 4H, 2 × CH
2), 1.54–1.46 (m, 4H, 2 × CH
2), 1.35–1.23 (m, 26H, 13 × CH
2), 0.93–0.84 (m, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.3, 173.8, 74.0, 34.7, 34.2, 34.1, 33.9, 31.9, 31.3, 29.5, 29.5, 29.3, 29.1, 29.0, 25.3, 25.2, 24.8, 24.6, 22.7, 22.3, 14.1, 13.9; HRMS (ESI
+):
m/
z calcd for C
24H
46NaO
4+: 421.3288; [M + Na]
+ found: 421.3286.
9-(Oleoyloxy)octadecanoic acid (
17b) [
57]. Colorless oil; Yield: 82%;
1H-NMR (400 MHz, CDCl
3):
δ 5.50–5.22 (m, 2H, 2 × =CH), 4.94–4.78 (m, 1H, COOCH), 2.34 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.27 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.12–1.89 (m, 4H, 2 × =CHC
H2), 1.66–1.59 (m, 4H, 2 × CH
2), 1.55–1.46 (m, 4H, 2 × CH
2), 1.36–1.22 (m, 42H, 21 × CH
2), 0.88 (t,
J = 6.7 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 178.6, 173.7, 130.0, 129.8, 74.0, 34.7, 34.2, 34.1, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.1, 29.1, 29.1, 29.0, 27.2, 27.2, 25.3, 25.3, 25.2, 24.6, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
36H
68NaO
4+: 587.5010; [M + Na]
+ found: 587.5010.
10-(Hexanoyloxy)octadecanoic acid (17c). Colorless oil; Yield: 84%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.77 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.58–1.39 (m, 4H, 2 × CH2), 1.33–1.23 (m, 26H, 13 × CH2), 0.93–0.82 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.0, 173.8, 74.1, 34.7, 34.1, 33.9, 31.9, 31.9, 31.3, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.1, 29.1, 29.0, 25.3, 25.3, 24.8, 24.7, 24.6, 22.7, 22.7, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3286.
10-(Palmitoyloxy)octadecanoic acid (
17d) [
58]. White solid; Yield: 86%; m.p.: 36–37 °C;
1H-NMR (400 MHz, CDCl
3):
δ 4.94–4.78 (m, 1H, COOCH), 2.34 (t,
J = 7.5 Hz, 2H, COCH
2), 2.27 (t,
J = 7.5 Hz, 2H, COCH
2), 1.67–1.57 (m, 4H, 2 × CH
2), 1.56–1.42 (m, 4H, 2 × CH
2), 1.37–1.22 (m, 46H, 23 × CH
2), 0.88 (t,
J = 6.6 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 179.0, 173.8, 74.1, 34.8, 34.2, 33.9, 31.9, 31.9, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 25.3, 25.3, 25.2, 24.7, 22.7, 22.7, 14.1, 14.1; HRMS (ESI
+):
m/
z calcd for C
34H
66NaO
4+: 561.4853; [M + Na]
+ found: 561.4858.
10-(Oleoyloxy)octadecanoic acid (
17e) [
20]. Colorless oil; Yield: 83%;
1H-NMR (400 MHz, CDCl
3):
δ 5.49–5.25 (m, 2H, 2 × =CH), 4.93–4.78 (m, 1H, COOCH), 2.34 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.27 (t,
J = 7.5 Hz, 2H, CH
2COO), 2.17–1.82 (m, 4H, 2 × =CHC
H2), 1.67–1.57 (m, 4H, 2 × CH
2), 1.55–1.44 (m, 4H, 2 × CH
2), 1.36–1.22 (m, 42H, 21 × CH
2), 0.88 (t,
J = 6.5 Hz, 6H, 2 × CH
3);
13C-NMR (100 MHz, CDCl
3):
δ 178.8, 173.7, 130.0, 129.8, 74.1, 34.7, 34.2, 33.8, 31.9, 31.9, 29.8, 29.7, 29.7, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 29.0, 27.2, 27.2, 25.3, 25.3, 25.2, 24.7, 22.7, 22.7, 14.1; HRMS (ESI
+):
m/
z calcd for C
36H
68NaO
4+: 587.5010; [M + Na]
+ found: 587.5010.
11-(Hexanoyloxy)octadecanoic acid (17f). Colorless oil; Yield: 80%; 1H-NMR (400 MHz, CDCl3): δ 4.93–480 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.66–1.59 (m, 4H, 2 × CH2), 1.54–1.46 (m, 4H, 2 × CH2), 1.36–1.24 (m, 26H, 13 × CH2), 0.93–0.84 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.8, 74.1, 34.7, 34.2, 33.9, 31.8, 31.3, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.0, 25.3, 25.3, 24.9, 24.7, 22.6, 22.3, 14.1, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3288.
11-(Palmitoyloxy)octadecanoic acid (17g). Colorless oil; Yield: 85%; 1H-NMR (400 MHz, CDCl3): δ 4.92–4.81 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.65–1.59 (m, 4H, 2 × CH2), 1.53–1.47 (m, 4H, 2 × CH2), 1.34–1.23 (m, 46H, 23 × CH2), 0.88 (t, J = 6.6 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.8, 74.1, 34.8, 34.2, 33.9, 31.9, 31.8, 29.7, 29.7, 29.6, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.0, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4863.
11-(Oleoyloxy)octadecanoic acid (17h). Colorless oil; Yield: 86%; 1H-NMR (400 MHz, CDCl3): δ 5.47–5.23 (m, 2H, 2 × =CH), 4.92–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.14–1.87 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.56–1.46 (m, 4H, 2 × CH2), 1.37–1.22 (m, 42H, 21 × CH2), 0.88 (t, J = 6.4 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.0, 173.7, 130.0, 129.8, 74.1, 34.7, 34.2, 33.9, 31.9, 31.8, 29.8, 29.7, 29.7, 29.5, 29.5, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 29.0, 27.2, 27.2, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.1; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5009.
13-(Hexanoyloxy)octadecanoic acid (17i). Colorless oil; Yield: 82%; 1H-NMR (400 MHz, CDCl3): δ 4.93–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.1 Hz, 2H, COCH2), 2.27 (t, J = 7.1 Hz, 2H, COCH2), 1.68–1.57 (m, 4H, 2 × CH2), 1.55–1.44 (m, 4H, 2 × CH2), 1.38–1.19 (m, 26H, 13 × CH2), 0.94–0.81 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.7, 173.8, 74.1, 34.7, 34.1, 34.1, 34.0, 31.7, 31.3, 29.5, 29.4, 29.2, 29.0, 25.3, 25.0, 24.8, 24.7, 22.5, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3287.
13-(Palmitoyloxy)octadecanoic acid (17j). White solid; Yield: 89%; m.p.: 39–41 °C; 1H-NMR (400 MHz, CDCl3): δ 4.91–4.82 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.27 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.59 (m, 4H, 2 × CH2), 1.54–1.47 (m, 4H, 2 × CH2), 1.35–1.23 (m, 46H, 23 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.3, 173.8, 74.1, 34.8, 34.2, 34.1, 33.9, 31.9, 31.7, 29.7, 29.7, 29.6, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 29.0, 25.3, 25.2, 25.0, 24.7, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M + Na]+ found: 561.4879.
13-(Oleoyloxy)octadecanoic acid (17k). Colorless oil; Yield: 87%; 1H-NMR (400 MHz, CDCl3): δ 5.42–5.26 (m, 2H, 2 × =CH), 4.90–4.83 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.27 (t, J = 7.5 Hz, 2H, CH2COO), 2.10–1.87 (m, 4H, 2 × =CHCH2), 1.66–1.59 (m, 4H, 2 × CH2), 1.53–1.46 (m, 4H, 2 × CH2), 1.34–1.24 (m, 42H, 21 × CH2), 0.88 (t, J = 6.3 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.7, 130.0, 129.7, 74.1, 34.7, 34.2, 34.1, 34.0, 31.9, 31.7, 29.8, 29.7, 29.6, 29.6, 29.5, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.1, 29.0, 29.0, 27.2, 27.2, 25.3, 25.2, 25.0, 24.7, 22.7, 22.5, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5010.
14-(Hexanoyloxy)octadecanoic acid (17l). Colorless oil; Yield: 81%; 1H-NMR (400 MHz, CDCl3): δ 4.95–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.55–1.46 (m, 4H, 2 × CH2), 1.38–1.21 (m, 26H, 13 × CH2), 0.94–0.83 (m, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.5, 173.8, 74.1, 34.7, 34.2, 34.0, 33.8, 31.3, 29.6, 29.5, 29.5, 29.4, 29.2, 29.0, 27.5, 25.3, 24.8, 24.7, 22.6, 22.3, 14.0, 13.9; HRMS (ESI+): m/z calcd for C24H46NaO4+: 421.3288; [M + Na]+ found: 421.3288.
14-(Palmitoyloxy)octadecanoic acid (17m). White solid; Yield: 80%; m. p.: 41–42 °C; 1H-NMR (400 MHz, CDCl3): δ 4.94–4.80 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, COCH2), 2.28 (t, J = 7.5 Hz, 2H, COCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.54–1.47 (m, 4H, 2 × CH2), 1.39–1.20 (m, 46H, 23 × CH2), 0.88 (t, J = 6.5 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.1, 173.8, 74.1, 34.8, 34.2, 33.9, 33.9, 31.9, 29.7, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.4, 29.3, 29.2, 29.2, 29.1, 27.5, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C34H66NaO4+: 561.4853; [M+Na]+ found: 561.4853.
14-(Oleoyloxy)octadecanoic acid (17n). Colorless oil; Yield: 85%; 1H-NMR (400 MHz, CDCl3): δ 5.49–5.23 (m, 2H, 2 × =CH), 4.95–4.79 (m, 1H, COOCH), 2.34 (t, J = 7.5 Hz, 2H, CH2COO), 2.28 (t, J = 7.5 Hz, 2H, CH2COO), 2.17–1.81 (m, 4H, 2 × =CHCH2), 1.67–1.58 (m, 4H, 2 × CH2), 1.56–1.46 (m, 4H, 2 × CH2), 1.37–1.16 (m, 42H, 21 × CH2), 0.88 (t, J = 5.8 Hz, 6H, 2 × CH3); 13C-NMR (100 MHz, CDCl3): δ 179.2, 173.8, 130.0, 129.7, 74.1, 34.7, 34.2, 33.9, 33.8, 31.9, 29.8, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.2, 29.2, 29.1, 29.1, 27.5, 27.2, 27.2, 25.3, 25.2, 24.7, 22.7, 22.6, 14.1, 14.0; HRMS (ESI+): m/z calcd for C36H68NaO4+: 587.5010; [M + Na]+ found: 587.5007.