Control of Nanoparticle Size of Intrinsically Fluorescent PET (Polyethylene Terephthalate) Particles Produced Through Nanoprecipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PET Nanoparticles Preparation
2.3. Spectroscopic Measurements
2.4. PET Nanoparticles Characterization
3. Results and Discussion
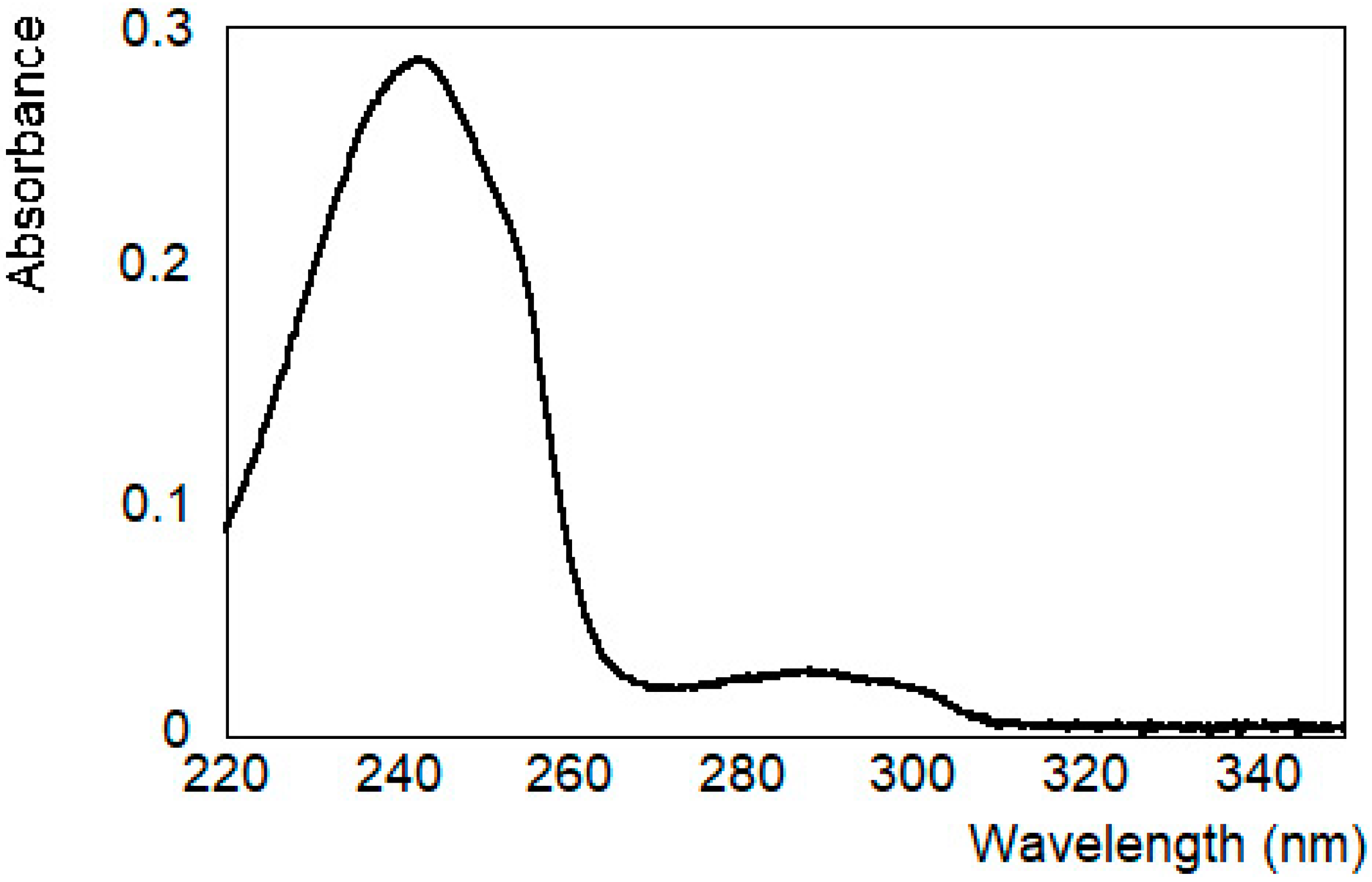
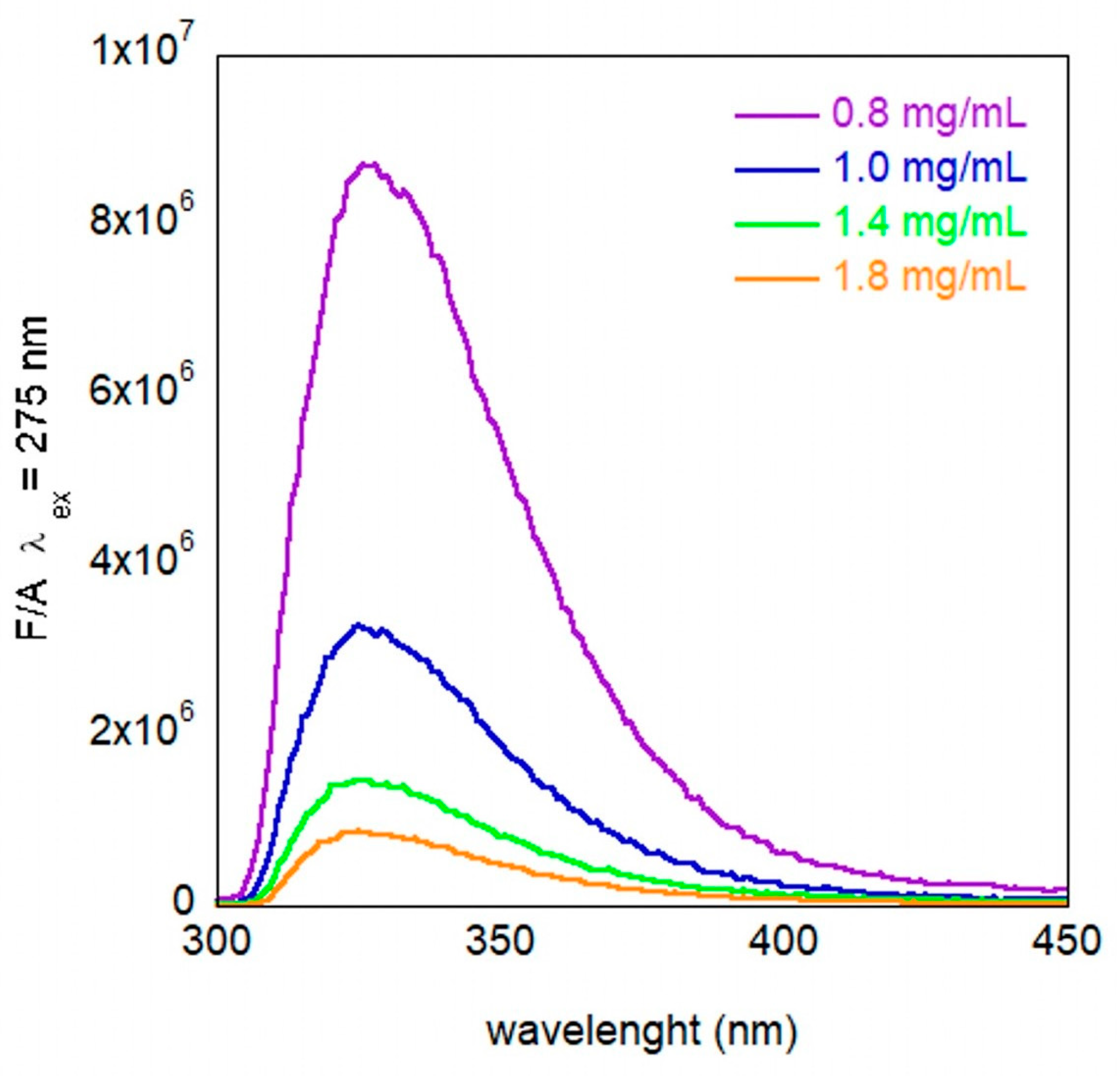
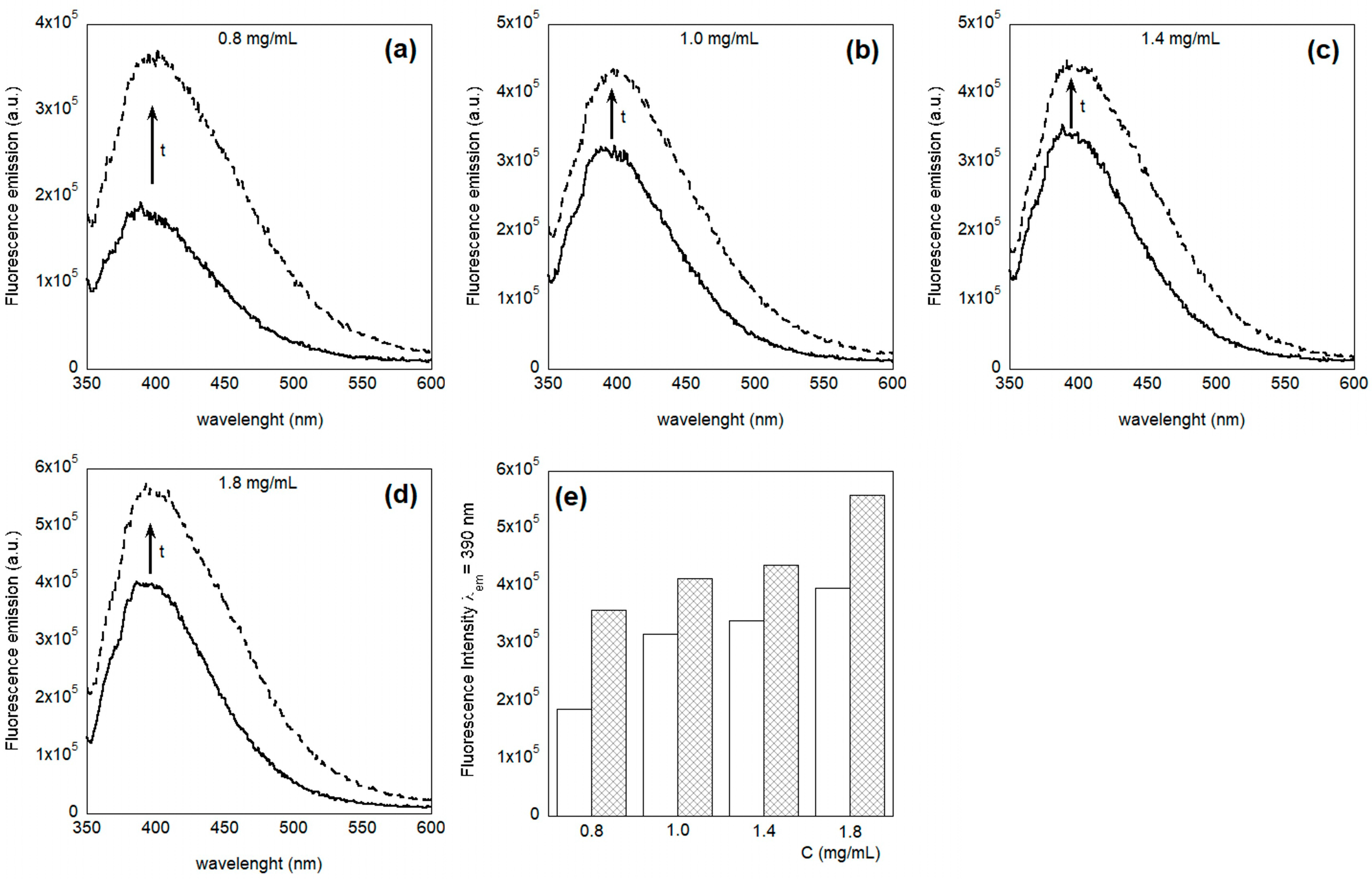
3.1. Characterization of the System in Solution
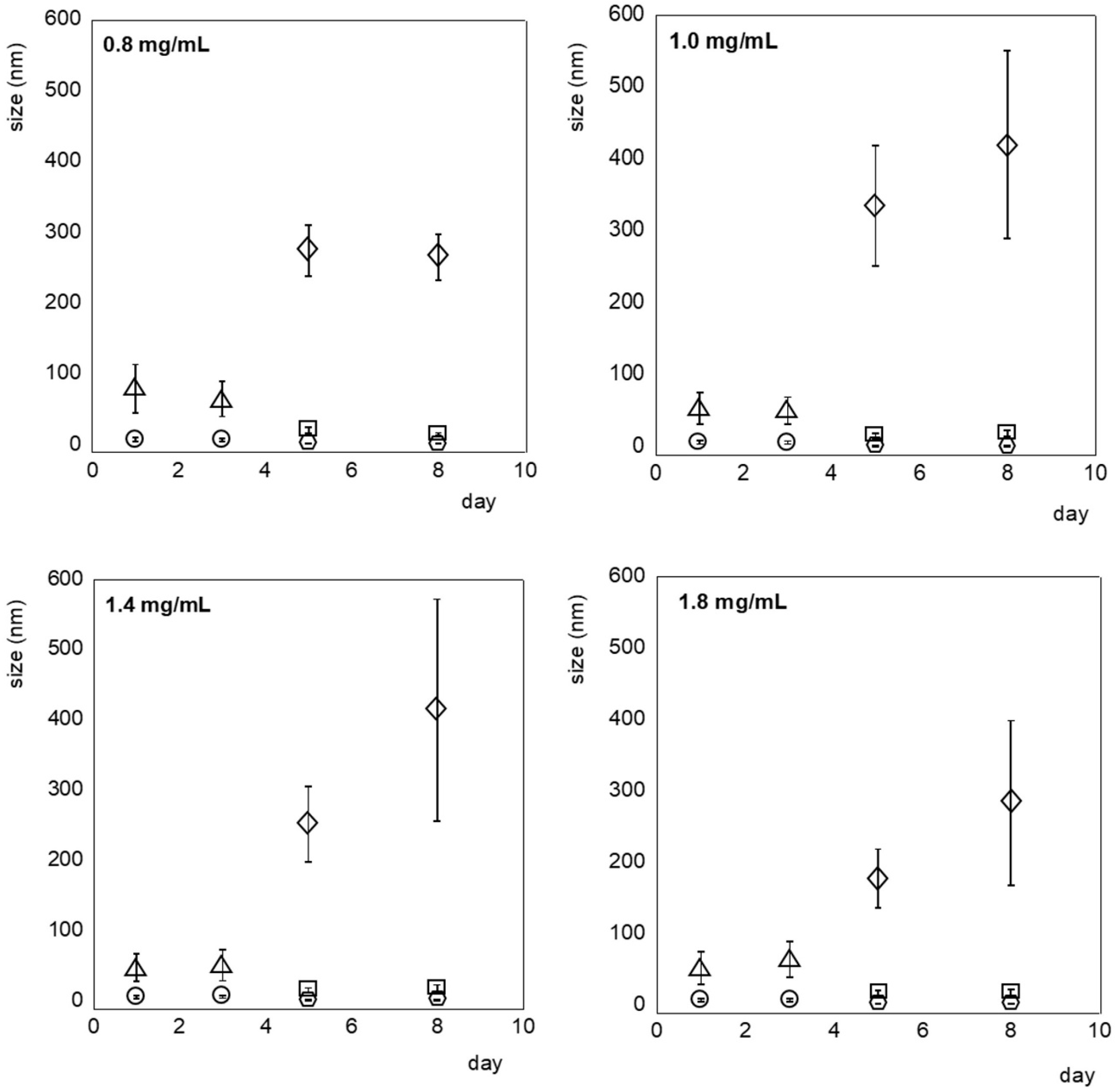
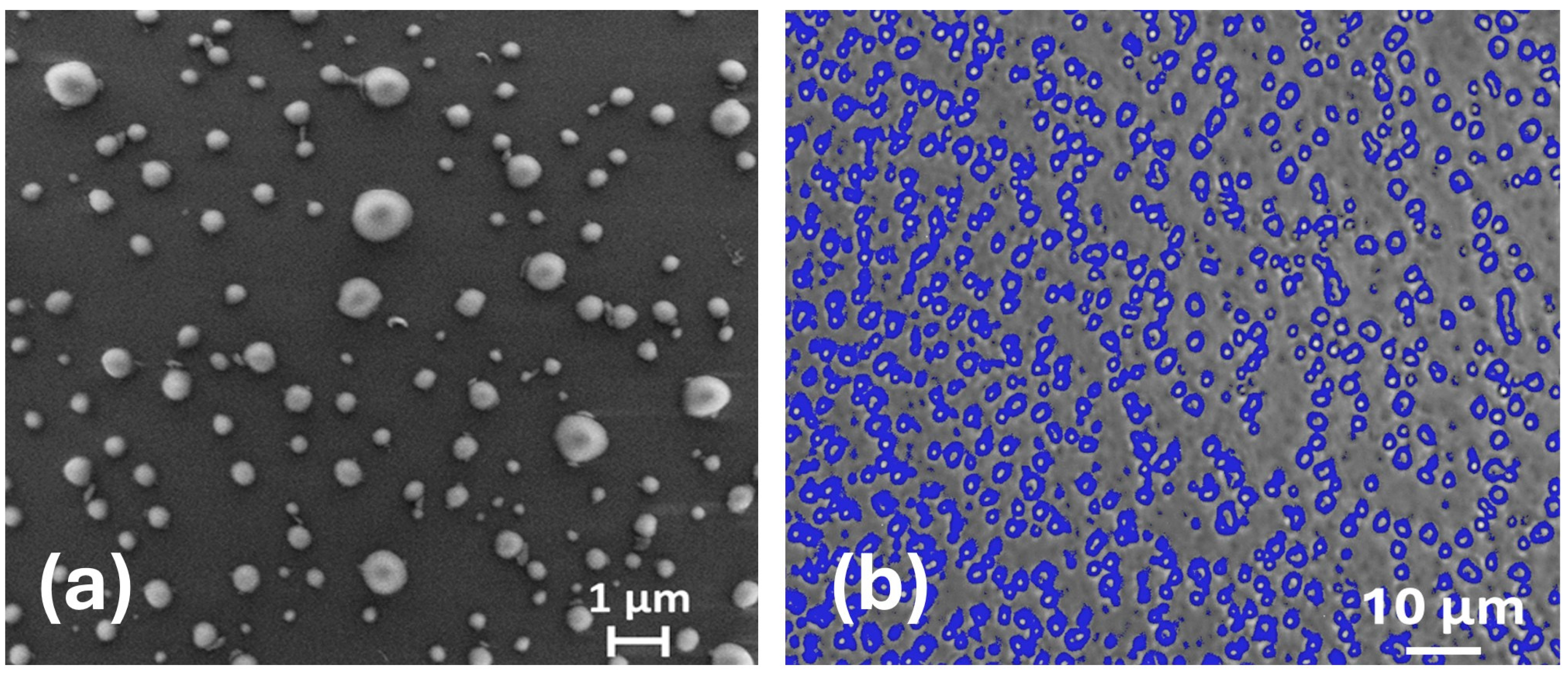
3.2. Characterization of PET NPs on Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2023; Plastics Europe: Brussels, Belgium, 2023. [Google Scholar]
- Cressey, D. Bottles, bags, ropes and toothbrushes: The struggle to track ocean plastics. Nature 2016, 536, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The Complex Mixture, Fate and Toxicity of Chemicals Associated with Plastic Debris in the Marine Environment, in Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 117–140. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Lotze, H.K.; Jubinville, I.; Wilcox, C.; Jambeck, J. Plastic as a persistent marine pollutant. Annu. Rev. Environ. Resour. 2017, 42, 1–26. [Google Scholar] [CrossRef]
- Monteiro, R.C.P.; Ivar, J.A.; Costa, M.F. Plastic pollution in islands of the Atlantic Ocean. Environ. Pollut. 2018, 238, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Passmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Proc. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.Y.; Kim, H.; Jang, J. Microplastic pollution in soil and groundwater: A review. Env. Chem. Lett. 2021, 19, 4211–4224. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 11, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Plastic in Cosmetics: Are We Polluting the Environment Through our Personal Care: Plastic Ingredients that Contribute to Marine Microplastic Litter; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2015. Available online: https://www.unep.org/resources/report/plastic-cosmetics-are-we-polluting-environment-through-our-personal-care (accessed on 10 February 2024).
- Da Costa, J.P.; Santos, P.S.; Duarte, A.C.; RochaSantos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Chou, S.-H.; Jhang, Y.-J.; Wu, T.-S.; Lin, L.-X.; Soo, Y.-L.; Hsiao, I.-L. Extraction method development for nanoplastics from oyster and fish tissues. Sci. Total Environ. 2022, 814, 152675. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X.; Li, H. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci. Total Environ. 2020, 708, 134841. [Google Scholar] [CrossRef]
- Senathirajah, K.; Palanisami, T. How Much Microplastics Are We Ingesting? Estimation of the Mass of Microplastics Ingested; Report for WWF Singapore; The University of Newcastle: Callaghan, Australia, 2019. [Google Scholar]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Besley, A.; Vijver, M.G.; Behrens, P.; Bosker, T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 2017, 114, 77–83. [Google Scholar] [CrossRef]
- Yang, T.; Luo, J.; Nowack, B. Characterization of Nanoplastics, Fibrils, and Microplastics Released during Washing and Abrasion of Polyester Textiles. Environ. Sci. Technol. 2021, 55, 15873–15881. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2021, 297, 118773. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 495–607. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Vélez-Escamilla, L.Y.; Contreras-Torres, F.F. Latest Advances and Developments to Detection of Micro- and Nanoplastics Using Surface-Enhanced Raman Spectroscopy. Part. Part. Syst. Charact. 2022, 39, 2100217. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Guan, B. Separation and identification of nanoplastics in tap water. Environ. Res. 2022, 204, 112134. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Lionetto, F.; Lionetto, M.G.; Mele, C.; Corcione, C.E.; Bagheri, S.; Udayan, G.; Maffezzoli, A. Autofluorescence of Model Polyethylene Terephthalate Nanoplastics for Cell Interaction Studies. Nanomaterials 2022, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef]
- Colomer, I.; Chamberlain, A.; Haughey, M.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 0088. [Google Scholar] [CrossRef]
- Johnson, L.M.; Mecham, J.B.; Krovi, S.A.; Moreno Caffaro, M.M.; Aravamudhan, S.; Kovach, A.L.; Fennell, T.R.; Mortensen, N.P. Fabrication of polyethylene terephthalate (PET) nanoparticles with fluorescent tracers for studies in mammalian cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef]
- Chikazumi, N.; Mukoyama, Y.; Sugitani, H. High-performance size-exclusion chromatography of poly- and oligoethylene terephthalate using a mixture of hexafluoroisopropanol and chloroform as the mobile phase. J. Chromatogr. A 1989, 479, 85–95. [Google Scholar] [CrossRef]
- Ouchi, I.; Miyamura, R.; Sakaguchi, M.; Hosaka, S.; Kitagawa, M. Excitation and emission spectra of polyethylene terephthalate and polyethylene 2,6-naphthalate films. Polym. Adv. Technol. 1999, 10, 195–198. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Roland, C.M. Absorption and fluorescence spectra of poly(ethylene terephthalate) dimers. Polymer 1990, 31, 2023–2026. [Google Scholar] [CrossRef]
- Ouchi, I. Anisotropic Absorption and Reflection Spectra of Poly(ethylene terephthalate) Films in Ultraviolet Region. Polymer J. 1983, 15, 225–243. [Google Scholar] [CrossRef][Green Version]
- Merrill, R.G.; Roberts, C.W. Photophysical Processes and Interactions Between Poly(ethylene Terephthalate) and 1-Amino-2-(2-methoxyet hoxy)-4-hydroxy-9,10-Anthraquinone. J. Appl. Polym. Sci. 1979, 21, 2745–2768. [Google Scholar] [CrossRef]
- Padhye, M.R.; Tamhane, P.S. Luminescence studies in Poly(Ethylene Terephtalate) Films at 77K. Angew. Macromol. Chem. 1978, 69, 33–45. [Google Scholar] [CrossRef]
- Cheung, P.S.R.; Roberts, C.W.; Wagener, K.B. Synthesis, Photodegradation, and Energy Transfer in a Series of Poly(Ethylene Terephthalate-co-2,6-Naphthalenedicarboxylate) Copolymers. J. Appl. Polym. Sci. 1979, 24, 1809–1830. [Google Scholar] [CrossRef]
- Allen, N.S.; McKellar, J.F. Luminescent species in poly(ethylene terephthalate). Makromol. Chem. 1978, 179, 523–526. [Google Scholar] [CrossRef]
- Hemker, D.; Frank, C.; Thomas Jule, W. Photophysical studies of amorphous orientation in poly(ethylene terephthalate) films. Polymer 1988, 29, 437–447. [Google Scholar] [CrossRef]
- Henneke, M.; Kurz, K.; Fuhrmann, J. Orientation of hot-drawn poly(butylene terephthalate) films as determined from the intrinsic polarized fluorescence. Macromolecules 1992, 25, 6190. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Muñoz-Tabares, J.A.; Aguilar-Guzmán, J.C.; Vazquez-Duhalt, R. A novel and simple method for polyethylene terephthalate (PET) nanoparticle production. Environ. Sci. Nano 2019, 6, 2031–2036. [Google Scholar] [CrossRef]
- Bombelli, C.; Bordi, F.; Borocci, S.; Diociaiuti, M.; Lettieri, R.; Limongelli, F.; Mancini, G.; Sennato, S. New Pyrenyl Fluorescent Amphiphiles: Synthesis and Aggregation Properties. Soft Matter 2011, 7, 8525–8534. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, R.; Mudassir, M.; Domenici, F.; Salina, A.; Venanzi, M.; D’Ottavi, C.; Di Bartolomeo, E.; Gatto, E. Control of Nanoparticle Size of Intrinsically Fluorescent PET (Polyethylene Terephthalate) Particles Produced Through Nanoprecipitation. Molecules 2025, 30, 282. https://doi.org/10.3390/molecules30020282
Lettieri R, Mudassir M, Domenici F, Salina A, Venanzi M, D’Ottavi C, Di Bartolomeo E, Gatto E. Control of Nanoparticle Size of Intrinsically Fluorescent PET (Polyethylene Terephthalate) Particles Produced Through Nanoprecipitation. Molecules. 2025; 30(2):282. https://doi.org/10.3390/molecules30020282
Chicago/Turabian StyleLettieri, Raffaella, Muhammad Mudassir, Fabio Domenici, Andrea Salina, Mariano Venanzi, Cadia D’Ottavi, Elisabetta Di Bartolomeo, and Emanuela Gatto. 2025. "Control of Nanoparticle Size of Intrinsically Fluorescent PET (Polyethylene Terephthalate) Particles Produced Through Nanoprecipitation" Molecules 30, no. 2: 282. https://doi.org/10.3390/molecules30020282
APA StyleLettieri, R., Mudassir, M., Domenici, F., Salina, A., Venanzi, M., D’Ottavi, C., Di Bartolomeo, E., & Gatto, E. (2025). Control of Nanoparticle Size of Intrinsically Fluorescent PET (Polyethylene Terephthalate) Particles Produced Through Nanoprecipitation. Molecules, 30(2), 282. https://doi.org/10.3390/molecules30020282











