In Vitro and In Vivo Evaluation of the Antischistosomal Activity of Polygodial and 9-Deoxymuzigadial Isolated from Drimys brasiliensis Branches
Abstract
1. Introduction
2. Results
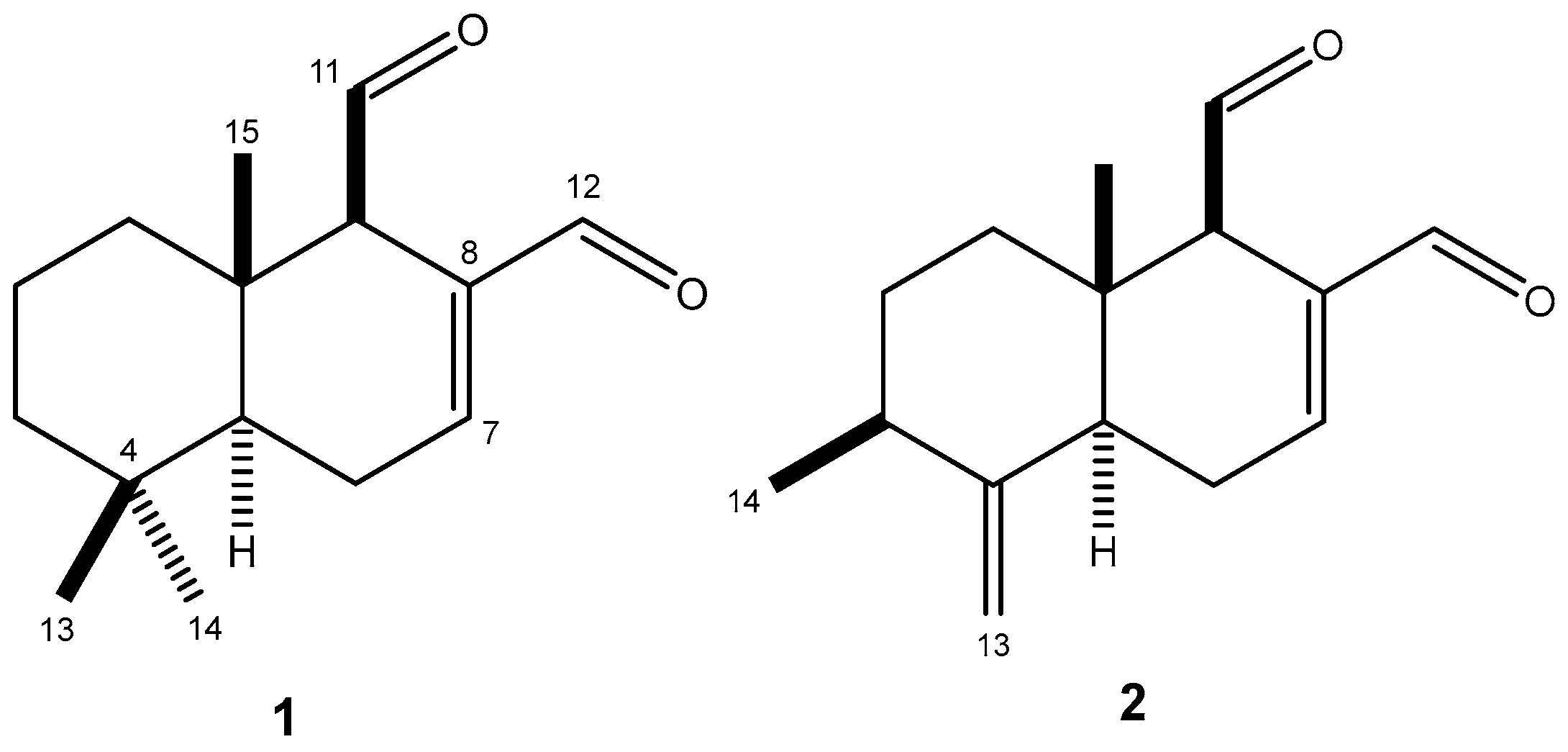
2.1. Chemical Characterization of Polygodial (1) and 9-Deoxymuzigadial (2)
2.2. In Vitro Evaluation of Polygodial (1) and 9-Deoxymuzigadial (2)
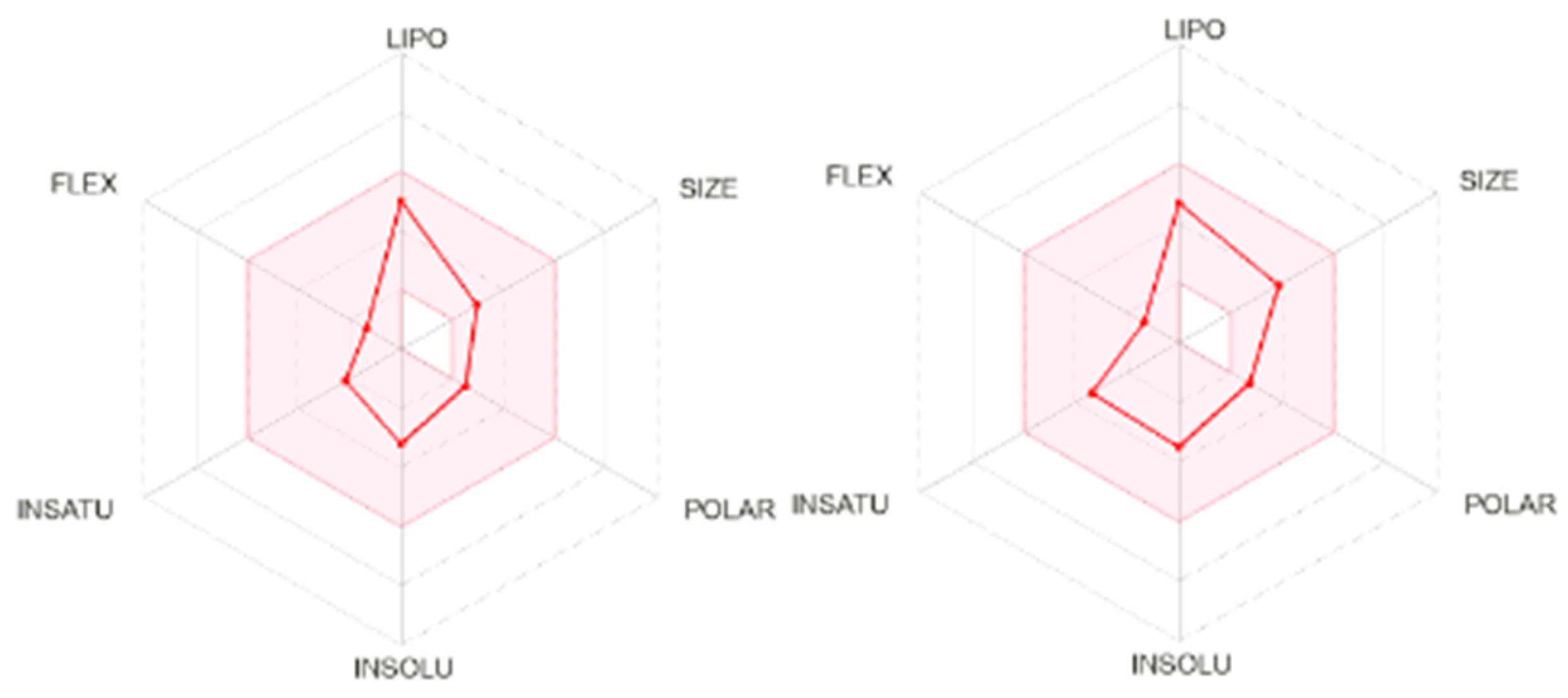
2.3. In Silico Evaluation of Polygodial (1)
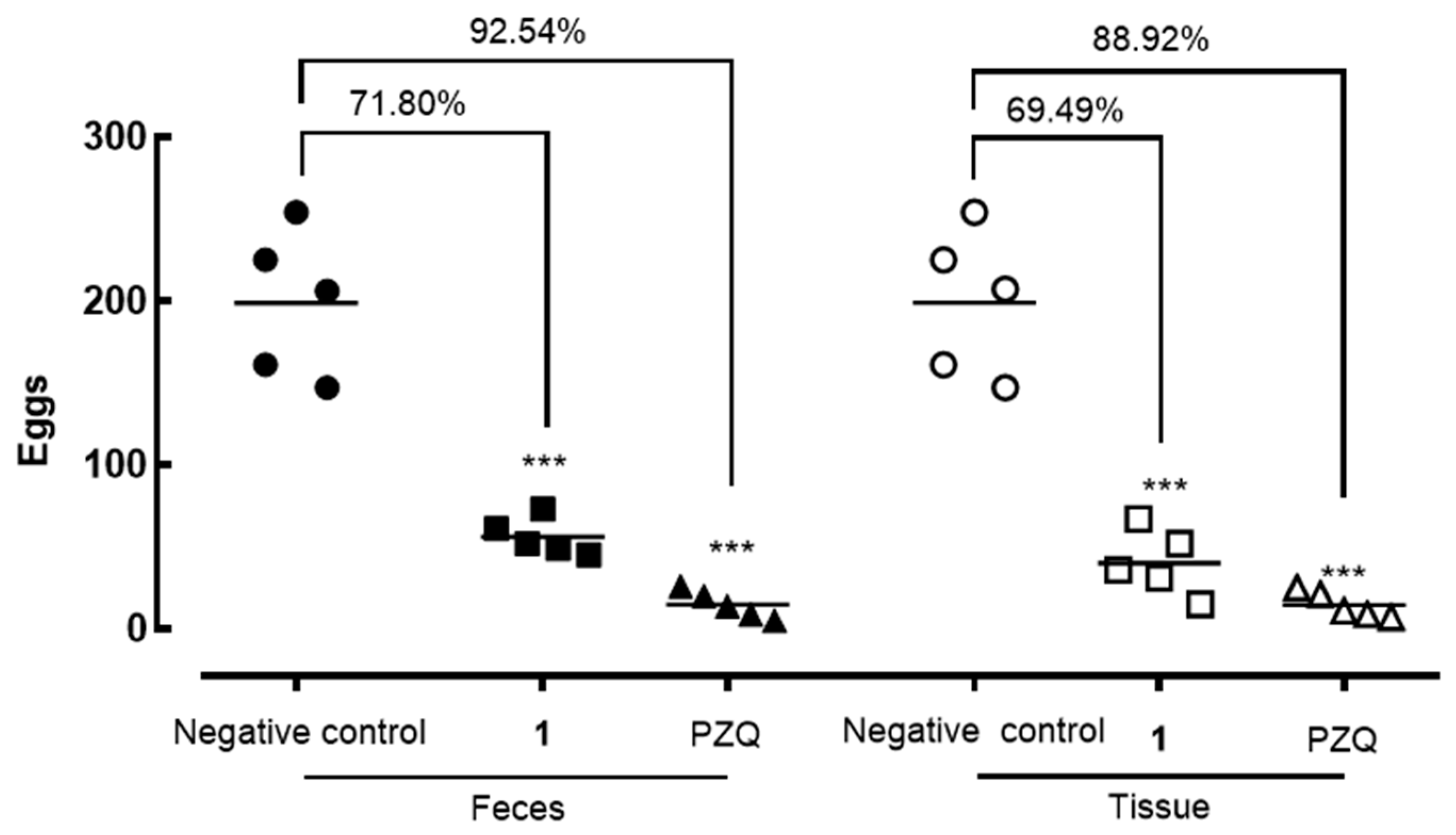
2.4. In Vivo Evaluation
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Plant Material
4.3. Extraction
4.4. Isolation of Polygodial (1) and 9-Deoxymuzigadial (2)
4.5. In Silico Analysis
4.6. Animals, Parasites, and Cells
4.7. In Vitro Antiparasitic Assay
4.8. In Vitro Cytotoxicity Assay
4.9. In Vivo Efficacy in Mice Infected with S. mansoni
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The Role of Natural Products in Drug Discovery and Development Against Neglected Tropical Diseases. Molecules 2016, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- LoVerde, P.T.; Alwan, S.N.; Taylor, A.B.; Rhodes, J.; Chevalier, F.D.; Anderson, T.J.; McHardy, S.F. Rational approach to drug discovery for human schistosomiasis. Int. J. Parasitol. Drugs Drug. Resist. 2021, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Filho, J.T.; Silva, A.C.; Dantas, R.F.; Gomes, B.F.; Souza-Neto, L.R.; Brandao-Neto, J.; Owens, R.J.; Furnham, N.; Neves, B.J.; Silva-Junior, F.P.; et al. Schistosomiasis Drug Discovery in the Era of Automation and Artificial Intelligence. Front. Immunol. 2021, 12, 642383. [Google Scholar] [CrossRef] [PubMed]
- Fratoni, E.; Claudino, V.D.; Yunes, R.A.; Franchi, G.C., Jr.; Nowill, A.E.; Filho, V.C.; Monache, F.D.; Malheiros, A. Further Drimane Sesquiterpenes from Drimys brasiliensis Stem Barks with Cytotoxic Potential. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, A.; Cechinel Filho, V.; Schmitt, C.B.; Yunes, R.A.; Escalante, A.; Svetaz, L.; Zacchino, S.; Delle Monache, F. Antifungal Activity of Drimane Sesquiterpenes from Drimys brasiliensis using Bioassay-guided Fractionation. J. Pharm. Sci. 2005, 8, 335–339. [Google Scholar]
- Ferreira, B.A.; Norton Filho, A.F.; Deconte, S.R.; Tomiosso, T.C.; Thevenard, F.; Andrade, S.P.; Lago, J.H.G.; Araújo, F.A. Sesquiterpene Polygodial from Drimys brasiliensis (Winteraceae) Down-Regulates Implant-Induced Inflammation and Fibrogenesis in Mice. J. Nat. Prod. 2020, 83, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.H.G.; Carvalho, L.A.C.; da Silva, F.S.; Toyama, D.O.; Fávero, O.A.; Romoff, P. Chemical Composition and Anti-Inflammatory Evaluation of Essential Oils from Leaves and Steam Barks from Drimys brasiliensis Miers (Winteraceae). J. Braz. Chem. Soc. 2010, 21, 1760–1765. [Google Scholar] [CrossRef]
- Claudino, V.D.; da Silva, K.C.; Cechinel Filho, V.; Yunes, R.A.; Delle Monache, F.; Giménez, A.; Salamanca, E.; Gutierrez-Yapu, D.; Malheiros, A. Drimanes from Drimys brasiliensis with Leishmanicidal and Antimalarial Activity. Mem. Inst. Oswaldo Cruz. 2013, 108, 140–144. [Google Scholar] [CrossRef]
- Umehara, E.; Teixeira, T.R.; Cajás, R.A.; Amaro, M.C.; de Moraes, J.; Lago, J.H.G. EDBD-3,6-Epidioxy-1,10-Bisaboladiene-An Endoperoxide Sesquiterpene Obtained from Drimys brasiliensis (Winteraceae) Exhibited Potent Preclinical Efficacy against Schistosoma mansoni Infection. Antibiotics 2024, 13, 779. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, M.S.; El-Khawaja, S.M.; El-Feraly, F.S.; Hufford, C.D. 9-Deoxy Drimane Sesquiterpenes from Canella winterana. Phytochemistry 1990, 29, 975–977. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Costa, J.M.C. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, e02582. [Google Scholar] [CrossRef]
- Talele, T.T. Natural-Products-Inspired Use of the gem-Dimethyl Group in Medicinal Chemistry. J. Med. Chem. 2018, 61, 2166–2210. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Valiante, V. Chemical Diversity and Biosynthesis of Drimane-Type Sesquiterpenes in the Fungal Kingdom. Chembiochem 2022, 23, 202200173. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.C.; Cajas, R.A.; Andrade-De-Siqueira, A.I.; Almeida, R.B.P.; Godoy-Silva, J.; Gonçalves, M.M.; Lago, J.H.G.; de Moraes, J. Evaluating the Antischistosomal Activity of Dehydrodieugenol B and Its Methyl Ether Isolated from Nectandra leucantha—A Preclinical Study against Schistosoma mansoni Infection. ACS Omega 2023, 8, 40890–40897. [Google Scholar] [CrossRef]
- Silva, M.P.; Oliveira, G.L.; de Carvalho, R.B.; de Sousa, D.P.; Freitas, R.M.; Pinto, P.L.; de Moraes, J. Antischistosomal Activity of the Terpene Nerolidol. Molecules 2014, 19, 3793–3803. [Google Scholar] [CrossRef]
- Mogre, S.S.; Brown, A.I.; Koslover, E.F. Getting Around the Cell: Physical Transport in the Intracellular World. Phys. Biol. 2020, 17, 61003. [Google Scholar] [CrossRef] [PubMed]
- Lopéz-Lopéz, D.; Razo-Hernández, R.S.; Millán-Pacheco, C.; Levya-Peralta, M.A.; Peña-Morán, O.A.; Sánchez-Carranza, J.N.; Rodríguez-López, V. Ligand-Based Drug Design of Genipin Derivatives with Cytotoxic Activity against HeLa Cell Line: A Structural and Theoretical Study. Pharmaceuticals 2023, 16, 1647. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Roldán, A.O.; López-Cardoso, E.M.; Rosas-Valdez, M.E.; Roman-Bravo, P.P.; Vargas-Pineda, D.G.; Cea-Olivares, R.; Acevedo-Quiroz, M.; Razo-Hernández, R.S.; Alvarez-Fitz, P.; Jancik, V. Synthesis, characterization, antimicrobial and theoretical studies of the first main group tris(ephedrinedithiocarbamate) complexes of As(III), Sb(III), Bi(III), Ga(III) and In(III). Polyhedron 2017, 134, 221–229. [Google Scholar] [CrossRef]
- Morais, C.S.; Mengarda, A.C.; Miguel, F.B.; Enes, K.B.; Rodrigues, V.C.; Espírito-Santo, M.C.C.; Siyadatpanah, A.; Wilairatana, P.; Couri, M.R.C.; de Moraes, J. Pyrazoline Derivatives as Promising Novel Antischistosomal Agents. Sci. Rep. 2021, 11, 23437. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.C.S.; Totini, C.H.; Cajás, R.A.; Teixeira, T.R.; Oliveira, E.A.; Cirino, M.E.; Souza, M.C.; Salvadori, M.C.; Teixeira, F.S.; de Moraes, J.; et al. In Vivo Antischistosomal Efficacy of Porcelia ponderosa γ-lactones. Phytomedicine 2024, 135, 156045. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Luz Filho, G.C.; Bueno-Silva, B.; de Moraes, J. Brazilian red propolis exhibits antiparasitic properties in vitro and reduces worm burden and egg production in an mouse model harboring either early or chronic Schistosoma mansoni infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef] [PubMed]
- Sessa, D.P.; Mengarda, A.C.; Simplicio, P.E.; Antar, G.M.; Lago, J.H.G.; de Moraes, J. 15β-Senecioyl-oxy-ent-kaur-16-en-19-oic Acid, a Diterpene Isolated from Baccharis lateralis, as Promising Oral Compound for the Treatment of Schistosomiasis. J. Nat. Prod. 2020, 83, 3744–3750. [Google Scholar] [CrossRef]
- Roquini, V.; Mengarda, A.C.; Cajas, R.A.; Martins-da-Silva, M.F.; Godoy-Silva, J.; Santos, G.A.; Espírito-Santo, M.C.C.; Pavani, T.F.A.; Melo, V.A.; Salvadori, M.C.; et al. The existing drug nifuroxazide as an antischistosomal agent: In vitro, in vivo, and in silico studies of macromolecular targets. Microbiol. Spectr. 2023, 11, e0139323. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Silva, M.P.; Cirino, M.E.; Morais, T.R.; Conserva, G.A.A.; Lago, J.H.G.; de Moraes, J. Licarin A, a neolignan isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), exhibited moderate preclinical efficacy against Schistosoma mansoni infection. Phytother. Res. 2021, 35, 5154–5162. [Google Scholar] [PubMed]
- Roquini, D.B.; Cogo, R.M.; Mengarda, A.C.; Mazloum, S.F.; Morais, C.S.; Xavier, R.P.; Salvadori, M.C.; Teixeira, F.S.; Ferreira, L.E.; Pinto, P.L.; et al. Promethazine exhibits antiparasitic properties in vitro and reduces worm burden, egg production, hepato- and splenomegaly in a schistosomiasis animal model. Antimicrob. Agents Chemother. 2019, 63, e01208-19. [Google Scholar] [CrossRef] [PubMed]

| Compound | S. mansoni EC50/μM | Vero EC50/μM | SI | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| polygodial (1) | 9.6 ± 0.5 | 9.3 ± 1.7 | >200 | >21 | >22 |
| 9-deoxymuzigadial (2) | NA | NA | >200 | - | - |
| praziquantel | 1.1 ± 0.7 | 1.3 ± 0.8 | >200 | >182 | >154 |
| Parameters | Polygodial (1) | Praziquantel |
|---|---|---|
| Molecular Weight (Da) | 234.33 | 312.41 |
| TPSA (Å) | 34.14 | 40.62 |
| log Po/w | 3.23 | 3.00 |
| log S | −3.20 | −3.52 |
| Gastrointestinal absorption | High | High |
| Lipinksi | Yes; 0 violation | Yes; 0 violation |
| Ghose | Yes | Yes |
| Veber | Yes | Yes |
| Egan | Yes | Yes |
| Muegge | Yes | Yes |
| Bioavaliability Score | 0.55 | 0.55 |
| PAINS | 0 alert | 0 alert |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umehara, E.; Cajas, R.A.; Conceição, G.B.; Antar, G.M.; Andricopulo, A.D.; Moraes, J.d.; Lago, J.H.G. In Vitro and In Vivo Evaluation of the Antischistosomal Activity of Polygodial and 9-Deoxymuzigadial Isolated from Drimys brasiliensis Branches. Molecules 2025, 30, 267. https://doi.org/10.3390/molecules30020267
Umehara E, Cajas RA, Conceição GB, Antar GM, Andricopulo AD, Moraes Jd, Lago JHG. In Vitro and In Vivo Evaluation of the Antischistosomal Activity of Polygodial and 9-Deoxymuzigadial Isolated from Drimys brasiliensis Branches. Molecules. 2025; 30(2):267. https://doi.org/10.3390/molecules30020267
Chicago/Turabian StyleUmehara, Eric, Rayssa A. Cajas, Gabriel B. Conceição, Guilherme M. Antar, Adriano D. Andricopulo, Josué de Moraes, and João Henrique G. Lago. 2025. "In Vitro and In Vivo Evaluation of the Antischistosomal Activity of Polygodial and 9-Deoxymuzigadial Isolated from Drimys brasiliensis Branches" Molecules 30, no. 2: 267. https://doi.org/10.3390/molecules30020267
APA StyleUmehara, E., Cajas, R. A., Conceição, G. B., Antar, G. M., Andricopulo, A. D., Moraes, J. d., & Lago, J. H. G. (2025). In Vitro and In Vivo Evaluation of the Antischistosomal Activity of Polygodial and 9-Deoxymuzigadial Isolated from Drimys brasiliensis Branches. Molecules, 30(2), 267. https://doi.org/10.3390/molecules30020267







