Guanidines Conjugated with Cell-Penetrating Peptides: A New Approach for the Development of Antileishmanial Molecules
Abstract
1. Introduction
2. Results and Discussion
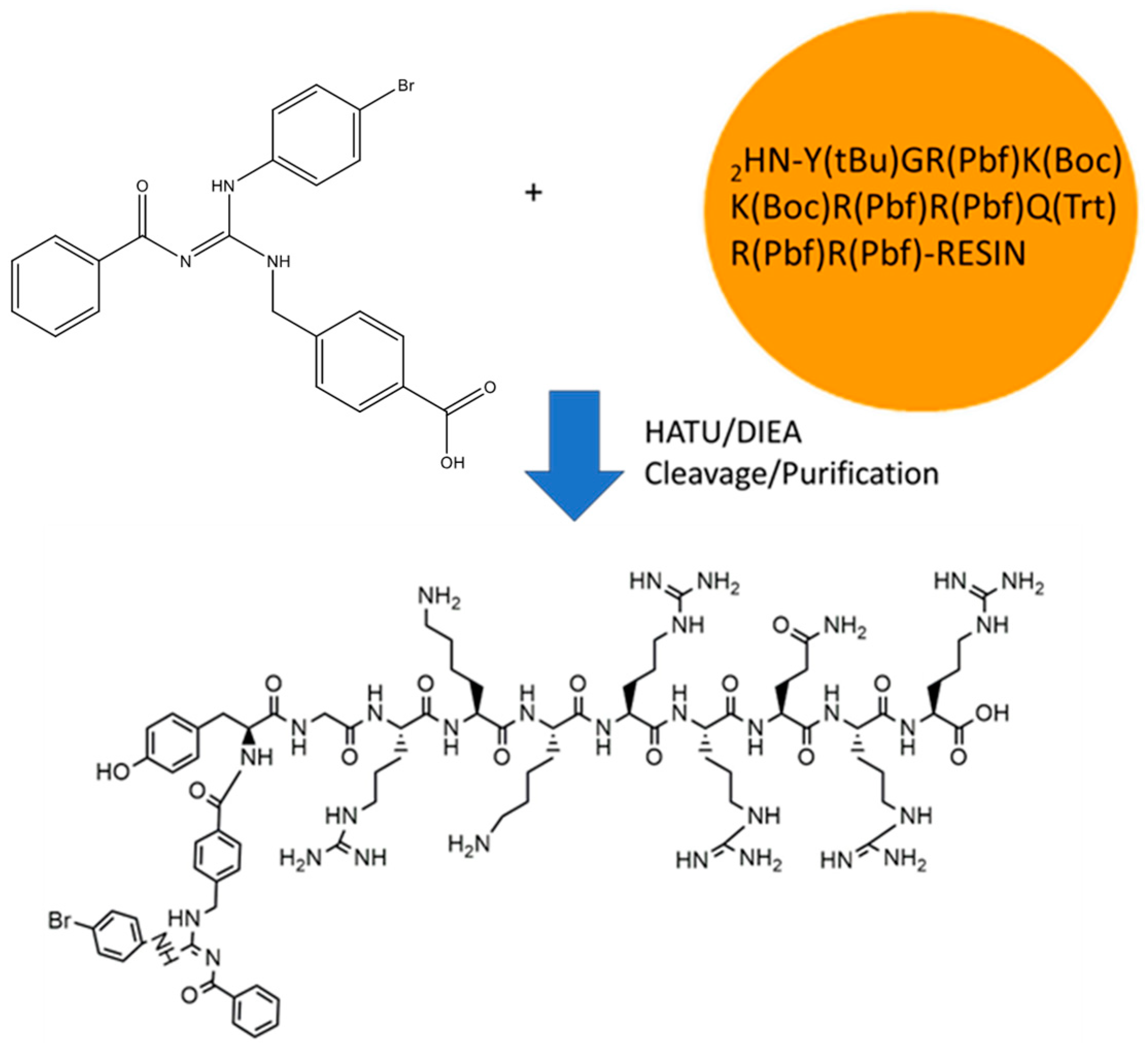
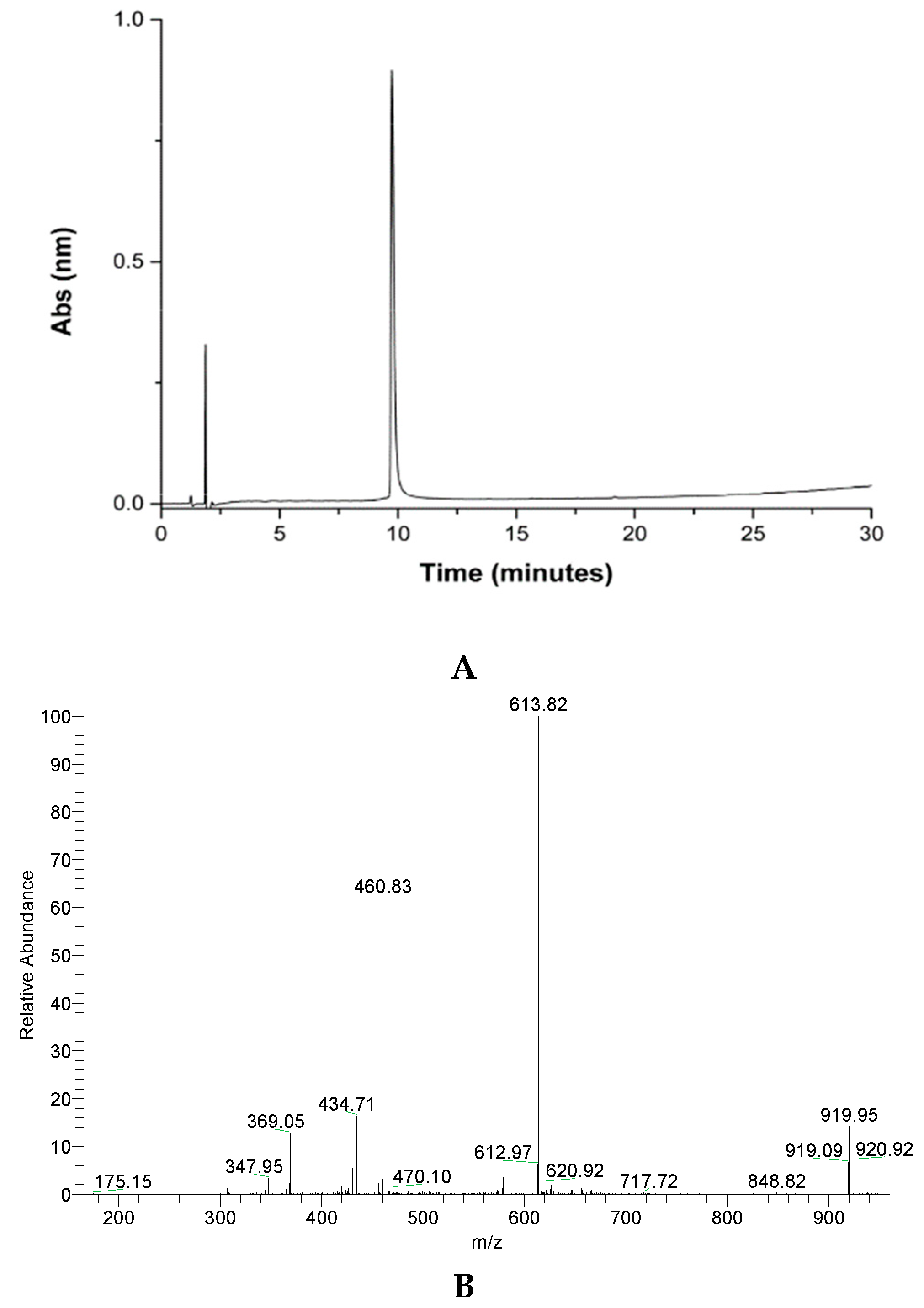
2.1. GVL1-TAT Synthesis and Characterization
2.2. Biological Assays
2.2.1. Evaluation of Leishmanicidal and Cytotoxic Activities
2.2.2. Stability Assay with Active Fetal Bovine Serum
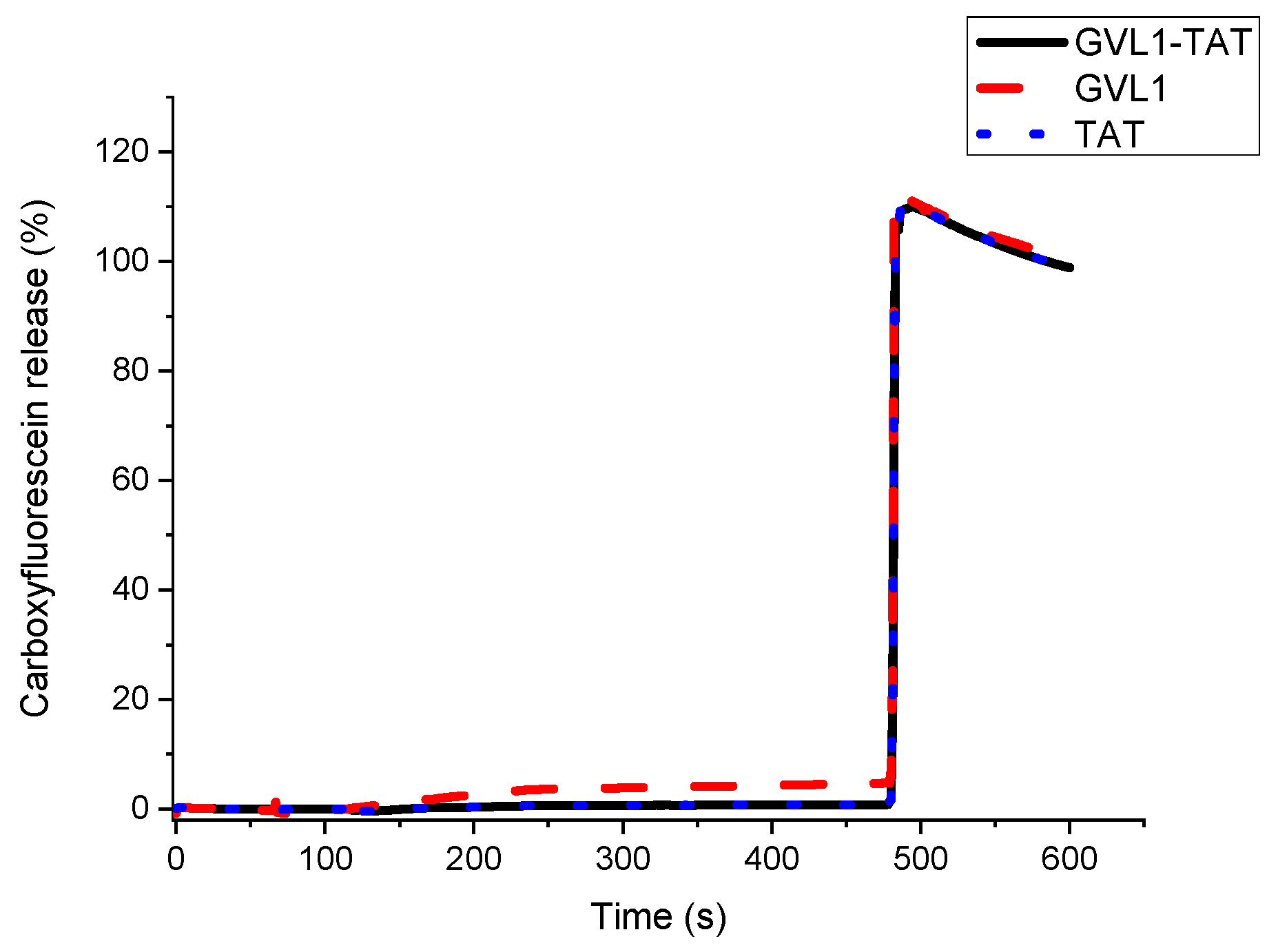
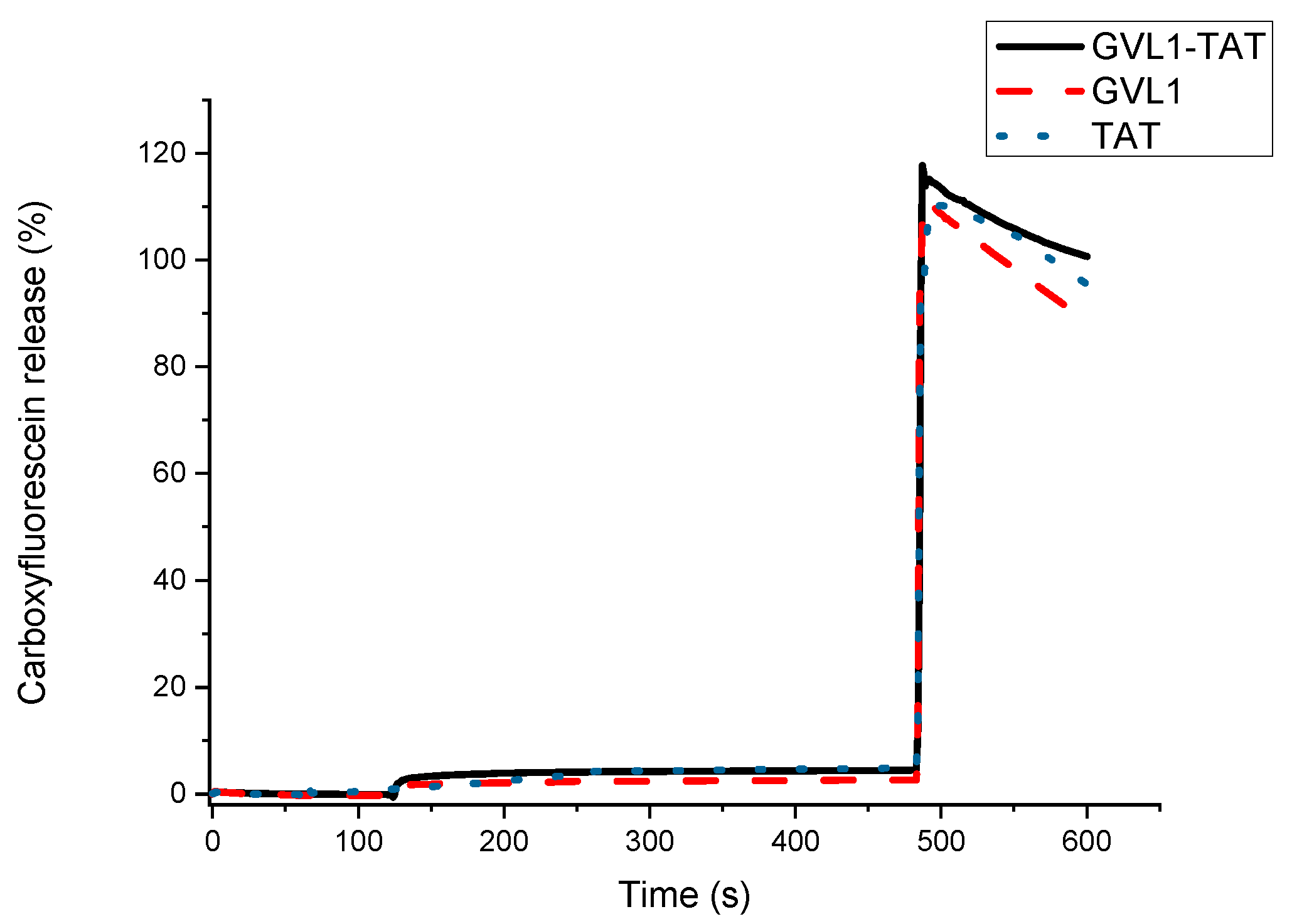
2.3. Vesicle Permeabilization
2.4. Cysteine Protease Enzyme Inhibition
2.5. Docking Studies
3. Materials and Methods
3.1. Peptide Synthesis
3.2. Biological Assays
3.3. Serum Stability
3.4. Vesicle Preparation and Permeabilization Assay
3.5. Cysteine Protease (CPB) Enzyme Inhibition Assay
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawit, G.; Girma, Z.; Simenew, K. A Review on Biology, Epidemiology and Public Health Significance of Leishmaniasis. J. Bacteriol. Parasitol. 2013, 4, 1000166. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, T.J. Visceral Leishmaniasis. Die Chir. 2019, 90, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of Amphotericin B Resistance in Clinical Isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Kopeček, J. Polymer-Drug Conjugates: Origins, Progress to Date and Future Directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef]
- Tassone, G.; Mazzorana, M.; Pozzi, C. Structural Basis of Parasitic HSP90 ATPase Inhibition by Small Molecules. Pharmaceuticals 2022, 15, 1341. [Google Scholar] [CrossRef]
- Martins, L.F.; Mesquita, J.T.; Pinto, E.G.; Costa-Silva, T.A.; Borborema, S.E.; Galisteo Junior, A.J.; Neves, B.J.; Andrade, C.H.; Shuhaib, Z.A.; Bennett, E.L.; et al. Analogues of Marine Guanidine Alkaloids are In Vitro Effective Against Trypanosoma cruzi and Selectively Eliminate Leishmania (L.) infantum Intracellular Amastigotes. J. Nat. Prod. 2016, 79, 2202–2210. [Google Scholar] [CrossRef]
- Varghese, S.; Srivastava, A.; Wong, S.W.; Le, T.; Pitcher, N.; Mesnard, M.; Lallemand, C.; Rahmani, R.; Moawad, S.R.; Huang, F.; et al. Novel Aroyl Guanidine Anti-Trypanosomal Compounds that Exert Opposing Effects on Parasite Energy Metabolism. Eur. J. Med. Chem. 2024, 268, 116162. [Google Scholar] [CrossRef]
- do Espírito Santo, R.D.; Velásquez, Á.; Passianoto, L.V.G.; Sepulveda, A.A.L.; da Costa Clementino, L.; Assis, R.P.; Baviera, A.M.; Kalaba, P.; Dos Santos, F.N.; Éberlin, M.N.; et al. N, N’, N″-Trisubstituted Guanidines: Synthesis, Characterization and Evaluation of Their Leishmanicidal Activity. Eur. J. Med. Chem. 2019, 171, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Bertonha, A.F.; Takaki, M.; Rodriguez, J.P.G. The Chemistry and Biology of Guanidine Natural Products. Nat. Prod. Rep. 2017, 34, 1264–1301. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.P.; da Silva Mela, M.F.; Anjos, L.R.D.; Saraiva, L.F.; Arenas Velásquez, A.M.; Kalaba, P.; Fabisiková, A.; Clementino, L.D.C.; Aufy, M.; Studenik, C.; et al. Novel Selective and Low-Toxic Inhibitor of LmCPB2.8ΔCTE (CPB) One Important Cysteine Protease for Leishmania Virulence. Biomolecules 2022, 12, 1903. [Google Scholar] [CrossRef]
- Rawat, A.; Roy, M.; Jyoti, A.; Kaushik, S.; Verma, K.; Srivastava, V.K. Cysteine Proteases: Battling Pathogenic Parasitic Protozoans with Omnipresent Enzymes. Microbiol. Res. 2021, 249, 126784. [Google Scholar] [CrossRef]
- Alexander, J.; Coombs, G.H.; Mottram, J.C. Leishmania mexicana Cysteine Proteinase-Deficient Mutants Have Attenuated Virulence for Mice and Potentiate a Th1 Response. J. Immunol. 1998, 161, 6794–6801. [Google Scholar] [CrossRef]
- Costa, N.C.S.; Dos Anjos, L.R.; de Souza, J.V.M.; Brasil, M.C.O.A.; Moreira, V.P.; Graminha, M.A.S.; Lubec, G.; Gonzalez, E.R.P.; Cilli, E.M. Development of New Leishmanicidal Compounds via Bioconjugation of Antimicrobial Peptides and Antileishmanial Guanidines. ACS Omega 2023, 8, 34008–34016. [Google Scholar] [CrossRef]
- Miwa, A.; Kamiya, K. Cell-Penetrating Peptide-Mediated Biomolecule Transportation in Artificial Lipid Vesicles and Living Cells. Molecules 2024, 29, 3339. [Google Scholar] [CrossRef]
- Charnay, N.; Ivanyi-Nagy, R.; Soto-Rifo, R.; Ohlmann, T.; López-Lastra, M.; Darlix, J.L. Mechanism of HIV-1 Tat RNA Translation and Its Activation by the Tat Protein. Retrovirology 2009, 6, 74. [Google Scholar] [CrossRef]
- Birch, D.; Christensen, M.V.; Staerk, D.; Franzyk, H.; Nielsen, H.M. Fluorophore Labeling of a Cell-Penetrating Peptide Induces Differential Effects on Its Cellular Distribution and Affects Cell Viability. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2483–2494. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Vunk, B.; Langel, Ü. Status Update in the Use of Cell-Penetrating Peptides for the Delivery of Macromolecular Therapeutics. Expert. Opin. Biol. Ther. 2021, 21, 361–370. [Google Scholar] [CrossRef]
- Illa, O.; Olivares, J.A.; Gaztelumendi, N.; Martínez-Castro, L.; Ospina, J.; Abengozar, M.; Sciortino, G.; Maréchal, J.D.; Nogués, C.; Royo, M.; et al. Chiral Cyclobutane-Containing Cell-Penetrating Peptides as Selective Vectors for Anti-Leishmania Drug Delivery Systems. Int. J. Mol. Sci. 2020, 21, 7502. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Hornillos, V.; Luque-Ortega, J.R.; Abengózar, M.A.; Amat-Guerri, F.; Acuña, A.U.; Rivas, L.; Andreu, D. A BODIPY-Embedding Miltefosine Analog Linked to Cell-Penetrating Tat(48-60) Peptide Favors Intracellular Delivery and Visualization of the Antiparasitic Drug. Amino Acids 2014, 46, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Recent Updates and Perspectives on Leishmaniasis. J. Infect. Dev. Ctries. 2015, 9, 588–596. [Google Scholar] [CrossRef]
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-Trypanosomatid Drug Discovery: Progress and Challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and Emerging Medications for the Treatment of Leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef]
- Sundar, S. Drug Resistance in Indian Visceral Leishmaniasis. Trop. Med. Int. Health 2001, 6, 849–854. [Google Scholar] [CrossRef]
- Qvit, N.; Rubin, S.J.S. Peptide Therapeutics: Scientific Approaches, Current Development Trends, and Future Directions. Curr. Top. Med. Chem. 2020, 20, 2903. [Google Scholar] [CrossRef]
- Raja, Z.; André, S.; Abbassi, F.; Humblot, V.; Lequin, O.; Bouceba, T.; Correia, I.; Casale, S.; Foulon, T.; Sereno, D.; et al. Insight into the Mechanism of Action of Temporin-SHa, a New Broad-Spectrum Antiparasitic and Antibacterial Agent. PLoS ONE 2017, 12, e0174024. [Google Scholar] [CrossRef]
- Valentim Silva, J.R.; de Barros, N.B.; Aragão Macedo, S.R.; Ferreira, A.D.S.; Moreira Dill, L.S.; Zanchi, F.B.; do Nascimento, J.R.; Fernandes do Nascimento, F.R.; Lourenzoni, M.R.; de Azevedo Calderon, L.; et al. A Natural Cell-Penetrating Nanopeptide Combined with Pentavalent Antimonial as Experimental Therapy Against Cutaneous Leishmaniasis. Exp. Parasitol. 2020, 217, 107934. [Google Scholar] [CrossRef]
- Cruz, G.S.; Santos, A.T.D.; Brito, E.H.S.; Rádis-Baptista, G. Cell-Penetrating Antimicrobial Peptides with Anti-Infective Activity Against Intracellular Pathogens. Antibiotics 2022, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Oualha, R.; Barhoumi, M.; Marzouki, S.; Harigua-Souiai, E.; Ben Ahmed, M.; Guizani, I. Infection of Human Neutrophils with Leishmania infantum or Leishmania major Strains Triggers Activation and Differential Cytokines Release. Front. Cell Infect. Microbiol. 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Palombi, I.R.; Lawrence, N.; White, A.M.; Gare, C.L.; Craik, D.J.; McMorran, B.J.; Malins, L.R. Development of Antiplasmodial Peptide-Drug Conjugates Using a Human Protein-Derived Cell-Penetrating Peptide with Selectivity for Infected Cells. Bioconjug. Chem. 2023, 34, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Dorlo, T.P.; Diro, E.; Costa, C.; Burza, S. The Status of Combination Therapy for Visceral Leishmaniasis: An Updated Review. Lancet Infect. Dis. 2024, 24, e36–e46. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Huang, W.; Shi, W.; Qian, H. Source and Exploration of the Peptides Used to Construct Peptide-Drug Conjugates. Eur. J. Med. Chem. 2021, 224, 113712. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Lorenzon, E.N.; Piccoli, J.P.; Santos-Filho, N.A.; Cilli, E.M. Dimerization of Antimicrobial Peptides: A Promising Strategy to Enhance Antimicrobial Peptide Activity. Protein Pept. Lett. 2019, 26, 98–107. [Google Scholar] [CrossRef]
- Santos, C.; Rodrigues, G.R.; Lima, L.F.; Dos Reis, M.C.G.; Cunha, N.B.; Dias, S.C.; Franco, O.L. Advances and Perspectives for Antimicrobial Peptide and Combinatory Therapies. Front. Bioeng. Biotechnol. 2022, 10, 1051456. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides-Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Wimley, W.C.; Hristova, K. The Mechanism of Membrane Permeabilization by Peptides: Still an Enigma. Aust. J. Chem. 2019, 73, 96–103. [Google Scholar] [CrossRef]
- Cameron, P.; McGachy, A.; Anderson, M.; Paul, A.; Coombs, G.H.; Mottram, J.C.; Alexander, J.; Plevin, R. Inhibition of Lipopolysaccharide-Induced Macrophage IL-12 Production by Leishmania mexicana Amastigotes: The Role of Cysteine Peptidases and the NF-kappaB Signaling Pathway. J. Immunol. 2004, 173, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Clementino, L.D.C.; Fernandes, G.F.S.; Prokopczyk, I.M.; Laurindo, W.C.; Toyama, D.; Motta, B.P.; Baviera, A.M.; Henrique-Silva, F.; Santos, J.L.D.; Graminha, M.A.S. Design, Synthesis and Biological Evaluation of N-Oxide Derivatives with Potent In Vivo Antileishmanial Activity. PLoS ONE 2021, 16, e0259008. [Google Scholar] [CrossRef] [PubMed]
- Mottram, J.C.; Coombs, G.H.; Alexander, J. Cysteine Peptidases as Virulence Factors of Leishmania. Curr. Opin. Microbiol. 2004, 7, 375–381. [Google Scholar] [CrossRef]
- Pollock, K.G.; McNeil, K.S.; Mottram, J.C.; Lyons, R.E.; Brewer, J.M.; Scott, P.; Coombs, G.H.; Alexander, J. The Leishmania mexicana Cysteine Protease, CPB2.8, Induces Potent Th2 Responses. J. Immunol. 2003, 170, 1746–1753. [Google Scholar] [CrossRef]
- Zaiou, M.; Nizet, V.; Gallo, R.L. Antimicrobial and Protease Inhibitory Functions of the Human Cathelicidin (hCAP18/LL-37) Prosequence. J. Invest. Dermatol. 2003, 120, 810–816. [Google Scholar] [CrossRef]
- Carneiro, P.P.; Conceição, J.; Macedo, M.; Magalhães, V.; Carvalho, E.M.; Bacellar, O. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. PLoS ONE 2016, 11, e0148084. [Google Scholar] [CrossRef]
- Wanasen, N.; Soong, L. L-Arginine Metabolism and Its Impact on Host Immunity Against Leishmania Infection. Immunol. Res. 2008, 41, 15–25. [Google Scholar] [CrossRef]
- Zabala-Peñafiel, A.; Dias-Lopes, G.; Souza-Silva, F.; Miranda, L.F.C.; Conceição-Silva, F.; Alves, C.R. Assessing the Effect of Antimony Pressure on Trypanothione Reductase Activity in Leishmania (Viannia) braziliensis. Biochimie 2023, 208, 86–92. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Lin, Y.C.; Chiang, S.C.; Lee, S.T. Divergence of Trypanothione-Dependent Tryparedoxin Cascade into Cytosolic and Mitochondrial Pathways in Arsenite-Resistant Variants of Leishmania amazonensis. Mol. Biochem. Parasitol. 2008, 157, 193–204. [Google Scholar] [CrossRef]
- Spiwoková, P.; Horn, M.; Fanfrlík, J.; Jílková, A.; Fajtová, P.; Leontovyč, A.; Houštecká, R.; Bieliková, L.; Brynda, J.; Chanová, M.; et al. Nature-Inspired Gallinamides Are Potent Antischistosomal Agents: Inhibition of the Cathepsin B1 Protease Target and Binding Mode Analysis. ACS Infect. Dis. 2024, 10, 1935–1948. [Google Scholar] [CrossRef]
- Maveyraud, L.; Mourey, L. Protein X-ray Crystallography and Drug Discovery. Molecules 2020, 25, 1030. [Google Scholar] [CrossRef] [PubMed]
- Ros-Lucas, A.; Martinez-Peinado, N.; Bastida, J.; Gascón, J.; Alonso-Padilla, J. The Use of AlphaFold for In Silico Exploration of Drug Targets in the Parasite Trypanosoma cruzi. Front. Cell Infect. Microbiol. 2022, 12, 944748. [Google Scholar] [CrossRef]
- Lasakosvitsch, F.; Gentil, L.G.; dos Santos, M.R.; da Silveira, J.F.; Barbiéri, C.L. Cloning and Characterisation of a Cysteine Proteinase Gene Expressed in Amastigotes of Leishmania (L.) amazonensis. Int. J. Parasitol. 2003, 33, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Possart, K.; Herrmann, F.C.; Jose, J.; Schmidt, T.J. In Silico and In Vitro Search for Dual Inhibitors of the Trypanosoma brucei and Leishmania major Pteridine Reductase 1 and Dihydrofolate Reductase. Molecules 2023, 28, 7526. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.; Sarma, M.; Kanaujia, S.P.; Dubey, V.K. Dual-Target Drugs Against Leishmania donovani for Potential Novel Therapeutics. Sci. Rep. 2023, 13, 18363. [Google Scholar] [CrossRef]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs Against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef]
- Fiorillo, A.; Colotti, G.; Exertier, C.; Liuzzi, A.; Seghetti, F.; Salerno, A.; Caciolla, J.; Ilari, A. Innovative Approach for a Classic Target: Fragment Screening on Trypanothione Reductase Reveals New Opportunities for Drug Design. Front. Mol. Biosci. 2022, 9, 900882. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid-Phase Peptide Synthesis. 3. An Improved Synthesis of Bradykinin. Biochemistry 1964, 3, 1385–1390. [Google Scholar] [CrossRef]
- Velásquez, A.M.A.; Ribeiro, W.C.; Venn, V.; Castelli, S.; Camargo, M.S.; de Assis, R.P.; de Souza, R.A.; Ribeiro, A.R.; Passalacqua, T.G.; da Rosa, J.A.; et al. Efficacy of a Binuclear Cyclopalladated Compound Therapy for Cutaneous Leishmaniasis in the Murine Model of Infection with Leishmania amazonensis and Its Inhibitory Effect on Topoisomerase 1B. Antimicrob. Agents Chemother. 2017, 61, e00688-17. [Google Scholar] [CrossRef]
- de Almeida, L.; Passalacqua, T.G.; Dutra, L.A.; Fonseca, J.N.V.D.; Nascimento, R.F.Q.; Imamura, K.B.; de Andrade, C.R.; Dos Santos, J.L.; Graminha, M.A.S. In Vivo Antileishmanial Activity and histopathological Evaluation in Leishmania infantum Infected Hamsters After Treatment with a Furoxan Derivative. Biomed. Pharmacother. 2017, 95, 536–547. [Google Scholar] [CrossRef]
- Velásquez, A.M.A.; Bartlett, P.J.; Linares, I.A.P.; Passalacqua, T.G.; Teodoro, D.D.L.; Imamura, K.B.; Virgilio, S.; Tosi, L.R.O.; Leite, A.L.; Buzalaf, M.A.R.; et al. New Insights into the Mechanism of Action of the Cyclopalladated Complex (CP2) in Leishmania: Calcium Dysregulation, Mitochondrial Dysfunction, and Cell Death. Antimicrob. Agents Chemother. 2022, 66, e0076721. [Google Scholar] [CrossRef] [PubMed]
- Lorenzón, E.N.; Piccoli, J.P.; Cilli, E.M. Interaction Between the Antimicrobial Peptide Aurein 1.2 Dimer and Mannans. Amino Acids 2014, 46, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Coudert, E.; Gehant, S.; de Castro, E.; Pozzato, M.; Baratin, D.; Neto, T.; Sigrist, C.J.A.; Redaschi, N.; Bridge, A.; Consortium, U. Annotation of Biologically Relevant Ligands in UniProtKB Using ChEBI. Bioinformatics 2023, 39, btac793. [Google Scholar] [CrossRef]
- Schöning-Stierand, K.; Diedrich, K.; Ehrt, C.; Flachsenberg, F.; Graef, J.; Sieg, J.; Penner, P.; Poppinga, M.; Ungethüm, A.; Rarey, M. ProteinsPlus: A Comprehensive Collection of Web-Based Molecular Modeling Tools. Nucleic Acids Res. 2022, 50, 611–615. [Google Scholar] [CrossRef]
- Graef, J.; Ehrt, C.; Rarey, M. Binding Site Detection Remastered: Enabling Fast, Robust, and Reliable Binding Site Detection and Descriptor Calculation with DoGSite3. J. Chem. Inf. Model. 2023, 63, 3128–3137. [Google Scholar] [CrossRef]
- Volkamer, A.; Griewel, A.; Grombacher, T.; Rarey, M. Analyzing the Topology of Active Sites: On the Prediction of Pockets and Subpockets. J. Chem. Inf. Model. 2010, 50, 2041–2052. [Google Scholar] [CrossRef]
- Company C. Bio Molegro Virtual Docker-User Manual; CLC Bio Company: Aarhus, Denmark, 2013. [Google Scholar]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]

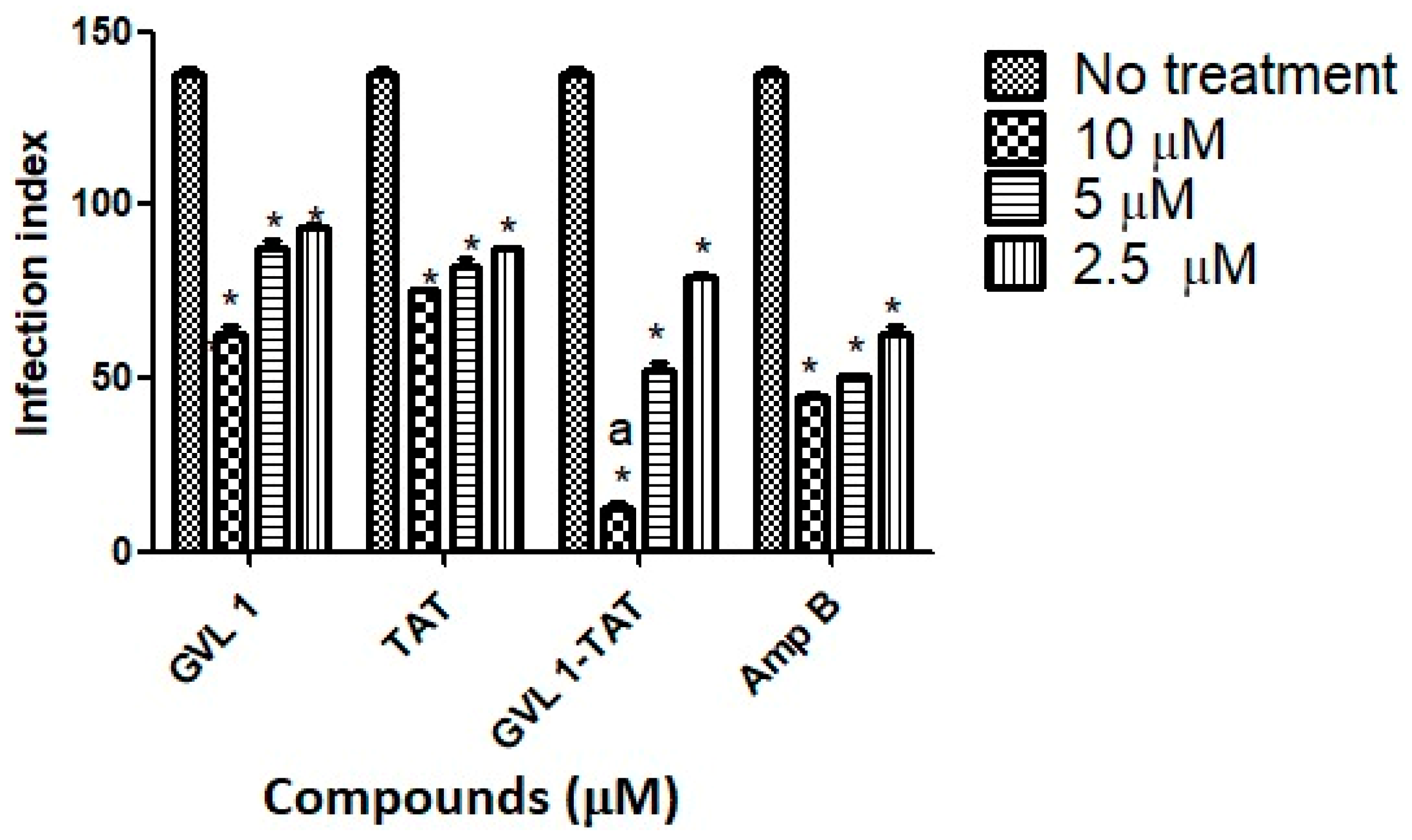
| Compound | Promastigote IC50 (μM) | Peritoneal Macrophages CC50 (μM) | Amastigote IC50 (μM) | SI |
|---|---|---|---|---|
| TAT | NA | >1000 ± 0.15 | NA | NA |
| GVL1-TAT | 5.32 ± 0.74 | >1000 ± 0.65 | 0.80 ± 0.06 | >1250 |
| GVL1 | 51.8 ± 0.10 | >1000 ± 0.013 | 7.5 ± 3.53 | >267 |
| Amp B | 3.3 ± 0.2 | 99.8 ± 3.7 | 0.75 ± 0.1 | >131 |
| Compound | Promastigote IC50 (μM) | Peritoneal Macrophages CC50 (μM) | Amastigote IC50 (μM) | SI |
|---|---|---|---|---|
| TAT | NA | >2000 ± 0.01 | NA | NA |
| GVL1-TAT | 1.2 ± 0.3 | >2000 ± 0.65 | 3.8 ± 0.3 | >526 |
| GVL1 | 50.8 ± 2.2 | >2000 ± 0.01 | 9.5 ± 2.5 | >210 |
| Amp B | 5.4 ± 1.4 | 24 ± 0.1 | 1.25 ± 0.5 | 19 |
| Compounds | Promastigote IC50 (µM) L. amazonensis | Promastigote IC50 (µM) L. infantum |
|---|---|---|
| GVL1 | 65 ± 2.4 | 50 ± 1.2 |
| TAT | NA | NA |
| GVL1-TAT | 2.6 ± 0.8 | 1.2 ± 0.3 |
| Amp B | 0.5 ± 0.1 | 0.3 ± 0.1 |
| Compound | CPB Inhibitory Activity 20 μM (%) | IC50 (μmol L−1) |
|---|---|---|
| TAT | 9.42 ± 0.01 | - |
| GVL1-TAT | 91.97 ± 1.70 | 3.78 ± 0.1 |
| GVL1 | 13.98 ± 2.8 | - |
| Compounds | Cysteine Protease | Trypanothione Reductase |
|---|---|---|
| GVL1 | −98.136 | −151.151 |
| GVL1-TAT | −173.934 | −188.683 |
| TAT | −136.364 | −179.726 |
| Amp B | −195.256 | −242.512 |
| Molecules | Hydrogens Bonds | Hydrophobic Interactions |
|---|---|---|
| GVL1 | - | Ala 100, Lys 101, Leu 104, Asn 105, Pro 106, Asp 107, Tyr 108, Ser 111, Glu 189, Asn 192 |
| TAT | - | Leu 149, Gln 179, Asn 192, Tyr 217, Thr 220, Gly 223, Gly 224, Thr 225, Pro 227, His 230 |
| GVL1-TAT | Ala 100, Lys 101, Leu 102, Tyr 103, Leu 104, Asn 105, Asp 107, Tyr 108, Lys 145, Gln 147, Gly 148, Leu 149, Cys 150, Cys 153, Phe 156, Gly 160, Glu 189, Cys 191, Asn 192, Asn 288, His 289, Gly 290, Val 291 | Lys 90, Phe 91, Leu 94, Pro 106, Tyr 109, Ser 111, His 112, Pro 143, Val 144, Gly 151, Ser 157, Ile 159, Asn 161, Glu 178, Gly 193, Gly 194, Val 262, Ala 263, Val 264, Leu 292, Lys 308, Asn 309, Trp 311, Gly 312 |
| Amp B 1 | Lys 101, Leu 104, Asn 105, Pro 106, Asp 107, tyr 109, Thr 110, Gly 151, Cys 191, Asn 192, Gly 194, Thr 269, Ser 286, Asn 288 | Pro 96, Ala 100, Leu 102, Tyr 103, Tyr 108, Leu 149, Gly 193, Leu 287, His 289 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, J.V.M.; Costa, N.C.S.; Brasil, M.C.O.A.; dos Anjos, L.R.; de Menezes, R.P.B.; Zampieri, E.H.; de Lima, J.S.; Velasquez, A.M.A.; Scotti, L.; Scotti, M.T.; et al. Guanidines Conjugated with Cell-Penetrating Peptides: A New Approach for the Development of Antileishmanial Molecules. Molecules 2025, 30, 264. https://doi.org/10.3390/molecules30020264
de Souza JVM, Costa NCS, Brasil MCOA, dos Anjos LR, de Menezes RPB, Zampieri EH, de Lima JS, Velasquez AMA, Scotti L, Scotti MT, et al. Guanidines Conjugated with Cell-Penetrating Peptides: A New Approach for the Development of Antileishmanial Molecules. Molecules. 2025; 30(2):264. https://doi.org/10.3390/molecules30020264
Chicago/Turabian Stylede Souza, João Victor Marcelino, Natalia C. S. Costa, Maria C. O. Arruda Brasil, Luana Ribeiro dos Anjos, Renata Priscila Barros de Menezes, Eduardo Henrique Zampieri, Jhonatan Santos de Lima, Angela Maria Arenas Velasquez, Luciana Scotti, Marcus Tullius Scotti, and et al. 2025. "Guanidines Conjugated with Cell-Penetrating Peptides: A New Approach for the Development of Antileishmanial Molecules" Molecules 30, no. 2: 264. https://doi.org/10.3390/molecules30020264
APA Stylede Souza, J. V. M., Costa, N. C. S., Brasil, M. C. O. A., dos Anjos, L. R., de Menezes, R. P. B., Zampieri, E. H., de Lima, J. S., Velasquez, A. M. A., Scotti, L., Scotti, M. T., Graminha, M. A. S., Gonzalez, E. R. P., & Cilli, E. M. (2025). Guanidines Conjugated with Cell-Penetrating Peptides: A New Approach for the Development of Antileishmanial Molecules. Molecules, 30(2), 264. https://doi.org/10.3390/molecules30020264











