Physicochemical Characterization and Asymmetric Catalytic Properties of New Biobased Organocatalytic Surfactants
Abstract
1. Introduction
2. Results and Discussion
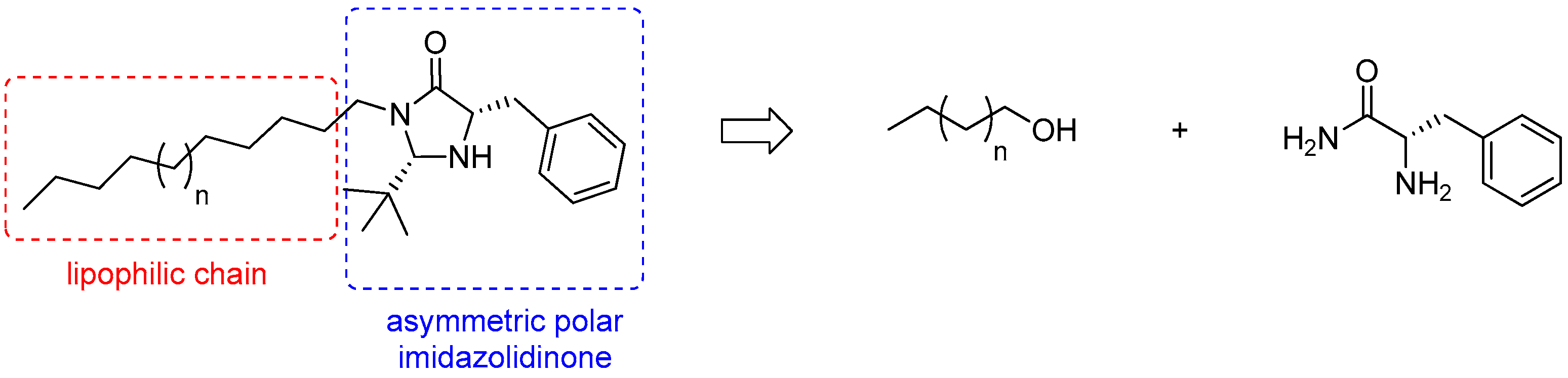
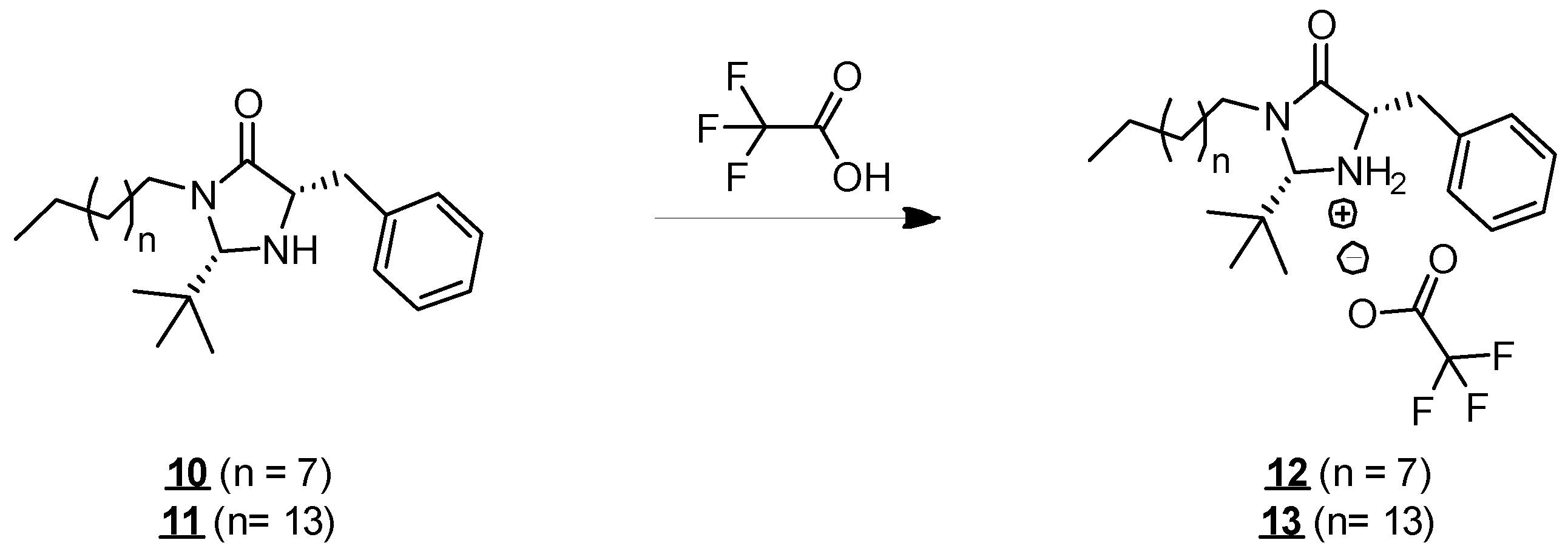
2.1. Synthesis
2.2. Solubility in Water
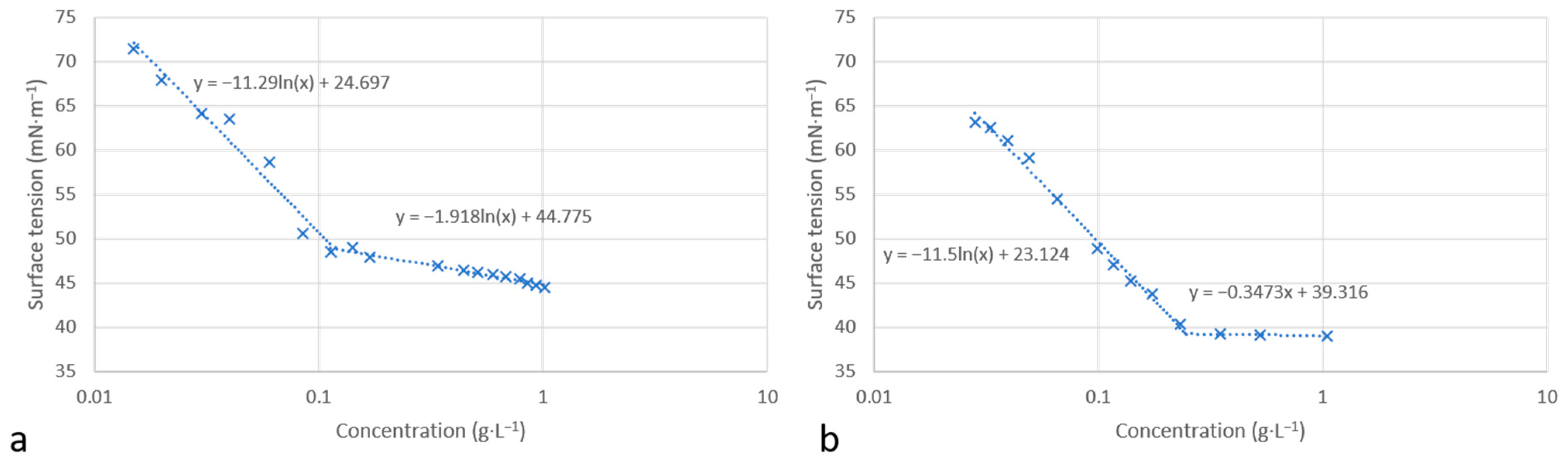
2.3. Surface Tension
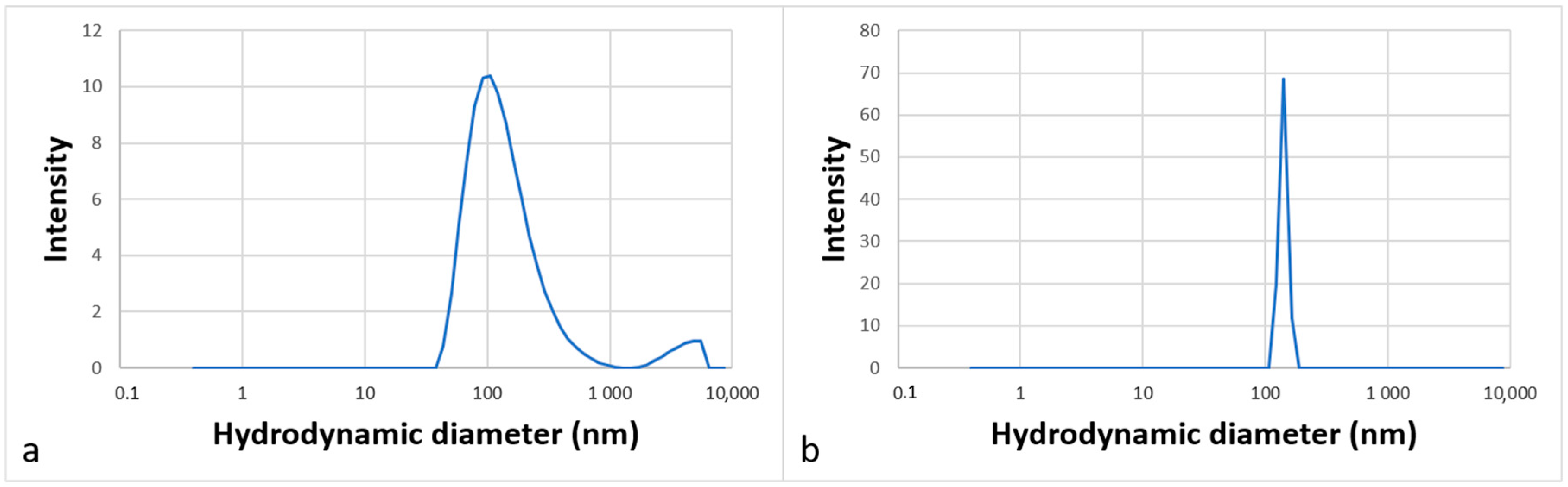
2.4. Aggregation Behavior in Water
2.5. Asymmetric Catalytic Activity of Compounds 12 and 13 in Water
3. Materials and Methods
3.1. Experimental Apparatus
3.2. Synthesis Experimental Procedure
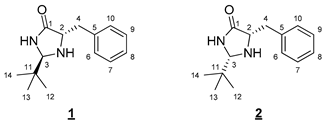
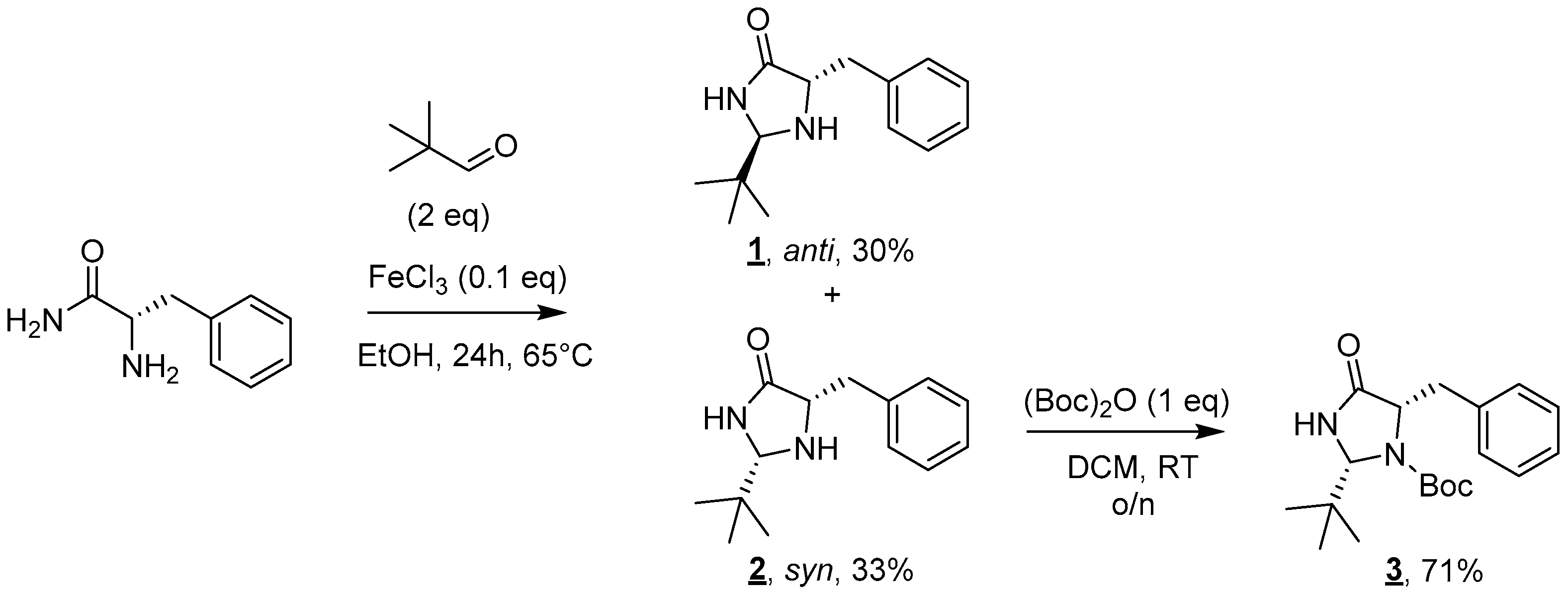
- (2R,5S)-5-Benzyl-2-(tert-butyl)imidazolidin-4-one (1) and (2S,5S)-5-benzyl-2-(tert-butyl)imidazolidin-4-one (2)
- Anti 1 diastereoisomer (Rf 0.40 (cyclohexane/EtOAc 5:5 (v/v)): 1H NMR (300 MHz, CDCl3) δ 7.40 (br s, 1H, NH), 7.35–7.21 (m, 5H, H6–10), 4.03 (d, J = 1.9 Hz, 1H, H3), 3.80 (ddd, J = 6.6, 4.4, 1.9 Hz, 1H, H2), 3.10 (dd, J = 14.1, 4.4 Hz, 1H, H4), 2.94 (dd, J = 14.1, 6.6 Hz, 1H, H4), 0.86 (s, 9H, H12–14). 13C NMR (75 MHz, CDCl3) δ 177.8 (C1), 137.2 (C5), 129.5 (C6, C 10), 128.7 (C7, C9), 126.8 (C8), 77.8 (C3), 60.0 (C2), 38.0 (C4), 36.1 (C11), 24.2 (s, C12–14). IR (KBr): 3357, 3196, 2967, 2866, 1688, 1454, 1376, 756, 700 cm−1. HRMS (DCI-CH4) Calculated for C14H21N2O 233.1654 [M]+, found 233.1642.
- Syn 2 diastereoisomer (Rf 0.15 (cyclohexane/EtOAc 5:5 (v/v)): 1H NMR (300 MHz, CDCl3) δ 7.81 (br s, 1H, NH), 7.36–7.17 (m, 5H, H6–10) 4.25 (d, J = 1.5 Hz, 1H, H3), 3.81 (ddd, J = 7.5, 4.0, 1.5 Hz, 1H, H2), 3.12 (dd, J = 13.7, 4.0 Hz, 1H, H4), 2.92 (dd, J = 13.7, 7.5 Hz, 1H, H4), 0.80 (s, 9H, H12–14). 13C NMR (75 MHz, CDCl3) δ 177.6 (C1), 137.9 (C5), 129.6 (C6, C10), 128.6 (C7, C9), 126.7 (C8), 77.3 (C3), 60.3 (C2), 37.7 (C4), 34.0 (C11), 24.3 (C12–14). IR (KBr): 3342, 3229, 2955, 2865, 1705, 1454, 1341, 730, 699 cm−1. HRMS (DCI-CH4): Calculated for C14H21N2O 233.1654 [M]+, found 233.1650.
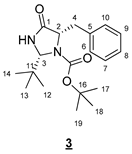
- tert-Butyl (2R,5S)-5-benzyl-2-(tert-butyl)-4-oxoimidazolidine-1-carboxylate (3)
- Rf 0.40 (cyclohexane/EtOAc 6:4 (v/v)). 1H NMR (300 MHz, CDCl3) δ 7.35–7.20 (m, 5H, H6–10), 6.68 (br s, 1H, NH), 5.02 (s, 1H, H3), 4.34 (t, J = 6.7 Hz, 1H, H2), 3.17 (dd, J = 13.8, 6.7 Hz, 1H, H4), 3.03 (dd, J = 13.8, 6.7 Hz, 1H, H4), 1.30 (s, 9H, H17–19), 0.98 (s, 9H, H12–14). 13C NMR (75 MHz, CDCl3) δ 173.7 (C1), 156.1 (C15), 138.1 (C5), 129.7 (C6, C10), 128.3 (C7, C9), 126.5 (C8), 81.3 (C16), 77.5 (C3), 61.6 (C2), 39.6 (C4), 36.7 (C3), 28.0 (C17–19), 25.8 (C12–14). IR (KBr): 3294, 2975, 1721, 1709, 1371, 1357, 1304, 1167, 1076, 953, 805, 780, 749, 737, 702 cm−1. HRMS (DCI-CH4) Calculated for C19H29N2O3 333.2178 [M+H]+, found 333.2174.
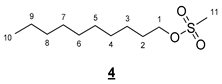
- Decyl methanesulfonate (4)
- 1H NMR (300 MHz, CDCl3) δ 4.22 (t, J = 6.6 Hz, 2H, H1), 3.00 (s, 3H, H11), 1.86–1.65 (m, 2H, H2), 1.46–1.16 (m, 14H, H3–9), 0.88 (t, J = 6.6 Hz, 3H, H10). IR (KBr): 2929, 2856, 1467, 1354, 1177, 975, 836, 746, 722 cm−1.

- Hexadecyl methanesulfonate (5)
- 1H NMR (300 MHz, CDCl3) δ 4.22 (t, J = 6.6 Hz, 2H, H1), 3.00 (s, 3H, H17), 1.86–1.64 (m, 2H, H2), 1.46–1.16 (m, 26H, H3–15), 0.88 (t, J = 6.7 Hz, 3H, H16). IR (KBr): 2918, 2851, 1474, 1344, 1328, 1168, 1160, 984, 942, 851, 751, 718 cm−1.
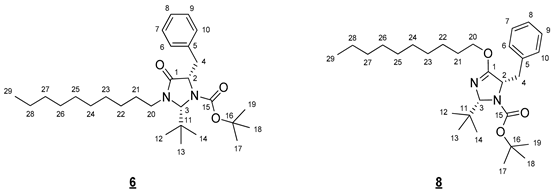
- tert-Butyl (2R,5S)-5-benzyl-2-(tert-butyl)-4-(decyloxy)-2,5-dihydro-1H-imidazole-1-carboxylate (6) et tert-butyl (2R,5S)-5-benzyl-2-(tert-butyl)-3-decyl-4-oxoimidazolidine-1-carboxylate (8)
- Compound 6 (Rf 0.19 (cyclohexane/AcOEt 9:1 (v/v))): 1H NMR (300 MHz, CDCl3) δ 7.36–7.17 (m, 5H, H6–10), 5.12 (s, 1H, H3), 4.32 (dd, J = 7.2, 6.2 Hz, 1H, H2), 3.92 (ddd, J = 14.1, 9.3, 7.2 Hz, 1H, H20), 3.20 (dd, J = 14.1, 6.2 Hz, 1H, H4), 3.03 (ddd, J = 9.3, 7.2, 3.4 Hz, 1H, H20), 2.98 (dd, J = 13.8, 7.2 Hz, 1H, H4), 1.35–1.22 (m, 23H, H22–28, H17–19), 1.06 (s, 9H, H12–14), 0.87 (t, J = 6.7 Hz, 3H, H29). 13C NMR (75 MHz, CDCl3) δ 171.5 (C1), 156.0 (C15), 138.3 (C5), 129.7 (C6, C10), 128.4 (C7, C9), 126.5 (C8), 81.1 (C16), 79.2 (C3), 61.9 (C2), 42.8 (C20), 39.8 (C4), 37.4 (C11), 31.9 (CH2), 29.5 (CH2), 29.5 (CH2), 29.3 (CH2), 29.2 (CH2), 28.0 (C17–19), 27.0 (C12–14), 26.7 (CH2), 26.5 (C21), 22.7 (CH2), 14.1 (C29). IR (KBr) 2927, 2856, 1708, 1366, 1167, 786, 751, 699 cm−1. HRMS (DCI-CH4) Calculated for C29H49N2O3 473.3743 [M+H]+, found 473.3761.
- Compound 8 (Rf 0.35 (cyclohexane/AcOEt 9:1 (v/v))): 1H NMR (300 MHz, CDCl3) δ 7.33–7.14 (m, 5H, H6–10), 5.19 (s, 1H, H3), 4.52 (dd, J = 7.8, 5.7 Hz, 1H, H2), 4.24 (dt, J = 10.5, 6.5 Hz, 1H, H20), 4.03 (dt, J = 10.5, 6.6 Hz, 1H, H20), 3.07 (dd, J = 13.6, 5.7 Hz, 1H, H4), 2.93 (dd, J = 13.6, 7.8 Hz, 1H, H4), 1.68–1.54 (m, 2H, H21), 1.36 (s, 9H, H17–19), 1.32–1.22 (m, 14H, H22–28), 0.98 (s, 9H, H12–14), 0.88 (t, J = 6.7 Hz, 3H, H29). 13C NMR (75 MHz, CDCl3) δ 167.6 (C1), 156.1 (C15), 138.5 (C5), 129.6 (C6, C10), 128.2 (C7, C9), 126.3 (C8), 90.1 (C3), 80.3 (C16), 68.3 (C20), 63.9 (C2), 40.2 (C4), 36.6 (C11), 31.9 (CH2), 29.6 (CH2), 29.5 (CH2), 29.3 (CH2), 29.3 (CH2), 28.7 (C21), 28.3 (C17–19), 26.7 (C12–14), 25.9 (CH2), 22.7 (CH2), 14.1 (C29). IR (KBr) 3416, 2927, 2856, 1712, 1667, 1366, 1331, 1163, 786, 745, 699 cm−1. HRMS (DCI-CH4) Calculated for C29H49N2O3 473.3743 [M+H]+, found 473.3751.
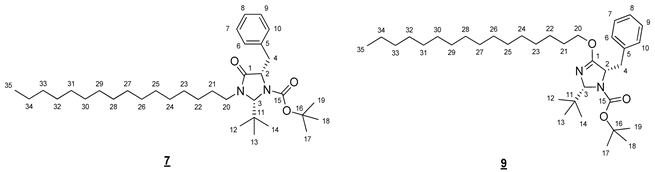
- tert-Butyl (2R,5S)-5-benzyl-2-(tert-butyl)-4-(hexadecyloxy)-2,5-dihydro-1H-imidazole-1-carboxylate (7) and tert-butyl (2R,5S)-5-benzyl-2-(tert-butyl)-3-hexadecyl-4-oxoimidazolidine-1-carboxylate (9)
- Compound 7 (Rf 0.17 (cyclohexane/AcOEt 9:1 (v/v))): 1H NMR (300 MHz, CDCl3) δ 7.36–7.14 (m, 5H, H6–10), 5.12 (s, 1H, H3), 4.39–4.27 (m, 1H, H2), 3.92 (ddd, J = 14.2, 9.3, 7.3 Hz, 1H, H20), 3.20 (dd, J = 13.8, 6.0 Hz, 1H, H4), 3.03 (ddd, J = 9.3, 6.7, 3.4 Hz, 1H, H20), 2.98 (dd, J = 13.8, 7.2 Hz, 1H, H4), 1.67 (m, 2H, H21), 1.38–1.15 (m, 26H, H22–34), 1.26 (s, 9H, H17–19) 1.06 (s, 9H, H12–14), 0.88 (t, J = 6.7 Hz, 3H, H35). 13C NMR (75 MHz, CDCl3) δ 171.5 (C1), 156.0 (C15), 138.3 (C5), 129.7 (C6, C10), 128.3 (C7, C9), 126.5 (C8), 81.1 (C16), 79.2 (C3), 61.9 (C2), 42.7 (C20), 39.8 (C4), 37.4 (C11), 31.9 (CH2), 29.7 (3 * CH2), 29.7 (2 * CH2), 29.6 (CH2), 29.5 (2 * CH2), 29.4 (CH2), 29.2 (CH2), 28,0 (C17–19), 27.0 (C12–14), 26.7 (C22), 26.5 (C21), 22.7 (CH2), 14.1 (C35). IR (KBr) 2926, 2854, 1708, 1367, 1167, 786, 750, 698 cm−1. HRMS (DCI-CH4) Calculated for C35H61N2O3 557.4682 [M+H]+, found 557.4688.
- Compound 9 (Rf 0.38 (cyclohexane/AcOEt 9:1 (v/v))): 1H NMR (300 MHz, CDCl3) δ 7.35–7.13 (m, 5H, H6–10), 5.19 (s, 1H, H3), 4.53 (dd, J = 7.7, 5.8 Hz, 1H, H2), 4.24 (dt, J = 10.5, 6.5 Hz, 1H, H20), 4.03 (dt, J = 10.5, 6.6 Hz, 1H, H20), 3.07 (dd, J = 13.6, 5.6 Hz, 1H, H4), 2.93 (dd, J = 13.6, 7.9 Hz, 1H, H4), 1.68–1.55 (m, 2H, H21), 1.36 (s, 9H, H17–19), 1.32–1.20 (m, 26H, H22–34), 0.98 (s, 9H, H12–14), 0.88 (t, J = 6.7 Hz, 3H, H35). 13C NMR (75 MHz, CDCl3) δ 167.6 (C1), 156.1 (C15), 138.5 (C5), 129.6 (C6, C10), 128.1 (C7, C9), 126.3 (C8), 90.1 (C3), 80.2 (C16), 68.3 (C20), 63.9 (C2), 40.2 (C4), 36.6 (C11), 32.0 (C33), 29.7 (4 * CH2), 29.7 (2 * CH2), 29.6 (CH2), 29.6 (CH2), 29.4 (CH2), 29.3 (CH2), 28.7 (C21), 28.3 (C17–19), 26.7 (C12–14), 25.9 (C22), 22.7 (CH2), 14.1 (C35). IR (KBr) 2925, 2855, 1712, 1666, 1365, 1331, 1175, 786, 745, 698 cm−1. HRMS (DCI-CH4) Calculated for C35H61N2O3 557.4682 [M+H]+, found 557.4700.

- (2S,5S)-5-benzyl-2-(tert-butyl)-3-decylimidazolidin-4-one (10)
- Rf 0.33 (cyclohexane/AcOEt 7:3 (v/v)). 1H NMR (500 MHz, CDCl3) δ 7.31–7.19 (m, 5H, H6–10), 4.23 (s, 1H, H3), 3.77 (ddd, J = 14.0, 9.7, 7.0 Hz, 1H, H15), 3.72–3.65 (m, 1H, H2), 3.15 (dd, J = 13.7, 3.9 Hz, 1H, H4), 3.05 (ddd, J = 14.0, 9.4, 4.6 Hz, 1H, H15), 2.91 (dd, J = 13.7, 7.7 Hz, 1H, H4), 1.63–1.54 (m, 1H, H16), 1.52–1.44 (m, 1H, H16), 1.34–1.20 (m, 14H, H17–23), 0.88 (t, J = 7.0 Hz, 3H, H24), 0.84 (s, 9H, H12–14). 13C NMR (126 MHz, CDCl3) δ 175.3 (C1), 138.0 (C5), 129.6 (C6, C10), 128.5 (C7, C9), 126.6 (C8), 79.6 (C3), 59.3 (C2), 42.6 (C15), 38.2 (C4), 35.4 (C11), 31.9 (CH2), 29.6 (CH2), 29.5 (CH2), 29.3 (2 * CH2), 27.0 (CH2), 26.9 (C16), 25.5 (C12–14), 22.7 (CH2), 14.1 (C24). IR (KBr) 2927, 2855, 1698, 1455, 751, 721, 700 cm−1. HRMS (DCI-CH4) Calculated for C24H41N2O 373.3219 [M+H]+, found 373.3213.

- (2S,5S)-5-benzyl-2-(tert-butyl)-3-hexadecylimidazolidin-4-one (11)
- Rf 0.37 (cyclohexane/AcOEt 7:3 (v/v)). 1H NMR (500 MHz, CDCl3) δ 7.31–7.19 (m, 5H, H6–10), 4.23 (s, 1H, H3), 3.77 (ddd, J = 14.1, 9.7, 7.1 Hz, 1H, H15), 3.72–3.65 (m, 1H, H2), 3.15 (dd, J = 13.7, 3.9 Hz, 1H, H4), 3.05 (ddd, J = 14.1, 9.3, 4.5 Hz, 1H, H15), 2.91 (dd, J = 13.7, 7.7 Hz, 1H, H4), 1.63–1.54 (m, 1H, H16), 1.51–1.44 (m, 1H, H16), 1.34–1.19 (s, 26H, H17–29), 0.88 (t, J = 6.9 Hz, 3H, H30), 0.84 (s, 9H, H12–14). 13C NMR (126 MHz, CDCl3) δ 175.3 (C1), 138.1 (C5), 129.6 (C6, C10), 128.5 (C7, C9), 126.6 (C8), 79.6 (C3), 59.3 (C2), 42.6 (C15), 38.3 (C4), 35.4 (C11), 31.9 (CH2), 29.7 (3 * CH2), 29.7 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.6 (CH2), 29.4 (CH2), 29.3 (CH2), 27.0 (CH2), 26.9 (C16), 25.5 (C12–14), 22.7 (CH2), 14.1 (C30). IR (KBr) 2926, 2854, 1699, 1455, 699 cm−1. HRMS (DCI-CH4) Calculated for C30H53N2O 457.4158 [M+H]+, found 457.4177.
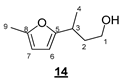
- 3-(5-Methylfuran-2-yl)butan-1-ol (14)
- Rf 0.37 (cyclohexane/AcOEt 7:3 (v/v)). 1H NMR (500 MHz, CDCl3) δ 5.86 (d, J = 3.0 Hz, 1H, H6), 5.84 (d, J = 3.0 Hz, 1H, H7), 3.71–3.61 (m, 2H, H1), 2.94 (m, 1H, H3), 2.25 (s, 3H, H9), 1.89 (ddt, J = 14.0, 8.0, 6.2 Hz, 1H, H2), 1.77 (td, J = 14.0, 6.6 Hz, 1H, H2), 1.25 (d, J = 7.0 Hz, 3H, H4). 13C NMR (126 MHz, CDCl3) δ 158.1 (C5), 150.3 (C8), 105.6 (C6), 104.3 (C7), 61.0 (C1), 38.8 (C2), 29.9 (C3), 19.4 (C4), 13.5 (C9). HRMS (DCI-CH4) Calculated for C9H15O2 155.1072 [M+H]+, found 155.1073.
3.3. Surface Tension Analysis
3.4. Dynamic Light Scattering Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pace, V.; Hoyos, P.; Castoldi, L.; Dominguez De Maria, P.; Alcantara, A. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Lelais, G.; MacMillan, D. History and Perspective of Chiral Organic Catalysts; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- La Sorella, G.; Strukul, G.; Scarso, A. Recent advances in catalysis in micellar media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Aratake, S.; Itoh, T.; Okano, T.; Nagae, N.; Sumiya, T.; Shoi, M.; Hayashi, Y. Highly Diastereo- and Enantioselective Direct Aldol Reactions of Aldehydes and Ketones Catalyzed by Siloxyproline in the Presence of Water. Chem. A Eur. J. 2007, 13, 10246–10256. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Guo, C.-S.; Xie, J.; Zhu, H.-L.; Huang, Z.-Z. Efficient and Highly Enantioselective Michael Addition of Aldehydes to Nitroalkenes Catalyzed by a Surfactant-type Organocatalyst in the Presence of Water. Chem. Lett. 2010, 39, 412–414. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, L. Efficient Enantioselective Michael Addition of Nitroalkenes Catalyzed by a Surfactant-Type Bifunctional Thiourea Organocatalyst in the Presence of Water. Lett. Org. Chem. 2012, 9, 51–55. [Google Scholar] [CrossRef]
- Wu, C.; Fu, X.; Ma, X.; Li, S.; Li, C. Threonine-surfactant organocatalysts for the highly diastereo- and enantioselective direct anti-Mannich reactions of hydroxyacetone. Tetrahedron Lett. 2010, 51, 5775–5777. [Google Scholar] [CrossRef]
- Wu, C.; Fu, X.; Ma, X.; Li, S. One-step, efficient synthesis of combined threonine–surfactant organocatalysts for the highly enantioselective direct aldol reactions of cyclic ketones with aromatic aldehydes in the presence of water. Tetrahedron Asymmetry 2010, 21, 2465–2470. [Google Scholar] [CrossRef]
- Kagan, H.B.; Tabart, M. Chiralité et synthèse asymétrique en chimie thérapeutique. L'actual. Chim. 2015, 393, 31–38. [Google Scholar]
- Mac Millan, D. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Bauta, J.; Calbrix, E.; Capblancq, S.; Cecutti, C.; Peydecastaing, J.; Delgado Raynaud, C.; Rouilly, A.; Simon, V.; Vaca-Medina, G.; Vandenbosshe, V.; et al. Global chemical characterization of sargassum spp. seaweeds from different locations on Caribbean islands: A screening of organic compounds and heavy metals contents. Phycology 2024, 4, 190–212. [Google Scholar] [CrossRef]
- Giry, C.; Bertrand, D.; Cecutti, C.; Brossard, C.; Moreau, E.; Thiébaud-Roux, S.; Vaca-Garcia, C.; Vedrenne, E. Green Optimization of the First Steps for the Synthesis of a Novel Surfactant: Towards the Elimination of CMR Solvents and the Drastic Reduction of the Used Solvent Volume. ChemistrySelect 2019, 4, 8621–8625. [Google Scholar] [CrossRef]
- Giry, C.; Bertrand, D.; Pierret, A.; Vedrenne, E.; Lacaze-Dufaure, C.; Fabre, J.-F.; Thiébaud-Roux, S.; Vaca-Garcia, C.; Cecutti, C. Synthesis and Characterization of a New Organocatalytic Biosourced Surfactant. Sustain. Chem. 2021, 2, 335–342. [Google Scholar] [CrossRef]
- Paras, N.A.; MacMillan, D.W.C. The Enantioselective Organocatalytic 1,4-Addition of Electron-Rich Benzenes to α,β-Unsaturated Aldehydes. J. Am. Chem. Soc. 2002, 124, 7894–7895. [Google Scholar] [CrossRef] [PubMed]
- Shendage, D.M.; Fröhlich, R.; Haufe, G. Highly Efficient Stereoconservative Amidation and Deamidation of α-Amino Acids. Org. Lett. 2004, 6, 3675–3678. [Google Scholar] [CrossRef] [PubMed]
- Taveira, A.F.; Hyaric, M.L.; Reis, E.F.C.; Araújo, D.P.; Ferreira, A.P.; de Souza, M.A.; Alves, L.L.; Lourenço, M.C.S.; Vicente, F.R.C.; de Almeida, M.V. Preparation and Antitubercular Activities of Alkylated Amino Alcohols and Their Glycosylated Derivatives. Bioorg. Med. Chem. 2007, 15, 7789–7794. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, C.; Turtle, E.D.; Kim, B.; O’Mahony, D.J.R.; Shiau, T.P.; Low, E.; Alvarez, N.J.; Celeri, C.E.; D’Lima, L.; Friedman, L.C.; et al. Novel N-Chloroheterocyclic Antimicrobials. Bioorg. Med. Chem. Lett. 2011, 21, 3029–3033. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, H.; Bi, J.; Zhang, C.; Zhang, H.; Bai, C.; Hu, Y.; Zhang, X. Pyridine–Oxazoline and Quinoline–Oxazoline Ligated Cobalt Complexes: Synthesis, Characterization, and 1,3-Butadiene Polymerization Behaviors. Inorg. Chim. Acta 2015, 435, 305–312. [Google Scholar] [CrossRef]
- Ashworth, I.W.; Cox, B.G.; Meyrick, B. Kinetics and Mechanism of N-Boc Cleavage: Evidence of a Second-Order Dependence upon Acid Concentration. J. Org. Chem. 2010, 75, 8117–8125. [Google Scholar] [CrossRef]
- Teunissen, H.P. Hexadecylamine (Cetylamine). Recl. Trav. Chim. Pays-Bas 1927, 46, 208–211. [Google Scholar] [CrossRef]
- El-Dossoki, F.I.; Gomaa, E.A.; Hamza, O.K. Solvation Thermodynamic Parameters for Alkyl Benzyl Dimethyl Ammonium Chloride and Cetyl Trimethyl Ammonium Chloride Surfactants in Water and Alcoholic-Water Solvents. J. Chem. Eng. Data 2019, 64, 4482–4492. [Google Scholar] [CrossRef]
- Ralston, A.W.; Hoffman, E.J.; Hoerr, C.W.; Selby, W.M. Studies on High Molecular Weight Aliphatic Amines and their Salts. I. Behavior of the Hydrochlorides of Dodecylamine and Octadecylamine in Water. J. Am. Chem. Soc. 1941, 63, 1598–1601. [Google Scholar] [CrossRef]
- Church, J.; Willner, M.R.; Renfro, B.R.; Chen, Y.; Diaz, D.; Lee, W.H.; Dutcher, C.S.; Lundin, J.G.; Paynter, D.M. Impact of Interfacial Tension and Critical Micelle Concentration on Bilgewater Oil Separation. J. Water Process Eng. 2021, 39, 101684. [Google Scholar] [CrossRef]
- Blachechen, L.S.; Silva, J.O.; Barbosa, L.R.S.; Itri, R.; Petri, D.F.S. Hofmeister effects on the colloidal stability of poly(ethylene glycol)-decorated nanoparticles. Colloid Polym. Sci. 2012, 290, 1537–1546. [Google Scholar] [CrossRef]
- El-Aila Hisham, J.Y. Interaction of Nonionic Surfactant Triton-X-100 with Ionic Surfactants. J. Dispers. Sci. Technol. 2009, 30, 1277–1280. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Adsorption and Aggregation Properties of Some Polysorbates at Different Temperatures. J. Solut. Chem. 2018, 47, 1824–1840. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; Bhattarai, A. Interfacial and Micellization Behavior of Cetyltrimethylammonium Bromide (CTAB) in Water and methanol-Water Mixture at 298.15 to 323.15 K. J. Chem. 2020, 280, 4653092. [Google Scholar] [CrossRef]
- Geng, F.; Yu, L.; Lu, T.; Li, Z.; Zheng, L.; Li, G. Studies on the Effects of Additional Components on Micellization of CTAB via Surface Tension Measurements. J. Dispers. Sci. Technol. 2008, 29, 1209–1213. [Google Scholar] [CrossRef]
- Man, Z.; Wu, W. Study on the Synthesis, Surface Activity, and Self-Assembly Behaviour of Anionic Non-Ionic Gemini Surfactants. Molecules 2024, 29, 1725. [Google Scholar] [CrossRef]
- Saito, T.; Hayamizu, K.; Yanagisawa, M.; Yamamoto, O.; Wasada, N.; Kinugasa, S.; Tanabe, K.; Tamura, T. Integrated Spectral Data Base System of Organic Compounds; National Institute of Advanced Industrial Science and Technology: Tokyo, Japan, 2004.




| Fatty Alcohol | Base | Compound | Yield (%) |
|---|---|---|---|
| Decan-1-ol (n = 7) | Triethylamine | 4 | 95 |
| Hexandecan-1-ol (n = 13) | Pyridine | 5 | 70 |
| Surfactant | 12 | 13 | Tween 20 | Triton X 100 | CTAB |
|---|---|---|---|---|---|
| CACw (g·L−1) | 0.12 | 0.25 | 0.10 b | 0.27 c | 0.33 c |
| γw (mN·m−1) | 49 | 39 | 41 b | 30 a | 34 e–35 c |
| Γmax (µmol·m−2) | 2.32 | 2.28 | 2.8 d | 2.1 c | 2.4 f–2.6 c |
| Amin (A2·molecule−1) | 71.6 | 72.8 | 58 b–59.5 d | 68 c | 63 c–69.2 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calbrix, E.; de Caro, P.; Thiebaud-Roux, S.; Cecutti, C.; Vedrenne, E. Physicochemical Characterization and Asymmetric Catalytic Properties of New Biobased Organocatalytic Surfactants. Molecules 2025, 30, 216. https://doi.org/10.3390/molecules30020216
Calbrix E, de Caro P, Thiebaud-Roux S, Cecutti C, Vedrenne E. Physicochemical Characterization and Asymmetric Catalytic Properties of New Biobased Organocatalytic Surfactants. Molecules. 2025; 30(2):216. https://doi.org/10.3390/molecules30020216
Chicago/Turabian StyleCalbrix, Elliot, Pascale de Caro, Sophie Thiebaud-Roux, Christine Cecutti, and Emeline Vedrenne. 2025. "Physicochemical Characterization and Asymmetric Catalytic Properties of New Biobased Organocatalytic Surfactants" Molecules 30, no. 2: 216. https://doi.org/10.3390/molecules30020216
APA StyleCalbrix, E., de Caro, P., Thiebaud-Roux, S., Cecutti, C., & Vedrenne, E. (2025). Physicochemical Characterization and Asymmetric Catalytic Properties of New Biobased Organocatalytic Surfactants. Molecules, 30(2), 216. https://doi.org/10.3390/molecules30020216






