1. Introduction
As humans venture to explore deep space, we must surmount many challenges. Some of the greatest challenges are primary life support systems needed to ensure the long-term survival of astronauts working during extended missions. Functional life support systems must be capable at least of providing oxygen, water, and CO
2 removal [
1,
2]. Meeting these challenges is a significant hurdle in extraterrestrial exploration. Recycling processes are vital, but no process will have 100% efficiency, so there always will be the necessity to produce new water and oxygen. Using electrolysis, oxygen can be produced from water, similarly to methods used on military naval vessels, but liquid water is rare in our region of space, with most lunar water being concentrated as ice at the Moon’s poles [
3]. Various technologies have been proposed to deal with this concern in non-polar areas. One potential solution suggested has been separating metal oxides to access the embedded oxygen and then reacting it with hydrogen to create water [
4]. This method is thought to be viable due to the discovery that the Moon contains large quantities of various metal oxides, such as iron oxide and titanium oxide [
5].
A new approach to meeting oxygen requirements on the Moon is employing ionic liquids (ILs) to process metal oxides common in lunar regolith to create water by using the oxygen from metal oxides and hydrogen, which can be recycled. One particular IL, 1-ethyl-3 methylimidazolium hydrogen sulfate, has permitted the operating temperature for this process to be reduced to less than 200 °C [
6]. Water, however, will be introduced into and dilute the IL. The IL solution must be concentrated to remove the water before it is re-used. The separated water could then be converted with electrolysis to generate oxygen and permit the hydrogen gas to be recycled back into the system. Direct contact membrane distillation (DCMD) was this work’s selected method for exploring this process. DCMD has potential for removing water from the diluted IL stream, allowing the IL to be concentrated during the process and lessening the need for system purging.
DCMD is a new method for separating water from aqueous solutions. DCMD has been suggested for processes such as desalination plants and dairy processing. The basis for the system is a temperature difference between hot aqueous solutions being passed on the bottom of a thin and porous hydrophobic membrane, with a cold water stream being passed on the top [
7]. During the DCMD process, the temperature gradient creates a vapor pressure difference. The polymer membrane enables water vapor to pass through from the high-temperature stream to condense in the low-temperature water stream. The membrane’s hydrophobic nature would be expected to allow only water vapor to pass through the membrane, owing to the IL solution being repulsed by the membrane or the solute molecules being too big to pass through the membrane. DCMD’s main advantage is that it does not require the IL solution to be completely vaporized. The complexity of the process is thus reduced significantly, decreasing the construction volume of the module and/or the energy requirements. This is in contrast with the more common method of separation, in which all or most of the liquid is vaporized and then condensed to produce a solute-rich base and a water-rich condensate. Typical distillation permits some contaminants to be entrained in the condensate [
8]. Traditional distillation methods work by either providing energy, in the form of heat, to the solution to vaporize it or by reducing the pressure with a vacuum system to cause vaporization at a lower temperature [
7]. Nevertheless, these operations have serious drawbacks, since heat-based vaporization is energy-intensive and also requires extra equipment. Vacuum-based methods require a complex system of interconnected pumps that may vary in operation and individual process control systems, which require either a batch-based method or a complex process to allow for continuous processing, increasing possible failure points.
DCMD provides the ability to separate water from aqueous streams in a manner that is energy-efficient and has reduced spatial requirements [
9]. DCMD separations do not require full vaporization, so complex, large distillation tower systems are not needed for water reclamation. DCMD also provides the benefits of vacuum distillation without its complexity, because recycled or waste heat sources could be used. For these reasons, the amount of energy that needs to be fed into the system could be reduced, increasing the energy efficiency of the process [
7].
A few membrane-based distillation methods have shown adequate results since the 1960s [
10]; however, their implementation in the past was considered ineffective due to issues with polymer technology. The underlying basis of the DCMD method relies on hydrophobic polymer-based membranes, with their effectiveness drastically shifting the effectiveness of the process. Advances in membrane and polymer technology have expanded the useful applications of membrane-based systems, but technological issues still remain that must be addressed. Typical concerns include the membranes’ costs and quality. Membrane quality difficulties can result in the degradation of the membrane, with rates dependent on the type of aqueous solution being processed and the quantity that is being pumped across the membrane. More research is required to understand mass and energy transfer across membranes with reference to varying molecule sizes in the solution [
10].
The present work studied the ability of a DCMD system in separating water from a diluted IL solution, since concentrated IL will be needed for use in acquiring the oxygen in metal oxides from lunar regolith [
6]. Little information on using DCMD on diluted IL is available in the literature, so this research is novel [
11]. Two of the most commonly used membranes for DCMD are polytetrafluoroethylene (PTFE) and polyvinylidene (PVDF), so these membranes were chosen for the experimental work [
12].
3. Materials and Methods
The IL tested was 1-ethyl-3 methylimidazolium hydrogen sulfate with a stock concentration of 95% (Sigma-Aldrich, St. Louis, MO, USA, S/N:49615761, CAS # 412009-61-1). Water content was assumed to be 5%. For the scope of this research project, it was assumed that the processing of metal oxides from lunar regolith had already been conducted and that it had successfully resulted in an aqueous IL solution. The solution’s initial desired concentration was chosen to be 20%. This percentage was chosen because it was the concentration used in previous studies [
6].
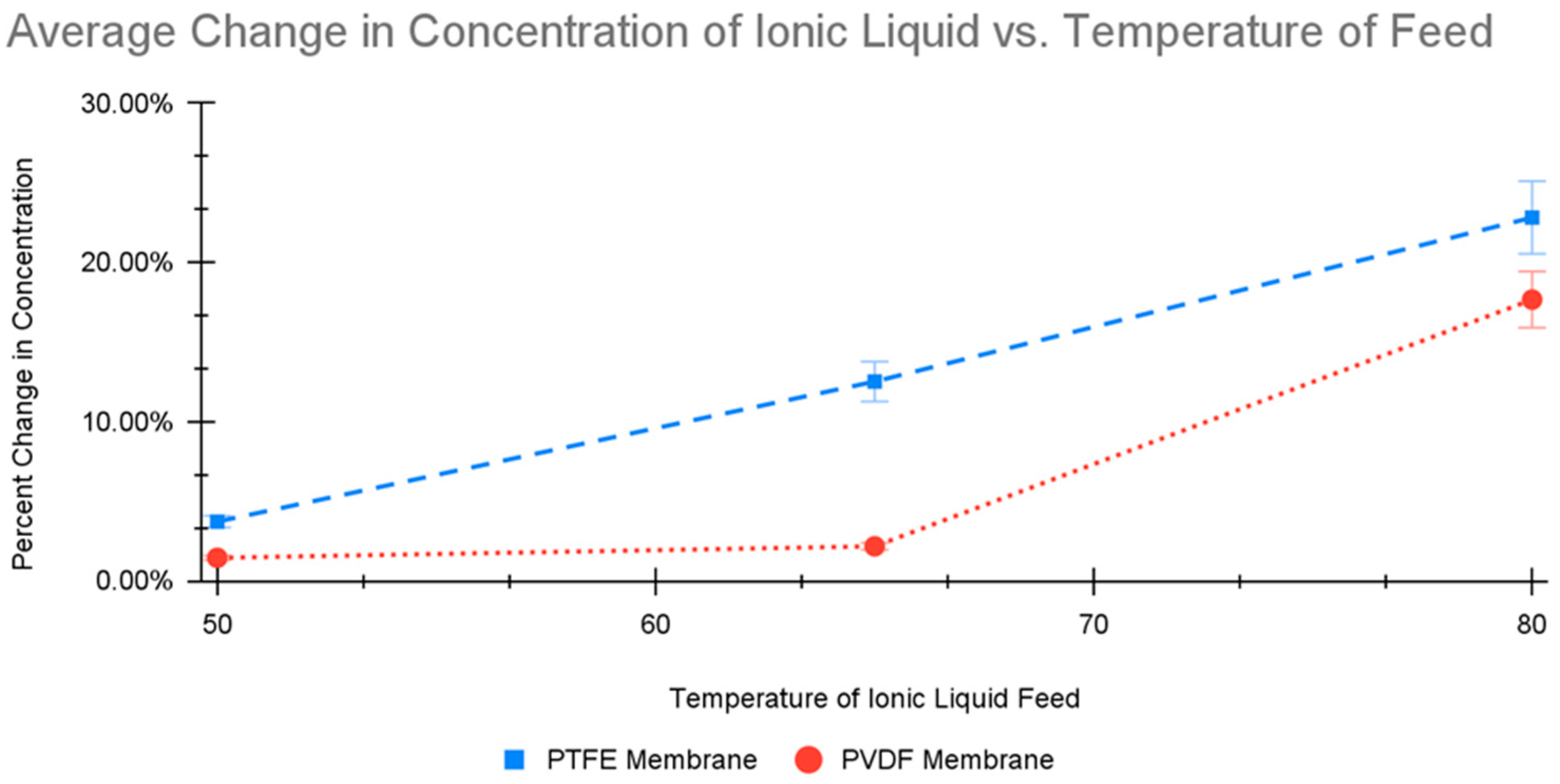
The solutions were processed using DCMD. The setup included a heated IL solution peristaltically pumped underneath the bottom of the polymer membrane and cooled water peristaltically pumped above the top layer of the polymer membrane. The temperatures of the two solutions (cooled and heated) were kept constant using water baths. The temperature of the cooled water was kept constant using a water bath at 5 °C. The heated water bath’s temperature was varied to 50 °C, 65 °C, and 80 °C. The reading error for temperature was ±1 °C, while the accuracy of the temperature reading was 0.1 °C. Two runs were performed at each temperature for 2 h with feed rates of 200 mL/min on each side of the membrane. After two tests at each of the three operating temperatures, the membrane used was changed from a PTFE to a PVDF polymer-based membrane (Sterlitech, Kent, WA, USA: 0.45 Micron PTFE Flat Sheet Membrane, with Polypropylene Netting Backer Laminated, Sepa, Lot#: J000014897 11-1; and Novamem 0.1 Micron Flat Sheet Membrane, PVDF100, MF, Sepa, Lot#: 624096PVDF100). The operation was then replicated to test how different membranes would interact with the IL.
During DCMD, 50 mL samples were removed at 30 min intervals from the hot IL solution. Samples were also taken initially before beginning the process and after it had finished. The final water obtained from the cold side was retained to test for the transfer of ions across the membrane. The mass of water from the cold side before and after each experiment was weighed and recorded. This was performed to find the total flux of the water during the experiment. It was observed that for the trials with the PTFE membrane, after 90 min of operation the change in the solution was negligible at the lowest temperature and therefore the DCMD operation was only performed for 90 min. The experimental duration was increased to 2 h when the data recorded indicated that an increase in time was required to reach significant changes with increased feed temperature. An analysis of samples was performed with two methods, conductivity and UV-Visible spectroscopy analysis.
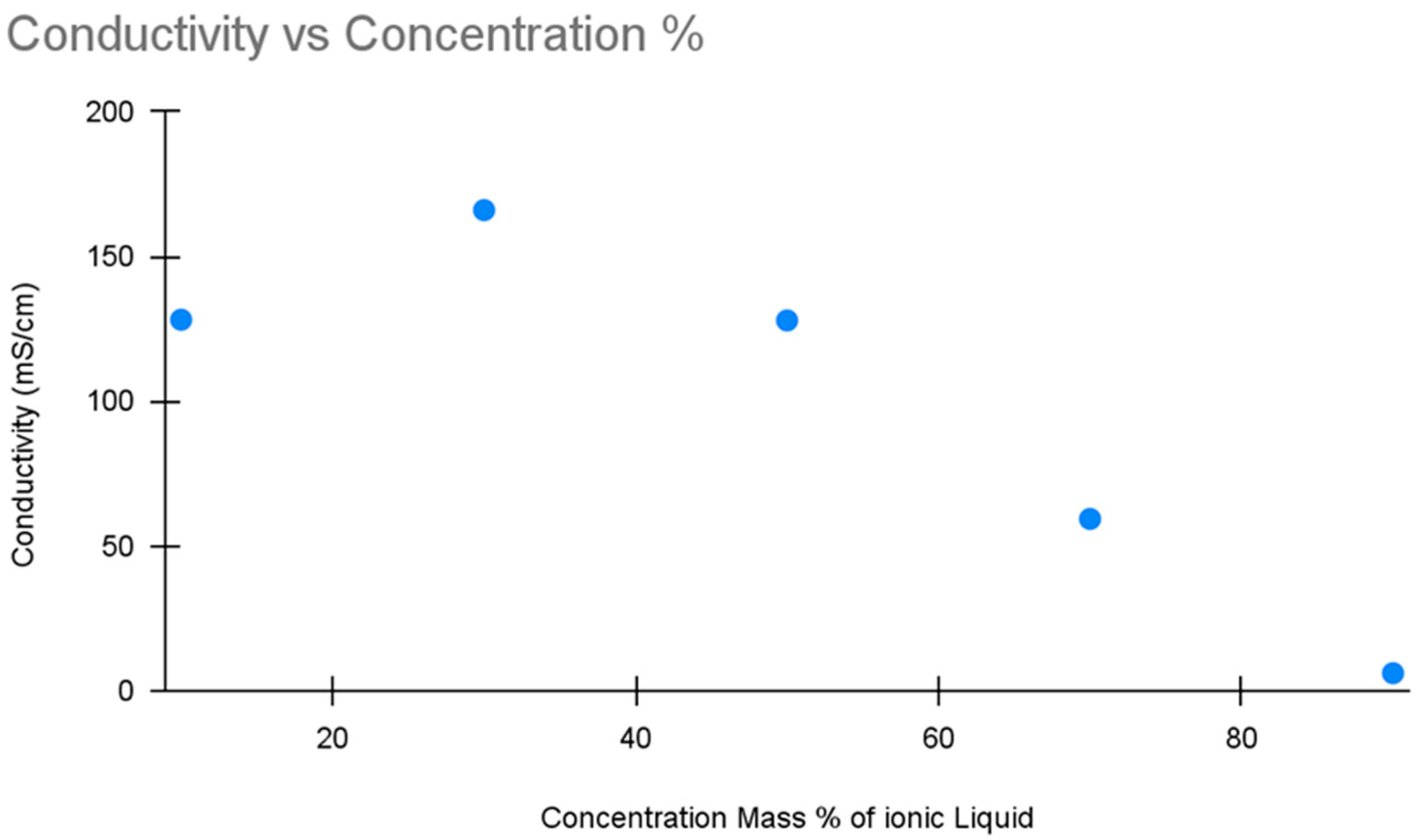
Conductivity tests were performed using 50 mL samples and read from an Ohaus brand conductivity meter (Parsippany, NJ, USA: Model ST20C-B). All samples were created by diluting the stock solution with water prepared with reverse osmosis. An initial calibration curve was created using a 5-point calibration curve ranging from 10% to 90% concentrations at 20% intervals. The DCMD samples were then tested using the same equipment and methodology.
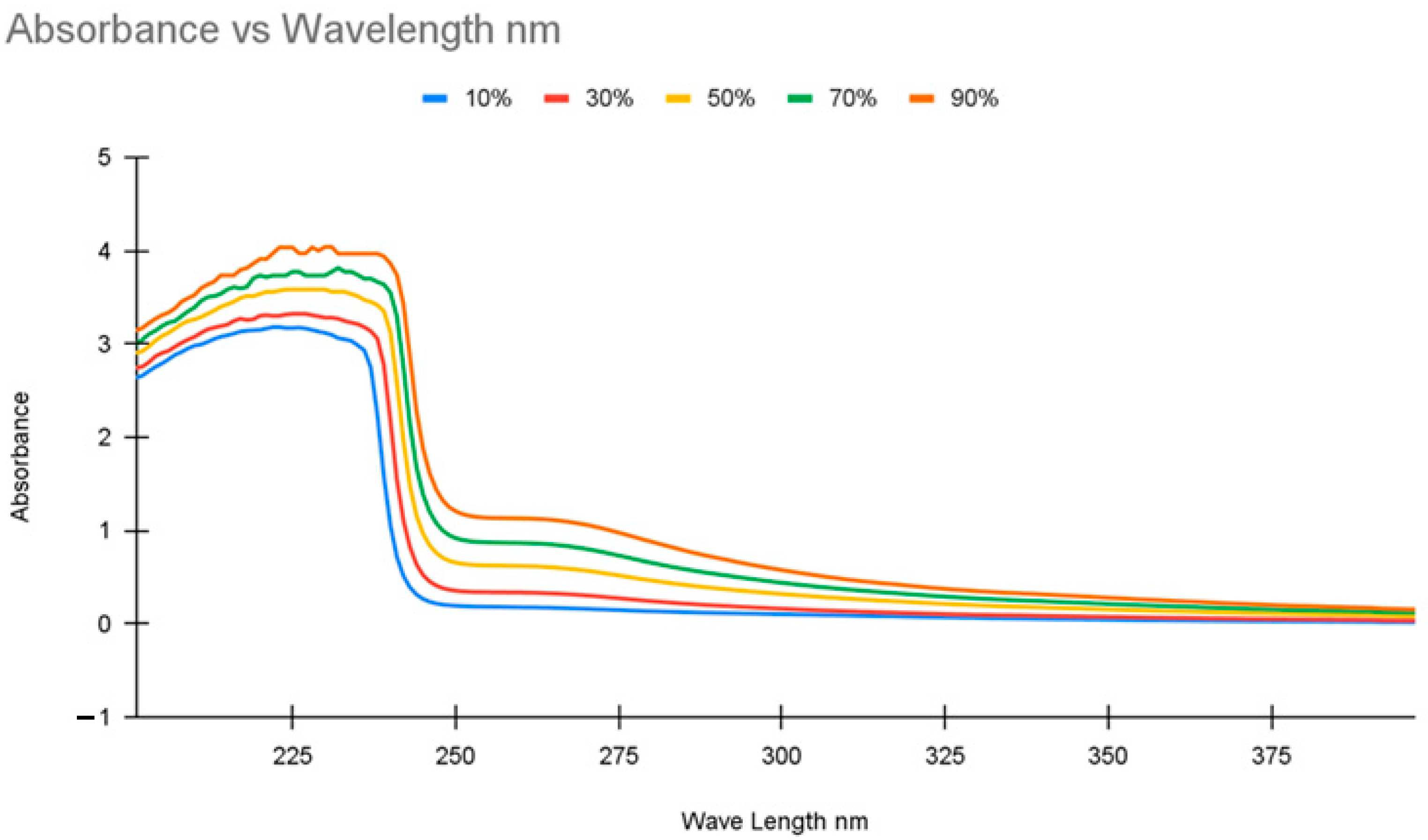
UV-Visible spectroscopy (UV-Vis) functions by sending a stable beam of light through a sample to examine the change in the absorbance of the light beam after it passes through the sample. The light beam used varies within a selected range of wavelengths. The changes in absorbance at different wavelengths depend on the compound being tested. As the concentration of compounds in a sample increases, absorbance increases. Thus, concentrations for samples with compounds that absorb light can be measured quantifiably.
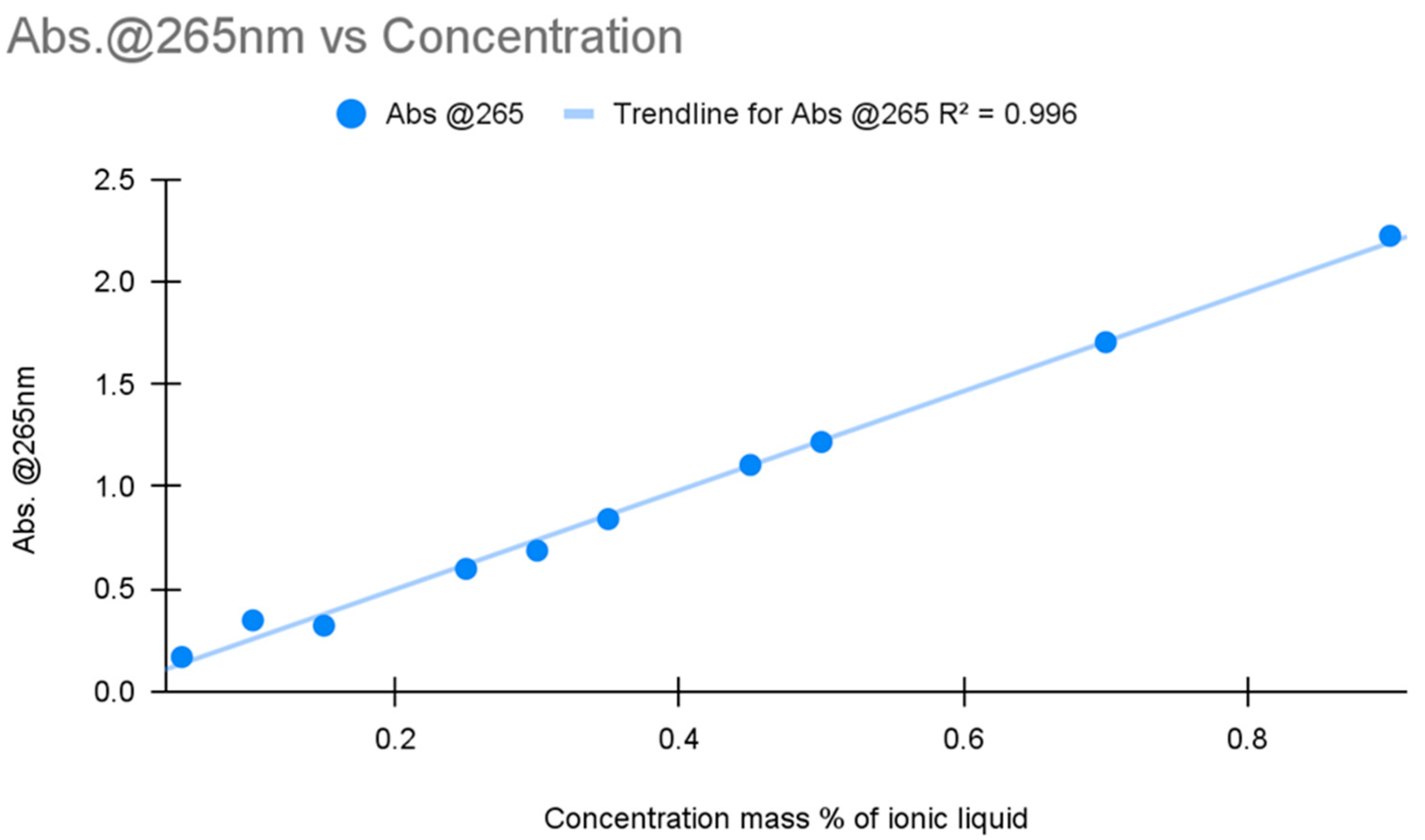
UV-Vis spectroscopy was performed on a Shimadzu spectrometer model no. UV-2401PC (Shimadzu Co., Kyoto, Japan) with a blank water sample as a baseline, and then 3 mL samples were used in quartz cuvettes. Two initial calibration curves were performed. The initial calibration was performed with the same solutions used to calibrate the conductivity meter, five solutions ranging from 10 to 90% concentration spaced out at 20% intervals. The second calibration was performed using five solutions ranging from 5 to 45% concentration spaced at 10% intervals. In the
Supplementary Materials, Figures S1–S4 give more detailed UV-Vis data.
4. Conclusions
Overall, the tests performed indicate the potential for DCMD technology to be employed in operating a water recovery system for ILs after use in lunar regolith processing. Both membranes tested offered advantages and drawbacks for use in water recovery depending on the water permeate’s end purpose. For purposes where the water quality can be flexible, the PTFE membrane operating at a high temperature would be preferable. These DCDM operating conditions offer a larger change in the concentration of the IL, permitting a faster recovery period before it can be reused in the initial DCMD process. However, this option would have the drawback of contaminating the permeate at a significantly faster rate than for the PVDF counterpart. The disadvantage of water contamination would have to be considered. In situations when high water quality must be maintained, such as for drinking water, the PVDF membrane would be preferred. This membrane gave a decreased water recovery rate but also provided a lower rate of contamination of the permeate. Both membranes did offer viability in the ability to recover water from ILs that have been diluted, resulting in a product stream of increased IL concentration.
Studying the life span viability of a variety of membrane materials will be essential in future work. Sulfuric acid has been reported to increase the pore size of PVDF membranes when exposed to it over a period of weeks [
16]. Hydrogen sulfate or bisulfate is considered a weaker form of sulfuric acid. When incorporated in an IL, hydrogen sulfate’s activity may be curtailed due to bonds with 1-ethyl-3 methylimidazolium [
17]. This study showed the viability of PTFE and PVDF membranes for performing the task of recovering water from IL streams. If put into practice for lunar missions, the resupply of IL and membranes could be reduced. Even so, a significant quantity of membranes would be required. This can only be defined by understanding the effective life spans of the membranes. This study had each membrane operate for approximately 12 h per membrane. In an industrial-level application, these membranes will need to be tested with the IL on the order of weeks of continual operating time