Targeted Enrichment and Characterization of Diester Diterpenoid Alkaloids in Aconitum Herbs Using Gas–Liquid Microextraction Coupled with High-Resolution Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
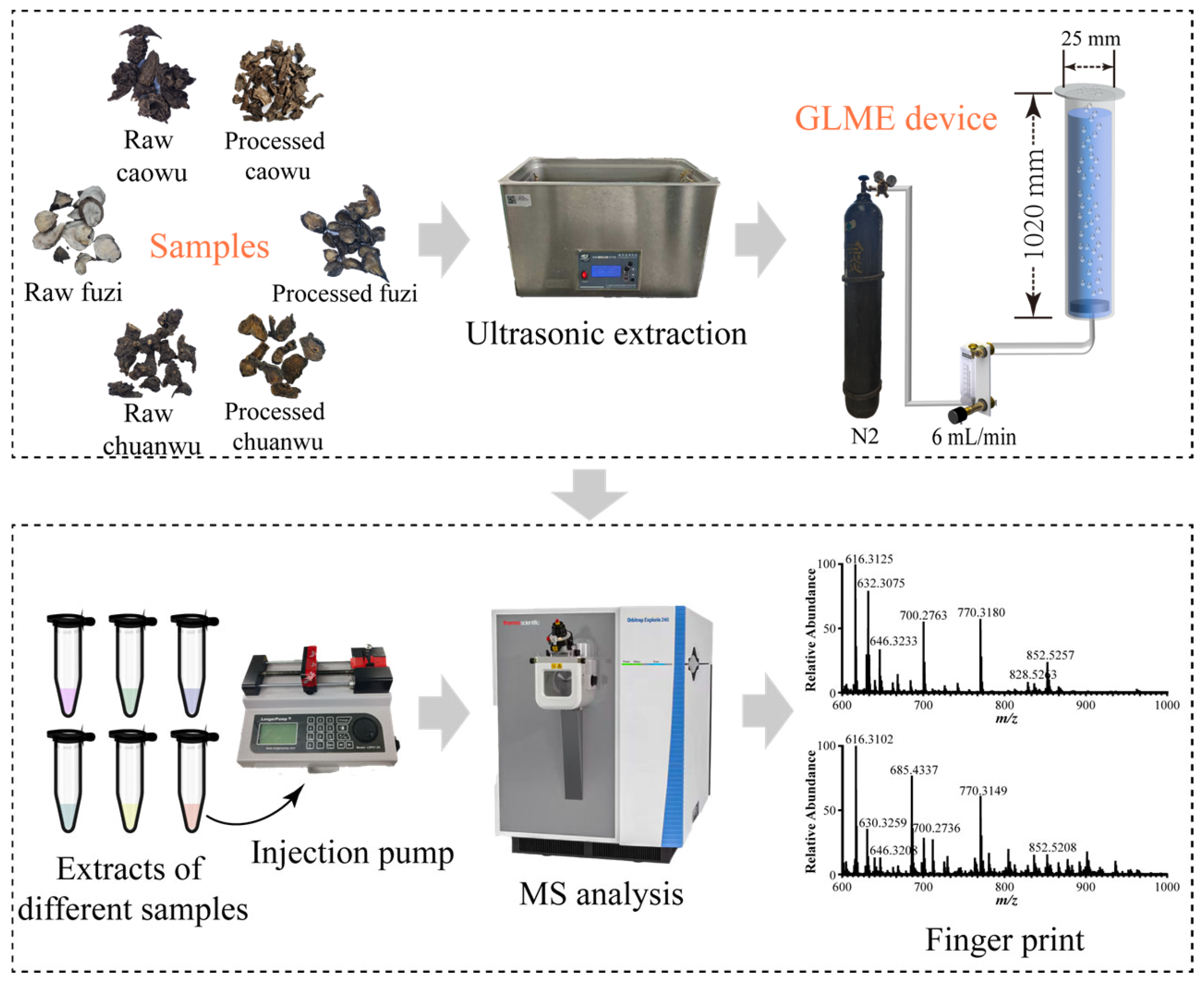
2.1. Construction and Optimization Conditions of GLME–Mass Spectrometry Device
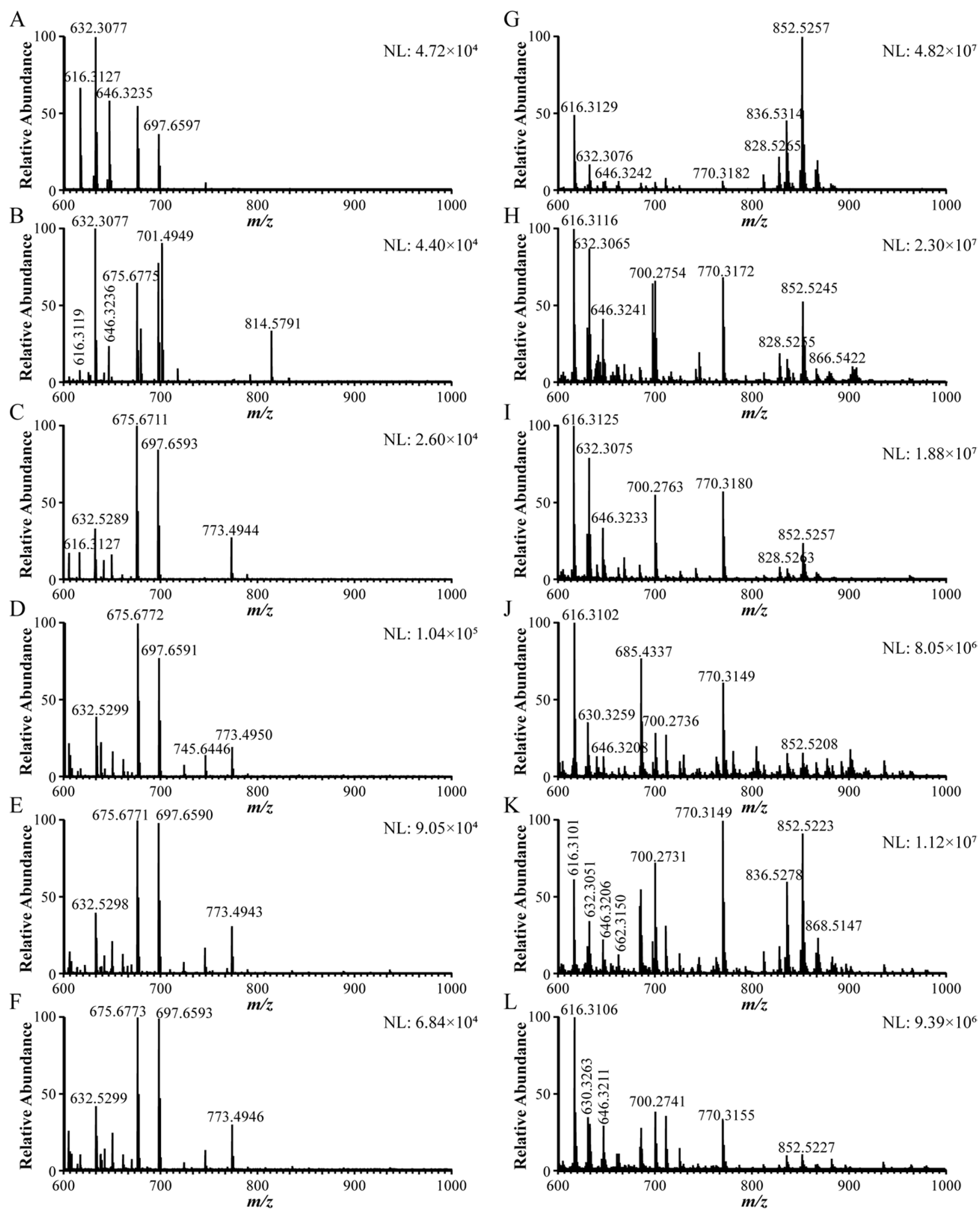
2.2. Difference Analysis of Mass Spectrometry Before/After GLME
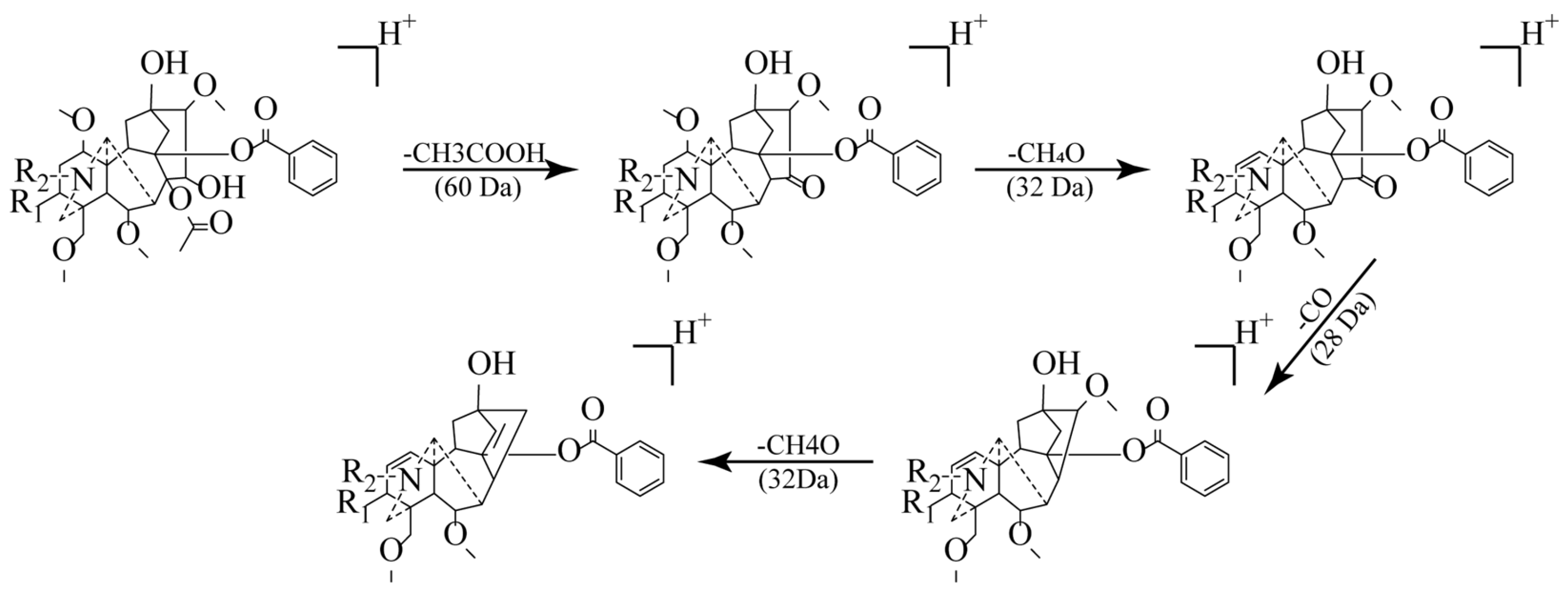
2.3. Identification of Chemical Constituents
| Type | No. | Formula | m/z | Error (ppm) | MS/MS | Compound Name | Reference |
|---|---|---|---|---|---|---|---|
| DDAs | 1 | C33H45NO9 | 600.3181 | −2.3 | 540.2971, 522.2844, 490.2584, 480.2760, 458.2321, 448.2493 | Delphinine | [28] |
| 2 | C32H43NO10 | 602.2963 | −0.7 | 542.2750, 510.2487, 482.2559, 450.2281 | N-demethylhypaconitine | [38] | |
| 3 | C34H47NO9 | 614.3335 | −1.8 | 554.3127, 522.2862, 494.2910, 462.2650 | Chasmaconitine | [38] | |
| 4 | C33H45NO10 | 616.3132 | −2.6 | 556.2919, 524.2656, 496.2707, 464.2443 | Hypaconitine * | [30] | |
| 5 | C34H47NO10 | 630.3287 | −2.2 | 570.3072, 538.2811, 510.2863, 478.2596 | 3-Deoxyaconitine | [35] | |
| 6 | C33H45NO11 | 632.3082 | −2.7 | 572.2867, 540.2607, 512.2655, 480.2393 | Mesaconitine * | [30,32] | |
| 7 | C35H49NO10 | 644.3447 | −2.8 | 584.2872, 552.2608, 524.2659, 492.2394 | Crassicauline A | [32] | |
| 8 | C34H47NO11 | 646.3242 | −3.1 | 586.3019, 554.2763, 526.2813, 494.2551 | Aconitine * | [33,39] | |
| 9 | C33H45NO12 | 648.3026 | −1.7 | 588.2816, 556.2555, 528.2607, 496.2358 | Beiwutine | [35] | |
| 10 | C35H49NO11 | 660.3391 | −1.9 | 600.3167, 568.2913, 540.2607, 508.2346 | Yunaconitine | [40] | |
| 11 | C34H47NO12 | 662.3187 | −2.4 | 602.2976, 570.2712, 542.2758, 510.2499 | 10-Hydroxy aconitine | [39] | |
| 12 | C34H45NO10 | 628.3104 | 1.9 | 568.2889, 536.2631, 508.2680, 476.2421 | Anhydroaconitine | [41] | |
| 13 | C36H49NO8 | 624.3541 | −1.6 | 564.3349, 532.3078, 504.2972, 472.2705 | 15-Ethyl-13-deoxyanhydroaconitine | - | |
| 14 | C36H49NO9 | 640.3494 | −2.2 | 580.3279, 548.3018, 520.3072, 488.2805 | 13-Hydroxy-15-ethylanhydroaconitine | - | |
| Other | 15 | C39H41NO11 | 700.2765 | −1.9 | 640.2556, 578.2397 | Trifoliolasine E | [39,42] |
| 16 | C43H47NO12 | 770.3180 | −1.4 | 648.2811, 500.2074, 378.1707, 318.1491 | (-)-1β,11a-diacetoxy-2α,13α-dibenzoyloxy-7β-hydroxy-15α-isobutanoyloxy-N-methyl-N,19-secohetisan-19-al | [39] | |
| lipo-alkaloids | 17 | C48H75NO10 | 826.5501 | −4.6 | 570.3065, 538,2805, 510.2856 | 8-palmitic-benzoyldeoxyaconitine | [43] |
| 18 | C48H76NO10 | 828.5258 | −0.2 | 572.2861, 540.2601, 512.2648 | 8-palmitic-benzoylmesaconine | [43] | |
| 19 | C47H73NO11 | 834.5154 | −0.4 | 556.2901, 524.2646, 496.2703 | 8-linolenic-benzoylhypaconine | [43] | |
| 20 | C49H71NO10 | 836.5308 | −0.1 | 556.2911, 524.2647, 496.2700 | 8-linoleic-benzoylhypaconine | [44] | |
| 21 | C49H73NO10 | 842.5416 | −0.4 | 586.3015, 554.2753, 526.2807 | 8-palmitic-benzoylaconine | [43] | |
| 22 | C48H75NO11 | 848.5314 | −0.8 | 570.3069, 538.2806, 510.2857 | 8-linolenic-benzoyldeoxyaconitine | [43] | |
| 23 | C50H73NO10 | 850.5478 | −1.8 | 570.3065, 538.2806, 510.2857 | 8-linoleic-benzoyldeoxyaconitine | [43] | |
| 24 | C50H75NO10 | 852.5250 | 0.7 | 572.2859, 540.2600, 512.2650 | 8-palmitic-benzoylmesaconine | [44] | |
| 25 | C49H73NO11 | 866.5428 | −1.8 | 586.3016, 554.2753, 526.2805 | 8-linoleic-benzoylaconine | [44] | |
| 26 | C47H73NO10 | 812.5318 | −1.4 | 556.2913, 524.2653, 496.2701 | 8-palmitic-benzoylaconine | [44] | |
| 27 | C51H72NO11 | 874.5108 | −1.0 | 572.2865, 540.2606, 512.2654 | 8-eicosapentaenoic-benzoylmesaconine | - |
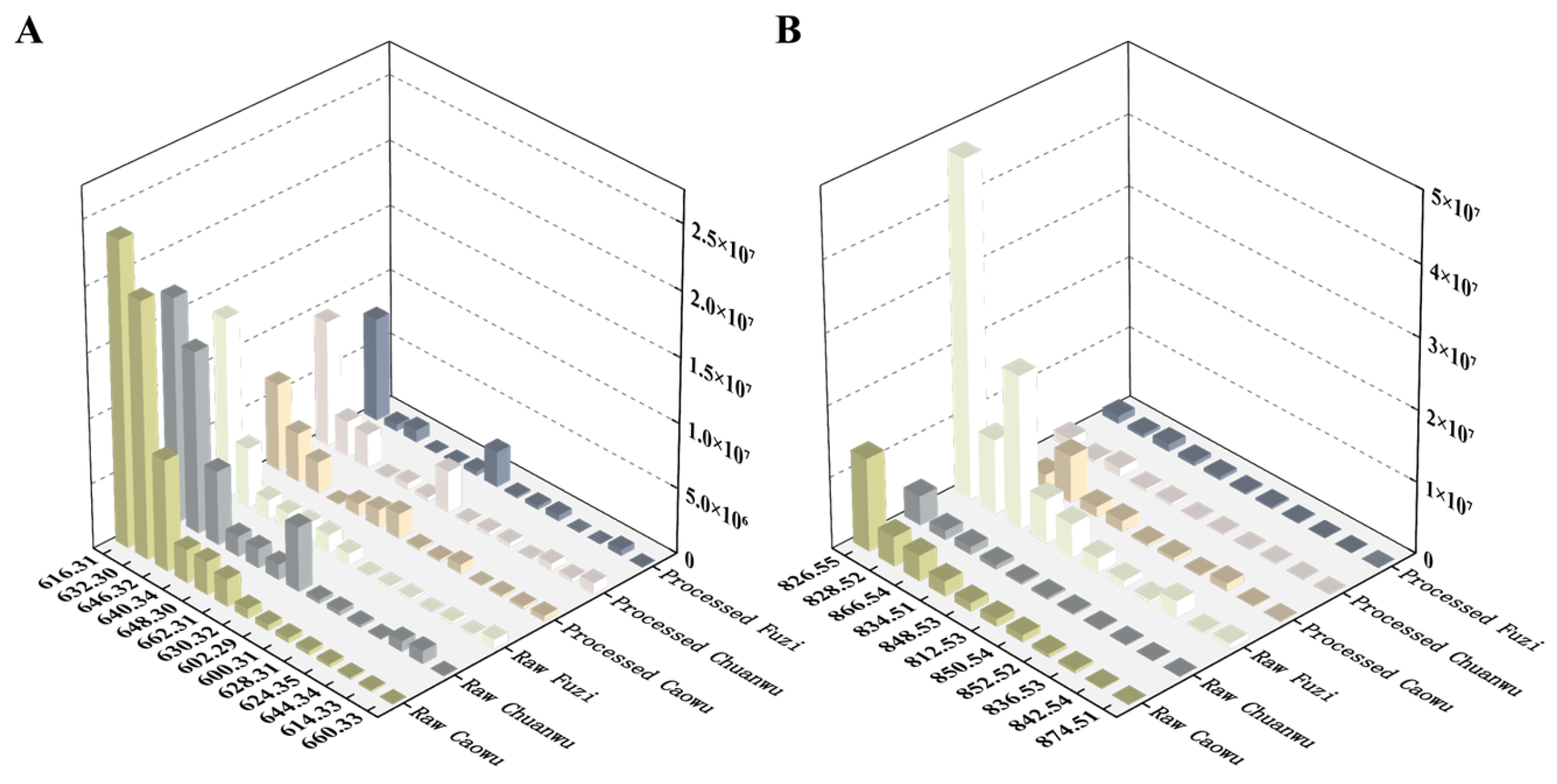
2.4. Difference Analysis of Six Kinds of Aconitum Herbs
2.4.1. DDAs
2.4.2. Lipo-Alkaloids
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Parameters of GLME Device
3.3. Optimization of Mass Spectrometry Conditions
3.4. Orbitrap MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Guo, L.; Zhang, X.; Yu, L.; Sun, J. Species of Chinese materia medica resources based on the fourth national survey of Chinese materia medica resources. Sci. Tradit. Chin. Med. 2024, 49, 3409–3413. [Google Scholar]
- Zheng, Y.; Wei, N.; Lu, C.; Li, W.; Jia, X.; Chen, L.; Chen, R.; Chen, Z. Mechanistic insights into honey-boiled detoxification of ChuanWu: A study on alkaloid transformation and supramolecular aggregation. J. Pharm. Anal. 2025, 15, 101205. [Google Scholar]
- He, Y.; Wei, Z.; Xie, Y.; Yi, X.; Zeng, Y.; Li, Y.; Liu, C. Potential synergic mechanism of Wutou-Gancao herb-pair by inhibiting efflux transporter P-glycoprotein. J. Pharm. Anal. 2020, 10, 178–186. [Google Scholar]
- He, G.; Wang, X.; Liu, W.; Li, Y.; Shao, Y.; Liu, W.; Liang, X.; Bao, X. Chemical constituents, pharmacological effects, toxicology, processing and compatibility of Fuzi (lateral root of Aconitum carmichaelii Debx): A review. J. Ethnopharmacol. 2023, 307, 116160. [Google Scholar] [CrossRef]
- Fu, Y.-P.; Li, C.-Y.; Peng, X.; Zou, Y.-F.; Rise, F.; Paulsen, B.S.; Wangensteen, H.; Inngjerdingen, K.T. Polysaccharides from Aconitum carmichaelii leaves: Structure, immunomodulatory and anti-inflammatory activities. Carbohydr. Polym. 2022, 291, 119655. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shao, S.; Guo, Q.; Xu, C.; Xia, H.; Zhang, T.; Shi, J. Aconicatisulfonines A and B, Analgesic Zwitterionic C20-Diterpenoid Alkaloids with a Rearranged Atisane Skeleton from Aconitum carmichaelii. Org. Lett. 2019, 21, 6850–6854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, H.; Qiu, L.-Z.; Yue, L.-X.; Zhang, G.-J.; Deng, H.-F.; Ni, Y.-H.; Gao, Y. Cardiac efficacy and toxicity of aconitine: A new frontier for the ancient poison. Med. Res. Rev. 2021, 41, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cai, S.; Qin, L.; Feng, Y.; Ding, M.; Luo, Z.; Shan, J.; Di, L. Alkaloids of Aconiti Lateralis Radix Praeparata inhibit growth of non-small cell lung cancer by regulating PI3K/Akt-mTOR signaling and glycolysis. Commun. Biol. 2024, 7, 1118. [Google Scholar]
- Qiu, Z.-D.; Wei, X.-Y.; Sun, R.-Q.; Chen, J.-L.; Tan, T.; Xu, J.-Q.; Cui, G.-H.; Chen, T.; Guo, J.; Lai, C.-J.-S.; et al. Limitation standard of toxic aconitines in Aconitum proprietary Chinese medicines using on-line extraction electrospray ionization mass spectrometry. Acta Pharm. Sin. B 2020, 10, 1511–1520. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Li, Y.; Li, W.; Xu, J.; Du, H. A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in Traditional Chinese Medicine. J. Ethnopharmacol. 2017, 207, 237–250. [Google Scholar] [CrossRef]
- Zhao, F.; Li, J. Recent Progress in Research of Alkaloid in Aconitum Plants. Chin. J. Mod. Appl. Pharm. 2010, 27, 1177–1182. [Google Scholar]
- Chunbei, Z.; Shusheng, L.; Jiang, T.; Linda, Q.; Huihui, L.; Yue, Z. Poisoning Associated with Consumption of a Homemade Medicinal Liquor—Chongqing, China, 2018. Morb. Mortal. Wkly. Rep. 2022, 71, 569–573. [Google Scholar]
- Gong, X.; Lu, Y.; Chen, X.; Qiu, F.; Chen, Q. Determination of Diester Alkaloid Limit in Mugua Pills by SPE-HPLC. China Pharm. 2017, 26, 17–19. [Google Scholar]
- Liu, Z. Study on Extraction Process and Hydrolysis Reaction of Aconite Diester Diterpene Alkaloids. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2012. [Google Scholar]
- Ali, N.; Rampazzo, R.d.C.P.; Costa, A.D.T.; Krieger, M.A. Current Nucleic Acid Extraction Methods and Their Implications to Point-of-Care Diagnostics. BioMed Res. Int. 2017, 2017, 9306564. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, J.; An, J.; Li, P. Application of turbulent flow chromatography in the analysis of biological samples. Chin. J. Chromatogr. 2010, 28, 168–174. [Google Scholar] [CrossRef]
- Wang, X.; Qi, W.; Zhao, X.; Lv, T.; Wang, X.; Zheng, L.; Yan, Y.; You, J. Determination of four phenolic endocrine disruptors in environmental water samples by high performance liquid chromatography-fluorescence detection using dispersive liquid-liquid microextraction coupled with derivatization. Chin. J. Chromatogr. 2014, 32, 623–628. [Google Scholar] [CrossRef]
- Gao, Y.; He, Q.; Guo, C.; Chen, W.; Pan, Y. Carbon Dioxide Microbubble Bursting Ionization Mass Spectrometry. Anal. Chem. 2022, 94, 17360–17364. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, H.; Xia, B.; He, L.; Yang, C.; Zhao, L.; Pan, Y. Ultrasensitive and Green Bubbling Extraction Strategies: An Extensible Solvent-Free Re-Enrichment Approach for Ultratrace Pollutants in Aqueous Samples. Anal. Chem. 2023, 95, 13683–13689. [Google Scholar] [CrossRef]
- Qiu, Z.; Wei, C.; Kang, L.; Zhou, L.; Lai, C.; Li, X.; Yan, B.; Xu, J.; Wang, S.; Huang, L. Sensitive quantitation of ultra-trace toxic aconitines in complex matrices by perfusion nano-electrospray ionization mass spectrometry combined with gas-liquid microextraction. Talanta 2024, 269, 125402. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, Y.; Guo, Y.; Jin, X.; Li, D.; Zhao, J. Determination of 16 polycyclic aromatic hydrocarbons in seaweed by ultrasound-assisted gas-liquid microextraction coupled with gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2025, 43, 950–958. [Google Scholar] [CrossRef]
- An, S.; Ranaweera, R.; Luo, L. Harnessing bubble behaviors for developing new analytical strategies. Analyst 2020, 145, 7782–7795. [Google Scholar] [CrossRef] [PubMed]
- Kou, W.; Zhang, H.; Konstantin, C.; Chen, H.-W. Charged Bubble Extractive Ionization Mass Spectrometry for Protein Analysis. Chin. J. Anal. Chem. 2017, 45, 1937–1943. [Google Scholar] [CrossRef]
- Wei, C.; Kang, L.; Zhao, Y.; Zhou, L.; Li, X.; Wang, S.; Huang, L.; Qiu, Z. Rapid toxicity characterization of Aconitum herbal medicines using perfusion nano-electrospray ionization mass spectrometry. J. Pharm. Anal. 2024, 14, 101016. [Google Scholar] [CrossRef] [PubMed]
- Cochran, R.E.; Jayarathne, T.; Stone, E.A.; Grassian, V.H. Selectivity Across the Interface: A Test of Surface Activity in the Composition of Organic-Enriched Aerosols from Bubble Bursting. J. Phys. Chem. Lett. 2016, 7, 1692–1696. [Google Scholar] [CrossRef]
- Chingin, K.; Yan, R.; Zhong, D.; Chen, H. Enrichment of Surface-Active Compounds in Bursting Bubble Aerosols. ACS Omega 2018, 3, 8709–8717. [Google Scholar] [CrossRef]
- Chingin, K.; Cai, Y.; Liang, J.; Chen, H. Simultaneous Preconcentration and Desalting of Organic Solutes in Aqueous Solutions by Bubble Bursting. Anal. Chem. 2016, 88, 5033–5036. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, J.; Qi, L.-W.; Li, P.; Jing, S.-L.; Li, H.-J. Structural characterization and identification of C19- and C20-diterpenoid alkaloids in roots of Aconitum carmichaeli by rapid-resolution liquid chromatography coupled with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1619–1635. [Google Scholar] [CrossRef]
- Yu, J.; Xia, J.; Xu, J.; Chen, S.; Zhang, Y.; Yin, F.; Fang, J.; Cai, L.; Zhang, B.; Zhan, Y.; et al. Chemical profile, anti-hepatoma activity, anti-acetylcholinesterase and antioxidant activity of aerial part of Aconitum carmichaeli Debx. Nat. Prod. Res. 2023, 37, 3884–3888. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Sun, M.; Liu, J. Fragmentation pathways of six aconitine alkaloids using HPLC-Q-TOF-MS technology. Chin. Tradit. Herb. Drugs 2016, 47, 2827–2831. [Google Scholar]
- Song, S.; Lan, X.; Xu, J.; Cui, Y.; Zhou, H.; Zheng, J.; Dai, S.; Zhang, J. Systematic analysis and identification of diterpenoid alkaloids from Aconitum carmichaeli Debx. by UHPLC-Q-Exactive Orbitrap MS. Chin. J. Pharm. Anal. 2023, 43, 918–929. [Google Scholar]
- Jiayu, Z.; Xu, J.; Cui, Y.; Wang, B.; Zhou, H.; Lan, X.; Song, S.; Dai, L.; Zhang, J. Comparative analysis of alkaloids in Aconiti Radix and Aconiti kusnezoffii Radix by UHPLC-Q-Exactive Orbitrap MS/MS. China J. Tradit. Chin. Med. Pharm. 2023, 38, 3285–3295. [Google Scholar]
- Bu, R.; Gao, C.; Ma, G.; Lian, H.; Bai, W. Rapid identification of chemical components in Aconiti kusnezoffii Radix and processed products based on UHPLC-Q-Exactive Orbitrap MS/MS combined technology. Chem. Res. Appl. 2025, 37, 854–863. [Google Scholar]
- Sun, H.; Wang, M.; Zhang, A.; Ni, B.; Dong, H.; Wang, X. UPLC–Q-TOF–HDMS Analysis of Constituents in the Root of Two Kinds of Aconitum Using a Metabolomics Approach. Phytochem. Anal. 2013, 24, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.; Liang, Z.; Ho, A.; Wong, L.; Yong, P.; Chen, H.; Zhao, Z. Distribution of toxic alkaloids in tissues from three herbal medicine Aconitum species using laser micro-dissection, UHPLC–QTOF MS and LC–MS/MS techniques. Phytochemistry 2014, 107, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, D.; Zhu, Y.; Hao, L. Study on the Fragmentation Behavior and Alcoholysates of Aconitum Alkaloids in Aconitum carmichaeli Debx. J. Emerg. Tradit. Chin. Med. 2016, 25, 2278–2282. [Google Scholar]
- Chen, Y.; Wang, H.; Fan, L. Mass spectrometry fragmentation behavior analysis of aconitine. Sci. Technol. Innov. Her. 2010, 21, 7–8. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, X.-J.; Guo, H.-M.; Wang, R.-L.; Song, R.; Tian, Y.; Zhang, Z.-J. Identification and comparative analysis of the major chemical constituents in the extracts of single Fuzi herb and Fuzi-Gancao herb-pair by UFLC-IT-TOF/MS. Chin. J. Nat. Med. 2014, 12, 542–553. [Google Scholar] [CrossRef]
- Gao, W.; Liu, X.-G.; Liu, L.; Li, P.; Yang, H. Targeted profiling and relative quantification of benzoyl diterpene alkaloids in Aconitum roots by using LC–MS/MS with precursor ion scan. J. Sep. Sci. 2018, 41, 3515–3526. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Liu, X.; Zhao, Z.-L.; Chen, S.-L.; Wang, Z.-T.; Xu, H.-X. Rapid determination of yunaconitine and related alkaloids in aconites and aconite-containing drugs by ultra high-performance liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 1567–1574. [Google Scholar] [CrossRef]
- Dunstan, W.R.; Carr, F.H. LXIX.—Contributions to our knowledge of the aconite alkaloïds. Part VI. Conversion of aconitine into isaconitine. J. Chem. Soc. Trans. 1893, 63, 991–994. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Chen, D.-L.; Chen, Q.-H.; Wang, F.-P. C20-Diterpenoid Alkaloids from Delphinium trifoliolatum. J. Nat. Prod. 2005, 68, 1076–1079. [Google Scholar] [CrossRef]
- Yue, H.; Pi, Z.; Song, F.; Liu, Z.; Cai, Z.; Liu, S. Studies on the aconitine-type alkaloids in the roots of Aconitum carmichaeli Debx. by HPLC/ESIMS/MSn. Talanta 2009, 77, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-D.; Zhang, X.; Wei, X.-Y.; Chingin, K.; Xu, J.-Q.; Gao, W.; Yang, B.; Wang, S.-L.; Tan, T.; Liu, E.H.; et al. Online discovery of the molecular mechanism for directionally detoxification of Fuzi using real-time extractive electrospray ionization mass spectrometry. J. Ethnopharmacol. 2021, 277, 114216. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qiu, Z.; Chen, J.; Sun, R.; Huang, L.; Lai, C. Research advancement in mechanisms of processing and compatibility for detoxication of Aconitums. China J. Chin. Mater. Medica 2019, 44, 3695–3704. [Google Scholar]
- Tian, Y. Research status of toxicity of Aconiti kusnezoffii Radix. J. Med. Inf. 2015, 1, 371. [Google Scholar] [CrossRef]
- Sun, W.; Liu, F.; Yuan, Q.; Shan, L.; Xiao, L.; Huang, J. Effect of Decocting Time on Toxicity of Aconite. J. Emerg. Tradit. Chin. Med. 2018, 27, 761–764+768. [Google Scholar]
- Wu, P.; Zhou, Y.; Liu, Y.; Yang, L.; Kuai, M.; Zhang, Z. Pre-decoction and long time-decoction of toxic traditional Chinese medicine in 2020 Edition of Chinese Pharmacopeia. Cent. South Pharm. 2021, 19, 762–768. [Google Scholar]
- Luan, S.; Gao, Y.; Liang, X.; Zhang, L.; Wu, Q.; Hu, Y.; Yin, L.; He, C.; Liu, S. Aconitine linoleate, a natural lipo-diterpenoid alkaloid, stimulates anti-proliferative activity reversing doxorubicin resistance in MCF-7/ADR breast cancer cells as a selective topoisomerase IIα inhibitor. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 65–76. [Google Scholar] [CrossRef]
- Lin, J.; Yan, J.; Xu, Q. Study of rapid extraction and chemical properties of lipo-alkaloids from processed Aconiti lateralis Radix Praeparata. China J. Tradit. Chin. Med. Pharm. 2020, 35, 5148–5152. [Google Scholar]
- Qiu, Z.; Wei, C.; Li, X.; Lai, C.; Zhan, Z.; Jin, Y.; Zhou, L.; Hao, Q.; Yang, J.; Wang, S.; et al. Rapid authentication of different herbal medicines by heating online extraction electrospray ionization mass spectrometry. J. Pharm. Anal. 2023, 13, 296–304. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wu, J.; Ma, X.; Zhou, L.; Li, X.; Ma, B.; Qiu, Z.; Kang, L. Development and challenges of mass spectrometry database for traditional Chinese medicine: A review. Sci. Tradit. Chin. Med. 2025, 3, 210–221. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Miao, C.; Wu, J.; Hua, Y.; Li, X.; Kang, L.; Qiu, Z. Targeted Enrichment and Characterization of Diester Diterpenoid Alkaloids in Aconitum Herbs Using Gas–Liquid Microextraction Coupled with High-Resolution Mass Spectrometry. Molecules 2025, 30, 4029. https://doi.org/10.3390/molecules30194029
Wang Y, Miao C, Wu J, Hua Y, Li X, Kang L, Qiu Z. Targeted Enrichment and Characterization of Diester Diterpenoid Alkaloids in Aconitum Herbs Using Gas–Liquid Microextraction Coupled with High-Resolution Mass Spectrometry. Molecules. 2025; 30(19):4029. https://doi.org/10.3390/molecules30194029
Chicago/Turabian StyleWang, Yijun, Ceyu Miao, Junxian Wu, Yutong Hua, Xiang Li, Liping Kang, and Zidong Qiu. 2025. "Targeted Enrichment and Characterization of Diester Diterpenoid Alkaloids in Aconitum Herbs Using Gas–Liquid Microextraction Coupled with High-Resolution Mass Spectrometry" Molecules 30, no. 19: 4029. https://doi.org/10.3390/molecules30194029
APA StyleWang, Y., Miao, C., Wu, J., Hua, Y., Li, X., Kang, L., & Qiu, Z. (2025). Targeted Enrichment and Characterization of Diester Diterpenoid Alkaloids in Aconitum Herbs Using Gas–Liquid Microextraction Coupled with High-Resolution Mass Spectrometry. Molecules, 30(19), 4029. https://doi.org/10.3390/molecules30194029





