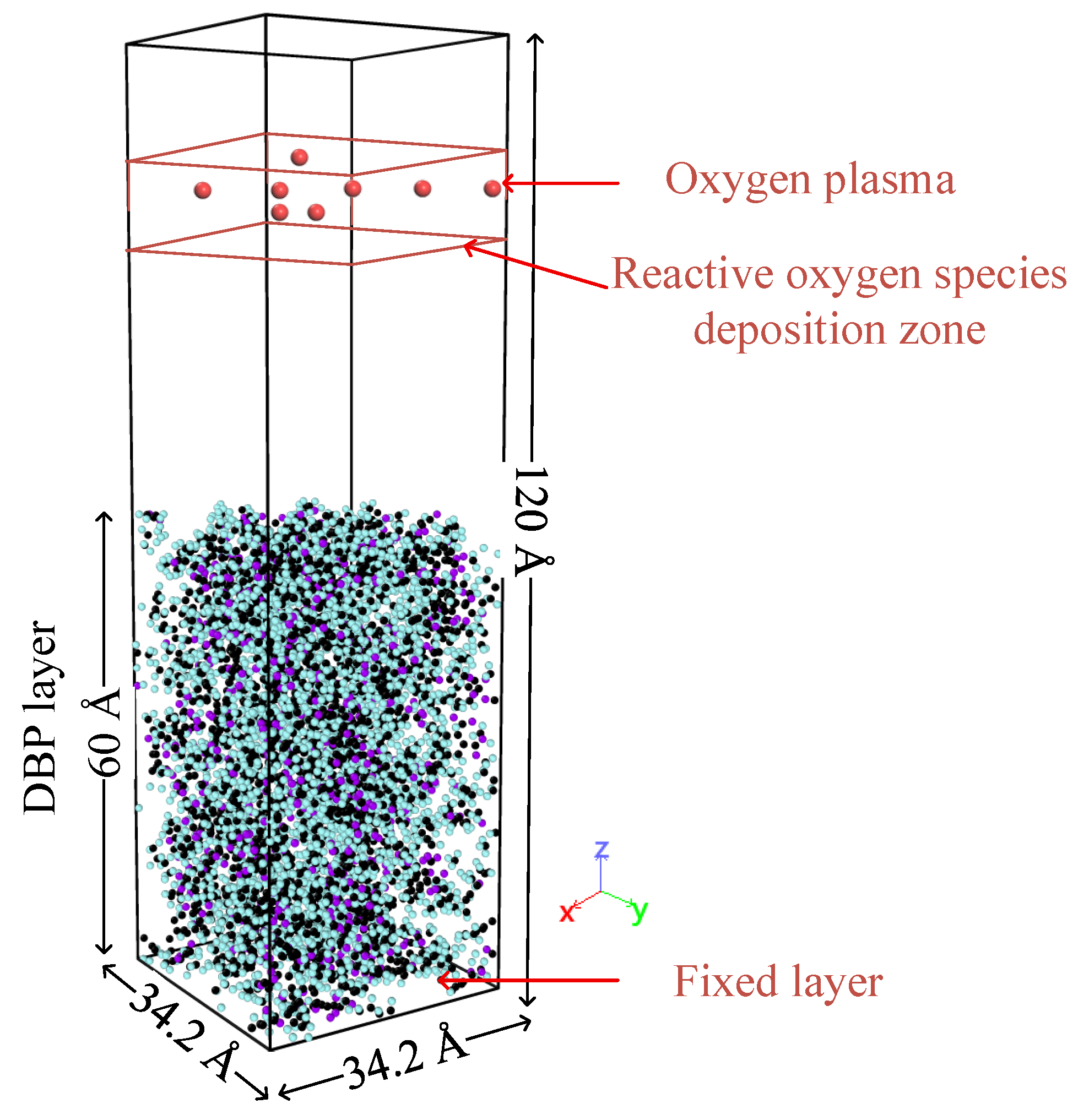
2.2. The Core Influence of the Initial Kinetic Energy and Other Parameters of Reactive Oxygen Species on the Cleaning Reaction
Having understood the specific pathways and characterization methods for the decomposition reaction by which ROS in the plasma remove DBP, we can proceed to quantitatively analyze the extent of the influence of the initial kinetic energy of the plasma on the selection of reaction pathways and the cleaning efficiency.
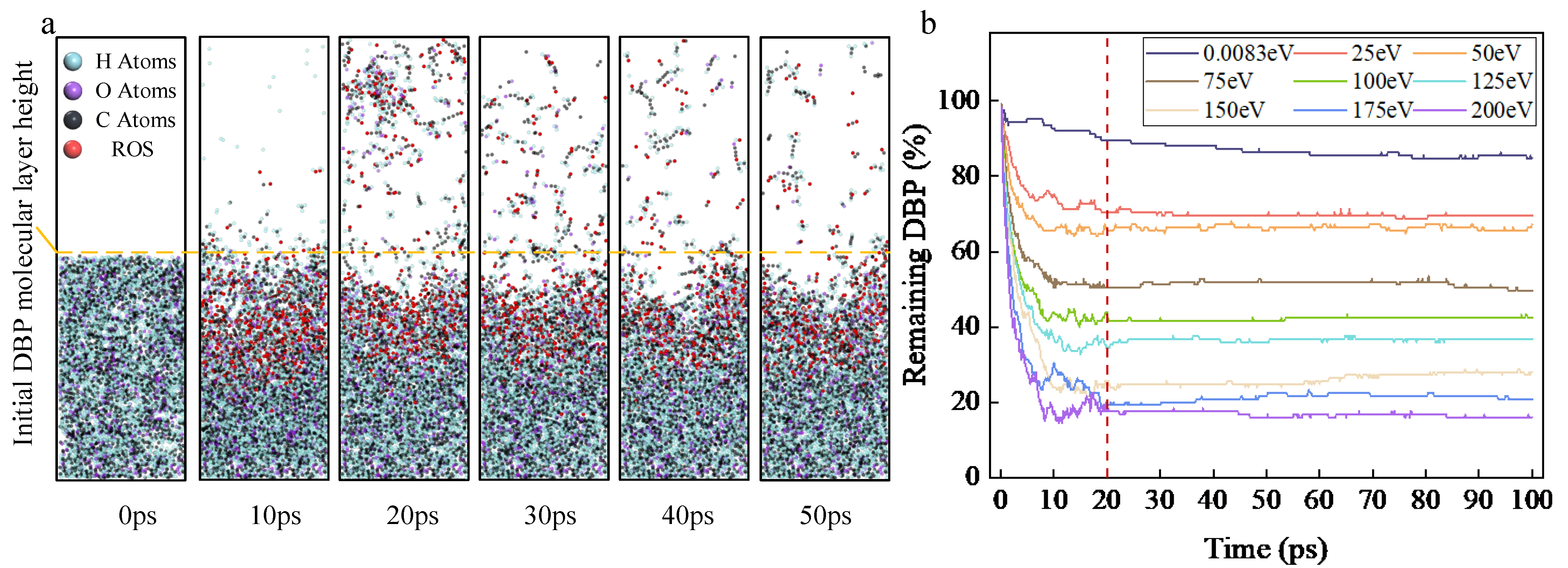
As shown in
Figure 1a, when the initial kinetic energy is zero, the DBP removal rate is relatively uniform between 0–100 ps, with decomposition reactions continuously occurring as the ROS diffuse and come into contact with DBP; the average reaction rate is 0.48 s
−1 (number of DBP decompositions per unit time). After imparting initial kinetic energy to the ROS, the ROS are uniformly deposited on the surface of the organic contaminant layer within 0–20 ps. Compared to the zero-kinetic-energy group, it is evident that the DBP decomposition reaction predominantly occurs within 0–20 ps, and as the initial kinetic energy increases, the DBP decomposition rate (2.43, 2.62, 4.05, 4.61, 5.06, 5.76, 6.34, and 6.70 s
−1, respectively) rises rapidly. Even with the minimum initial kinetic energy (25 eV), the DBP decomposition rate increased by 406.3% compared to the zero-kinetic-energy group. At the maximum DBP decomposition rate (maximum initial kinetic energy of 200 eV), it increased by 1310% relative to the zero-kinetic-energy group.
In the zero-kinetic-energy group, upon completion of the cleaning reaction, the residue ratio of DBP was 84.8%. After imparting initial kinetic energy to the ROS, the DBP residue ratio decreased rapidly with increasing initial kinetic energy (69.6%, 67.2%, 49.6%, 42.4%, 36.8%, 28%, 20.8%, and 16%, respectively). Compared to the zero-kinetic-energy group, even with the minimum initial kinetic energy, the DBP residue ratio decreased by 17.92%, while at the lowest DBP residue ratio (maximum initial kinetic energy of 200 eV), it decreased by 81.13%. This comparative data indicates that ROS with initial kinetic energy contacts DBP more rapidly, inducing its decomposition reaction, and can substantially enhance the DBP decomposition rate and removal efficiency.
Knowing the significant promoting effect of the initial kinetic energy of ROS on the DBP decomposition reaction, the next step is to define the mechanism by which initial kinetic energy influences the cleaning reaction. To more accurately quantify the factors affected by initial kinetic energy, the instantaneous abundance is first defined: for any target species in the reaction system, the instantaneous abundance is recorded as the ratio of the number of molecules of the target species at time t with incident kinetic energy E to the initial number of DBP molecules in the system (the normalization reference), used to represent the relative conversion rate. This data can quantify the dominance of a reaction route and, through normalization, eliminates statistical drift caused by system expansion, enabling comparison of pathway weights under different initial kinetic energies.
Furthermore, it is noted that during the reaction, ROS may combine with H2 or hydrogen atoms dissociated in the system or undergo further oxidation, generating various hydrogen-oxygen compounds. Therefore, the oxygen-containing fraction ϕHO is defined: ϕHO is defined as the instantaneous fraction of oxygen-bearing species among molecules containing only H and O elements. A high ϕHO represents the degree of secondary oxidation of hydrogen atoms or H2 in the system. It can measure the re-oxidation capability of oxygen radicals towards hydrogen radicals and serve as a discriminator of oxygen atom activity.
Subsequently, to assess the selective proportion among different reaction pathways, the reaction pathway weight is defined: the proportion of the number of molecules of a characteristic product of a specific reaction pathway to the total number of molecules of all characteristic products at a given time. The higher this weight coefficient, the greater the contribution of the corresponding reaction pathway in the DBP decomposition reaction. The calculation formulas for the above parameters are as follows:
In the equation, n(C12, t, E) denotes the instantaneous abundance of C12-based products at time t and initial kinetic energy E; n(C12HxOy) represents the number of C12-based product molecules in the simulation system at time t and initial kinetic energy E; and N(C16H22O4) indicates the initial number of DBP molecules in the simulation system. ϕHO(t, E) represents the oxygen-containing fraction of the system at time t and initial kinetic energy E. RSB, RDB, and RPh denote the weight coefficients of the reaction pathway for mono-butyl side chain cleavage, dual-butyl side chain cleavage, and benzene ring cleavage, respectively.
Thus, the variations in the instantaneous abundance of each characteristic product, the oxygen-containing fraction of the system, and the weight coefficients of each reaction pathway with respect to time and kinetic energy are shown in
Figure 3,
Figure 4 and
Figure 5, respectively.
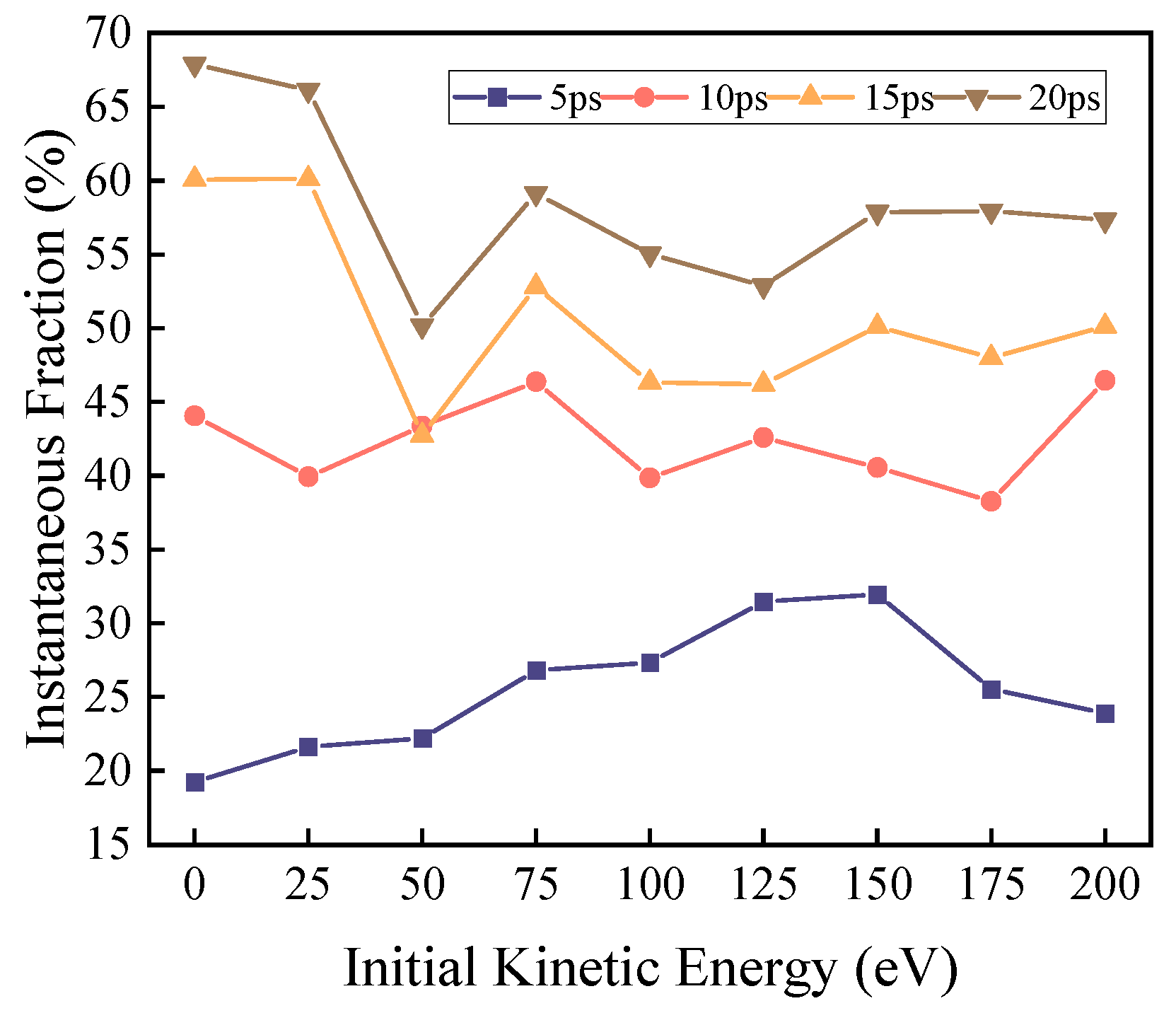
According to the experimental setup, reactive oxygen atoms are uniformly deposited within the 0–20 ps timeframe, and the DBP decomposition reaction primarily occurs within 0–20 ps. Therefore, the analysis of products and reaction pathway weights focuses mainly on the 0–20 ps period. The characteristic products corresponding to different reaction pathways are observed separately.
The zero-kinetic-energy group, which excludes the physical effects of kinetic energy, reflects the pure chemical effect of the reaction between reactive oxygen atoms and DBP and can serve as a control group. Hence, the reaction behavior of the zero-kinetic-energy group is analyzed first.
Under zero kinetic energy conditions, the weight of the mono-side chain cleavage reaction remains consistently high. Considering that the reactive oxygen atoms in the zero-kinetic-energy group require time to diffuse within the system and make extensive contact with DBP after 5 ps, the decomposition reaction occurs comprehensively. At this point, the weight of the mono-side chain cleavage reaction remains above 80%, while the weights of the dual-side chain cleavage reaction and benzene ring cleavage reaction continue to decrease. This indicates that for the zero-kinetic-energy group, the reactive oxygen atoms have low activation energy and weak oxidation capacity, resulting in incomplete DBP decomposition.
It is noted that ϕHO increased by 47% at 20 ps compared to 5 ps and continues to rise over time, indicating that a large number of reactive oxygen atoms are captured by free hydrogen atoms, forming stable small molecules such as H2O, and are unable to oxidize and decompose DBP. This indirectly demonstrates the weak oxidation capacity of reactive oxygen atoms in the absence of kinetic energy.
The yield of C12 exhibits a single peak at t = 5 ps, with the peak located around 25 eV. Meanwhile, its yield decreases with increasing kinetic energy, and the weight of the mono-side chain cleavage reaction pathway continuously decreases as kinetic energy rises. The peak yield is primarily due to the fact that even with a very low initial kinetic energy (25 eV) imparted to the reactive oxygen atoms, the reaction rate is significantly increased compared to the zero-kinetic-energy group. Thus, the C12 generation rate of the 25 eV group increased by 26.8% compared to the zero-kinetic-energy group within the same timeframe. As kinetic energy further increases, the weight of the mono-side chain cleavage reaction decreases, leading to a decline in C12 yield.
The yield of C8 shows a single peak at t = 5 ps, with the peak located around 150 eV. Meanwhile, its yield initially increases and then decreases with rising kinetic energy. The weight of the dual-side chain cleavage reaction pathway generally exhibits an increasing trend with increasing kinetic energy, reaching a plateau at 75 eV and subsequently maintaining fluctuations. At this point, this reaction pathway approaches saturation.
The yield of C6 displays a single peak at t = 5 ps, with the peak located around 175 eV. Meanwhile, its yield initially increases and then decreases with rising kinetic energy. The weight of the benzene ring cleavage reaction pathway generally shows an increasing trend with increasing kinetic energy, fluctuating within the 125–200 eV range, while increasing overall.
The yield of small molecules continuously increases over time at the same time point. The oxygen-containing fraction ϕHO gradually increases within the 0–150 eV range at the same time point, peaks at 150 eV, and then gradually decreases. Under the same kinetic energy (any kinetic energy), ϕHO continuously increases over time.
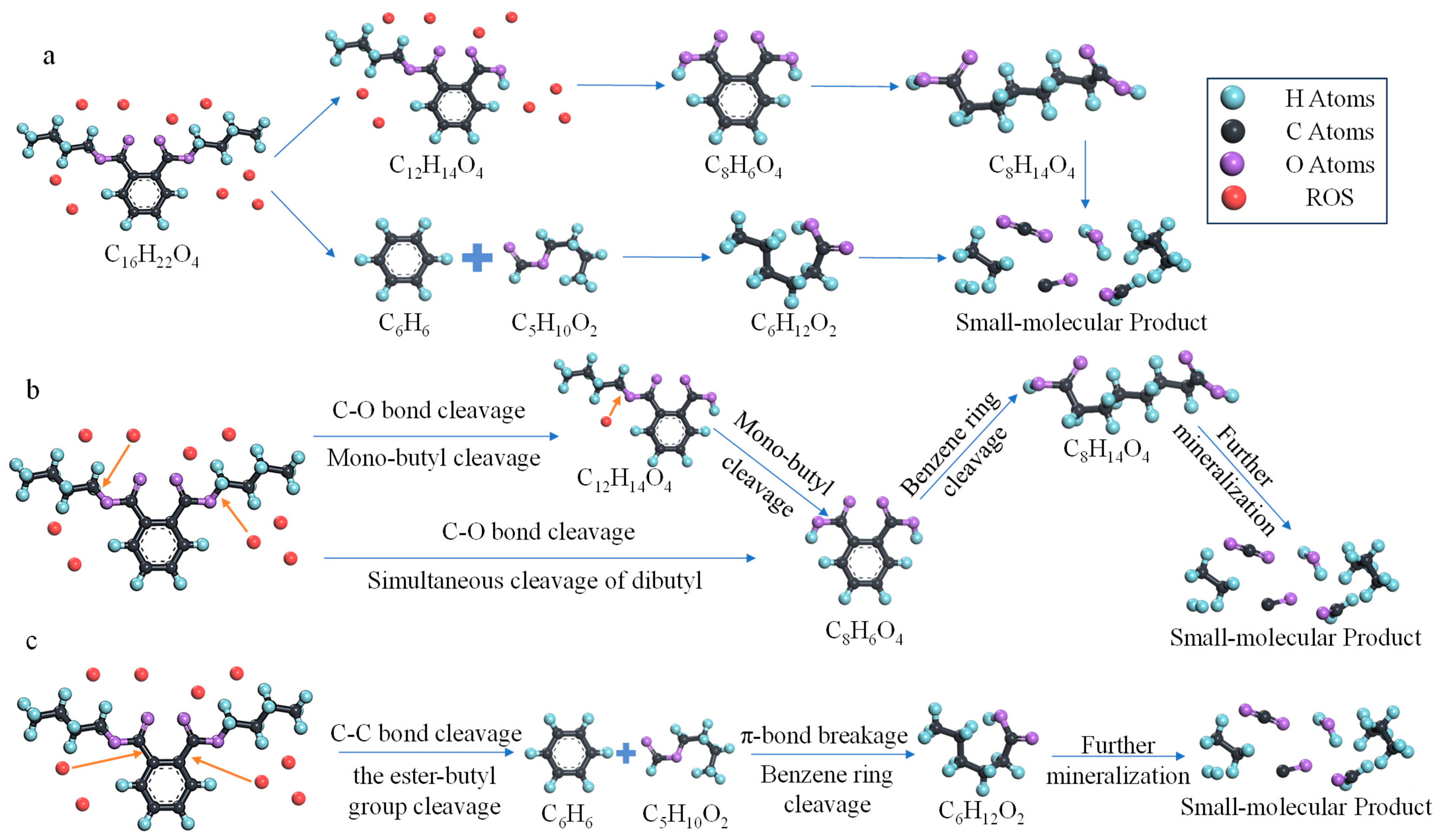
Analysis of the simulation results indicates that the initial kinetic energy of the ROS does not linearly enhance all decomposition pathways. Instead, it selectively activates different reaction channels by overcoming the energy barriers of various reaction pathways, governing the entire process from initial cleavage to deep decomposition of DBP molecules. Overall, an activation sequence of mono-butyl side chain cleavage → dual-butyl side chain cleavage → direct aromatic ring cleavage is observed. This suggests that as the initial kinetic energy of ROS increases from 0.0083 eV to 200 eV, the reaction system sequentially surmounts three increasing reaction energy barriers. The specific reaction pathway analysis is as follows.
First, the variation in the instantaneous abundance of C12 (characteristic product of mono-butyl side chain cleavage) with time and initial kinetic energy indicates that initial kinetic energy within a relatively low range (25 eV) can significantly promote mono-butyl side chain cleavage (compared to the zero-kinetic-energy group). At this stage, this reaction pathway is the most sensitive to kinetic energy and is activated first. This may originate from the spatial exposure of the butyl side chain, which has minimal shielding and undergoes strong collisions first. Moreover, the C-O single bond connecting the ester group and the butyl group is relatively weak, making it the most vulnerable point for cleavage under low-energy perturbations. The aromatic ring skeleton, located inward within the molecule and surrounded by a strong electron cloud, requires higher kinetic energy to provide sufficient energy transfer for the reaction to occur. Thus, kinetic energy significantly increases the collision efficiency between ROS and the C-O single bond near the ester group, overcoming the reaction energy barrier and more readily initiating oxidative cleavage. However, as kinetic energy further increases (25–200 eV), the C12 yield continuously decreases, and its reaction weight declines within the 0–200 eV range. This phenomenon indicates that at higher initial kinetic energies, other reaction pathways are activated, leading to a shift in reaction pathways: mono-side chain cleavage is replaced by other pathways, such as simultaneous dual-side chain cleavage or direct benzene ring cleavage. Furthermore, as initial kinetic energy increases, the activity of ROS further enhances, and C12 products are further decomposed, unable to exist stably as reaction intermediates. Consequently, their weight and current yield decrease. The changes in the instantaneous abundance and reaction weight of C12 products vividly demonstrate the catalytic effect of physical action on specific chemical reactions.
Second, the instantaneous abundance of C8 (characteristic product of dual-butyl side chain cleavage) generally shows an increasing trend within the 100–150 eV kinetic energy range, indicating that increased kinetic energy effectively promotes dual-side chain cleavage. The corresponding trend in reaction weight further demonstrates that within the 0–75 eV range, the dual-side chain cleavage pathway is effectively activated in this kinetic energy interval and gradually becomes the dominant process as initial kinetic energy increases. Compared to the mono-side chain cleavage pathway, the dual-side chain cleavage pathway has a higher energy barrier, thus requiring higher initial kinetic energy for activation. Moreover, compared to low-kinetic-energy conditions, higher kinetic energy results in stronger ROS activity, which does not diminish after reacting with a single side chain. After the initial kinetic energy exceeds 75 eV, the reaction weight reaches a plateau, indicating that the dual-side chain cleavage pathway saturates beyond 75 eV. This may be because increased kinetic energy only promotes a higher overall decomposition reaction rate without further enhancing the selectivity of this pathway, while the benzene ring cleavage reaction is activated, leading to a balanced reaction weight for dual-side chain cleavage. The changes in the instantaneous abundance and reaction weight of C8 products illustrate that as kinetic energy increases, higher energy input drives the reaction pathway toward more intense and thorough decomposition. Simultaneously, the physical impact of initial kinetic energy makes the molecular structure of DBP more susceptible to destruction, which is difficult to achieve with pure chemical etching in the zero-kinetic-energy group.
Third, the instantaneous abundance of C6 (characteristic product of direct benzene ring cleavage) generally increases and exhibits fluctuations within the 0–175 eV kinetic energy range. This reflects the dynamic competition among reaction pathways. The slight decreases in reaction weight at 75 eV and 125 eV may correspond to the peak of dual-butyl side chain cleavage, where dual-side chain cleavage consumes some ROS, reducing the probability of direct reaction with the benzene ring. After 125 eV, the weight of the benzene ring cleavage pathway increases and reaches its peak. This indicates that higher initial kinetic energy significantly promotes direct benzene ring cleavage, making it an important deep decomposition pathway. This is because the benzene ring, due to its conjugated system, is highly stable and difficult to directly cleave at low and medium kinetic energies. Thus, higher energy is required to break the aromatic ring. Increased kinetic energy enables ROS to effectively insert into π-bonds, causing ring opening and generating ring-opening products. This trend is consistent with the mechanism of high-energy particle-induced aromatic ring cleavage, where the kinetic energy of ROS helps overcome steric hindrance and electron cloud repulsion. This clearly demonstrates that high-kinetic-energy physical bombardment is an effective means to overcome high reaction energy barriers and achieve complete decomposition of contaminants.
Small molecule products, as characteristic indicators of the complete decomposition of organic contaminants, show a continuous increase in instantaneous abundance over time, consistent with the gradual nature of oxidative decomposition. Regardless of the initial kinetic energy, the continuous generation of small molecules indicates that DBP decomposition ultimately tends toward complete decomposition. Once larger product fragments are generated, they continue to break down into subsequent chain oxidation processes, producing final products such as CO2 and H2O. Increased initial kinetic energy only enhances the generation rate and quantity of small molecules without involving the selection of reaction pathways. This indicates that regardless of the intermediate pathway chosen, higher energy input will eventually lead to decomposition into small molecules, and increasing the initial kinetic energy of ROS remains an effective way to achieve complete decomposition of organic contaminants.
The variation of ϕHO provides a measure of the system’s oxidation degree. At the same time point, ϕHO gradually increases within the 0–150 eV kinetic energy range, peaks at 150 eV, and then gradually decreases. This indicates that increased initial kinetic energy facilitates oxidation reactions. The oxidative decomposition of DBP is essentially a dehydrogenation and oxidation process. High-kinetic-energy ROS possesses higher collision energy, more effectively breaking C-H bonds in molecules for dehydrogenation, and the detached hydrogen atoms rapidly combine with reactive oxygen. Moreover, H2O and H2O2 are typically final products of deep oxidation, and their increased proportion indicates that the reaction is progressing toward complete decomposition. The decrease in ϕHO after further increases in kinetic energy may be due to overly intense decomposition reactions caused by high-energy collisions, generating a large number of hydrogen-rich free radicals and producing H2. Meanwhile, ROS are further captured by DBP molecules and react, leading to a decrease in the oxygen-containing proportion. The continuous increase in ϕHO over time at the same kinetic energy also illustrates that DBP decomposition ultimately tends toward complete decomposition, with released H atoms continuously reacting with ROS to form H2O and H2O2.
The aforementioned research has confirmed that the initial kinetic energy of ROS serves as a key physical parameter playing a decisive role in the degradation process of DBP. However, in actual plasma cleaning processes, the reaction is coordinately regulated by plasma parameters (flux, irradiation dose NROS) and environmental parameters (ambient temperature). To systematically reveal the multi-parameter synergistic mechanism, this study investigates the quantitative relationship between irradiation flux, irradiation dose, ambient temperature, and the efficiency and extent of DBP removal, exploring how these parameters synergize with the initial kinetic energy of ROS to influence the decomposition reaction efficiency.
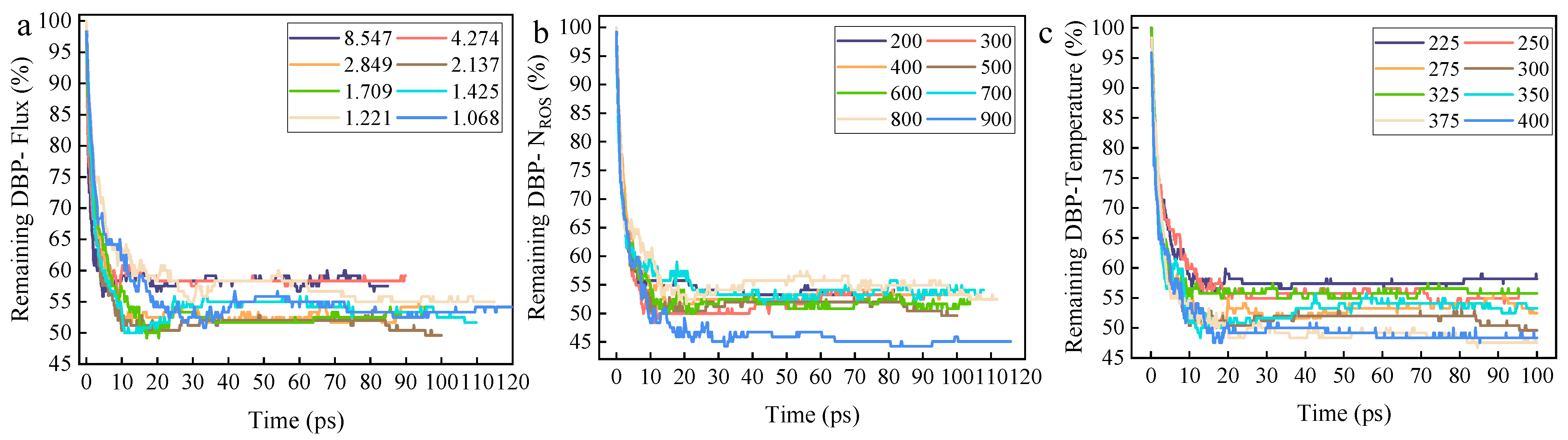
First, according to the selected experimental design, a stepped experiment with eight gradients was conducted for each characteristic parameter. The DBP residue ratio for each group was calculated, and the data are plotted in
Figure 6a–c. Data analysis reveals that the final residue ratio of DBP initially decreases and then increases with rising irradiation flux, while it decreases approximately monotonically with increasing irradiation dose and ambient temperature.
The non-monotonic change in DBP residue with irradiation flux—first decreasing and then increasing—indicates that there exists an optimal window of irradiation flux for enhancing DBP removal rate. In the lower flux range, increasing the flux significantly improves the coverage of active sites per unit time and the advancement rate of the reaction front. Combined with the deep penetration capability of high-kinetic-energy ROS, this accelerates the reaction through a spatiotemporal synergistic effect: high-kinetic-energy ROS are responsible for in-depth breakthrough, while high flux ensures sufficient active particles to fill and clean the opened reaction space, thereby significantly reducing the residue. However, when the flux exceeds the optimal value, the residue increases instead of decreasing. This may stem from surface passivation or competitive consumption induced by over-irradiation. An excessively high flux may cause a sharp increase in the concentration of ROS in the surface region, potentially leading to self-recombination reactions between ROS, resulting in the ineffectual consumption of active species; high-frequency particle bombardment may cause excessive cross-linking or carbonization of surface organic matter, forming a dense inert carbon layer that blocks contact between internal DBP and ROS.
Secondly, irradiation dose exhibits a monotonic negative correlation with DBP residue, reflecting the cumulative effect of reaction energy input. Regardless of the kinetic energy of individual ROS, an increase in the total dose directly implies an increase in total energy input and the total number of active particles. In the initial stage of the reaction, ROS first react with molecules on the surface of the organic contaminant layer, while the high initial kinetic energy of ROS allows them to penetrate the DBP molecular layer, forming reaction channels. Subsequently, sustained irradiation with a sufficient number of ROS utilizes these reaction channels to continue advancing into the interior of the DBP molecular layer, reacting with a larger area of DBP molecules to complete the cleaning reaction and significantly reduce the DBP residue ratio. The irradiation dose ensures the thoroughness of the reaction, effectively complementing the reaction rate promoted by kinetic energy.
Finally, an increase in ambient temperature monotonically promotes DBP removal, indicating a positive synergistic effect between thermal effects and particle kinetic energy effects. Firstly, the temperature rise provides an additional thermodynamic driving force for all reaction pathways (especially the high-energy-barrier benzene ring opening reaction), reducing the effective energy barrier that ROS need to overcome, enabling even ROS with relatively low kinetic energy to initiate more profound decomposition reactions. Secondly, the temperature increase significantly amplifies the thermal motion amplitude of DBP molecular chains, promoting the desorption and diffusion of reaction products and preventing them from blocking the reaction interface. Therefore, the coupling of the thermal kinetic energy provided by the ambient temperature and the initial kinetic energy of ROS collectively determines the effective energy per collision, more effectively exciting the reaction pathways.
The aforementioned simulation results can also be compared and validated against our team’s previous experimental findings [
10,
17,
22]. Experimentally, it was observed that the decomposition rate and efficiency of DBP significantly improved with the optimization of plasma power, yet the underlying mechanism behind how power enhancement influences the decomposition process could not be elucidated. Molecular dynamics simulations similarly reflect this trend and, moreover, provide a rational analysis demonstrating that the kinetic energy of plasma is a critical factor affecting the decomposition reaction. Experimentally, even after cleaning, residual carbon signals were detected on the sample surface by XPS, which can be attributed to stable aromatic intermediates. The simulations reveal that cleavage of the benzene ring requires a higher energy barrier compared to side-chain cleavage. The simulation results thereby explain why complete carbon removal is so challenging in experiments. Similarly, the variation in spectral excitation intensity across different measurement regions and differing irradiation times in experiments corresponds, respectively, to variations in plasma flux and irradiation dose in the simulations, and their influence on the DBP removal rate and efficiency follows identical trends. Thus, although certain parameters in the molecular dynamics simulations were scaled to ensure computational feasibility, the consistency in trends between the simulation and experimental outcomes supports the reliability of the model.
2.3. Analysis of the Reaction Mechanism of ROS Based on Diffusion and Energy Characteristics
The aforementioned research results indicate that the initial kinetic energy of ROS is a key physical parameter governing the selectivity of their reaction pathways with organic contaminants. However, the influence of kinetic energy does not exist in isolation; the final macroscopic reaction efficiency and selectivity are the microscopic outcome of the combined effects of the transport and diffusion behavior of ROS within the contaminant matrix and the local reaction energy. To deeply reveal the essence of how initial kinetic energy influences the reaction mechanism, this section will further integrate the mean square displacement (MSD) of oxygen plasma components, the penetration depth, and the evolution of the system’s kinetic and potential energy. From the perspectives of diffusion dynamics and system energy distribution, a comprehensive analysis of the intrinsic relationship between the transport capacity of ROS, their energy state, and the ultimate reaction efficiency and pathway selection will be conducted.
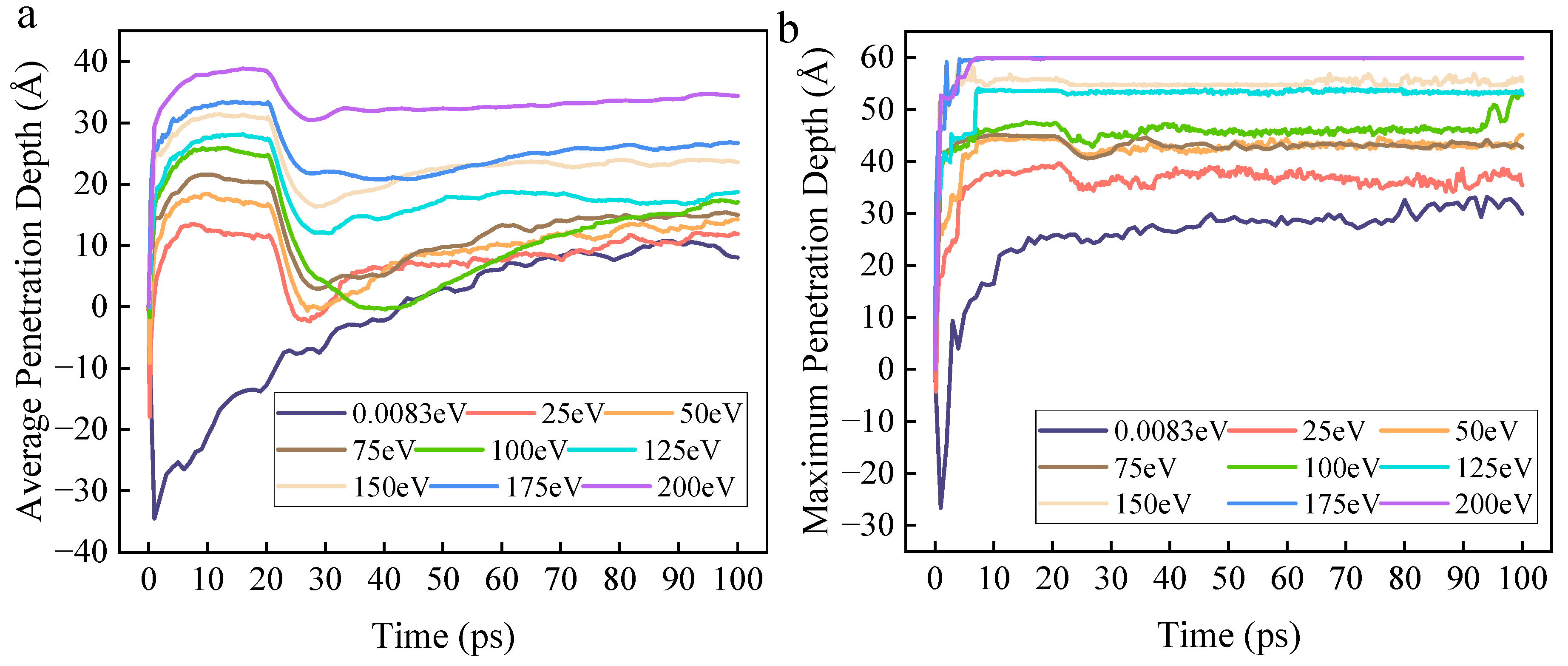
Firstly, based on atomic coordinates, the penetration depth of ROS into the organic contaminant molecular layer can be calculated. The relationships of the average penetration depth and the maximum penetration depth with time and initial kinetic energy are shown in
Figure 7a,b, respectively. Since ROS are incident from a fixed height above the DBP molecular layer, the penetration depth is negative at the beginning of the reaction. The data results show that the average penetration depth of the zero-kinetic-energy group increases from 0 to 100 ps and eventually stabilizes. For the groups with kinetic energy, the average penetration depth increases rapidly within 0–10 ps, enters a plateau phase between 10–20 ps, then decreases rapidly, and finally increases slowly toward a steady state. Moreover, as the kinetic energy increases, the average penetration depth gradually increases. The maximum penetration depth increases progressively over time and with increasing kinetic energy.
Specifically, the average penetration depth of the zero-kinetic-energy group increases monotonically with time and eventually stabilizes. This is because the initial kinetic energy of zero causes ROS to rely primarily on concentration gradient-driven, relatively slow random diffusion until they achieve a uniform distribution in the simulation system, resulting in limited penetration capability into the organic contaminant molecular layer. In stark contrast, the average penetration depth of the groups with initial kinetic energy exhibits a complex pattern characterized by “rapid rise-plateau-decline-slow increase.” Within the 0–10 ps range, high-kinetic-energy ROS utilize their initial kinetic energy to overcome intermolecular forces and directionally penetrate the DBP molecular layer, with higher kinetic energy leading to greater penetration depth. This stage is dominated by physical effects, with chemical reactions being unable to compete. During the 10–20 ps range, a transient balance is achieved between the physical bombardment effect and the consumption of ROS by chemical reactions. At this stage, ROS have penetrated to a certain depth within the DBP molecular layer, significantly increasing the contact area with DBP molecules and drastically raising the probability of reaction. This leads to the consumption of a large number of ROS by reactions before they can reach their potential maximum penetration depth, causing the growth of the average penetration depth to stagnate.
After passing the plateau phase (when ROS insertion stops), the average penetration depth decreases rapidly. The rate of longitudinal diffusion of ROS begins to fall below the consumption rate due to reactions in the surface region, causing the reaction front to recede toward the surface of the molecular layer. Simultaneously, ROS that have reacted to form small molecules gradually escape from the simulation system due to diffusion, macroscopically manifesting as a decrease in average penetration depth. Subsequently, as the reactant concentration decreases due to consumption, the simulation system gradually enters a stable reaction period. A large number of ROS that have participated in reactions and become small molecules gradually escape, and the kinetic energy of the ROS is largely depleted, preventing further penetration into deeper layers. This results in a slow increase in the average penetration depth.
The continuous increase in maximum penetration depth with time and kinetic energy demonstrates that, although the average penetration depth fluctuates and exhibits plateaus due to chemical reactions, an increase in kinetic energy enables some ROS to break through the barrier of reaction consumption and reach the deepest part of the contaminant molecular layer. This greatly increases the collision frequency between ROS and deep-layer DBP molecules, as well as the probability of chemical reactions occurring. Furthermore, this avoids the scenario where the reaction depth of ROS is too shallow, causing subsequently irradiated ROS to only further decompose surface reaction intermediates rather than decompose more DBP molecules. This further contributes to an increase in the DBP decomposition rate.
In summary, an increase in initial kinetic energy enhances the initial injection capability of ROS, rapidly expanding the early reaction interface. Although intense decomposition reaction consumption limits the increase in penetration depth, the strong penetration capability conferred by high kinetic energy ensures that ROS can react with deep-layer DBP molecules, preventing reaction stagnation due to diffusion limitations.
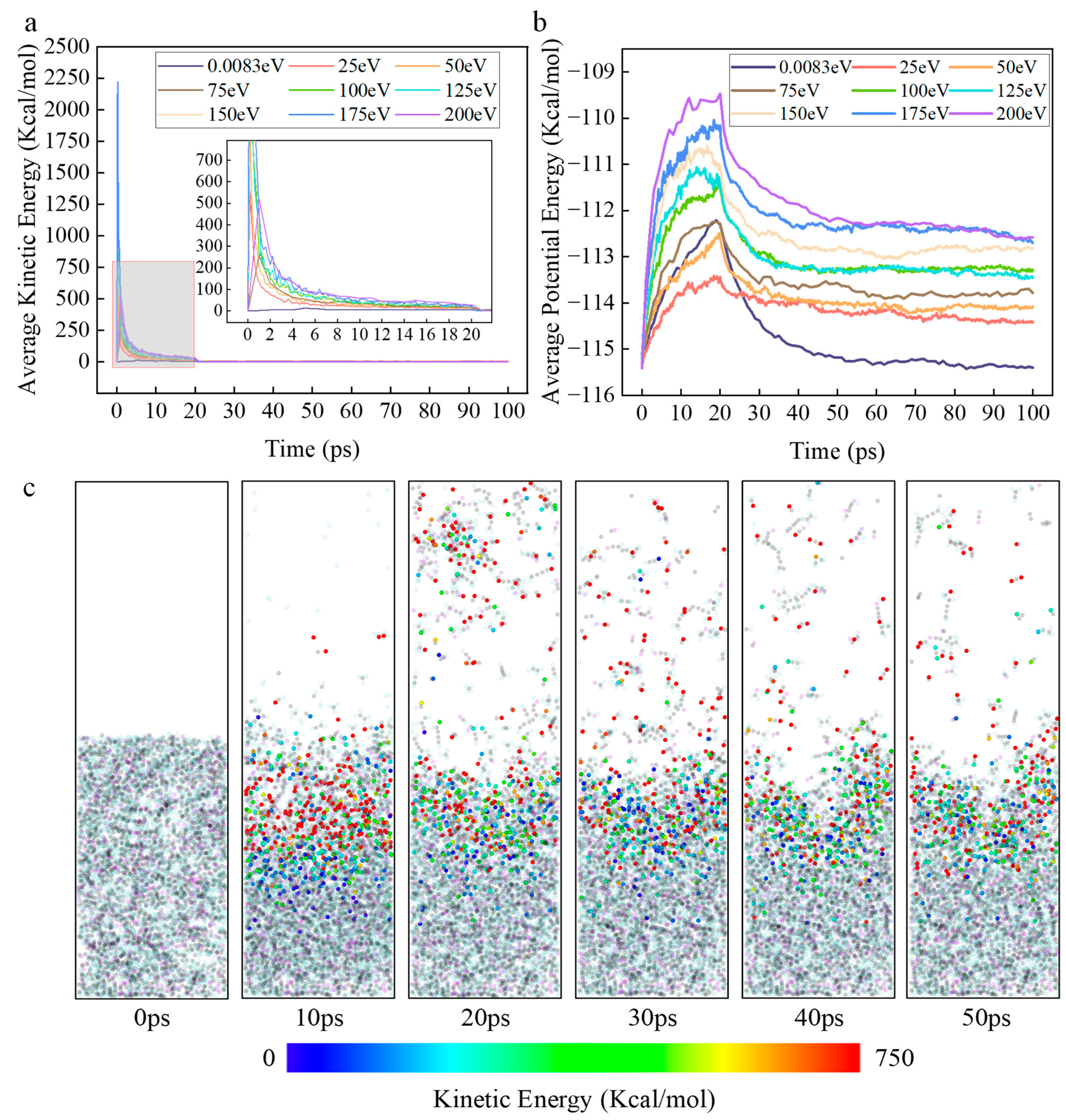
Secondly, the variation in the average kinetic energy of ROS in the simulation system with time and initial kinetic energy, and the variation in the potential energy of the simulation system with time and initial kinetic energy, obtained through software processing, are shown in
Figure 8a,b, respectively.
Figure 8c displays the kinetic energy cloud map illustrating the change in kinetic energy of ROS particles over time during the simulated reaction process.
It was experimentally observed that the average kinetic energy of ROS in all groups with kinetic energy decreases sharply in the initial stage (0–20 ps) and eventually approaches zero, and the higher the initial kinetic energy, the slower the kinetic energy decay rate. This phenomenon clearly indicates that intense energy exchange and dissipation occur between the initial kinetic energy of ROS and the DBP system. The mechanism is that high-kinetic-energy ROS undergo inelastic collisions with DBP, converting their kinetic energy into two forms of energy: collisions cause vibration, stretching, and torsion of chemical bonds in DBP molecules, directly leading to an increase in the system’s potential energy, providing activation energy for chemical reactions; kinetic energy is dissipated by exciting molecular translation and rotation, equivalently raising the local instantaneous temperature of the simulation system.
Between 0–20 ps, the system potential energy increases uniformly and is positively correlated with the initial kinetic energy of ROS. This stage corresponds to the process of ROS being inserted stepwise and undergoing numerous collisions with DBP molecules. The higher the initial kinetic energy, the greater the total energy flux injected, so more energy is converted into potential energy within chemical bonds, exciting the simulation system to a higher energy barrier.
After 20 ps, the system potential energy of the zero-kinetic-energy group decreases more rapidly compared to the groups with kinetic energy and stabilizes at a lower steady-state value. This indicates that its energy release process is primarily completed through reactions after ROS diffusion with the surface of the DBP molecular layer, where the reaction area is limited, and the system quickly reaches energy balance. In contrast, after imparting initial kinetic energy, the system’s potential energy is maintained at a higher steady-state level. This phenomenon indicates that high initial kinetic energy endows ROS with high penetration capability, enabling reactions to occur not only on the surface. High kinetic energy results in an overall higher system potential energy level, meaning more DBP molecules are elevated above the reaction energy barrier. This directly leads to a faster reaction rate. Meanwhile, the slower the kinetic energy decay, the more sustained the energy supply, continuously driving decomposition and further increasing the decomposition rate. Secondly, the higher system of potential energy creates conditions for reaction pathways requiring higher energy activation. This is consistent with the previous analysis. For example, the ring-opening reaction of the benzene ring requires far more energy than the cleavage of the butyl side chain. Due to the low overall potential energy level, reactions in the low-kinetic-energy group preferentially occur along low-energy-barrier pathways. In contrast, high kinetic energy maintains a high potential energy environment, allowing high-energy-barrier pathways to occur and gradually become the dominant reaction pathways.
Subsequently, the variation in the mean MSD of DBP molecules and the MSD of ROS with time and kinetic energy, calculated based on atomic coordinates, is shown in
Figure 9a,b, respectively. Under all incident kinetic energies, the MSD of DBP rises rapidly within 0–10 ps, exhibiting a significant transient peak at 30–45 ps. This peak shows a non-monotonic variation with kinetic energy: medium kinetic energies (approximately 100–125 eV) produce the highest overshoot, while lower (50–75 eV) and higher (150–200 eV) kinetic energies yield smaller peaks. After the peak, the MSD gradually decreases over approximately 20–40 ps, stabilizing for most cases by 80–100 ps. The mobility of ROS is significantly higher than that of DBP. Its MSD climbs rapidly within 0–10 ps. Multiple curves show an early plateau at 10–20 ps, followed by a decline and stabilization between 40–60 ps. As kinetic energy increases, the initial slope and steady-state level generally rise. The positive correlation between MSD of ROS and kinetic energy indicates enhanced transport that assists chemical reactions by increasing encounter frequency and penetration; however, the elementary removal steps remain chemical in nature. High-kinetic-energy ROS significantly enhance the effective collision frequency with DBP molecules through increased mobility (higher MSD slope and steady-state value), which is the primary reason for the macroscopic reaction rate increase with kinetic energy. Furthermore, the data indicate that medium kinetic energies (~100–125 eV) most effectively excite transient large-scale structural reorganization in DBP molecules, a phenomenon closely related to reaction pathway selection. This energy range most efficiently converts the kinetic energy of ROS into energetic excitation of DBP molecules, whereas excessively high kinetic energy may lead to over-penetration, dissipating energy before it is fully transferred to DBP; insufficient kinetic energy fails to effectively excite DBP motion. The mobility of ROS is markedly higher than that of DBP (MSD is 2–5 times greater), and this gap further widens at higher kinetic energies. This implies that ROS remains the driver of the reaction. This asymmetry in mobility allows the reaction front to advance rapidly into the material interior, while DBP fragments remain relatively localized. This explains why the reaction removal rate is higher under high kinetic energy conditions.