Design, Synthesis and Herbicidal Activity of 1,2,4-Oxadiazole Compounds as Novel Light-Dependent Protochlorophyllide Oxidoreductase Inhibitors
Abstract
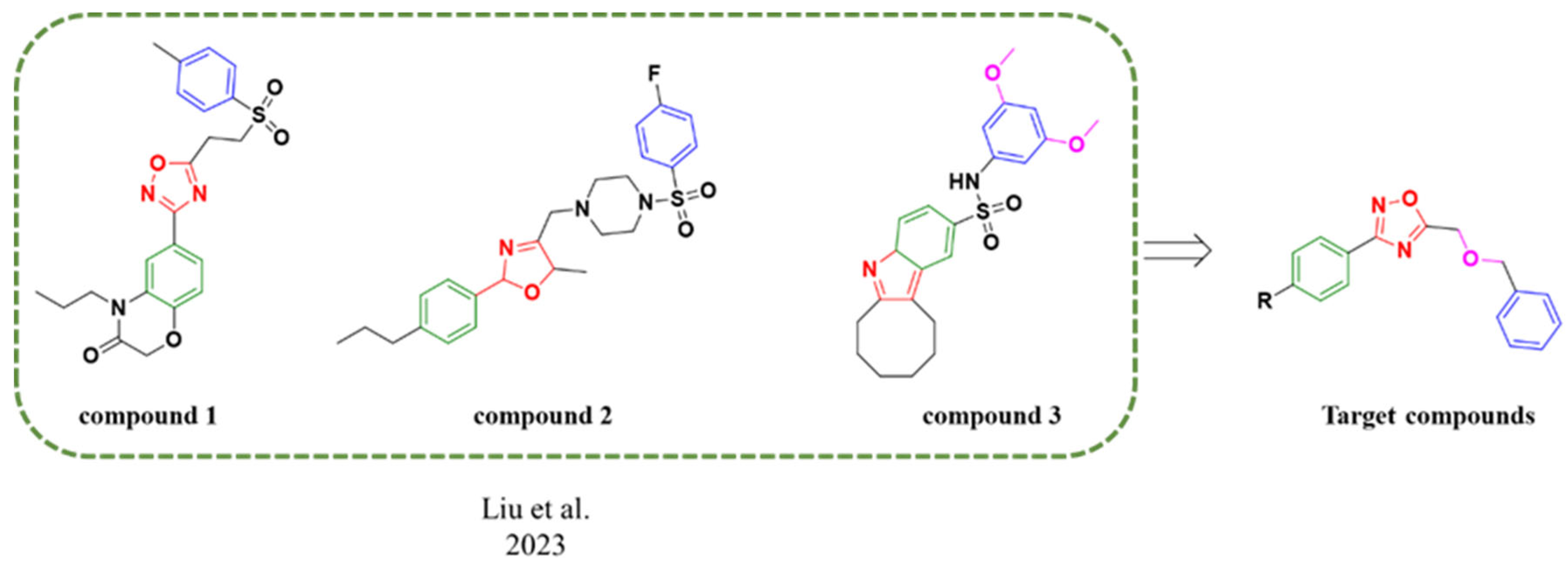
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Herbicidal Activity Assay and SAR
| Compound | Dosage (g ai/ha) | |||
|---|---|---|---|---|
| 37.5 | 75 | 150 | ||
| 5a | 4-OCH2CH3 | 37.5 ± 1.8 | 50.3 ± 1.1 | 66.1 ± 4.5 |
| 5b | 3-Cl | 48.2 ± 1.6 | 62.3 ± 3.8 | 83.5 ± 1.5 |
| 5c | 4-Cl | 45.4 ± 3.1 | 67.5 ± 3.8 | 75.5 ± 0.3 |
| 5d | 3-F | 38.3 ± 2.1 | 68.3 ± 2.1 | 74.6 ± 1.3 |
| 5e | 4-F | 37.5 ± 3.0 | 56.4 ± 3.3 | 68.5 ± 2.1 |
| 5f | 3-I | 32.3 ± 1.9 | 53.0 ± 2.3 | 62.2± 1.7 |
| 5g | 4-I | 28.1 ± 3.1 | 57.4 ± 1.1 | 70.3 ± 1.8 |
| 5h | 3-Br | 45.3 ± 0.9 | 40.0 ± 0.8 | 53.8 ± 2.8 |
| 5i | 4-Br | 47.2 ± 1.0 | 57.7 ± 1.8 | 57.4 ± 1.1 |
| 5j | 3-NO2 | 80.2 ± 1.4 | 92.6 ± 0.5 | 92.3 ± 2.6 |
| 5k | 4-NO2 | 82.3 ± 2.6 | 98.6 ± 1.4 | 94.6 ± 1.9 |
| 5l | - | 22.9 ± 0.8 | 27.4 ± 3.5 | 33.9 ± 2.8 |
| 5m | 4-SCH3 | 23.0 ± 1.3 | 28.7 ± 2.9 | 55.2 ± 1.3 |
| 5n | 4-CH3 | 16.1 ± 2.4 | 26.7 ± 0.8 | 40.4 ± 3.1 |
| 5o | 4-OCH3 | 27.9 ± 1.5 | 35.6 ± 3.1 | 43.6 ± 1.4 |
| 5p | 4-Ph | 44.6 ± 1.9 | 48.4 ± 0.8 | 53.2 ± 2.5 |
| 5q | 4-OCH2Ph | 88.9 ± 2.1 | 93.6 ± 1.2 | 92.3 ± 2.6 |
| 5r | 4-NCH3 | 12.3 ± 1.4 | 27.4 ± 3.5 | 34.2 ± 3.1 |
| 5s | 3,5-CH3 | 17.3 ± 2.6 | 25.7 ± 3.0 | 36.4 ± 4.0 |
| 5t | 3,5-OCH3 | 33.8 ± 1.8 | 35.5 ± 2.3 | 47.4 ± 2.9 |
| SUT | - | 83.8 ± 2.3 | 94.6 ± 1.9 | 94.6 ± 1.9 |
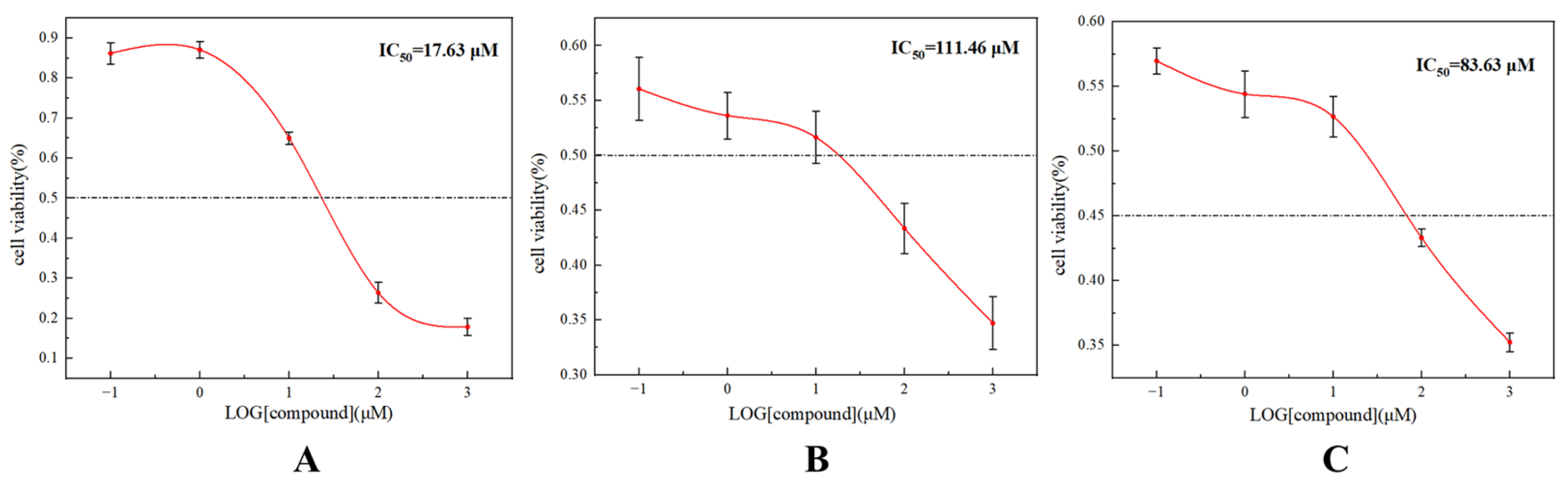
2.3. LPOR Inhibitory Experiments
2.4. Crop Selectivity Test
2.5. Molecular Docking
2.6. Molecular Dynamic Simulation
3. Materials and Methods
3.1. Equipment and Materials
3.2. Synthesis
3.2.1. General Procedures for Synthesis of Compound 2
3.2.2. General Procedures for Synthesis of Compound 4
3.2.3. General Procedures for Synthesis of Compound 5
3.3. Herbicidal Activity Assay
3.4. Determination of LPOR Enzyme Inhibitory Activity in Vitro
3.5. Crop Selectivity
3.6. Molecular Docking
3.7. Molecular Dynamic Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horvath, D.P.; Clay, S.A.; Swanton, C.J.; Anderson, J.V.; Chao, W.S. Weed-induced crop yield loss: A new paradigm and new challenges. Trends Plant Sci. 2023, 28, 567–582. [Google Scholar] [CrossRef]
- Zhao, L.X.; Chen, K.Y.; He, X.L.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and biological activity determination of novel phenylpyrazole protoporphyrinogen oxidase inhibitor herbicides containing five-membered heterocycles. J. Agric. Food Chem. 2023, 71, 14164–14178. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M. Current state of herbicides in herbicide-resistant crops. Pest Manage. Sci. 2014, 70, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.Q.; Qu, M.H.; Wang, G.; Ma, Z.Y.; Chen, X.G.; Zhou, Z.H.; Qi, J.T.; Gao, X.M.; Li, H.L.; Jia, H.L. Crop detection technologies, mechanical weeding executive parts and working performance of intelligent mechanical weeding: A review. Front. Plant Sci. 2024, 15, 1361002. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjo̷th, C.A.; de Weger, L.A.; Lommen, S.T.; Augustinus, B.A.; Bonini, M.; Karrer, G.; Sikoparija, B.; et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat. Commun. 2020, 11, 1745. [Google Scholar] [CrossRef]
- Morin, L. Progress in biological control of weeds with plant pathogens. Annu. Rev. Phytopathol. 2020, 58, 201–223. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef]
- Han, S.; Wang, S.; Fu, S.; Chen, K.; Gao, W.; Cheng, Y.; Liu, M.; Zhang, X.; Lei, K. Design, synthesis, and herbicidal activity of novel 5 acylbarbituric acid derivatives containing maleimide moieties and evaluation of their mode of action. J. Agric. Food Chem. 2025, 73, 11386–11398. [Google Scholar] [CrossRef]
- Qu, R.Y.; He, B.; Yang, J.F.; Lin, H.Y.; Yang, W.C.; Wu, Q.Y.; Li, Q.X.; Yang, G.F. Where are the new herbicides? Pest Manage. Sci. 2021, 77, 2620–2625. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manage. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Meng, Y. Theoretical and Experimental Studies on Plant Light-Dependent Protochlorophyllide Oxidoreductase as a Novel Target for Searching Potential Herbicides. J. Agric. Food Chem. 2023, 71, 11654–11666. [Google Scholar] [CrossRef]
- Liu, R.; Shang, J.; Gao, Z.; Miao, J.; Tian, Y.; Hu, X.; Lu, H. Photo/thermo bi-cube mechanism of proton-coupled electron transfer reaction catalyzed by plant light-dependent protochlorophyllide oxidoreductase. J. Catal. 2025, 448, 116158. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Q. Origin and evolution of the light-dependent protochlorophyllide oxidoreductase (LPOR) genes. Plant Biol. 2004, 6, 537–544. [Google Scholar] [CrossRef]
- Heyes, D.J.; Hunter, C.N. Making light work of enzyme catalysis: Protochlorophyllide oxidoreductase. Trends Biochem. Sci. 2005, 30, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Zhang, X.; He, B.; Diao, L.P.; Sheng, S.L.; Wang, J.L.; Guo, X.P.; Su, N.; Wang, L.F.; Jiang, L.; et al. A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol. 2007, 145, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Jiang, Y.; Xu, C.; Sun, X.Y.; Ma, C.; Xia, Z.; Zhao, H.Q. Oleanane-type triterpene conjugates with 1H-1,2,3-triazole possessing of fungicidal activity. Molecules 2022, 27, 4928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.G.; Liang, C.G.; Sun, Y.Q.; Teng, P.; Wang, J.Q.; Zhang, W.H. Design, synthesis and antifungal activities of novel pyrrole- and pyrazole-substituted coumarin derivatives. Mol. Divers. 2019, 23, 915–925. [Google Scholar] [CrossRef]
- Hu, B.Y.; Zhao, H.Q.; Chen, Z.L.; Xu, C.; Zhao, J.Z.; Zhao, W.T. Efficient synthesis and bioactivity of novel triazole derivatives. Molecules 2018, 23, 709. [Google Scholar] [CrossRef]
- Ke, S.Y.; Qian, X.H.; Liu, F.Y.; Wang, N.; Yang, Q.; Li, Z. Novel 4H-1,3,4-oxadiazin-5(6H)-ones with hydrophobic and long alkyl chains: Design, synthesis, and bioactive diversity on inhibition of monoamine oxidase, chitin biosynthesis and tumor cell. Eur. J. Med. Chem. 2009, 44, 2113–2121. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl Com 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Heterocyclic Compounds: Importance in Anticancer Drug Discovery. Anticancer Agents Med. Chem. 2022, 22, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Achhireddy, N.R.; Kirkwood, R.C.; Fletcher, W.W. Oxadiazon absorption, translocation, and metabolism in rice (Oryza sativa) and barnyardgrass (Echinochloa crus-galli). Weed Sci. 1984, 32, 727–731. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Growth response of direct-seeded rice to oxadiazon and bispyribac-sodium in aerobic and saturated soils. Weed Sci. 2011, 59, 119–122. [Google Scholar] [CrossRef]
- de Ferreira, F.R.; Schapira, M. A systematic analysis of atomic protein-ligand interactions in the PDB. Med. Chem. Commun. 2017, 8, 1970–1981. [Google Scholar] [CrossRef]
- Yu, L.; Yang, K.; Yao, L.; Wang, N.; Kang, H.; Yao, G.; Li, X.; Qin, B. Synthesis and Antifungal Activity of 1,2,4-Oxadiazole Derivatives. Molecules 2025, 30, 1851. [Google Scholar] [CrossRef]
- Sharma, M.; Patton, Z.E.; Shoemaker, C.R.; Bacsa, J.; Biegasiewicz, K.F. N-Halogenation by Vanadium-Dependent Haloperoxidases Enables 1,2,4-Oxadiazole Synthesis. Angew Chem. Int. Ed. Engl. 2024, 63, e202411387. [Google Scholar] [CrossRef]
- Fu, W.; Yuan, Q.; Zhang, H.; Li, X.; Lu, Y.; Yan, W.; Ye, Y.; Liu, X.; Li, Z.; Shao, X. Novel SDH Inhibitors as Antifungal Leads: From Azobenzene Derivatives to the 1,2,4-Oxadiazole Compounds. J. Agric. Food Chem. 2024, 72, 24272–24282. [Google Scholar] [CrossRef]
- Unadkat, V.; Rohit, S.; Parikh, P.; Sanna, V.; Singh, S. Rational design-aided discovery of novel 1,2,4-oxadiazole derivatives as potential EGFR inhibitors. Bioorg. Chem. 2021, 114, 105–124. [Google Scholar] [CrossRef]
- Ou, Y.; Guo, X.; Zhang, Q.; Zhang, W.; Gan, X. Design, synthesis, and nematicidal activity of novel 1,2,4-oxadiazole derivatives containing amide fragments. Mol Divers. 2025, 29, 2293–2304. [Google Scholar] [CrossRef]
- Chen, H.; Bai, Z.; Chu, Z.; Wen, Y. Study on the effect of the chiral herbicide imazethapyr on flowering initiation in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2025, 293, 118049. [Google Scholar] [CrossRef]
- He, B.; Hu, Y.; Liu, D.; Zang, X.; He, X.; Chen, W.; Yang, J.; Feng, M.; Chen, P.; Wei, L.; et al. Substrate-based discovery of α-hydroxycarboxylic acid derivatives as potential herbicides targeting dihydroxyacid dehydratase. Nat. Commun. 2025, 16, 5205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, C.; Shen, T.; Ma, D.; Gu, Y.C.; Xu, H.; Xi, Z. Functional Identification of 1-Deoxy-d-Xylulose-5-Phosphate Synthase (DXPS) as an Effective Herbicide Target for the Discovery of Novel Potent Inhibitors through Virtual Screening. J. Agric. Food Chem. 2025, 73, 14839–14849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Luo, K.; Guo, X.D.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Design, Synthesis, and Biological Activity Evaluation of Novel Phenoxypyridine Derivatives Containing Acylthiourea Fragments as Protoporphyrinogen Oxidase Inhibitor Herbicides. J. Agric. Food Chem. 2025, 73, 5020–5032. [Google Scholar] [CrossRef] [PubMed]
- Schmermund, L.; Bierbaumer, S.; Schein, V.K.; Winkler, C.K.; Kara, S.; Kroutil, W. Extending the Library of Light-Dependent Protochlorophyllide Oxidoreductases and their Solvent Tolerance, Stability in Light and Cofactor Flexibility. ChemCatChem. 2020, 12, 4044–4051. [Google Scholar] [CrossRef]
- Dong, C.S.; Zhang, W.L.; Wang, Q.; Li, Y.S.; Wang, X.; Zhang, M.; Liu, L. Crystal structures of cyanobacterial light-dependent protochlorophyllide oxidoreductase. Proc. Natl. Acad. Sci. USA 2020, 117, 8455–8461. [Google Scholar] [CrossRef]
- Zhang, S.W.; Godwin, A.R.F.; Taylor, A.; Hardman, S.J.O.; Jowitt, T.A.; Johannissen, L.O.; Hay, S.; Baldock, C.; Heyes, D.J.; Scrutton, N.S. Dual role of the active site ‘lid’ regions of protochlorophyllide oxidoreductase in photocatalysis and plant development. FEBS J. 2021, 288, 175–189. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Melo, A.A.; Kruk, J.; Frost, A.; Gabruk, M. Photocatalytic LPOR forms helical lattices that shape membranes for chlorophyll synthesis. Nat. Plants. 2021, 7, 437–444. [Google Scholar] [CrossRef]
- Yang, Q.; Shen, Z.; Chen, R.; Yao, C.; Pan, Z.; Dong, X. Identification of Potent CDK9 Inhibitors with Novel Skeletons via Virtual Screening, Biological Evaluation, and Molecular Dynamics Simulation. J. Med. Chem. Lett. 2024, 15, 1654–1661. [Google Scholar] [CrossRef]
- Pabis, A.; Geronimo, I.; York, D.M.; Paneth, P. Molecular Dynamics Simulation of Nitrobenzene Dioxygenase Using AMBER Force Field. J. Chem. Theory Comput. 2014, 10, 2246–2254. [Google Scholar] [CrossRef]

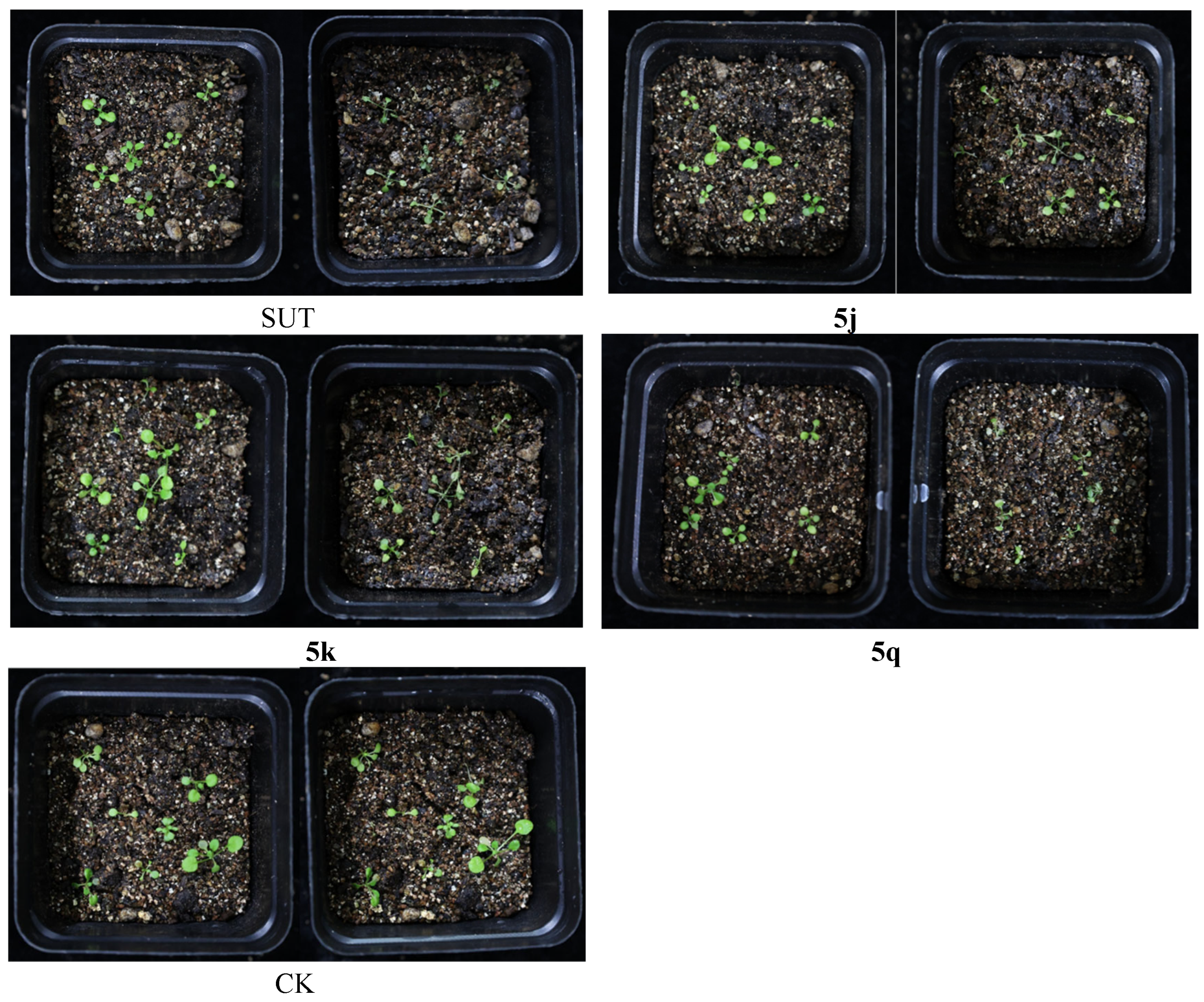


| Compound | Injury (%) | |||
|---|---|---|---|---|
| Wheat | Rice | Corn | Cotton | |
| SUT | 50 | 40 | 30 | 10 |
| 5q | 80 | 40 | 70 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Miao, J.; Tian, Y.; Luo, W.; Shang, J.; Liu, R.; Lu, H. Design, Synthesis and Herbicidal Activity of 1,2,4-Oxadiazole Compounds as Novel Light-Dependent Protochlorophyllide Oxidoreductase Inhibitors. Molecules 2025, 30, 3970. https://doi.org/10.3390/molecules30193970
Hu X, Miao J, Tian Y, Luo W, Shang J, Liu R, Lu H. Design, Synthesis and Herbicidal Activity of 1,2,4-Oxadiazole Compounds as Novel Light-Dependent Protochlorophyllide Oxidoreductase Inhibitors. Molecules. 2025; 30(19):3970. https://doi.org/10.3390/molecules30193970
Chicago/Turabian StyleHu, Xiao, Jing Miao, Yiyi Tian, Wennan Luo, Jixian Shang, Ruiyuan Liu, and Huizhe Lu. 2025. "Design, Synthesis and Herbicidal Activity of 1,2,4-Oxadiazole Compounds as Novel Light-Dependent Protochlorophyllide Oxidoreductase Inhibitors" Molecules 30, no. 19: 3970. https://doi.org/10.3390/molecules30193970
APA StyleHu, X., Miao, J., Tian, Y., Luo, W., Shang, J., Liu, R., & Lu, H. (2025). Design, Synthesis and Herbicidal Activity of 1,2,4-Oxadiazole Compounds as Novel Light-Dependent Protochlorophyllide Oxidoreductase Inhibitors. Molecules, 30(19), 3970. https://doi.org/10.3390/molecules30193970





