Pulsed Electric Fields Reshape the Malting Barley Metabolome: Insights from UHPLC-HRMS/MS
Abstract
1. Introduction
2. Results
2.1. Extraction Method Optimization and UHPLC-HRMS/MS Analysis
2.2. Data Processing Optimization
2.3. Chemometric Analysis
2.4. PEF-Related Biomarkers and Their Ontologies
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Treated Barley Material
4.3. PEF Treatment of Malting Barley
4.4. Malting Experiment Conditions
4.5. Preparation and Extraction of Malting Samples for Metabolomic Fingerprinting
4.6. The UHPLC-HRMS/MS Metabolomic Fingerprinting
4.7. Data Processing
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghoshal, G. Comprehensive Review on Pulsed Electric Field in Food Preservation: Gaps in Current Studies for Potential Future Research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Kamboj, A.; Chopra, R.; Singh, R.; Saxena, V.; GV, P.K. Effect of Pulsed Electric Field Parameters on the Alkaline Extraction of Valuable Compounds from Perilla Seed Meal and Optimization by Central Composite Design Approach. Appl. Food Res. 2022, 2, 100240. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Tabatabaei Yazdi, F.; Mortazavi, S.A.; Mohammadi, M. Using Pulsed Electric Field Pre-Treatment to Optimize Coriander Seeds Essential Oil Extraction and Evaluate Antimicrobial Properties, Antioxidant Activity, and Essential Oil Compositions. LWT 2023, 182, 114852. [Google Scholar] [CrossRef]
- Fan, R.; Wang, L.; Fan, J.; Sun, W.; Dong, H. The Pulsed Electric Field Assisted-Extraction Enhanced the Yield and the Physicochemical Properties of Soluble Dietary Fiber From Orange Peel. Front. Nutr. 2022, 9, 925642. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Pulsed Electric Field Improved Protein Digestion of Beef during In-Vitro Gastrointestinal Simulation. LWT 2019, 102, 45–51. [Google Scholar] [CrossRef]
- Chian, F.M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket. Foods 2021, 10, 512. [Google Scholar] [CrossRef]
- Lytras, F.; Psakis, G.; Gatt, R.; Cebrián, G.; Raso, J.; Valdramidis, V. Exploring the Efficacy of Pulsed Electric Fields (PEF) in Microbial Inactivation during Food Processing: A Deep Dive into the Microbial Cellular and Molecular Mechanisms. Innov. Food Sci. Emerg. Technol. 2024, 95, 103732. [Google Scholar] [CrossRef]
- Karki, R.; Oey, I.; Bremer, P.; Silcock, P. Understanding the Effect of Meat Electrical Conductivity on Pulsed Electric Field (PEF) Process Parameters and the Ability of PEF to Enhance the Quality and Shorten Sous Vide Processing for Beef Short Ribs. Food Res. Int. 2023, 163, 112251. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Pataro, G.; Ferrari, G.; Augusto, P.E.D.; Le-Bail, P.; Le-Bail, A. Pulsed Electric Fields (PEF) Treatment to Enhance Starch 3D Printing Application: Effect on Structure, Properties, and Functionality of Wheat and Cassava Starches. Innov. Food Sci. Emerg. Technol. 2021, 68, 102602. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A. Feasibility of Using Pulsed Electric Fields to Modify Biomacromolecules: A Review. Trends Food Sci. Technol. 2018, 72, 91–113. [Google Scholar] [CrossRef]
- Patras, A.; Choudhary, P.; Rawson, A. Recovery of Primary and Secondary Plant Metabolites by Pulsed Electric Field Treatment In BT—Handbook of Electroporation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 2517–2537. [Google Scholar] [CrossRef]
- Neri, L.; Giancaterino, M.; Rocchi, R.; Tylewicz, U.; Valbonetti, L.; Faieta, M.; Pittia, P. Pulsed Electric Fields (PEF) as Hot Air Drying Pre-Treatment: Effect on Quality and Functional Properties of Saffron (Crocus Sativus L.). Innov. Food Sci. Emerg. Technol. 2021, 67, 102592. [Google Scholar] [CrossRef]
- Stranska, M.; Prusova, N.; Behner, A.; Dzuman, Z.; Lazarek, M.; Tobolkova, A.; Chrpova, J.; Hajslova, J. Influence of Pulsed Electric Field Treatment on the Fate of Fusarium and Alternaria Mycotoxins Present in Malting Barley. Food Control 2023, 145, 109440. [Google Scholar] [CrossRef]
- Li, M.; Niu, M. New Technologies in Cereal Processing and Their Impact on the Physical Properties of Cereal Foods. Foods 2023, 12, 4008. [Google Scholar] [CrossRef] [PubMed]
- Bagarinao, N.C.; King, J.; Leong, S.Y.; Agyei, D.; Sutton, K.; Oey, I. Effect of Germination on Seed Protein Quality and Secondary Metabolites and Potential Modulation by Pulsed Electric Field Treatment. Foods 2024, 13, 1598. [Google Scholar] [CrossRef]
- Colen, L.; Swinnen, J. Economic Growth, Globalisation and Beer Consumption. J. Agric. Econ. 2016, 67, 186–207. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and Beer. Impact of Beer Production Process on Mycotoxin Contamination. A Review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef]
- Karabín, M.; Jelínek, L.; Průšová, N.; Ovesná, J.; Stránská, M. Pulsed Electric Field Treatment Applied to Barley before Malting Reduces Fusarium Pathogens without Compromising the Quality of the Final Malt. LWT 2024, 206, 116575. [Google Scholar] [CrossRef]
- Saxton, R.; Lahey, C.; Smith, B.; Hibberd, E.; Bevan, J.; Baumhoff, C.; Galant, A.; Young, J.; Meyer, B.; McDougal, O.M. Impact of Pulsed Electric Field Treatment on Barley Germination for Malting. LWT 2024, 197, 115882. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-Q.; Jiang, W.; Wu, M.; Rao, S.-Q.; Qian, J.-Y. Pulsed Electric Field as a Means to Elevate Activity and Expression of α-Amylase in Barley (Hordeum Vulgare, L.) Malting. Food Bioprocess Technol. 2019, 12, 1010–1020. [Google Scholar] [CrossRef]
- Polachini, T.C.; Norwood, E.-A.; Le-Bail, P.; Le-Bail, A.; Cárcel, J.A. Pulsed Electric Field (PEF) Application on Wheat Malting Process: Effect on Hydration Kinetics, Germination and Amylase Expression. Innov. Food Sci. Emerg. Technol. 2023, 86, 103375. [Google Scholar] [CrossRef]
- Vegi, A.; Schwarz, P.; Wolf-Hall, C.E. Quantification of Tri5 Gene, Expression, and Deoxynivalenol Production during the Malting of Barley. Int. J. Food Microbiol. 2011, 150, 150–156. [Google Scholar] [CrossRef]
- Behner, A.; Palicova, J.; Tobolkova, A.-H.; Prusova, N.; Stranska, M. Pulsed Electric Field Induces Significant Changes in the Metabolome of Fusarium Species and Decreases Their Viability and Toxigenicity. Toxins 2025, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Pyun, Y.-R.; Mok, C. Pulsed Electric Field (PEF) Technology for Microbial Inactivation in Low-Alcohol Red Wine. Food Sci. Biotechnol. 2018, 27, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Evrendilek, G.A.; Karatas, B.; Uzuner, S.; Tanasov, I. Design and Effectiveness of Pulsed Electric Fields towards Seed Disinfection. J. Sci. Food Agric. 2019, 99, 3475–3480. [Google Scholar] [CrossRef] [PubMed]
- Rakusanova, S.; Cajka, T. Tips and Tricks for LC–MS-Based Metabolomics and Lipidomics Analysis. TrAC Trends Anal. Chem. 2024, 180, 117940. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS Models in Metabolomics: The Impact of Permutation of Dataset Rows on the K-Fold Cross-Validation Quality Parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Huang, Y.; Liang, Q.; Cai, S.; Zhang, G. Time-Course Comparative Metabolome Analysis of Different Barley Varieties during Malting. J. Agric. Food Chem. 2022, 70, 2051–2059. [Google Scholar] [CrossRef]
- Van Norman, J.M.; Benfey, P.N. Arabidopsis Thaliana as a Model Organism in Systems Biology. WIREs Syst. Biol. Med. 2009, 1, 372–379. [Google Scholar] [CrossRef]
- Wu, S.; Tohge, T.; Cuadros-Inostroza, Á.; Tong, H.; Tenenboim, H.; Kooke, R.; Méret, M.; Keurentjes, J.B.; Nikoloski, Z.; Fernie, A.R.; et al. Mapping the Arabidopsis Metabolic Landscape by Untargeted Metabolomics at Different Environmental Conditions. Mol. Plant 2018, 11, 118–134. [Google Scholar] [CrossRef]
- Beale, M.H.; Sussman, M.R. Metabolomics of Arabidopsis Thaliana. Annu. Plant Rev. 2011, 43, 157–180. [Google Scholar] [CrossRef]
- Astorquiza, P.L.; Usorach, J.; Racagni, G.; Villasuso, A.L. Diacylglycerol Pyrophosphate Binds and Inhibits the Glyceraldehyde-3-Phosphate Dehydrogenase in Barley Aleurone. Plant Physiol. Biochem. 2016, 101, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dubots, E.; Botté, C.; Boudière, L.; Yamaryo-Botté, Y.; Jouhet, J.; Maréchal, E.; Block, M.A. Role of Phosphatidic Acid in Plant Galactolipid Synthesis. Biochimie 2012, 94, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yamaguchi, Y.; Hirano, T.; Yokoyama, T.; Uyehara, T.; Namai, T.; Yamanaka, S.; Harada, N. Unsaturated Hydroxy Fatty Acids, The Self Defensive Substances in Rice Plant Against Rice Blast Disease. Chem. Lett. 1984, 13, 409–412. [Google Scholar] [CrossRef]
- Naidu, S.V.; Gupta, P.; Kumar, P. Enantioselective Syntheses of (−)-Pinellic Acid, α- and β-Dimorphecolic Acid. Tetrahedron 2007, 63, 7624–7633. [Google Scholar] [CrossRef]
- Valent, B. The Impact of Blast Disease: Past, Present, and Future. Methods Mol. Biol. 2021, 2356, 1–18. [Google Scholar] [CrossRef]
- Floerl, S.; Majcherczyk, A.; Possienke, M.; Feussner, K.; Tappe, H.; Gatz, C.; Feussner, I.; Kües, U.; Polle, A. Verticillium Longisporum Infection Affects the Leaf Apoplastic Proteome, Metabolome, and Cell Wall Properties in Arabidopsis Thaliana. PLoS ONE 2012, 7, e31435. [Google Scholar] [CrossRef]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of Secondary Metabolites in Root Exudates of Arabidopsis Thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef]
- Cowley, T.; Walters, D. Local and Systemic Effects of Oxylipins on Powdery Mildew Infection in Barley. Pest Manag. Sci. 2005, 61, 572–576. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Quartacci, M.F.; Izzo, R. Free Fatty Acids, Neutral and Polar Lipids in Hordeum Vulgare Exposed to Long-Term Fumigation with SO2. Physiol. Plant. 1991, 81, 467–472. [Google Scholar] [CrossRef]
- Guo, H.-M.; Li, H.-C.; Zhou, S.-R.; Xue, H.-W.; Miao, X.-X. Cis-12-Oxo-Phytodienoic Acid Stimulates Rice Defense Response to a Piercing-Sucking Insect. Mol. Plant 2014, 7, 1683–1692. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, M.-S.; Zhu, L. 12-Oxo-Phytodienoic Acid Enhances Wheat Resistance to Hessian Fly (Diptera: Cecidomyiidae) Under Heat Stress. J. Econ. Entomol. 2018, 111, 917–922. [Google Scholar] [CrossRef]
- Varsani, S.; Grover, S.; Zhou, S.; Koch, K.G.; Huang, P.-C.; Kolomiets, M.V.; Williams, W.P.; Heng-Moss, T.; Sarath, G.; Luthe, D.S.; et al. 12-Oxo-Phytodienoic Acid Acts as a Regulator of Maize Defense against Corn Leaf Aphid. Plant Physiol. 2019, 179, 1402–1415. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Weber, H.; Reymond, P.; Browse, J.; Farmer, E.E. Plant Defense in the Absence of Jasmonic Acid: The Role of Cyclopentenones. Proc. Natl. Acad. Sci. USA 2001, 98, 12837–12842. [Google Scholar] [CrossRef] [PubMed]
- Maucher, H.; Stenzel, I.; Miersch, O.; Stein, N.; Prasad, M.; Zierold, U.; Schweizer, P.; Dorer, C.; Hause, B.; Wasternack, C. The Allene Oxide Cyclase of Barley (Hordeum Vulgare L.)—Cloning and Organ-Specific Expression. Phytochemistry 2004, 65, 801–811. [Google Scholar] [CrossRef]
- Hamany Djande, C.Y.; Steenkamp, P.A.; Piater, L.A.; Tugizimana, F.; Dubery, I.A. Hordatines and Associated Precursors Dominate Metabolite Profiles of Barley (Hordeum Vulgare L.) Seedlings: A Metabolomics Study of Five Cultivars. Metabolites 2022, 12, 310. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Ahmadabadi, M.; Asgari Lajayer, B.; Bagheri, N.; Chamani, M.; Gougerdchi, V.; Hamedpour-Darabi, M.; Shu, W.; Price, G.W.; Dell, B. Role of Neurotransmitters (Biomediators) in Plant Responses to Stress. Plants 2024, 13, 3134. [Google Scholar] [CrossRef]
- Wang, W.; Snooks, H.D.; Sang, S. The Chemistry and Health Benefits of Dietary Phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.-E.; Hong, S.-C.; Kim, S.-Y.; Kwon, H.C.; Hyun, C.-G.; Choi, J. Genome Analysis of Streptomyces Nojiriensis JCM 3382 and Distribution of Gene Clusters for Three Antibiotics and an Azasugar across the Genus Streptomyces. Microorganisms 2021, 9, 1802. [Google Scholar] [CrossRef]
- Ruparelia, J.; Rabari, A.; Mitra, D.; Panneerselvam, P.; Das-mohapatra, P.K.; Jha, C.K. Efficient Applications of Bacterial Secondary Metabolites for Management of Biotic Stress in Plants. Plant Stress 2022, 6, 100125. [Google Scholar] [CrossRef]
- de Araújo Silva, M.M.; dos Santos, D.Y.A.C.; Melo-de-Pinna, G.F.A.; Câmara, T.d.J.R.; Oliveira, A.F.M. Chemical Composition and Ultrastructure of the Foliar Cuticular Wax of Two Brazilian Cultivars of Castor Bean (Ricinus Communis L.). Ind. Crops Prod. 2017, 95, 558–563. [Google Scholar] [CrossRef]
- González, A.; Ayerbe, L. Effect of Terminal Water Stress on Leaf Epicuticular Wax Load, Residual Transpiration and Grain Yield in Barley. Euphytica 2010, 172, 341–349. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, C.-M. Cuticular Wax Biosynthesis as a Way of Inducing Drought Resistance. Plant Signal. Behav. 2011, 6, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, Y.; Guo, N.; Gao, J.; Ni, Y. Variations of the Composition of the Leaf Cuticular Wax among Chinese Populations of Plantago Major. Chem. Biodivers. 2015, 12, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells 2021, 10, 1284. [Google Scholar] [CrossRef]
- Ludwig, R.A.; Spencer, E.Y.; Unwin, C.H. An Antifungal Factor from Barley of Possible Significance in Disease Resistance. Can. J. Bot. 1960, 38, 21–29. [Google Scholar] [CrossRef]
- Pihlava, J.-M. Identification of Hordatines and Other Phenolamides in Barley (Hordeum Vulgare) and Beer by UPLC-QTOF-MS. J. Cereal Sci. 2014, 60, 645–652. [Google Scholar] [CrossRef]
- Stoessl, A.; Unwin, C.H. The Antifungal Factors in Barley. V. Antifungal Activity of the Hordatines. Can. J. Bot. 2011, 48, 465–470. [Google Scholar] [CrossRef]
- Prusova, N.; Karabin, M.; Jelinek, L.; Chrpova, J.; Ovesna, J.; Svoboda, P.; Dolezalova, T.; Behner, A.; Hajslova, J.; Stranska, M. Application of Pulsed Electric Field During Malting: Impact on Fusarium Species Growth and Mycotoxin Production. Toxins 2024, 16, 537. [Google Scholar] [CrossRef]
- Paznocht, L.; Kotíková, Z.; Šulc, M.; Lachman, J.; Orsák, M.; Eliášová, M.; Martinek, P. Free and Esterified Carotenoids in Pigmented Wheat, Tritordeum and Barley Grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef]
- Shi, M.F.; Fan, J.J.; Li, S.J.; Yu, X.L.; Liang, X.M. The Influence of High Voltage Electric Field for Barley Seed Germination and Its Mechanism. Appl. Mech. Mater. 2014, 675–677, 1142–1145. [Google Scholar] [CrossRef]
- Lynikiene, S.; Pozeliene, A. Effect of Electrical Field on Barley Seed Germination Stimulation. Available online: https://cigrjournal.org/index.php/Ejounral/article/view/408/403 (accessed on 8 September 2025).
- Heistek, J.C.; Douma, A.C. Induction of Lipase Activity in Germinating Barley. In Plant Lipid Metabolism; Springer: Dordrecht, The Netherlands, 1995; pp. 301–303. [Google Scholar] [CrossRef]
- Al-Taher, F.; Nemzer, B. Effect of Germination on Fatty Acid Composition in Cereal Grains. Foods 2023, 12, 3306. [Google Scholar] [CrossRef]
- Zhang, N.; Jones, B.L. Development of Proteolytic Activities during Barley Malting and Their Localization in the Green Malt Kernel. J. Cereal Sci. 1995, 22, 147–155. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme Activity during Germination of Different Cereals: A Review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Mannochio-Russo, H.; de Almeida, R.F.; Nunes, W.D.G.; Bueno, P.C.P.; Caraballo-Rodríguez, A.M.; Bauermeister, A.; Dorrestein, P.C.; Bolzani, V.S. Untargeted Metabolomics Sheds Light on the Diversity of Major Classes of Secondary Metabolites in the Malpighiaceae Botanical Family. Front. Plant Sci. 2022, 13, 854842. [Google Scholar] [CrossRef]
- Alseekh, S.; Fernie, A.R. Expanding Our Coverage: Strategies to Detect a Greater Range of Metabolites. Curr. Opin. Plant Biol. 2023, 73, 102335. [Google Scholar] [CrossRef]
- Stranska, M.; Behner, A.; Ovesna, J.; Svoboda, P.; Hajslova, J. What Happens Inside the Germinating Grain After Microbial Decontamination by Pulsed Electric Field? Data-Driven Multi-Omics Helps Find the Answer. Molecules 2025, 30, 924. [Google Scholar] [CrossRef]
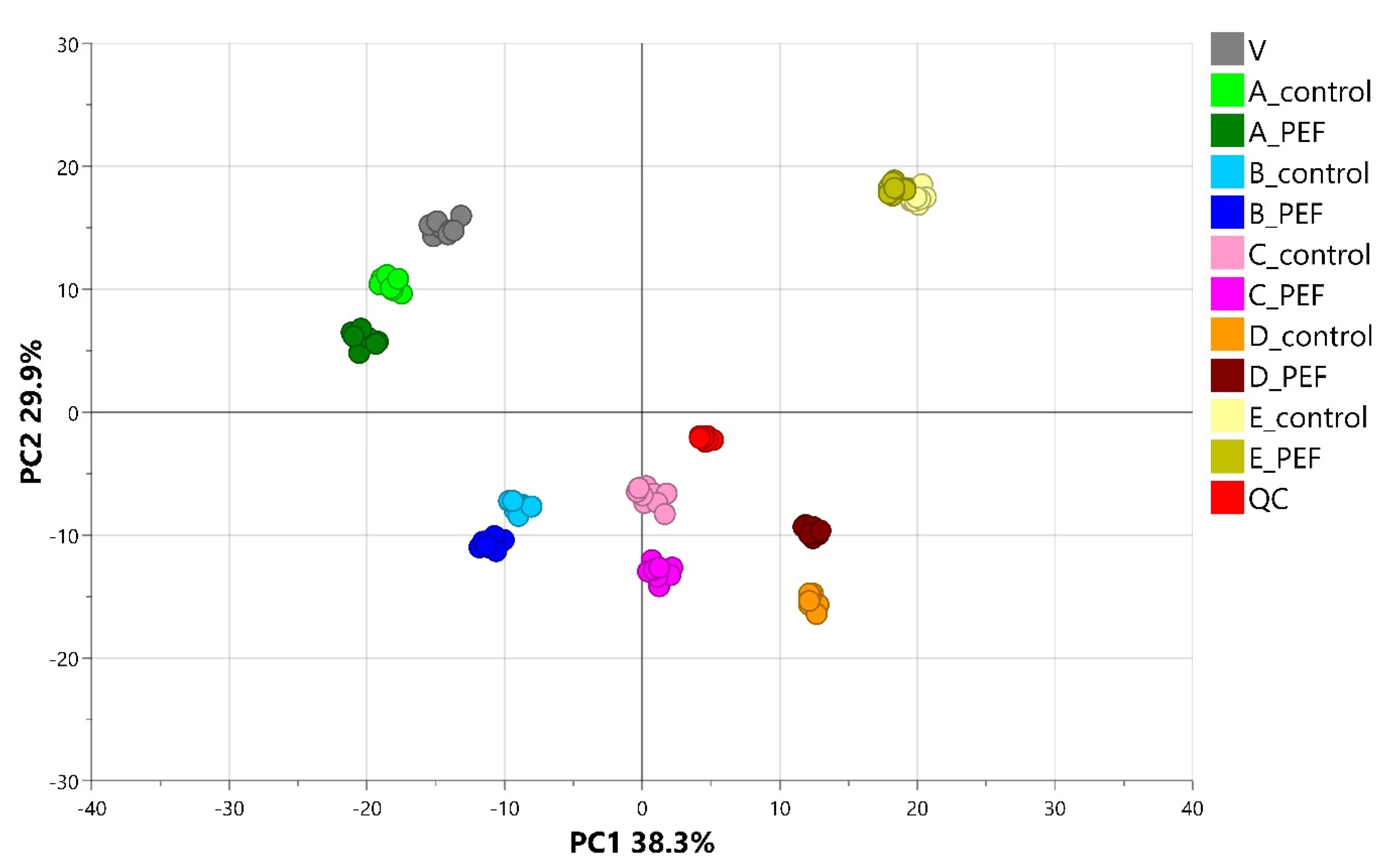
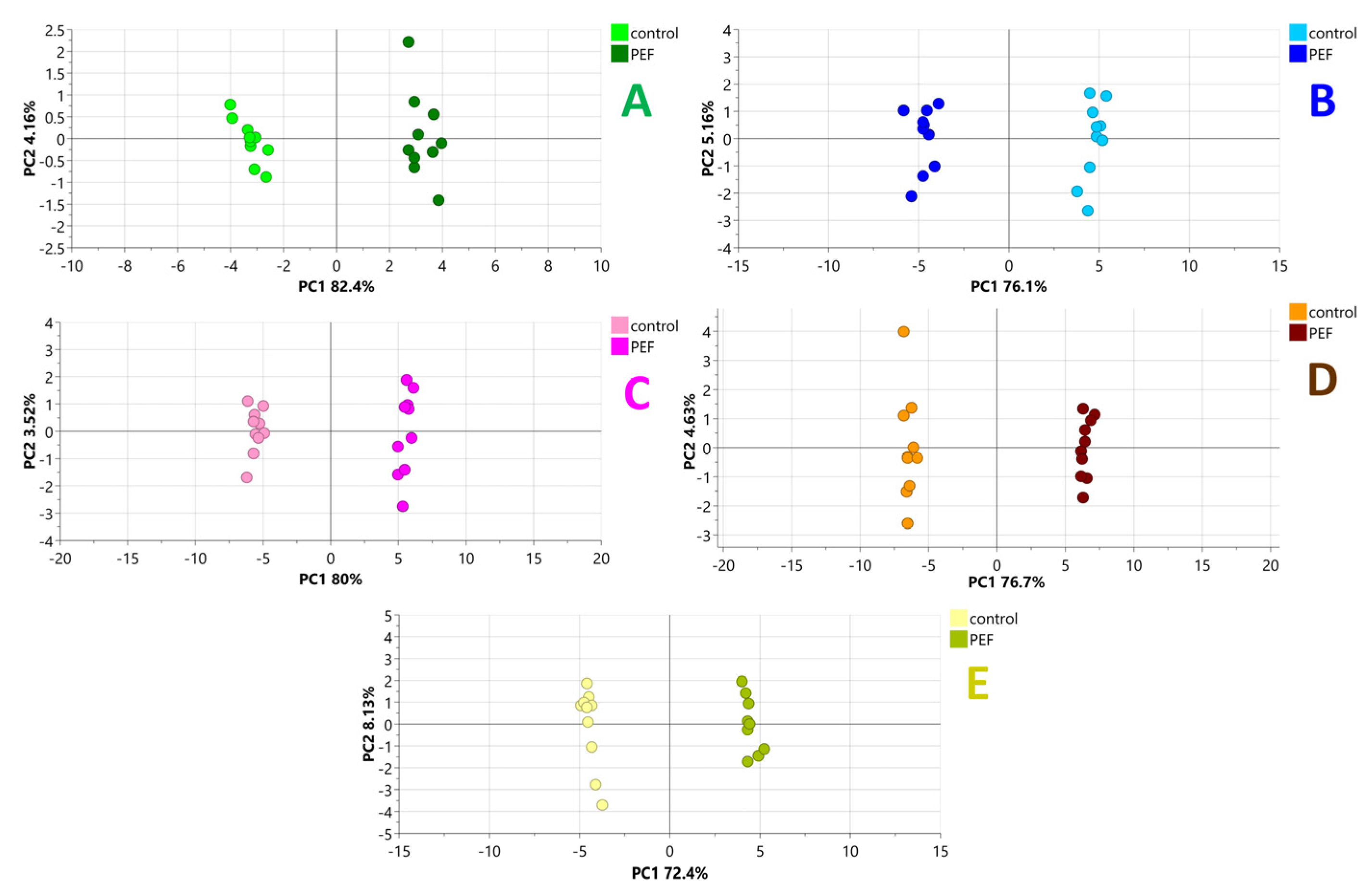

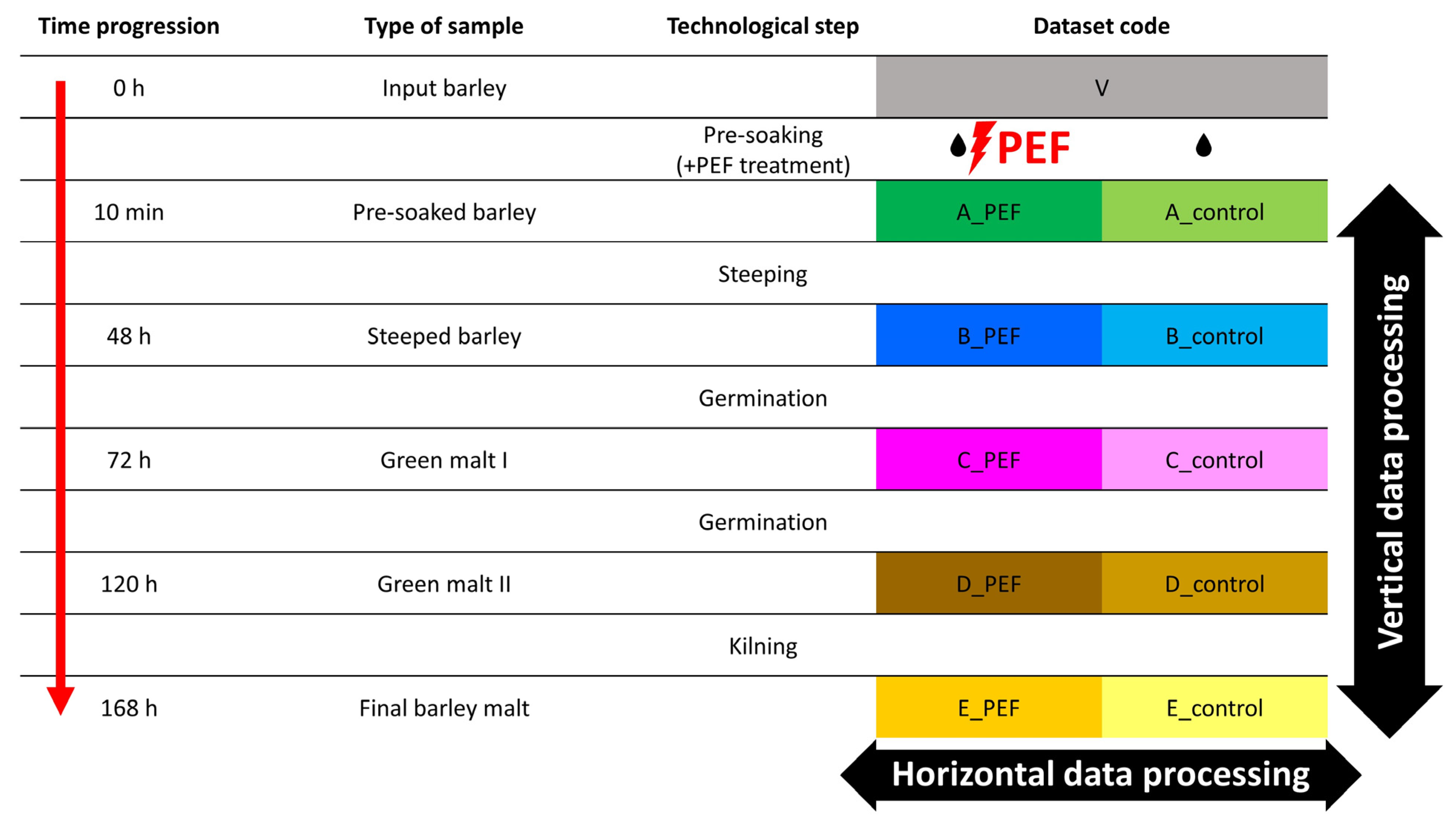
| Dataset | Type of Sample | PEF-Related Trend | Unique Ontology * |
|---|---|---|---|
| A | Pre-soaked barley | ↑ UP-regulated | 1,2-diacylglycerol-3-phosphates |
| B | Steeped barley | ↑ UP-regulated | Lineolic acids and derivatives; Triacylglycerols; Octadecanoids; Diterpenoids |
| B | Steeped barley | ↓ DOWN-regulated | 1-(1Z-alkenyl),2-acylglycerophosphoinositols; Furanoid fatty acids; Hybrid peptides; N-acylserotonins |
| C | Green malt I | ↑ UP-regulated | N-acyl-alpha amino acids and derivatives; Beta carbolines, N-acylserotonins, Triacylglycerols |
| D | Green malt II | ↓ DOWN-regulated | Sesquiterpenoids; Linoleic acids and derivatives; 1,2-diacylglycerols; Fatty acid esters; Fatty alcohols; Prostaglandins and related compounds |
| D | Green malt II | ↑ UP-regulated | Triacylglycerols; Very long-chain fatty acids |
| E | Final barley malt | ↓ DOWN-regulated | Acyclic terpenoids |
| E | Final barley malt | ↑ UP-regulated | Very long-chain fatty acids |
| Dataset | Trend | Unique Ontology * |
|---|---|---|
| PEF | ↑ 1-2-3-4 | Triterpenoids, Linoleic acids and derivatives, 2-arylbenzofuran flavonoids, Diterpene glycosides, Fatty acid esters, Glycosphingolipids, N-acyl amines, Xanthophylls, Long-chain ceramides/fatty acids/fatty alcohols |
| PEF | ↓ 4-3-2-1 | 1,2-diacylglycerol-3-phosphates, Triacylglycerols, Phosphocholines |
| control | ↑ 1-2-3-4 | Triterpenoids |
| control | ↓ 4-3-2-1 | 1,2-diacylglycerol-3-phosphates, 1-acyl-sn-glycero-3-phosphocholines, Fatty alcohols, Long-chain fatty acids |
| injection volume | 2 µL |
| autosampler temperature | 10 °C |
| column temperature | 60 °C |
| mobile phase composition | (A) 5 mM ammonium in milli-Q water/methanol (95:5, v/v) with 0.1% formic acid (v/v) (B) 5 mM ammonium in isopropanol/methanol/milli-Q water (65:30:5, v/v/v) with 0.1% formic acid (v/v) |
| elution gradient | 0.0 min (10% B; 0.4 mL/min) 1.0 min (50% B; 0.4 mL/min) 5.0 min (80% B; 0.4 mL/min) 11.0 min (100% B; 0.4 mL/min) 19.0 min (100% B; 0.4 mL/min) 19.01 min (10% B; 0.4 mL/min) 21.0 min (10% B; 0.4 mL/min) |
| curtain gas pressure | 35 psi |
| nebulizing gas pressure | 55 psi |
| drying gas pressure | 55 psi |
| temperature | 500 °C |
| capillary voltage | −4.5 kV (ESI-)/+4.5 kV (ESI+) |
| declustering potential | 80 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behner, A.; Prusova, N.; Karabin, M.; Jelinek, L.; Hajslova, J.; Stranska, M. Pulsed Electric Fields Reshape the Malting Barley Metabolome: Insights from UHPLC-HRMS/MS. Molecules 2025, 30, 3953. https://doi.org/10.3390/molecules30193953
Behner A, Prusova N, Karabin M, Jelinek L, Hajslova J, Stranska M. Pulsed Electric Fields Reshape the Malting Barley Metabolome: Insights from UHPLC-HRMS/MS. Molecules. 2025; 30(19):3953. https://doi.org/10.3390/molecules30193953
Chicago/Turabian StyleBehner, Adam, Nela Prusova, Marcel Karabin, Lukas Jelinek, Jana Hajslova, and Milena Stranska. 2025. "Pulsed Electric Fields Reshape the Malting Barley Metabolome: Insights from UHPLC-HRMS/MS" Molecules 30, no. 19: 3953. https://doi.org/10.3390/molecules30193953
APA StyleBehner, A., Prusova, N., Karabin, M., Jelinek, L., Hajslova, J., & Stranska, M. (2025). Pulsed Electric Fields Reshape the Malting Barley Metabolome: Insights from UHPLC-HRMS/MS. Molecules, 30(19), 3953. https://doi.org/10.3390/molecules30193953






