A Novel Method of Coupling In Situ Time-Resolved FTIR and Microwave Irradiation: Application to the Monitoring of Quinoxaline Derivatives Synthesis
Abstract
1. Introduction
2. Results and Discussion
- MeOH–Water and Water: The inclusion of water led to prolonged reaction times compared to MeOH alone, because pure water or water-rich mixtures strongly hydrogen-bond to the o-phenylenediamine. Protic solvents stabilise the amine’s lone pairs (lowering its energy) and thus reduce its nucleophilicity. Furthermore, the presence of a broad O-H peak in the solvent at 1630 cm−1 interfered with the accurate assessment of the reaction, particularly in the obstruction of the 1684 cm−1 C=O carbonyl peak.
- DMSO: Observations made under MW and non-MW conditions unveiled the formation of undesirable side products only after the influence of MW. Additionally, the reaction failed to reach completion in all conditions, as indicated by the persistence of the B substrate, corroborated by TLC and IR analyses. By-products may occur because strong absorbers, such as DMSO, have high dielectric losses (above 14.00), causing them to heat rapidly in a microwave chamber. DMSO, in particular, decomposes into toxic substances at high temperatures, which are quickly reached under microwave irradiation. This decomposition can produce sulfur dioxide, formaldehyde, methyl mercaptan, dimethyl sulfide, dimethyl disulfide, and bis(methylthio)methane.
- DCM: Rapid crystallisation was observed in the reaction mixture. Immediate solid formation hindered proper mixing of the reagents, and the reaction did not easily proceed even upon prolonged heating. The precipitated crystals remained insoluble under the applied conditions, thereby preventing further monitoring of the progression of the transformation.
- The use of Montmorillonite K10 as a catalyst in molar concentrations ranging from 10 to 40 mol% proved inefficient. Although increasing the catalyst loading led to a reduction in reaction time, the improvement was limited—even at 200 W microwave power, 40 mol% of Montmorillonite K10 resulted in a reaction completion time of 80 min, compared to 130 min at 10 mol% in MeCN.
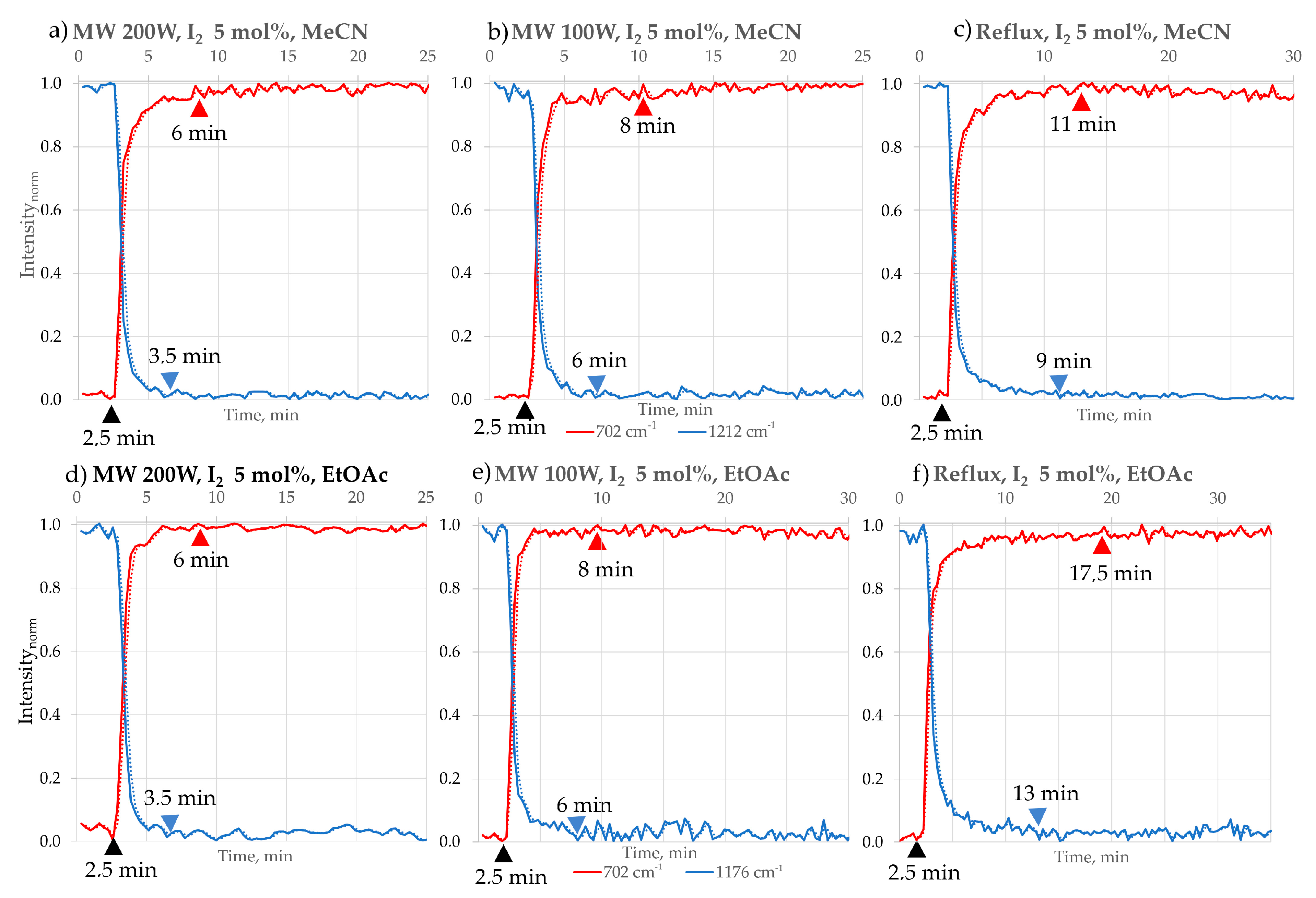
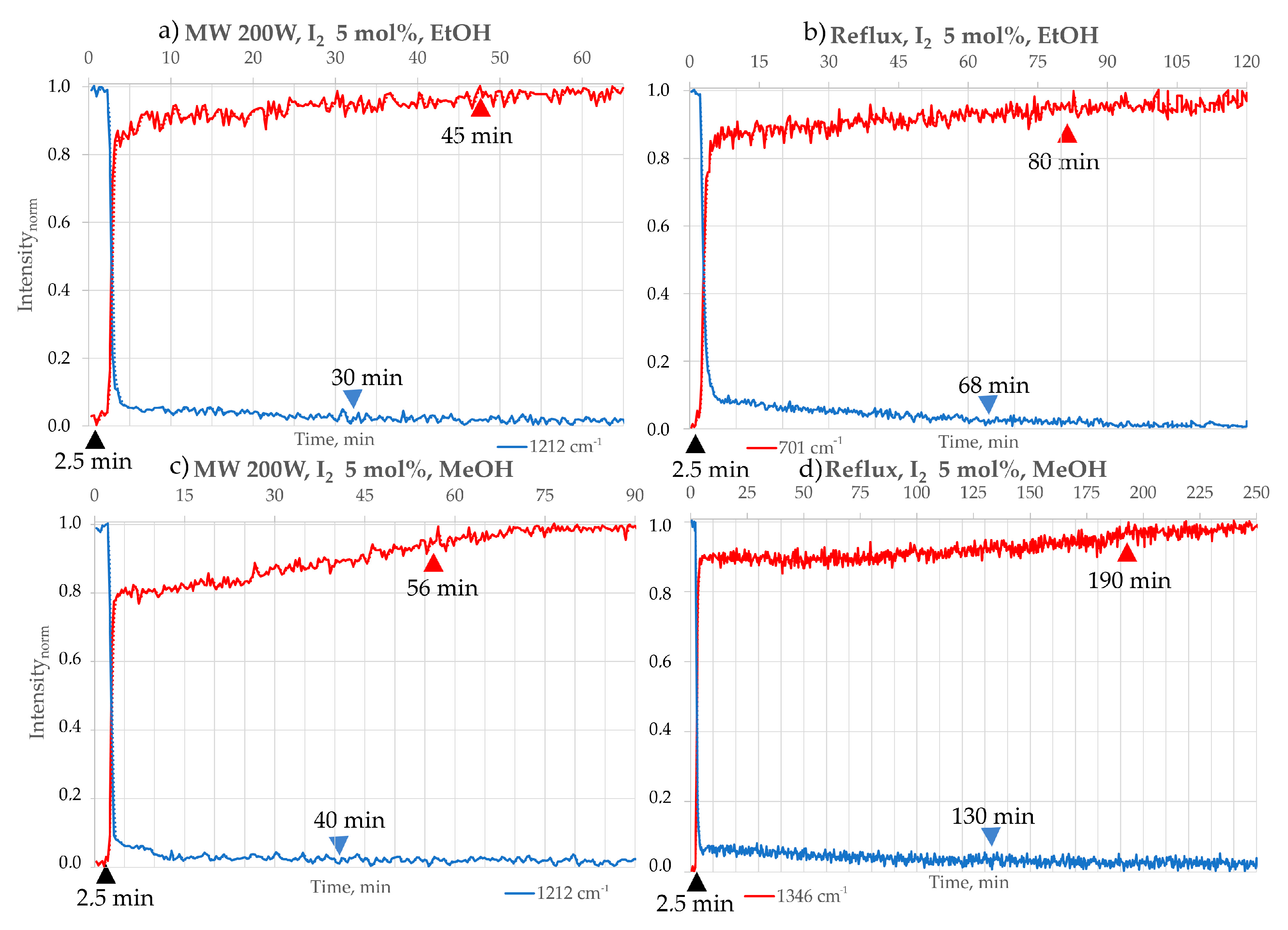
- The use of iodine (I2) at 10 mol% was found to be suboptimal compared to 5 mol% (Table 5), as the reaction reached completion more rapidly under the lower catalyst loading. Molecular iodine (I2) is well recognised as an efficient and sustainable catalyst in various organic transformations, including cycloadditions, Michael additions, esterifications, and heterocyclic syntheses. Its catalytic activity is primarily attributed to halogen bonding and its ability to act as a mild Lewis acid, thereby lowering activation barriers. In numerous reported protocols, small catalytic loadings (typically 5–10 mol%) can accelerate reactions and deliver excellent yields. However, the effect of iodine loading is known to be substrate- and reaction-dependent. For example, Zhang et al. reported that increasing I2 from 1 mol% to 5 mol% improved the yield of an annulation reaction to 86%, but further increase beyond 5 mol% resulted in no additional benefit [34]. In our system, we carefully repeated the reaction (up to five times under identical conditions to exclude experimental error) in MeOH, EtOH, and MeCN. We consistently observed that 5 mol% I2 afforded better reaction time than 10 mol%. We hypothesise that excess iodine may participate in undesired side processes. In particular, o-PDA, one of the key substrates, is prone to oxidation under oxidative conditions, and higher iodine loading may facilitate such competing pathways, lowering the overall yield. This observation is consistent with the data summarised in the table below:
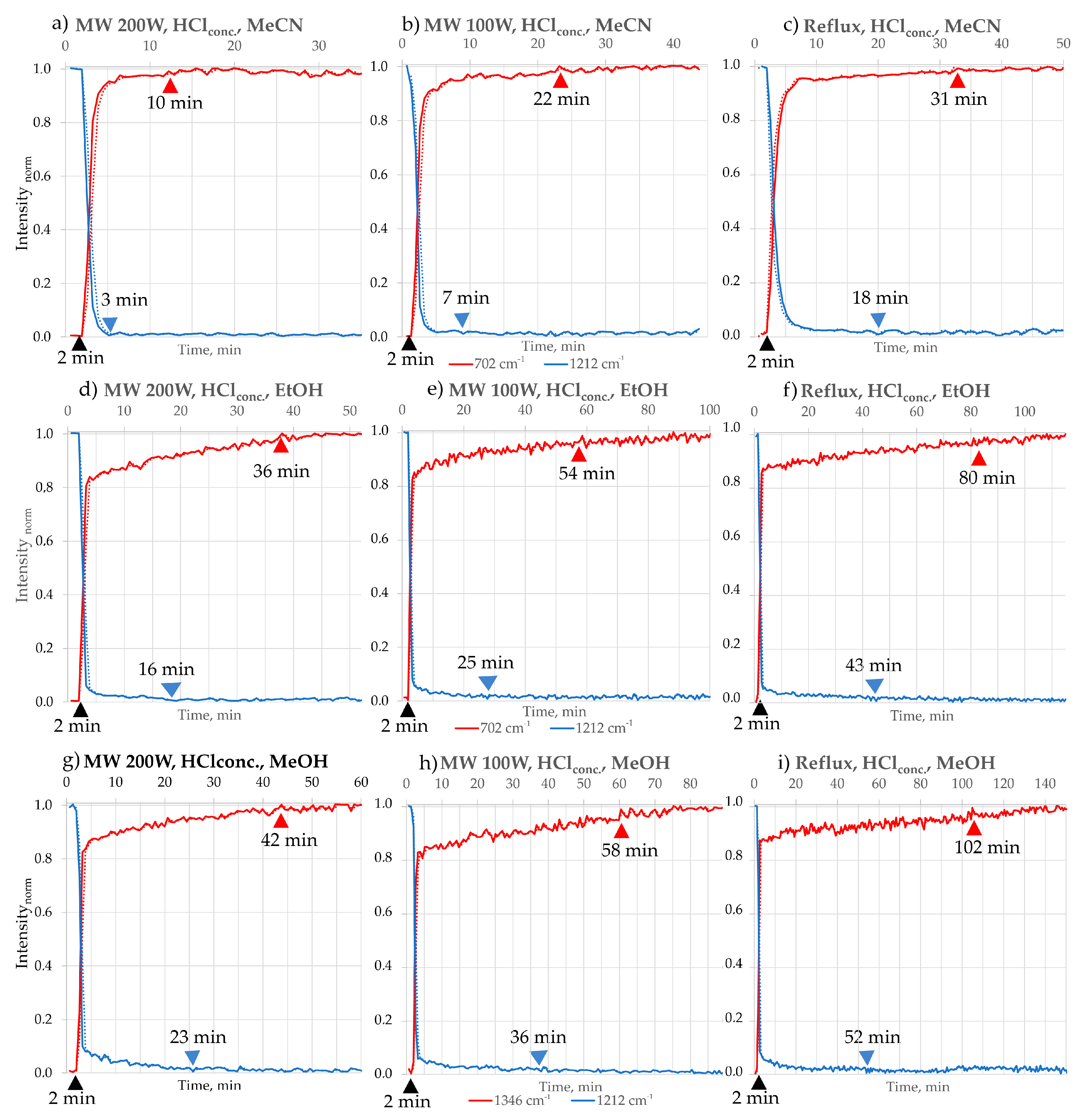
- Also, the negative influence of water on reaction kinetics was observed by employing a 10% HCl solution as a catalyst, applied in the same proportion as concentrated HCl (Figure 8). A marked increase in reaction time was noted under various conditions when using the diluted HCl solution, highlighting the critical role of water in modulating reaction efficiency. In MeOH under 200 W, the reaction time increased from 22 min with concentrated HCl to 46 min with 10% HCl. For EtOH, under 200 W, the reaction time increased from 18 to 49 min. The reaction between B and o-PDA, forming 2,3-DPQ, provides an illustrative case study for examining the influence of water on acid-catalysed transformations. This reaction typically proceeds via a condensation mechanism, where removing water as a by-product is crucial for driving the equilibrium toward product formation. When water is present in the reaction medium, either as part of the solvent system or introduced through dilution of the catalyst, the equilibrium is disrupted, favouring the reactants and significantly reducing the reaction rate. Additionally, water molecules can compete with the reactants for active catalytic sites, thereby hindering the catalytic activity of HCl. These findings emphasise the importance of minimising water content in acid-catalysed reactions involving B and o-PDA, particularly when aiming for high efficiency and reduced reaction times.
3. Materials and Methods
3.1. Equipment
3.2. Solvents and Chemicals
3.3. Synthetic Procedures
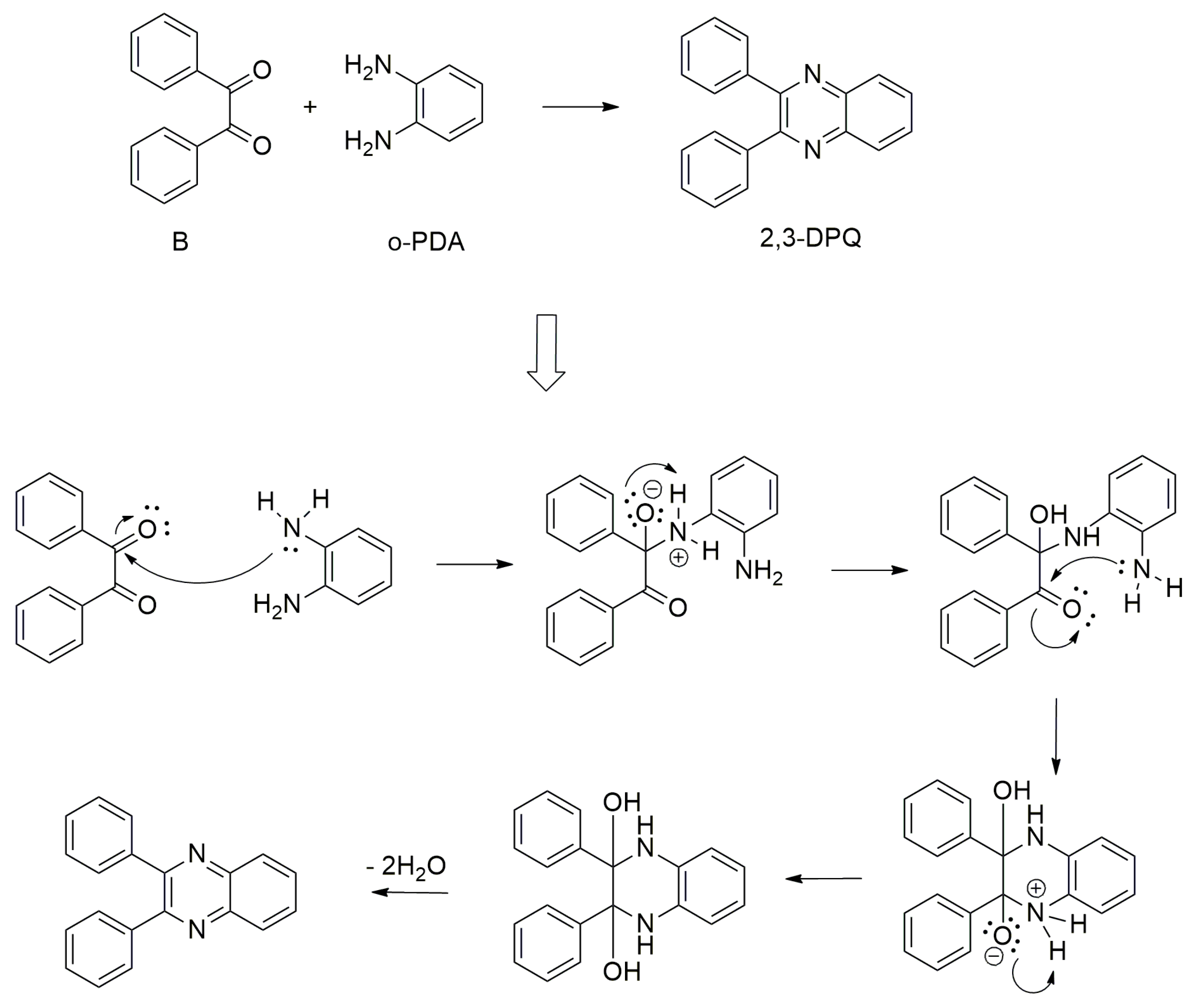
General Procedure of 2,3-DPQ Synthesis
- (1)
- MW method: A total of 10 mL of the selected solvent was placed in a reaction flask along with the catalyst, which consisted of 5 drops of concentrated hydrochloric acid (HCl) or 1 mL of 10% HCl aqueous solution or 5–10 mol% I2 or 10–40 mol% of Montmorillonite K10. The background spectra of the solvent and catalyst were recorded before adding reactants. Subsequently, 3.2 mmol (0.673 g, 1.0 eq) of B was introduced into the reaction flask containing the solvent and catalyst. The mixture was heated to facilitate the dissolution of B by microwave irradiation at either 100 W or 200 W power settings. Spectral data collection was initiated, and after 1.5–2 min of spectra recording, 3.2 mmol (0.346 g, 1.0 eq) of o-PDA was added to the reaction mixture. The reaction was allowed to proceed under continued microwave irradiation until completion (full conversion) under reflux, as monitored by in situ FTIR spectral analysis and TLC. Full conversion was not observed in DMSO, MeOH–Water and Water.
- (2)
- Conventional heating: A total of 10 mL of the selected solvent was placed in a reaction flask along with the catalyst, which consisted of either five drops of concentrated hydrochloric acid (HCl) or 1 mL of 10% HCl aqueous solution or 5–10 mol% I2 or 10–40 mol% of Montmorillonite K10. The background spectra of the solvent and catalyst were recorded before adding reactants. Subsequently, 3.2 mmol (0.673 g, 1.0 eq) of B was introduced into the reaction flask containing the solvent and catalyst. The mixture was heated to facilitate the dissolution of B. Spectral data collection was initiated. After 1.5–2 min of spectra recording, 3.2 mmol (0.346 g, 1.0 eq) of o-PDA was added to the reaction mixture. The reaction was allowed to proceed under continued heating until completion under reflux, as monitored by in situ FTIR spectral analysis (presented in Supplementary Materials) and TLC (after the finishing of MW irradiation). Complete conversion was not observed in DMSO, MeOH–Water and Water.
3.4. Analysis Setup Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacFarlane, D.R.; Zhang, X.; Kar, M. Measure and Control: Molecular Management Is a Key to the Sustainocene! Green Chem. 2016, 18, 5689–5692. [Google Scholar] [CrossRef]
- Cherniienko, A.; Lesyk, R.; Zaprutko, L.; Pawełczyk, A. IR-EcoSpectra: Exploring Sustainable Ex Situ and in Situ FTIR Applications for Green Chemical and Pharmaceutical Analysis. J. Pharm. Anal. 2024, 14, 100951. [Google Scholar] [CrossRef]
- Surapaneni, A.; Surapaneni, A.; Wu, J.; Bajaj, A.; Reyes, K.; Adwankar, R.; Njoo, E. Kinetic Monitoring and Fourier-Transform Infrared (FTIR) Spectroscopy of the Green Oxidation of (-)-Menthol to (-)-Menthone. J. Emerg. Investig. 2020, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Halim, S.M.; Mamdouh, M.A.; Eid, S.M.; Ibrahim, B.M.; Aly Labib, D.A.; Soliman, S.M. The Potential Synergistic Activity of Zolmitriptan Combined in New Self-Nanoemulsifying Drug Delivery Systems: ATR-FTIR Real-Time Fast Dissolution Monitoring and Pharmacodynamic Assessment. Int. J. Nanomed. 2021, 16, 6395–6412. [Google Scholar] [CrossRef]
- Reddy, K.S.; Siva, B.; Reddy, S.D.; Naresh, N.R.; Pratap, T.V.; Rao, B.V.; Hong, Y.-A.; Kumar, B.V.; Raju, A.K.; Reddy, P.M.; et al. In Situ FTIR Spectroscopic Monitoring of the Formation of the Arene Diazonium Salts and Its Applications to the Heck–Matsuda Reaction. Molecules 2020, 25, 2199. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Microwave (MW), Ultrasound (US) and Combined Synergic MW-US Strategies for Rapid Functionalization of Pharmaceutical Use Phenols. Molecules 2018, 23, 2360. [Google Scholar] [CrossRef]
- Keglevich, G. Microwaves as “Co-Catalysts” or as Substitute for Catalysts in Organophosphorus Chemistry. Molecules 2021, 26, 1196. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Green and Energy-Efficient Methods for the Production of Metallic Nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2354–2376. [Google Scholar] [CrossRef]
- Leadbeater, N.E. In Situ Reaction Monitoring of Microwave-Mediated Reactions Using IR Spectroscopy. Chem. Commun. 2010, 46, 6693. [Google Scholar] [CrossRef]
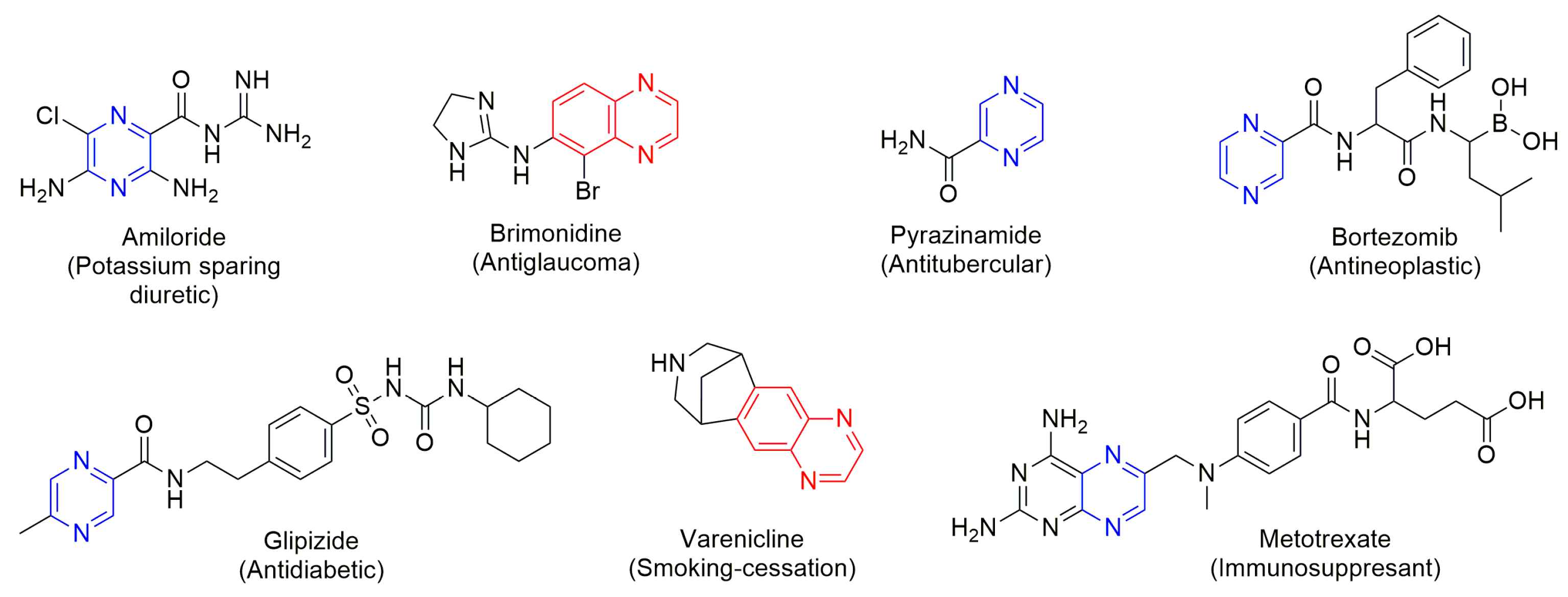
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Guo, H.-Y.; Quan, Z.-S.; Shen, Q.-K.; Li, X.; Luan, T. Natural Products–Pyrazine Hybrids: A Review of Developments in Medicinal Chemistry. Molecules 2023, 28, 7440. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C. The Cascade Annulation Approach to Quinoxalines by Radical NHC Catalysis. Green Synth. Catal. 2025, in press. [Google Scholar] [CrossRef]
- Ma, C.; Meng, H.; Li, J.; Yang, X.; Jiang, Y.; Yu, B. Photocatalytic Transition-Metal-Free Direct 3-Acetalation of Quinoxaline-2(1H)-Ones. Chin. J. Chem. 2022, 40, 2655–2662. [Google Scholar] [CrossRef]
- Montero, V.; Montana, M.; Carré, M.; Vanelle, P. Quinoxaline Derivatives: Recent Discoveries and Development Strategies towards Anticancer Agents. Eur. J. Med. Chem. 2024, 271, 116360. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Alqurashi, R.M.; Masaret, G.S.; Abdulwahab, H.G. Recent Methods for the Synthesis of Quinoxaline Derivatives and theirBiological Activities. Mini-Rev. Med. Chem. 2024, 24, 920–982. [Google Scholar] [CrossRef]
- Raphoko, L.A.; Lekgau, K.; Lebepe, C.M.; Leboho, T.C.; Matsebatlela, T.M.; Nxumalo, W. Synthesis of Novel Quinoxaline-Alkynyl Derivatives and Their Anti-Mycobacterium Tuberculosis Activity. Bioorg. Med. Chem. Lett. 2021, 35, 127784. [Google Scholar] [CrossRef]
- Umezu, Y.; Horiyama, E.; Nakai, Y.; Ninomiya, M.; Nishina, A.; Koketsu, M. Synthesis of Raloxifene-like Quinoxaline Derivatives by Intramolecular Electrophilic Cyclization with Disulfides. Bioorg. Med. Chem. Lett. 2023, 93, 129415. [Google Scholar] [CrossRef]
- Corona, P.; Gessi, S.; Ibba, R.; Merighi, S.; Mirandola, P.; Pinna, G.A.; Nigro, M.; Pozzi, G.; Asproni, B.; Travagli, A.; et al. Novel Oxadiazole-Quinoxalines as Hybrid Scaffolds with Antitumor Activity. Int. J. Mol. Sci. 2025, 26, 1439. [Google Scholar] [CrossRef] [PubMed]
- Miniyar, P.; Murumkar, P.; Patil, P.; Barmade, M.; Bothara, K. Unequivocal Role of Pyrazine Ring in Medicinally Important Compounds: A Review. Mini-Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic Biomolecules as Green Corrosion Inhibitors. J. Mol. Liq. 2021, 341, 117265. [Google Scholar] [CrossRef]
- Abad, N.; Sallam, H.H.; Al-Ostoot, F.H.; Khamees, H.A.; Al-horaibi, S.A.; A, S.M.; Khanum, S.A.; Madegowda, M.; Hafi, M.E.; Mague, J.T.; et al. Synthesis, Crystal Structure, DFT Calculations, Hirshfeld Surface Analysis, Energy Frameworks, Molecular Dynamics and Docking Studies of Novel Isoxazolequinoxaline Derivative (IZQ) as Anti-Cancer Drug. J. Mol. Struct. 2021, 1232, 130004. [Google Scholar] [CrossRef]
- Bassanini, I.; Braga, T.; Tognoli, C.; Vanoni, M.; Mazza, G.; Basilico, N.; Parapini, S.; Riva, S. A ChemoEnzymatic Approach for the Preparation of “Linear-Shaped” Diaryl Pyrazines as Potential Antiprotozoal Agents. Eur. J. Org. Chem. 2024, 27, e202400204. [Google Scholar] [CrossRef]
- Shaikh, S.K.J.; Kamble, R.R.; Bayannavar, P.K.; Kariduraganavar, M.Y. Benzils: A Review on Their Synthesis. Asian J. Org. Chem. 2022, 11, e202100650. [Google Scholar] [CrossRef]
- Kumar, A.; Dhameliya, T.M.; Sharma, K.; Patel, K.A.; Hirani, R.V.; Bhatt, A.J. Sustainable Approaches towards the Synthesis of Quinoxalines: An Update. J. Mol. Struct. 2022, 1259, 132732. [Google Scholar] [CrossRef]
- Zhao, Z.; Wisnoski, D.D.; Wolkenberg, S.E.; Leister, W.H.; Wang, Y.; Lindsley, C.W. General Microwave-Assisted Protocols for the Expedient Synthesis of Quinoxalines and Heterocyclic Pyrazines. Tetrahedron Lett. 2004, 45, 4873–4876. [Google Scholar] [CrossRef]
- Mohsenzadeh, F.; Aghapoor, K.; Darabi, H.R. Benign Approaches for the Microwave-Assisted Synthesis of Quinoxalines. J. Braz. Chem. Soc. 2007, 18, 297–303. [Google Scholar] [CrossRef]
- Wolkenberg, S.E.; Wisnoski, D.D.; Leister, W.H.; Wang, Y.; Zhao, Z.; Lindsley, C.W. Efficient Synthesis of Imidazoles from Aldehydes and 1,2-Diketones Using Microwave Irradiation. Org. Lett. 2004, 6, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Martak, F.; Cahyani, N.W.D.; Nugraheni, Z.V.; Utomo, W.P. Properties and Toxicity of Cobalt(II) Complex with 2,4,5-Triphenyl-1H-Imidazole Ligand. Indones. J. Chem. 2018, 16, 260. [Google Scholar] [CrossRef]
- Sigma-Aldrich. IR Spectrum Table & Chart; Sigma-Aldrich: St. Louis, MO, USA, 2019. [Google Scholar]
- Sayyah, S.M.; Khaliel, A.B.; Aboud, A.A.; Mohamed, S.M. Chemical Polymerization Kinetics of Poly-O-Phenylenediamine and Characterization of the Obtained Polymer in Aqueous Hydrochloric Acid Solution Using K2Cr2O7 as Oxidizing Agent. Int. J. Polym. Sci. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Muthuselvi, C.; Pandiaraja, S.S.; Ravikumar, B.; Athimoolam, S.; Srinivasan, N.; Krishnakum, R.V. FT-IR and FT-Raman Spectroscopic Analyzes of Indeno Quinoxaline Derivative Crystal. Asian J. Appl. Sci. 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Mahadik, P.; Jagwani, D.; Joshi, R. A Greener Chemistry Approach for Synthesis of 2,3-Diphenyl Quinoxaline. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 482–490. [Google Scholar]
- Guhanathan, S.; Padma, R.; Sathiya, M. Investigation on Photoluminescence Behaviour of 2, 3-Diphenylquinoxalin-6-Vinyl Benzaldehyde. SOJ Mater. Sci. Eng. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Wang, Y.; Liu, J.-Q.; Wang, X.-S. An Efficient Green Synthesis of 5H-Spiro[Benzo [4,5]Imidazo [1,2-c]Quinazoline-6,3′-Indolin]-2′-Ones Catalyzed by Iodine in Ionic Liquids. Heterocycl. Commun. 2017, 23, 385–388. [Google Scholar] [CrossRef]
- National Library of Medicine—2,3-Diphenylquinoxaline 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_3-Diphenylquinoxaline (accessed on 22 September 2025).
- Chen, C.-Y.; Hu, W.-P.; Liu, M.-C.; Yan, P.-C.; Wang, J.-J.; Chung, M.-I. Efficient Synthesis of Quinoxalines with Hypervalent Iodine as a Catalyst. Tetrahedron 2013, 69, 9735–9741. [Google Scholar] [CrossRef]
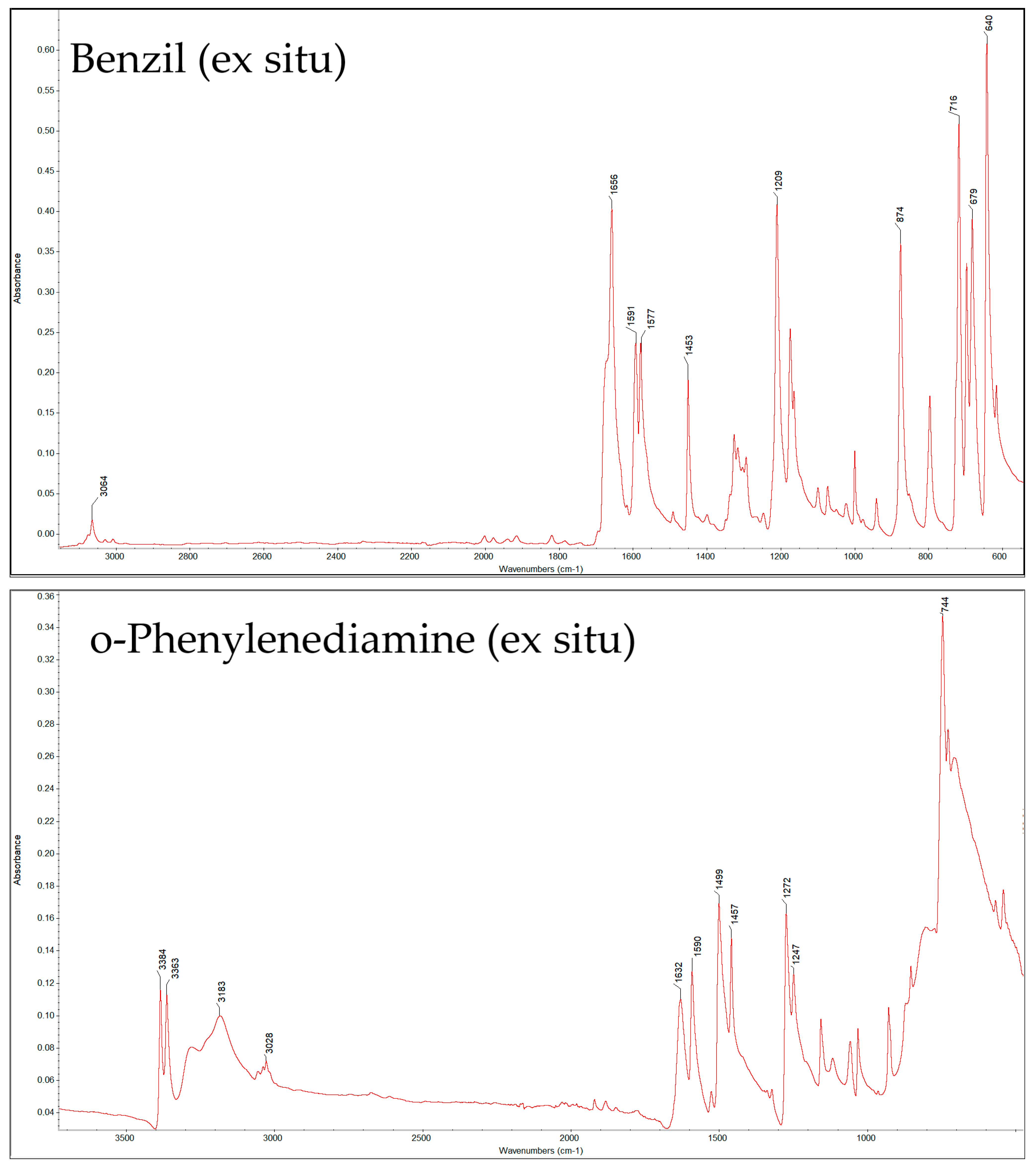
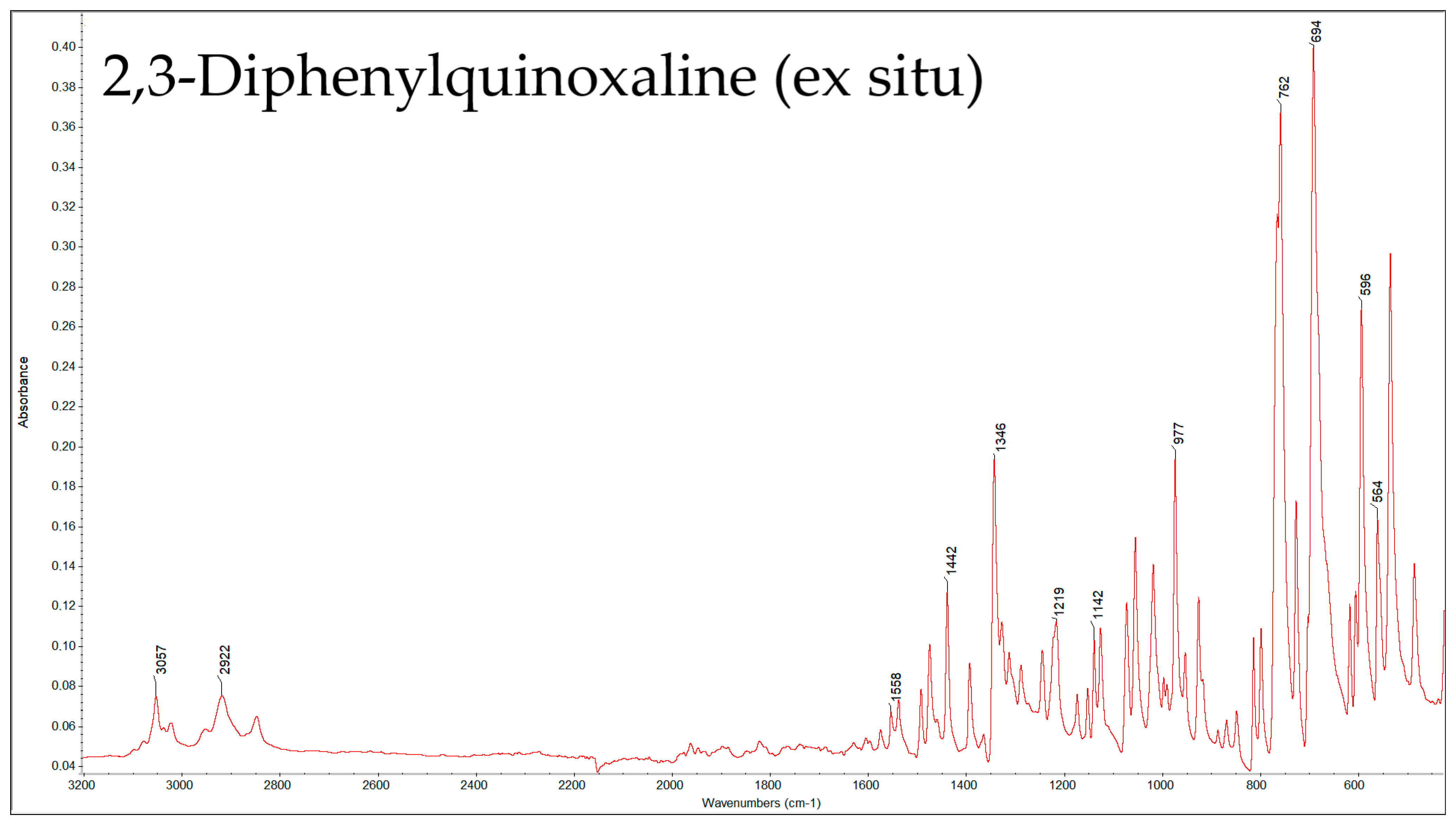
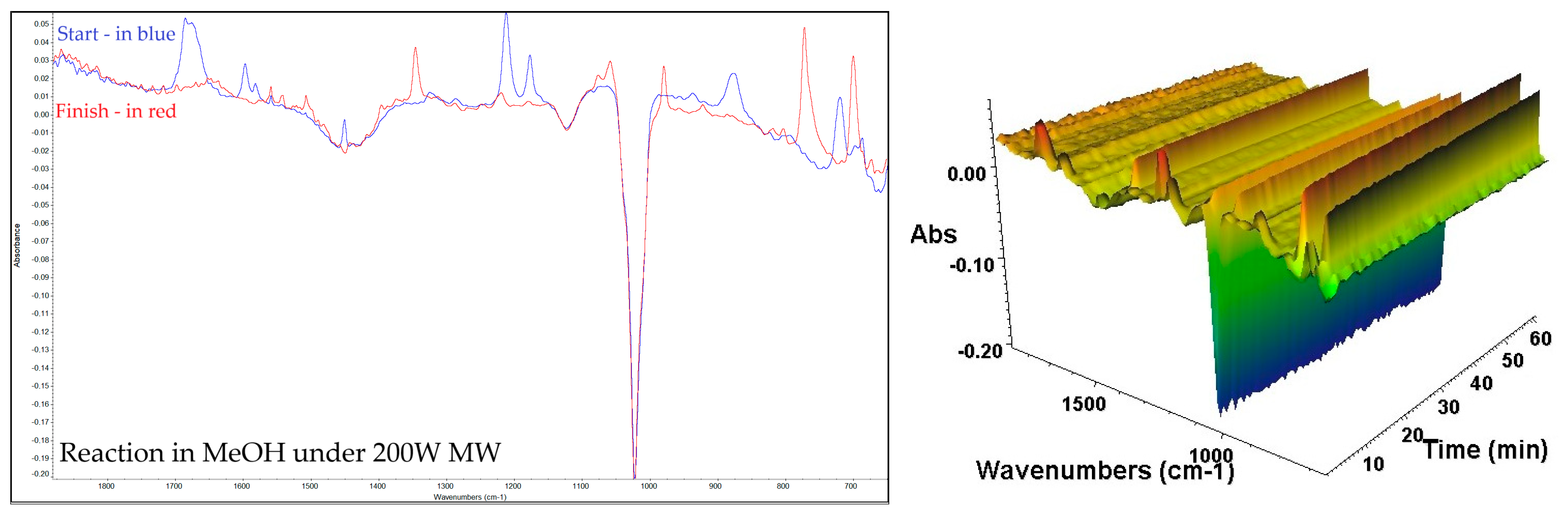


| Compound | Peak (cm−1) by Ex Situ FTIR | Characteristic Group | Literature Source |
|---|---|---|---|
| B | 3050 | =C–H stretch from the phenyl rings | [28,29] |
| 1656 | C=O stretching of the diketone | [28,29] | |
| 1591, 1577 | aromatic C=C in-ring stretches (phenyl moieties) | [28,29] | |
| 1453 | aromatic C–C stretching or C-H in-plane bending | [28,29] | |
| 1209 | C-C bend. of ketones/C–C stretches in the phenyl ring | [28] | |
| 1173 | C-C bend. of ketones/C–C stretches in the phenyl ring | [28] | |
| 874, 716, 679 | Out-of-plane C–H bending modes of monosubstituted benzene rings | [28] | |
| o-PDA | 3384, 3183 | N–H stretching vibrations of the primary amine | [29] |
| 3027 | Aromatic C–H stretching of the benzene ring | [29,30] | |
| 1636 | N–H bending (scissoring) in primary amines | [29,30] | |
| 1590, 1272, 1247 | C–N stretching vibrations of aromatic amines and may overlap with in-plane N–H bending or ring deformation modes | [29,30] | |
| 744 | out-of-plane C–H bending of ortho-substituted benzene | [29,30] | |
| 2,3-DPQ | 3057 | Aromatic =C–H stretch (sp2 C–H) | [29,31,32,33] |
| 1558, 1541 | C=N stretching of quinoxaline conjugated with aromatic C=C stretch | [33] | |
| 1442 | C-C=N/C=C | [32,33] | |
| 1346 | Aromatic C–N stretch or CH in-plane bend | [29,31,33] | |
| 1219 | C-N/in-plane CH bending region | [29,31,32] | |
| 1142 | C-N/in-plane CH bending region | [29,31] | |
| 977 | C-H | [29] | |
| 762 | out-of-plane =C–H bending for monosubstituted phenyl rings | [29,31,32,33] | |
| 694 | C-H (plane vibrations of the arom. ring) | [29,31] | |
| 596, 564 | C-C-N (skeletal ring deformation modes) | [31] |
| Peak (and Peak’s Borders) by In Situ FTIR, cm−1 | Functional Group | From Which Compound |
|---|---|---|
| 1636 (1694; 1583) | O-H | H2O |
| 1442 (1452; 1426) | C-C=N/C=C | DPQ |
| 1345 (1358; 1295) | C-N/C-C(phenyl) | DPQ |
| 1225 (1252; 1200) | C-N/C-H | DPQ |
| 1149 (1163; 1120) | C-N/C-H | DPQ |
| 809 (823; 792) | C-H (plane vibrations of the aromatic ring) | DPQ |
| 772 (792; 739) | C-H (monosubstituted phenyl ring) | DPQ |
| 700 (718; 677) | C-H (aromatic ring) | DPQ |
| Condition/Solvent | MW 200 W | MW 100 W | Reflux | Stirring at Room Temperature | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCl conc. | |||||||||||
| B Consumption Time [min] | 2,3-DPQ PGS * [min] | Yield *** [%] | B Consumption time [min] | 2,3-DPQ PGS * [min] | Yield *** [%] | B Consumption time [min] | 2,3-DPQ PGS * [min] | Yield *** [%] | Approximate Reaction Finish Time [h] | Yield *** [%] | |
| MeCN | 3 | 10 | 98 | 7 | 22 | 97 | 18 | 31 | 93 | 2 | 89 |
| EtOH | 16 | 36 | 96 | 25 | 54 | 95 | 43 | 80 | 91 | 3.2 | 86 |
| MeOH | 23 | 42 | 96 | 36 | 58 | 95 | 52 | 102 | 91 | 5 | 86 |
| I2 5 mol% | |||||||||||
| MeCN | 3.5 | 6 | 99 | 6 | 8 | 98 | 9.5 | 11 | 95 | 40 min | 91 |
| EtOAc | 3.5 | 6 | 98 | 6 | 8 | 97 | 13 | 17.5 | 93 | 45 min | 89 |
| EtOH | 30 | 45 | 95 | - ** | - ** | - ** | 68 | 80 | 91 | 3.5 | 88 |
| MeOH | 40 | 56 | 95 | - ** | - ** | - ** | 130 | 190 | 90 | 7.5 | 87 |
| Reaction Time [min] | Time reduction Factor in Different Reaction Conditions | |||
|---|---|---|---|---|
| b.p./Reflux | MW-100 W | MW-200 W | ||
| HCl | 31 | 22 | 10 | 3:2:1 |
| I2 | 11 | 8 | 6 | 2:1.3:1 |
| Time reduction overall factor in different solvents | 1:3 | 1:3 | 1:2 | |
| I2 Load at Room Temperature Mixing | ||
|---|---|---|
| 5 mol% | 10 mol% | |
| MeCN | 45 min | 4 h |
| EtOH | 3.5 h | Not complete |
| MeOH | 7.5 h | Not complete |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherniienko, A.; Kossakowski, K.; Zaprutko, L.; Lesyk, R.; Olender, D.; Pawełczyk, A. A Novel Method of Coupling In Situ Time-Resolved FTIR and Microwave Irradiation: Application to the Monitoring of Quinoxaline Derivatives Synthesis. Molecules 2025, 30, 3875. https://doi.org/10.3390/molecules30193875
Cherniienko A, Kossakowski K, Zaprutko L, Lesyk R, Olender D, Pawełczyk A. A Novel Method of Coupling In Situ Time-Resolved FTIR and Microwave Irradiation: Application to the Monitoring of Quinoxaline Derivatives Synthesis. Molecules. 2025; 30(19):3875. https://doi.org/10.3390/molecules30193875
Chicago/Turabian StyleCherniienko, Alina, Kacper Kossakowski, Lucjusz Zaprutko, Roman Lesyk, Dorota Olender, and Anna Pawełczyk. 2025. "A Novel Method of Coupling In Situ Time-Resolved FTIR and Microwave Irradiation: Application to the Monitoring of Quinoxaline Derivatives Synthesis" Molecules 30, no. 19: 3875. https://doi.org/10.3390/molecules30193875
APA StyleCherniienko, A., Kossakowski, K., Zaprutko, L., Lesyk, R., Olender, D., & Pawełczyk, A. (2025). A Novel Method of Coupling In Situ Time-Resolved FTIR and Microwave Irradiation: Application to the Monitoring of Quinoxaline Derivatives Synthesis. Molecules, 30(19), 3875. https://doi.org/10.3390/molecules30193875









