Preparation and Rheological Properties of Xanthoceras Sorbifolia Bunge Oil-Based Lubricating Oil Based on Ring-Opening Esterification Modification and Nano-C14MA/MMT Synergistic Strengthening
Abstract
1. Introduction
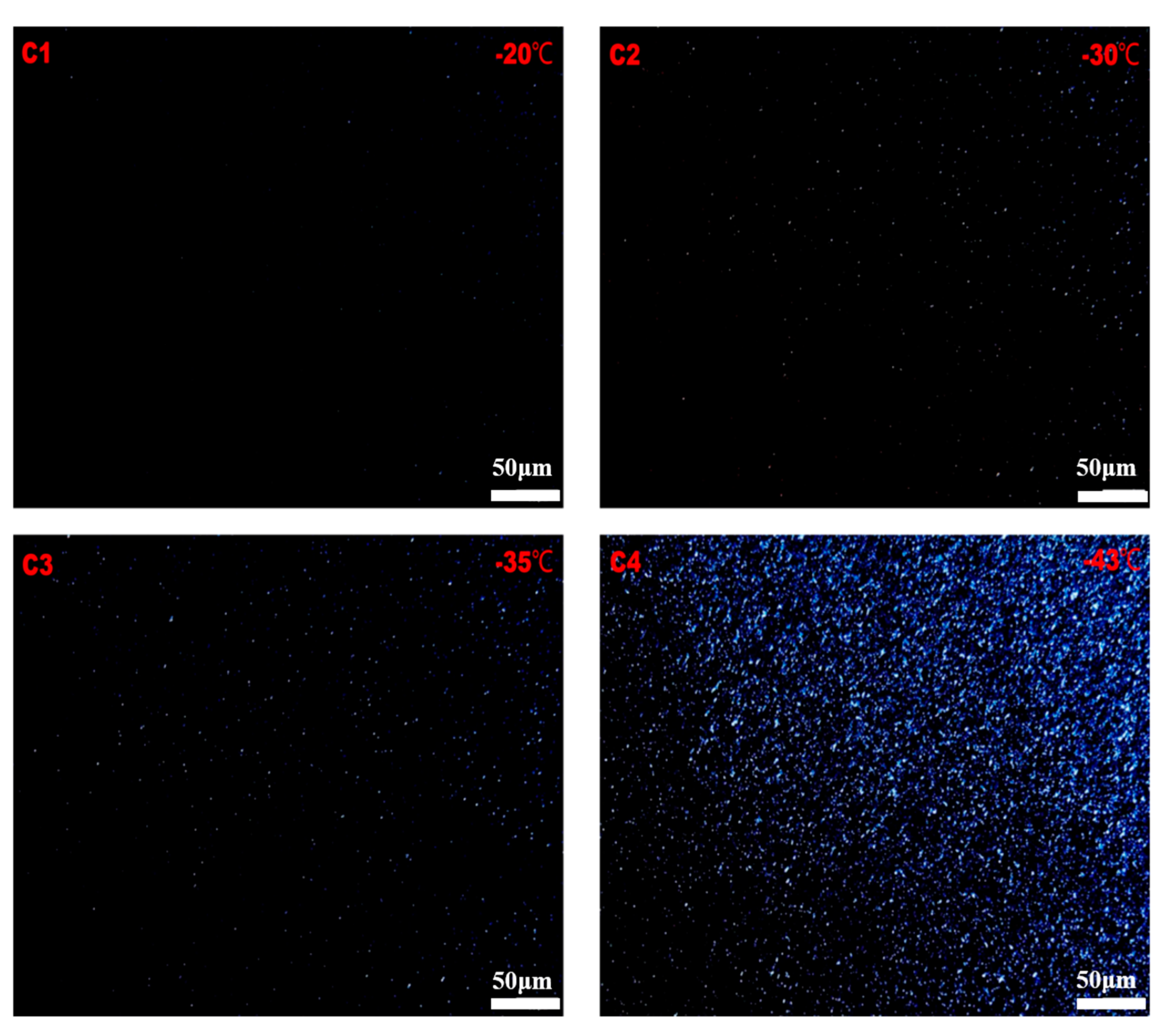
2. Results
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Montmorillonite/Tetradecyl Methacrylate Surfactant (Nano-C14MA/MMT)
3.3. Preparation of Ring-Opening Esterification of Modified Xanthoceras Sorbifolia Oil-Based Lubricant Base Oil
3.4. Preparation of Xanthoceras Sorbifolia Oil-Based Lubricating Oil Based on Synergistic Enhancement of Ring-Opening Esterification Modification and Nano-Depressant
3.5. Material Characterization
3.6. Xanthoceras Sorbifolia Oil-Based Lubricating Oil Performance Test
3.7. Test of Freezing Point, Pour Point, and Flash Point
3.8. Low-Temperature Crystal Morphology and Surface Wettability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinda, S.; Patwardhan, A.V.; Goud, V.V. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 2008, 99, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, M.S.; Kulkarni, V.M.; Patwardhan, A.V. Ultrasound-assisted chemoenzymatic epoxidation of soybean oil by using lipase as biocatalyst. Ultrason. Sonochemistry 2018, 40, 912–920. [Google Scholar] [CrossRef]
- Ju, Y.H.; Sari, N.N.F.; Go, A.W. Preparation of Epoxidized Fatty Acid Ethyl Ester from Tung Oil as a Bio-lubricant Base-Stock. Waste Biomass Valorization 2020, 11, 4145–4155. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, H.; Song, Y.; Sen, M.; Wei, X.; Chen, C.; Chen, B.; Xu, Z. Green preparation of branched biolubricant by chemically modifying waste cooking oil with lipase and ionic liquid. J. Clean. Prod. 2020, 274, 122918. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Yu, S.; Shen, Y.; Wu, Y.; Chen, B.; Nie, K.; Zhang, X. Modification and synthesis of low pour point plant-based lubricants with ionic liquid catalysis. Renew. Energy 2020, 153, 1320–1329. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Wang, T. Hydrogenolysis of Lignin-Derived Aryl Ethers over Heterogeneous Catalysts: A Review. Green Chem. 2021, 23, 5348–5365. [Google Scholar]
- Chen, J.; Li, S.; Xu, M. Graphene oxide functionalized via surface-Initiated raft polymerization for enhanced lubrication and pour point depression. ACS Appl. Mater. Interfaces 2020, 12, 17827–17835. [Google Scholar]
- Salih, N.; Salimon, J.; Yousif, E. The Physicochemical and Tribological Properties of Oleic Acid Based Triester Biolubricants. Ind. Crops Prod. 2011, 34, 1089–1096. [Google Scholar] [CrossRef]
- Ob-Eye, J.; Chaiendoo, K.; Itthibenchapong, V. Catalytic conversion of epoxidized palm fatty acids through oxirane ring opening combined with esterification and the properties of palm oil-based biolubricants. Ind. Eng. Chem. Res. 2021, 60, 15989–15998. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Zhou, S. Bio-based lubricant modification via epoxide ring-opening reaction coupled with graphene oxide nanohybrid pour point depressants. Ind. Crops Prod. 2021, 170, 113782. [Google Scholar]
- Guerin, T.F. Environmental liability and life-cycle management of used lubricating oils. J. Hazard. Mater. 2008, 160, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, T.; Zhao, C. Synthesis of branched bio-lubricant base oil from oleic acid. ChemSusChem 2020, 13, 5516–5522. [Google Scholar] [CrossRef]
- Agamuthu, P.; Abioye, O.P.; Aziz, A.A. Phytoremediation of soil contaminated with used lubricating oil using Jatropha curcas. J. Hazard. Mater. 2010, 179, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.T.; Quina, M.J.; Gando-Ferreira, L.M. Management of waste lubricant oil in Europe: A circular economy approach. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2015–2050. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.T.; Park, J.H. Remediation of soil contaminated with lubricating oil by extraction using subcritical water. J. Ind. Eng. Chem. 2014, 20, 1511–1516. [Google Scholar] [CrossRef]
- Tamires, M.; Renato, M.R.V.; Dionísio, B.; Galdino, A.; Martha, V.T.C.; Marcos, R.O.; Cristiani, B.; Maria, A.P.C. Sophorolipids production by candida bombicola ATCC 22214 and its potential application in soil bioremediation. Waste Biomass Valorization 2017, 8, 743–753. [Google Scholar]
- Panchal, T.M.; Ankit, P.D.D.; Chauhan, M.T.; Jigar, V.P. A methodological review on bio-lubricants from vegetable oil based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70. [Google Scholar] [CrossRef]
- Pawar, R.V.; Hulwan, D.B.; Mandale, M.B. Recent advancements in synthesis, rheological characterization, and tribological performance of vegetable oil-based lubricants enhanced with nanoparticles for sustainable lubrication. J. Clean. Prod. 2022, 378, 134454. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Xiaodong, X.; Bing, G.; Yuyu, L.; Jiale, L.; Lujia, H.; Xian, L. The effect of temperature on the identification of NIR animal fats and oils species and its mechanism. Vib. Spectrosc. 2023, 124, 103498. [Google Scholar] [CrossRef]
- Hilditch, T.P. Marine Animal Oils of Canada. Nature 1941, 147, 548–549. [Google Scholar] [CrossRef]
- Rajendra, U.P.D.; Shiva, K. A critical review on vegetable oil-based bio-lubricants: Preparation, characterization, and challenges. Environ. Dev. Sustain. 2022, 25, 9011–9046. [Google Scholar]
- Fountain, C.W.; Jennings, J.; McKie, C.K. Viscosity of Common Seed and Vegetable Oils. J. Chem. Educ. 1997, 74, 224. [Google Scholar] [CrossRef]
- Ayhan, D.; Abdullah, B.; Waqar, A.; Manzoor, S. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Taufiqurrahmi, N.; Bhatia, S. Catalytic cracking of edible and non-edible oils for the production of biofuels. Energy Environ. Sci. 2011, 4, 1087–1112. [Google Scholar] [CrossRef]
- Teresa, R.; Luis, L.; Olivia, F. Compressibilities and viscosities of reference and vegetable oils for their use as hydraulic fluids and lubricants. Green Chem. 2011, 13, 1293–1302. [Google Scholar] [CrossRef]
- Kržan, B.; Vižintin, J. Tribological properties of an environmentally adopted universal tractor transmission oil based on vegetable oil. Tribol. Int. 2003, 36, 827–833. [Google Scholar] [CrossRef]
- Wickramasinghe, K.C.; Sasahara, H.; Rahim, E.A.; Perera, G.I.P. Recent advances on high performance machining of aerospace materials and composites using vegetable oil-based metal working fluids. J. Clean. Prod. 2021, 310, 127459. [Google Scholar] [CrossRef]
- Pindit, K.; Thanapimmetha, A.; Saisriyoot, M.; Srinopakun, P. Biolubricant basestocks synthesis using 5-step reaction from jatropha oil, soybean oil, and palm fatty acid distillate. Ind. Crops Prod. 2021, 166, 113484. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Quadir, M.A.; Thaher, M.; Annamalai, S.N.; Mahata, C.; Hawari, A.H.; Al, J.H. A comparative physicochemical property assessment and techno-economic analysis of biolubricants produced using chemical modification and additive-based routes. Sci. Total Environ. 2022, 847, 157648. [Google Scholar] [CrossRef] [PubMed]
- MeiRong, C.; RuiSheng, G.; Feng, Z.; WeiMin, L. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Xu, B.-S.; Xu, Y.; Gao, F.; Shi, P.-J.; Wu, Y.-X. Cu nanoparticles effect on the tribological properties of hydrosilicate powders as lubricant additive for steel–steel contacts. Tribol. Int. 2011, 44, 878–886. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Wang, J.; Wu, Y.; An, D.; Xi, D. Experimental Study on the Lubrication and Cooling Effect of Graphene in Base Oil for Si3N4/Si3N4 Sliding Pairs. Micromachines 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, H.; Rastogi, R.B. Epoxidized Jatropha Oil as Base Stock with MoS2 Additives. Tribol. Lett. 2021, 69, 1–12. [Google Scholar]
- Guo, L.; Xie, G.X.; Luo, J.B. Nanodiamond-enhanced Lubrication in Vegetable Oils. Carbon 2019, 152, 548–556. [Google Scholar]
- Zhang, H.; Wei, P.F.; Liu, J.H. Borate Esters as Multifunctional Additives. Tribol. Int. 2023, 178, 108–115. [Google Scholar]
- He, H.; Frost, R.L.; Xi, Y. Modification of Montmorillonites with Thermally Stable Surfactants: Preparation and Characterization. J. Therm. Anal. Calorim. 2006, 84, 217–220. [Google Scholar]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming Montmorillonite Surfaces Using a Cationic Surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, H.; Zhang, Y. In situ free radical polymerization for fabricating graphene-based nanocomposite as efficient pour point depressant. Energy Fuels 2018, 32, 6785–6793. [Google Scholar]
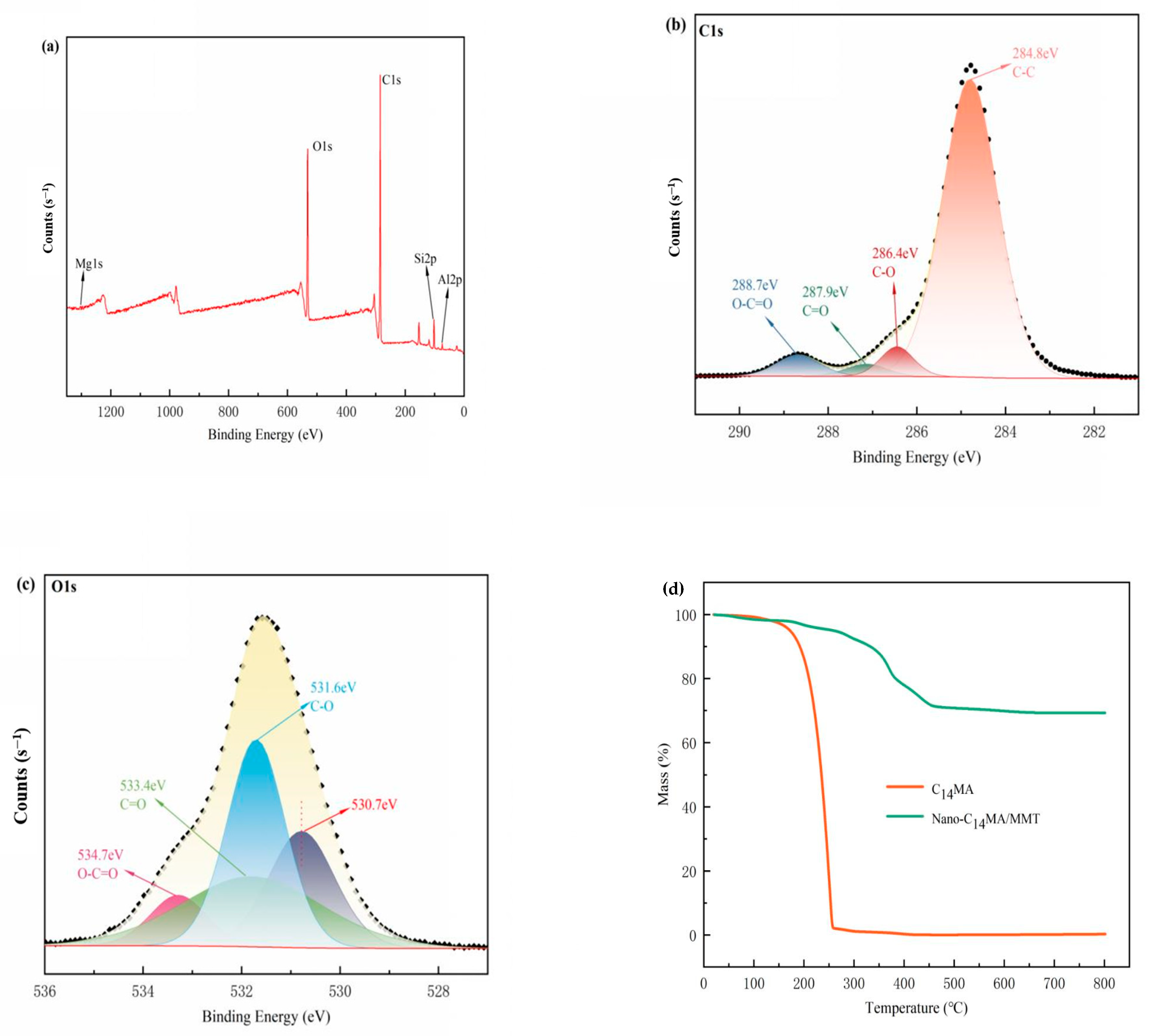
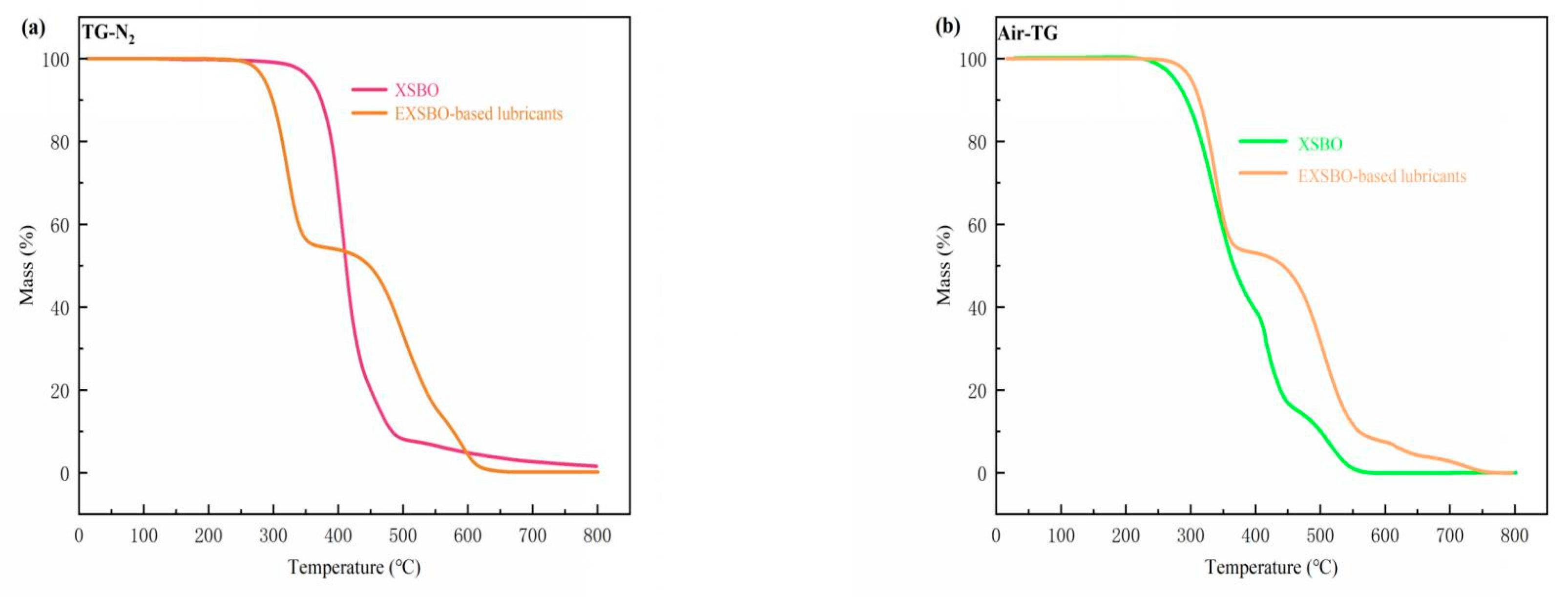
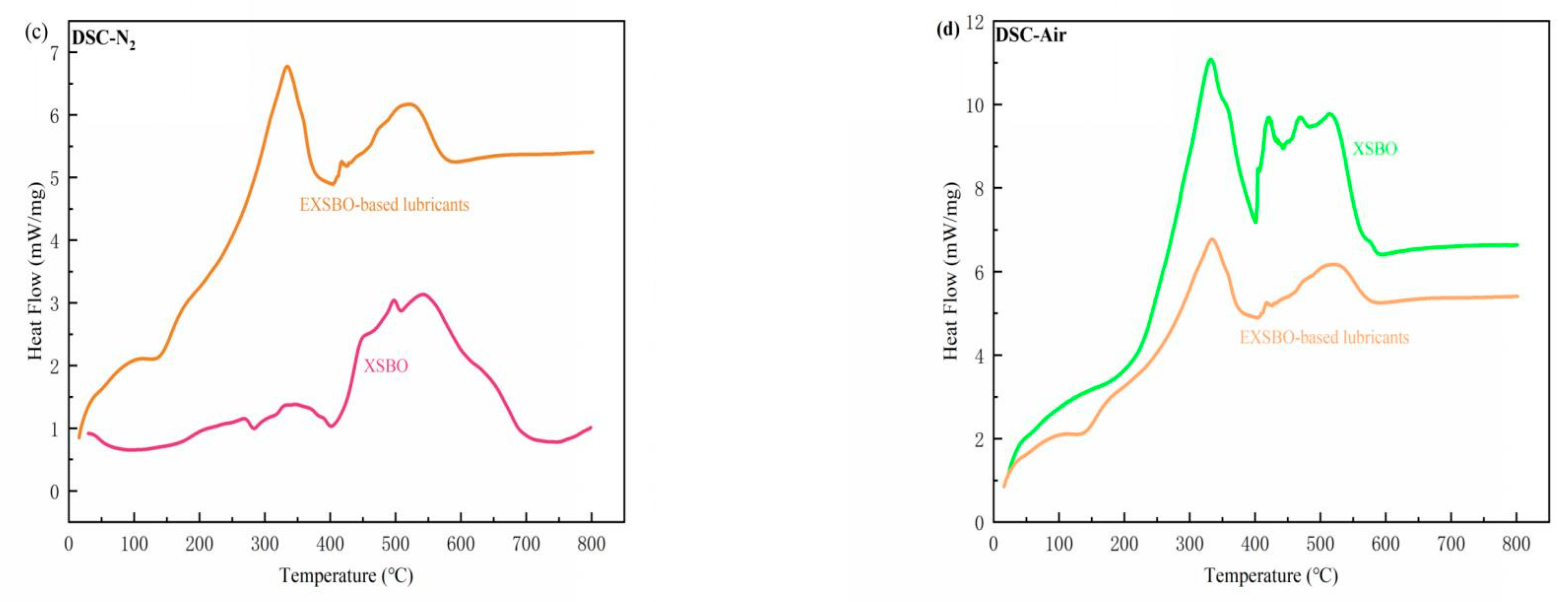
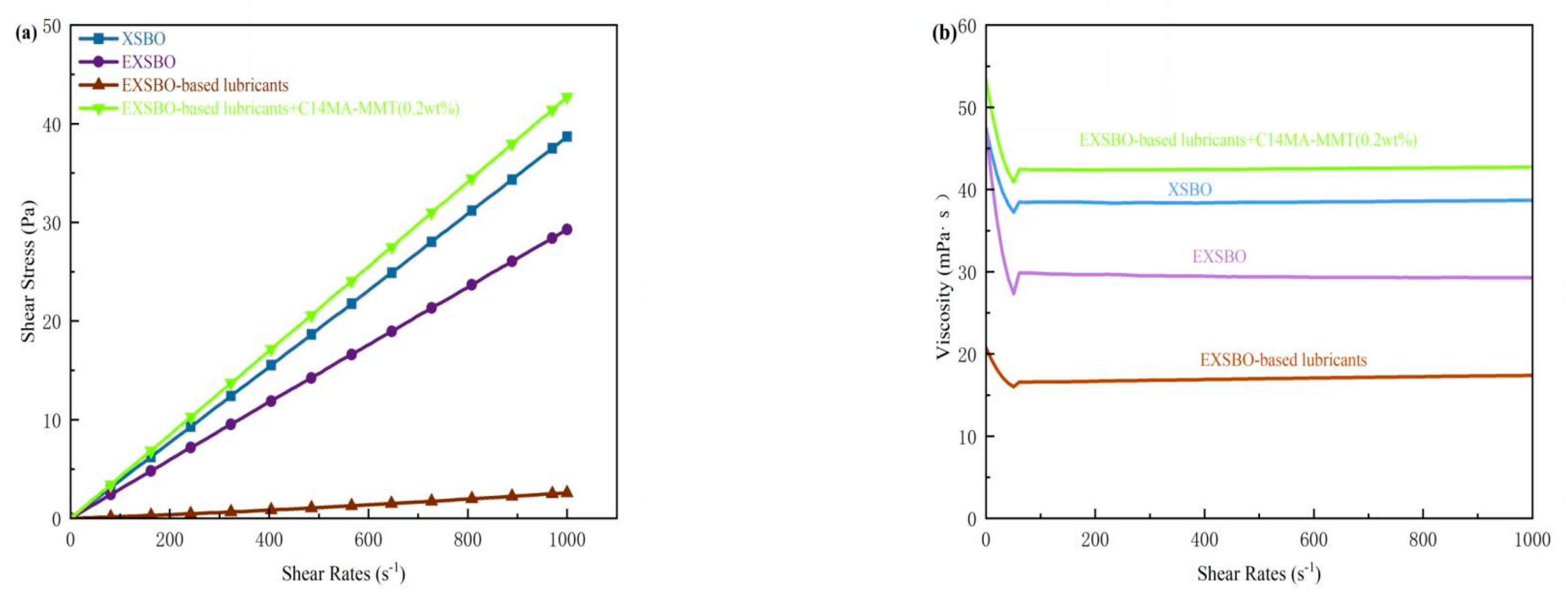


| Sample | Nano- C14MA/MMT (wt%) | KV40 (mm2/s) | KV100 (mm2/s) | VI | Solidifying Point (SP) (°C) | Flash Point (FP) (°C) |
|---|---|---|---|---|---|---|
| EXSBO-based lubricants (wt%Nano- C14MA/MMT) | 0 | 76.2 | 19.2 | 110.3 | −18 | 190 |
| 0.05 | 116.2 | 21.7 | 128.2 | −20 | 192 | |
| 0.1 | 161.7 | 25.5 | 133.7 | −23 | 201 | |
| 0.15 | 235.3 | 31.8 | 136.3 | −27 | 210 | |
| 0.2 | 293.3 | 36.5 | 141.1 | −30 | 237 | |
| 0.25 | 375.4 | 44.2 | 153.8 | −36 | 253 | |
| 0.3 | 424.1 | 50.8 | 180.7 | −43 | 264 | |
| 0.35 | 399.2 | 46.2 | 177.2 | −35 | 251 |
| Specimen | COF |
|---|---|
| XSBO | 0.099 |
| EXSBO-based lubricants (0.3 wt% Nano-C14MA/MMT) | 0.011 |
| Specimen | 1 | 2 | 3 | AWSD (mm) | |||
|---|---|---|---|---|---|---|---|
| X1 | Y1 | X2 | Y2 | X3 | Y3 | ||
| XSBO | 0.7 | 0.7 | 0.58 | 0.72 | 0.74 | 0.71 | 0.69 |
| EXSBO-based lubricants (0.3 wt% Nano-C14MA/MMT) | 0.43 | 0.45 | 0.41 | 0.42 | 0.48 | 0.45 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, K.; Wang, H.; Hou, T.; Lv, Z.; Li, W.; Wang, Z.; Hao, Y. Preparation and Rheological Properties of Xanthoceras Sorbifolia Bunge Oil-Based Lubricating Oil Based on Ring-Opening Esterification Modification and Nano-C14MA/MMT Synergistic Strengthening. Molecules 2025, 30, 3830. https://doi.org/10.3390/molecules30183830
Li Z, Zhang K, Wang H, Hou T, Lv Z, Li W, Wang Z, Hao Y. Preparation and Rheological Properties of Xanthoceras Sorbifolia Bunge Oil-Based Lubricating Oil Based on Ring-Opening Esterification Modification and Nano-C14MA/MMT Synergistic Strengthening. Molecules. 2025; 30(18):3830. https://doi.org/10.3390/molecules30183830
Chicago/Turabian StyleLi, Zexin, Kai Zhang, Haoyue Wang, Tao Hou, Zhuoyi Lv, Wencong Li, Zhenpeng Wang, and Yinan Hao. 2025. "Preparation and Rheological Properties of Xanthoceras Sorbifolia Bunge Oil-Based Lubricating Oil Based on Ring-Opening Esterification Modification and Nano-C14MA/MMT Synergistic Strengthening" Molecules 30, no. 18: 3830. https://doi.org/10.3390/molecules30183830
APA StyleLi, Z., Zhang, K., Wang, H., Hou, T., Lv, Z., Li, W., Wang, Z., & Hao, Y. (2025). Preparation and Rheological Properties of Xanthoceras Sorbifolia Bunge Oil-Based Lubricating Oil Based on Ring-Opening Esterification Modification and Nano-C14MA/MMT Synergistic Strengthening. Molecules, 30(18), 3830. https://doi.org/10.3390/molecules30183830





